Abstract
Intensive chemotherapy or chemotherapy plus irradiation and allogeneic stem cell transplantation can be curative for patients with hematologic diseases. Reduced intensity transplants can also achieve cure, and result in less treatment related mortality but higher relapse rates. Thus, optimizing the conditioning regimens used in allogeneic transplantation remains an important goal. We conducted a Phase I/II trial to determine the maximum tolerated dose (MTD) and dose limiting toxicities (DLT) of a continuous infusion of busulfan over 90 hours in conjunction with fludarabine followed by allogeneic related or unrelated donor transplant. Fifty-four patients with advanced hematologic malignancies were enrolled on this study. The MTD was identified as a 24 hour area under the curve (AUC) of approximately 7095 uMmin which represents a 43% increase over the standard total daily AUC dose of 4800 uMmin given by intermittent schedules. DLTs at doses over 8000 uMmin were identified as a desquamative skin rash and mucositis. No dose-related increase in hepatic, pulmonary or other organ toxicies were seen while efficacy appeared to be improved at higher dose levels. Continuous infusion busulfan with intermittent fludarabine provides an alternative treatment strategy that is generally well tolerated and permits an increase in total busulfan dose with encouraging efficacy.
Introduction
The use of high dose therapy with allogeneic stem cell transplantation has been shown to be curative in a number of hematologic diseases. Cure rates of 30 −70% in different populations of patients can be identified with failure being attributed either to relapse of the underlying disease or treatment related mortality.1,2 In some cases, the mortality and morbidity associated with treatment is a result of the conditioning regimen given prior to the infusion of donor stem cells, whereas in other situations it is a result of the immunologic dysregulation that results in graft rejection, infections or graft versus host disease (GVHD) from infused donor immune cells. With improvement in therapeutic regimens prior to transplant and prevention of GVHD, better antibiotic and transfusion support, and high resolution human leukocyte antigen (HLA) typing, an increasing percentage of treatment failures are the result of relapse of underlying disease.3,4,5 This shift towards higher disease relapse rates has also resulted from improvements in prognostic factors, and identification of residual disease in patients with acute and chronic leukemia. Increasingly, patients who are in better risk categories are not taken to transplant, as they have a significant cure rate with standard therapy.6 Relapse has also become an increasingly important source of treatment failure in the setting of patients undergoing therapy with either reduced intensity or non-ablative conditioning regimens, both of which rely significantly on the underlying graft-vs-tumor effect in order to maintain remission status post-transplant.7–9
While there is data suggesting the importance of co-morbidities, age, CMV status, disease risk and quality of donor/recipient match in outcomes 10–12, there is also data suggesting that conditioning regimen intensity is important in long-term control of the underlying malignancy.13–16, In some diseases such as acute lymphoblastic leukemia, there is general agreement that better disease control is provided by the use of total-body radiation (TBI) but that in the majority of patients with myeloid disease, any increased benefits in disease control with TBI are offset by a higher likelihood of treatment related morbidity and mortality.17–18 Nevertheless, because of the high relapse rate in these advanced malignancies, there continues to be value in identifying more effective conditioning regimens to control the underlying disease.
Pre-clinical data by Teicher and colleagues19 have demonstrated that continuous exposure of malignant cells to alkylating agents in vitro provides a greater cell kill than comparable AUC exposure delivered by intermittent schedules. There is also clinical data that this approach, as demonstrated by the prolonged infusion of anthracycline in the EPOCH and VAD regimens, may be associated with better outcomes and improved tumor control.20–22 Based on this work, we postulated that administering busulfan as a prolonged infusion might permit a higher total AUC with reduced toxicity as a result of lower peak concentrations, while still providing greater disease control. In the current report we describe the results of a Phase I/II study report that assessed the MTD, toxicities, and clinical outcomes following the administration of busulfan via a prolonged infusion schedule in allogeneic transplant patients with advanced hematologic malignancies.
Patients and Methods
Patients with advanced, refractory, or high-risk hematologic cancers who were deemed suitable for myeloablative conditioning and between the ages of 20–55 were eligible for enrollment. All patients provided appropriate informed consent according to UNC Institutional Review Board policies. Patients were stratified by disease risk according to ASBMT/Center for International Blood & Marrow Transplant Research (CIBMTR) criteria (23). Co-morbidity scores were assessed using the Sorror index (10) and severity of VOD was assessed according to the Bearman criteria (24). Patients with other malignancies that did not qualify for CIBMTR stratification required demonstration of high-risk features or advanced disease beyond complete response (CR1) for which no other curative therapy was available. Acute and Chronic GVHD scoring were as outlined by Glucksberg (25) and Shulman (26), respectively.
Busulfan Pharmacokinetic (PK) Analysis
A test dose of .8 mg/kg adjusted ideal body weight (IBW) busulfan was administered over 30’ followed by plasma levels at baseline, 30’, 1, 2,4, and 6 hours after start of the infusion. Based on the AUC and Clss values obtained with the test dose, targeted AUC dosing estimated to achieve the desired AUC dosing level per protocol was then undertaken.27–30 Within one week of the test dose, patients were admitted for the therapeutic dose and subsequent transplant. Busulfan plasma concentrations were collected before the start of the 90 hour infusion and then at 30’, 1, 2,4, 6, 12, 18, 24, 36, 48, 60, 72, 84, 90.5, 92 and 96 hours following the start of the infusion. A 90-hour infusion was chosen to reflect 15/16ths of a full 16 dose schedule while the test dose represented 1/16th of the full 16 dose schedule. All whole blood samples were centrifuged at 1000 x g for 10 minutes at 4° C and aliquots of plasma were collected and stored at −80° C until analysis. Busulfan concentrations were quantified at Emory University Hospital using high-pressure gas chromatography.30 The lower limit of quantitation was 0.1 umol/L and the assay was linear between 0.1 and 20.0 umol/L. AUC calculations were assessed for the test dose and on the first 6 hours of the therapeutic infusion, and dosages adjusted for hours 42–90 following return of the initial AUC values if they were more than 10% above or below the desired range.
Individual busulfan plasma concentrations were used to estimate the following PK parameters using a non-compartmental model on WinNonlin 4.0 (Pharsight Corp., Mountain View, CA): maximum plasma concentration (Cmax), area under the concentration-time curve through the last measurable time point (AUC); terminal half-life (t1/2), and whole blood clearance (Cl). The AUC was calculated using the log-linear trapezoid method. All AUC and clearance data was natural log-transformed and reported using descriptive statistics.
Statistical Considerations
In the Phase I portion of the study, patients were dosed in cohorts according to the five target AUC levels. Additional patients were enrolled at dose level one (standard dose) during the Phase I and II portions of the study if their insurance coverage did not allow enrollment onto a Phase I study. The maximum tolerated dose (MTD) was defined as the dose with the dose-limiting toxicity (DLT) rate of 0.25. A dose assignment strategy that allowed for delayed toxicity outcome was used.31 Initial escalation was in cohorts of three patients until at least one patient developed a DLT. After the initial dose escalation, patients were assigned to the current dose cohort if estimated DLT rate at the current dose was between 0.15 and 0.35. The dose was increased or decreased if the estimated DLT rate at the current dose was below 0.15 or higher than 0.35. The sample size for Phase I was set at 35 patients. An additional 25 patients were enrolled to the estimated MTD. During Phase II portion of the trial the rate of non-relapse mortality at day 100 was monitored using the Pocock boundary to stop the trial if the rate was too high.32 Similarly the rate of irreversible Grade 3 toxicity or Grade 4 toxicity lasting more than 2 weeks was monitored. The acceptable rate for each was set to 0.2.
Treatment
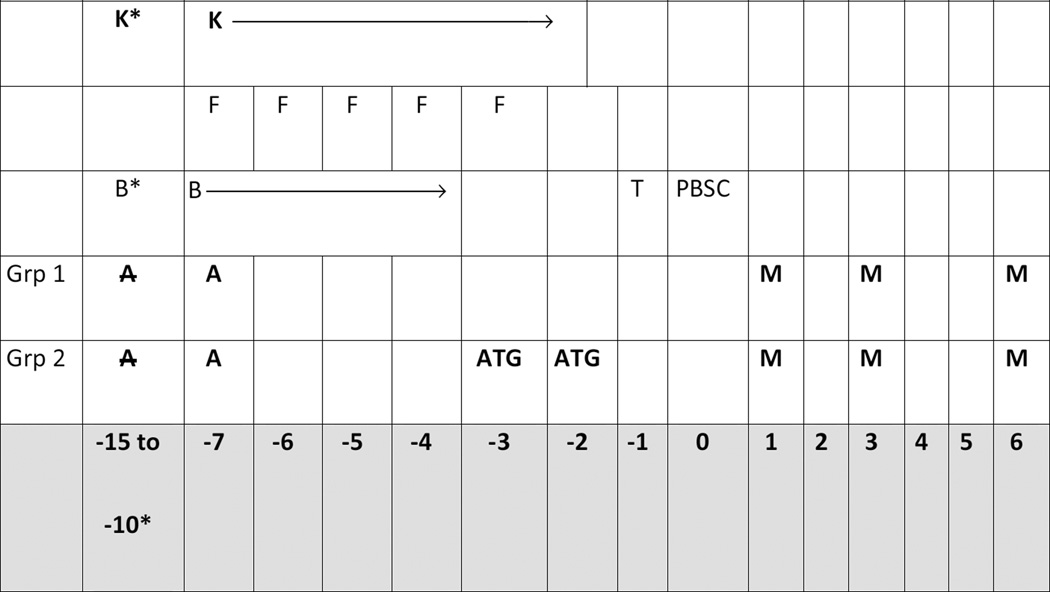
In addition to busulfan, all patients also received daily fludarabine at a dose of 30 mg/m2/d x 5 according to the schedule outlined in Figure 1. All patients received GVHD prophylaxis with tacrolimus starting on day −1 and targeted to maintain serum levels of 3–8 ug/dl. Patients received either alemtuzumab at a dose of 30 mg/d x 1 or 2 days depending on whether they were a matched related (1 day) or mismatched related or unrelated (2 days) donor/recipient pair. After the first 30 patients were enrolled, because of concerns over a high rate of viral infections, matched related patients received MTX and tacrolimus alone, or tacrolimus, MTX + ATG if they were mismatched related or unrelated. MTX was given at a dose of 5 mg/m2 on days +1, +3, and +6.33
Figure 1.
| K |
Keppra: 1 g bid to
start 24 hours prior to the test dose to continue through
day −2 for seizure prophylaxis. |
| F | Fludarabine: 30 mg/m2/day x 5 days IVPB over 30 minutes on Days −7 through −3. |
| B |
Busulfan: dose by
continuous IV infusion over 90 hours on Days −7 to
−4. Pts will receive a single dose of busulfan at 0.8 mg/kg over 2 hours between days –15 and −10 followed by the targeted 90 hour infusion on Days –7 to −4 as described in section 3.2.2. |
| ATG |
Rabbit anti-thymocyte
globulin: .5 mg/kg on day −3 and 2.5
mg/kg on day −2 (group 2 only) |
| A | Thirty patients received
alemtuzumab at a dose of 30 mg/d x 1 or 2 days depending
on whether they were a matched related (1 day) or mismatched related or unrelated (2 days) donor/recipient pair |
| M | Methotrexate: 5 mg/m2 on days +1, +3, and +6. |
| T |
Tacrolimus:
Tacrolimus target serum levels are 3–8 ng/mL. The
suggested starting dose was 0.03 mg/kg PO BID from Day – 1 to Day +120 and then taper by Day +180 |
Results
Demographics
54 patients with myeloid malignancy (36 patients), acute lymphoblastic leukemia (7 patients), non-Hodgkin’s or Hodgkin’s lymphoma (7 patients) and a variety of myeloproliferative diseases or prolymphocytic leukemia (4 patients), were enrolled on this study (Table 1). Patients received alemtuzumab in addition to tacrolimus (30 patients), tacrolimus plus ATG and methotrexate (18 patients) or methotrexate and tacrolimus alone (6 patients). 34 patients received transplants from matched unrelated donors and 20 received a matched related donor transplant. All recipients received peripheral blood stem cells. Median age of the entire patient population was 40, with 20 females and 34 males. Additional details of host and donor CMV status, donor/recipient sex, diagnosis, co-morbidity index, and disease risk are listed in Table 1. Descriptions of GVHD prophylaxis, and the incidence of acute and chronic GVHD grade, are listed in Table 3. The data is presented for each of three measured Busulfan AUC doses delivered and divided into low, intermediate and high AUC dose cohorts.
Table 1.
Demographics by Measured Busulfan AUC Dose
| Total # pts | AUC 5078 uM/min Low |
AUC 6372 uM/min Intermediate |
AUC 7605 uM/min High |
|
|---|---|---|---|---|
| # patients | 54 | 18 | 18 | 18 |
| M/F | 20 F, 34 M | 9 F, 9 M | 3 F, 15 M | 8 F, 10 M |
| Age (median) | 40 | 37 | 40 | 45 |
| Host CMV status | 18 Neg, 36 Pos | 8 Neg, 10 Pos | 4 Neg, 14 Pos | 6 Neg, 12 Pos |
| Donor/Recipient sex | 6 M/F 12 F/M 22 M/M 14F/F |
2 M/F 2 F/M 7 M/M 7 F/F |
1 M/F 6 F/M 9 M/M 2 F/F |
3 M/F 4 F/M 6 M/M 5 F/F |
| Disease Histology | ||||
| AML/MDS/CML | 26/8/1 | 5/5/0 | 9/2/0 | 12/1/1 |
| - ALL | 7 | 6 | 1 | 0 |
| - NHL/HD | 5/2 | 0/0 | 3/2 | 2/0 |
| - MF/CLL/CMML | 1/1/1 | 0/1/0 | 0/0/1 | 1/0/0 |
| - PCL | 2 | 1 | 0 | 1 |
| Type of transplant | ||||
| - MUD | 34 | 11 | 10 | 13 |
| - MRD | 20 | 7 | 8 | 5 |
| CMI (median) | 2 | 1 | 2 | 2 |
| Disease risk | ||||
| - Low | 16 | 8 | 4 | 4 |
| - Intermediate | 20 | 5 | 7 | 8 |
| - High | 18 | 5 | 7 | 6 |
Table 3.
Toxicities and Outcomes by Measured AUC
| AUC Tertile (n) | 54 | Low (18) | Intermediate (18) | High (18) |
|---|---|---|---|---|
| AUC Measured (uMmin) |
5078 (3933–5615) |
6372 (5639–6965) |
7605 (7054–8863) |
|
| Grade 4 mucositis | 4 | 1 | 1 | 2 |
| Grade 5 VOD/Liver Failure |
3 | 1 (sepsis/liver failure) |
1 (VOD) |
1 (VOD) |
| TRM (GVH, Infection, pneumonitis, VOD) |
18 (33%) | 6 (33%) | 6 (33%) | 6 (33%) |
| GVHD prophylaxis: Tacrolimus + |
||||
| Alemtuzumab | 30 | 15 | 10 | 5 |
| ATG + MTX | 18 | 3 | 6 | 9 |
| MTX | 6 | 0 | 2 | 4 |
| Acute GVHD grade ≥ 2 | 28 (52%) | 7 (39%) | 10 (56%) | 11 (61%) |
| Acute GVHD grade 3,4 | 9 (17%) | 2 (11%) | 4 (22%) | 3 (17%) |
| Chronic GVH; Intermediate/Severe |
12 (22%) | 4 (22%) | 6 (33%) | 2 (11%) |
| OS at 3 year | 0.42 | 0.28 | 0.39 | 0.55 |
| RFS at 3 year | 0.36 | 0.22 | 0.39 | 0.43 |
PK Measurements and MTD determinations
All 54 patients underwent test dose administration and PK measurements that were performed 4–7 days prior to initiating therapeutic dosing of busulfan. A total of 5 AUC levels ranging from a low dose of 4800 uMmin to a high of 8363 micromole-minutes (uMmin) were delivered. Dose level 3, at a targeted AUC of 6912 uMmin, was identified as the MTD for this regimen (Table 2). The actual average dose delivered by continuous infusion at dose level 3 was 7095 uMmin. Dose targeting precision was estimated by root mean squared error and was 10.2% for that dose. Within day and between day variability measured by coefficient of variation, were below 10% for all busulfan concentrations. Patients enrolled on each dose level were listed in Table 2 according to the targeted AUC administered. Fourteen patients were enrolled at dose level 1 including eleven who were enrolled after escalation to subsequent dose levels had occurred in order to capture PK and Phase II data on patients whose insurance companies precluded their participation in a Phase I or dose escalation study.
Table 2.
AUC and DLT by Planned Dose Level
| Dose Level |
n | Goal (actual range) |
90 hr mean AUC (uM*min) |
Precision | DLTs |
|---|---|---|---|---|---|
| 1 | 14 | 4800 (3933–6302) |
4973 | 11.7% | 1 |
| 2 | 7 | 5760 (5231–6081) |
5620 | 4.9% | 1 |
| 3 | 24 | 6912 (5705–8268) |
7095 | 10.2% | 1 |
| 4 | 7 | 7603 (6056–8255) |
7000 | 11.1% | 2 |
| 5 | 2 | 8363 (7243–8863) |
8680 | 15.9% | 2 |
Toxicity and MTD
The initial dose escalations were undertaken in five separate cohorts with AUC targeted ranges varying from a low of 4800 uMmin to a high of 8363 uMmin delivered per 24 hours. Because the purpose of this trial was to define an AUC-directed dose of chemotherapy by continuous infusion, toxicities were determined and reported for each of these cohorts during the initial dose escalation portion of the trial (Table 2). Two patients on the original 5th dose level experienced grade 4 DLT of mucositis and a desquamative, intertrigenous skin rash that developed between days 0 and +10. As a result, additional subjects were enrolled at dose level 4. When two additional DLT cases of grade 4 mucositis were identified at the original 4th dose level, an AUC goal of 7603 uMmin, it was determined that the 3rd dose level with a target AUC of 6912 uMmin and an actual AUC of 7095 uMmin was the MTD. Of the 24 patients treated at dose level 3, one grade 5 case of VOD was seen. No other grade 4 or 5 regimen related toxicities were observed at this dose level.
Since AUC levels varied between the groups, and the goal was to identify an actual achieved AUC based dose, additional outcomes analysis were undertaken by dividing patients into thirds according to the actual AUCs delivered rather than the original planned AUCs (Table 3). While we also considered AUC as a continuous covariate and estimated linear effect of AUC on the hazard function, the assumption that AUC affected the hazard function in a linear manner was incorrect and therefore we have presented results for the AUC levels divided into thirds. This post-hoc analysis provided larger numbers per group and allowed for more robust analysis and comparisons of both safety and efficacy according to the actual dose delivered rather than the planned dose level. With this analysis, the lowest third was comprised of 18 patients who received a median AUC dose of 5078 uMmin of busulfan. The middle third contained 18 patients who received a median dose of 6372 uMmin of busulfan and the highest third was comprised of 18 patients with a median AUC of 7605 uMmin. Grade 4 and 5 life threatening or fatal toxicities were seen in two patients in the lowest third who had veno-occlusive disease and severe mucositis. In the intermediate dose level, one patient had grade 4 mucositis and one had severe VOD, while in the highest third there was one fatal case of veno-occlusive disease and 2 cases of grade 4 mucositis. The individuals in the highest third with grade 4 mucositis both had AUCs in excess of 8600 uMmin. Engraftment was as expected in all cohorts with a median time to neutral count greater than 1000 of 13 days and platelet count greater than 20,000 without transfusion of 15 days.
Graft versus Host Disease
Thirty of the fifty-four patients were treated with alemtuzumab and 29 of them lived to day 100 and were fully evaluable for GVHD. Of these 29 patients, 11 (38%) developed grade 2 acute and two (7%) patients had grade 3 or 4 acute GVHD, while six (21%) patients developed extensive chronic GVHD and three (10%) had limited chronic GVHD (Table 3). Six (21%) patients had grade I GVHD and seven (24%) had no acute or chronic GVHD. Eighteen patients received ATG and two developed grade I acute GVHD, five (28%) developed grade II acute GVHD, two (11%) developed grade III acute GVHD, and three (17%) developed grade IV acute GVHD. Two patients developed chronic extensive GVHD. Four patients had no acute or chronic GVHD. Six additional patients received no ATG or alemtuzumab following transplantation with a matched related donor. Of these patients, two (33%) had grade I, two (33%) had grade II, one (17%) had grade III, and one (17%) had grade IV acute GVHD. One (17%) of these patients developed extensive chronic GVHD and none were without any GVHD.
Chimerism
Of the 54 enrolled and evaluable patients, 49 had whole blood chimerism and 46 had T cell chimerism measured at day 30 (Table 3). Forty-seven of 49 had ≥ 95% donor chimerism in the whole blood compartment and 30/45 had ≥ 95% donor chimerism in the T cell compartment. Of the remaining samples analyzed for whole blood chimerism, 2 were <95% donor (21 and 54%) and 15/45 had <95% donor T cells (range 27–94%). At day 100, 40/45 evaluable patients had ≥ 95% donor whole blood chimerism and 25/42 had > 95% donor in the T cell compartment. 17/42 patients were < 95% donor T cell chimeras (range 1–91%). There were no differences in chimerism results as a function of either the immunosuppression used or busulfan dose received.
Outcomes
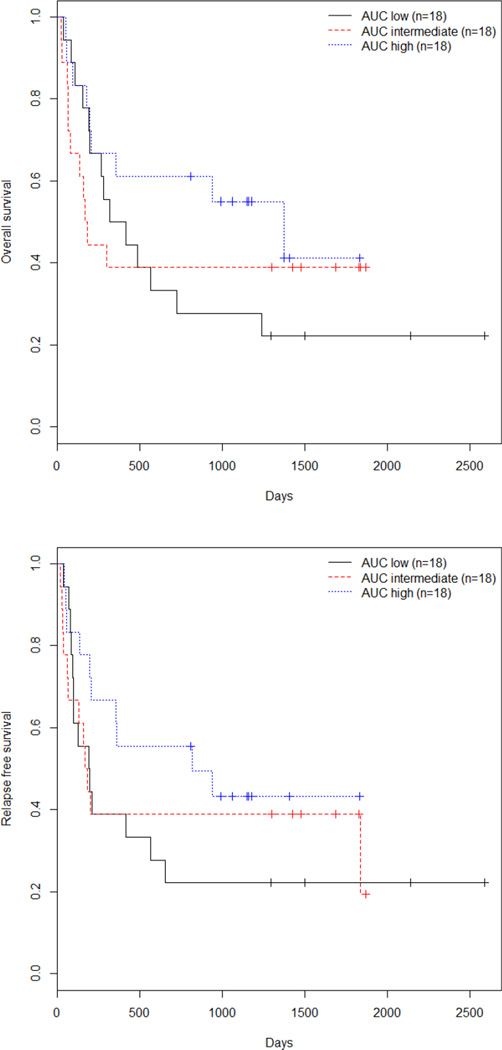
The purpose of this study was to identify the DLT and MTD along with identifying the potential value of higher doses of busulfan when administered as a prolonged IV infusion. Sixteen subjects had low, 20 had intermediate, and 18 had high-risk disease by CIBMTR criteria (Table 1). Unadjusted overall survival (OS) and relapse-free survival (RFS) for all 54 subjects were 0.42 and 0.36 respectively. When analyzed according to the AUC low (group 1, 18 pts), middle (group 2, 18 pts), or high (group 3, 18 pts) dose (Table 4), OS was 0.28, 0.39, and 0.55 and RFS was 0.22, 0.39, and 0.43, respectively (p=NS for all analyses). For the entire group, univariate analysis identified age, recipient CMV status, and disease risk as significant factors for OS and RFS (Table 3). Co-morbidity scores, diagnosis, GVH occurrence, donor/recipient sex and donor type (MUD or MRD) were not significant, nor were the non-relapse mortality rate or OS different between the three AUC groups in univariate analysis. The use of alemtuzumab vs ATG did not affect OS or relapse rates with HRs of 1.48 and 1.55 and p values of 0.27 and 0.21, respectively. Adjusted analysis by AUC group identified high vs low AUC dose (p=0.049) and disease risk (p=0.01) as significant for both OS and RFS (Table 4, Figure 2). Outcomes were similar between AUC groups 2 (intermediate) and 3 (high). Differences in OS and RFS were limited to the good and intermediate risk patients as outcomes for high-risk patients were poor for all AUC groups (1/18 OS and 0/18 RFS). Similar results were obtained when these analyses were performed according to targeted rather than measured AUC (data not shown).
Table 4.
Cox regression analysis for effect of univariable and multivariable risk factors on OS and RFS
| Model | Covariate | OS | RFS | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| AUC (2 vs 1) | 0.94 | (0.42, 2.08) | 0.88 | 0.90 | (0.41, 1.95) | 0.78 | |
| AUC (3 vs 1) | 0.59 | (0.25, 1.36) | 0.21 | 0.58 | (0.26, 1.31) | 0.19 | |
| AUC (3 vs 2) | 0.62 | (0.26, 1.51) | 0.30 | 0.64 | (0.28, 1.50) | 0.31 | |
| GVHD prophy | 0.64 | (0.32, 1.30) | 0.21 | 0.61 | (0.31, 1.21) | 0.16 | |
| Donor | 1.48 | (0.75, 2.93) | 0.26 | 1.73 | (0.89, 3.37) | 0.11 | |
| Univariate | Age | 1.04 | (1.00, 1.07) | 0.04 | 1.04 | (1.01, 1.08) | 0.01 |
| Analysis | CMV | 3.22 | (1.40, 7.43) | 0.01 | 2.98 | (1.34, 6.59) | 0.01 |
| GCa | 0.97 | (0.42, 2.23) | 0.94 | 0.89 | (0.40, 1.99) | 0.78 | |
| GVHD | 1.11 | (0.51, 2.41) | 0.78 | 1.06 | (0.51, 2.22) | 0.87 | |
| CMIb | 1.16 | (0.73, 1.84) | 0.54 | 1.12 | (0.72, 1.75) | 0.62 | |
| Diagnosisc | .81 | (0.41, 1.6) | 0.54 | 0.81 | (0.42, 1.58) | 0.54 | |
| Disease riskd | 2.42 | (1.51, 3.88) | <.01 | 2.93 | (1.78, 4.83) | <.01 | |
| AUC (2 vs 1) | 0.79 | (0.35, 1.76) | 0.56 | 0.57 | (0.25, 1.28) | 0.17 | |
| AUC (3 vs 1) | 0.42 | (0.18, 1.00) | 0.049 | 0.34 | (0.14, 0.79) | 0.01 | |
| Adjusted | AUC (3 vs 2) | 0.54 | (0.22, 1.33) | 0.18 | 0.59 | (0.25, 1.40) | 0.23 |
| Analysis | Age | 1.01 | (0.97, 1.05) | 0.65 | 1.02 | (0.99, 1.06) | 0.18 |
| CMV | 2.05 | (0.85, 4.94) | 0.11 | 1.97 | (0.86, 4.54) | 0.11 | |
| Disease risk | 2.16 | (1.24, 3.75) | 0.01 | 2.75 | (1.56, 4.88) | <.01 | |
GC: donor/recipient gender pairing
CMI: co-morbidity index
Diagnosis: AML + MDS vs others
Disease Risk according to ASBMT/CIBMTR criteria (ref 23)
Figure 2.
Kaplan Meier curves by : Top, OS; Bottom, RFS.
Discussion
The value of allogeneic stem cell transplantation has traditionally been derived from a combination of the capacity of the conditioning regimen to eradicate the underlying disease, permit durable donor engraftment, and confer an antitumor effect (graft vs tumor) from the donor cell infusion. In recent years, the use of reduced intensity regimens have permitted the expansion of allogeneic transplants into populations of older and less robust patients and provide cure for a significant population that had not previously been eligible for such therapy. The rates of cancer relapse after RIC HCT remains unacceptably high, however, 1,3,4 and appear higher compared to myeloablative regimens for certain diseases.13,14,16 Evidence suggests that more intensive regimens are associated with improved disease control for those patients who are in better health and able to tolerate these therapies, particularly for advanced diseases such as relapsed and refractory lymphomas or high-risk acute myeloid and lymphoid leukemias. While retrospective data has suggested that the overall survivals are comparable in patients over the age of 50 treated with fully ablative or reduced intensity regimens, a recent trial by the Bone Marrow Transplant Clinical Trials Network (CTN0901) was stopped early because of an apparent increase in early relapse rates in the population getting less intensive therapies. Details of this study are not yet available, but it implies that intensive regimens continue to be valuable, particularly since relapse remains the major cause of treatment failure in this patient population.
In the current study, a novel approach for administration of a highly effective chemotherapy agent, IV busulfan, along with the immunosuppressant, fludarabine, was utilized as a means to allow potential dose escalation of this drug for healthy patients under the age of 55 compared to four time a day, or once a day, administration schedules. Investigators at MD Anderson have identified an AUC MTD of 6000 by once daily infusion in conjunction with fludarabine as an appropriate dose for younger and healthier individuals. In our study, we were able to administer IV busulfan as a continuous infusion over 90 hours at a Phase II MTD of approximately 7000 uMmin AUC per 24 hours for a total AUC exposure of 26,250 uMmin plus the test dose of approximately 1200 uMmin. This resulted in a total busulfan dose of approximately 27,500 uMmin and represents a 15% increase over the AUC identified by the MD Anderson investigators and a 43% increase over a standard AUC of 4800 uMmin/day.17,34,35 In addition to this higher total dose of drug, the prolonged administration schema may result in improved cell kill as reported by Teicher and colleagues with preclinical testing of models using a variety of cell lines.19 While the results obtained in this trial do not permit conclusions regarding the anti-tumor efficacy of this approach, they indicate that a higher and potentially more effective dose can be administered safely with the continuous infusion schema described in this manuscript. It is also appreciated that the results at the lowest AUC may be poorer than expected, and that prolonged infusion may be less effective at standard doses than intermittent schedules, but with small numbers and disease heterogeneity, it is not possible to provide a definitive analysis of these outcomes.
It is important to point out that there did not appear to be a significant increase in toxicity associated with these higher doses of drugs until the delivery of AUC doses above 8000 uMmin/day at which time we identified mucositis and skin rash as the DLTs. An intertriginous rash associated with grade IV mucositis was seen in both patients treated at AUCs greater than 8000 uMmin over 24 hours, and suggests that GVHD prophylaxis regimens that avoid methotrexate or other mucositis enhancing agents may be useful in limiting this significant source of morbidity and mortality. If additional patients were enrolled at these levels it is likely that further organ toxicities such as hepatic or pulmonary damage would have been seen but it was notable that neither significant VOD of the liver nor interstitial pneumonitis were seen with an increased frequency at the higher dose levels. There was one case of severe at the lowest AUC cohort and one case at the highest dose level. A third case that was observed at the intermediate dose level was felt to represent hepatic failure in conjunction with sepsis and multi-organ failure. While early pulmonary complications without an identifiable infectious agent were contributors to the death of two subjects, whether this was from busulfan or other non-infectious causes was not discernible. Two long-term surviving patients have developed pulmonary complications in the presence of GVHD, but these were not felt to be due to busulfan. Other attendant morbidities such as GVHD and infectious complications were observed at a rate that is consistent with what would be expected using ablative regimens. There was no excess mortality associated with alemtuzumab, but its use was discontinued after the first 30 patients because of an accompanying increase in viral infections and related complications.
In summary, the use of prolonged infusion busulfan appears to allow for a higher MTD compared to intermittent busulfan infusion schedules. Further trials to refine and reduce the complications seen such as mucositis and skin rash at the highest dose levels are being considered along with efforts to optimize the efficacy of this approach. Other than the challenge of administering a continuous infusion of a drug that requires preparation 2–3 times a day, there were no significant complications seen with this approach that were not also attributable to the higher doses. The use of a test dose prior to initiation of treatment appears to be important to identify the appropriate range for administering this drug since our study determined that the general accuracy of the AUC based on the busulfan clearance was found to be in excess of 90%. This approach holds promise as a treatment regimen that is tolerable and may diminish relapse rates in this population of patients with high-risk hematologic cancers.
Highlights.
Determination of an AUC MTD with continuous infusion busulfan in allogeneic transplantation
Identification of rash and mucositis as the DLTs of high-dose continuous infusion busulfan
Potential increased efficacy with an increased AUC of busulfan given as a continuous infusion
Acknowledgements
This study was supported in part by National Institutes of Health grant CA016086 and a research grant from Otsuka Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented in part at the American Society of Bone Marrow Transplant Tandem Meetings in 2012.
Authorship
Study design: TS, CW, DG, JS and AI
Data: TS, GW, YR, AI, JS, JC, TC, PA, WA, KR, JS
Statistical Analysis: YC, AI
Manuscript Preparation: TS, CW, AI
Manuscript editing and review: TS, CW, YC, AI, JS, TC, JC, PA, WW, SS, JS
Disclosures: None
References
- 1.Gooley T, Chien MD, Pergam S, et al. Reduced mortality after allogeneic stem cell transplantation. New Eng J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hahn T, McCarthy P, Hassebroek A, et al. Significant improvements in survival after allogeneic stem cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013;31:2437–2449. doi: 10.1200/JCO.2012.46.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanate AS, Pasquini MC, Hari PN, Hamadani M. Allogeneic hematopoietic cell transplant for acute myeloid leukemia: Current state in 2013 and future directions. World J Stem Cells. 2014;6(2):69–81. doi: 10.4252/wjsc.v6.i2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horan JT, Logan BR, Agovi-Johnson MA, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011;29:805–813. doi: 10.1200/JCO.2010.32.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bejar R, Stevenson K, Caughey B, et al. Somatic mutations predict poor outcomes in patients with myelodysplastic syndrome after hematopoietic stem cell transplantation. J Clin Oncol. 2014;32:2691–2698. doi: 10.1200/JCO.2013.52.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ringden O, Labopin M, Ehninger G, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27:4570–4577. doi: 10.1200/JCO.2008.20.9692. [DOI] [PubMed] [Google Scholar]
- 8.Scott BL, Sandmaier BM, Storer B, et al. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia. 2006;20:128–135. doi: 10.1038/sj.leu.2404010. [DOI] [PubMed] [Google Scholar]
- 9.de Lima M, Anagnostopoulos A, Munsell M, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–872. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 10.Sorror ML, Giralt S, Sandmaier BM, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110:4606–4613. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SJ, Klein J, Haagenson M, et al. High resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4585. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 12.Gragert L, Eapen M, Williams E, et al. HLA match likelihood for hematopoietic stem cell grafts in the U.S. N Engl J Med. 2014;371:339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimoni A, Nagler A. Optimizing the conditioning regimen for allogeneic stem- cell transplantation in acute myeloid leukemia; dose intensity is still in need. Best Pract Res Clin Haematol. 2011;24:369–379. doi: 10.1016/j.beha.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Schlenk RF, Döhner K, Mack S, et al. Prospective Evaluation of Allogeneic Hematopoietic Stem-Cell Transplantation From Matched Related and Matched Unrelated Donors in Younger Adults With High-Risk Acute Myeloid Leukemia: German-Austrian Trial AMLHD98A. J Clin Oncol. 28:4642–4648. doi: 10.1200/JCO.2010.28.6856. [DOI] [PubMed] [Google Scholar]
- 15.Luger SM, Ringden O, Zhang MJ, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012;47:203–211. doi: 10.1038/bmt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alyea EP, Kim HT, Ho V, et al. Comparative outcome of non-myeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005;105:1810–1814. doi: 10.1182/blood-2004-05-1947. [DOI] [PubMed] [Google Scholar]
- 17.Copelan EA, Hamilton BK, Avalos B, et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood. 2013;122(24):3863–3870. doi: 10.1182/blood-2013-07-514448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bredeson C, LeRademacher J, Kato K, et al. Prospective cohort study comparing intravenous busulfan to total body irradiation in hematopoietic cell transplantation. Blood. 2013;122:3871–3878. doi: 10.1182/blood-2013-08-519009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teicher BA, Holden SA, Eder JP, et al. Influence of schedule on alkylating agent cytotoxicity in vitro and in vivo. Cancer Res. 1989;49:5994–5998. [PubMed] [Google Scholar]
- 20.Eder JP, Elias AD, Ayash L, et al. A phase I trial of continuous-infusion cyclophosphamide in refractory cancer patients. Cancer Chemother Pharmacol. 1991;29:61–65. doi: 10.1007/BF00686337. [DOI] [PubMed] [Google Scholar]
- 21.Wilson WH, Grossbard ML, Pittaluga S, et al. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharma- codynamic approach with high efficacy. Blood. 2002;99:2685–2693. doi: 10.1182/blood.v99.8.2685. [DOI] [PubMed] [Google Scholar]
- 22.Barlogie B, Alexanian R. Effective treatment of advanced multiple myeloma refractory to alkylating agents. NEJM. 1984;310:1353–1356. doi: 10.1056/NEJM198405243102104. [DOI] [PubMed] [Google Scholar]
- 23.CIBMTR risk stratification reference according to the ASBMT/CIBMTR standard reporting guidelines. http://c.ymcdn.com/sites/www.asbmt.org/resource/resmgr/RFI/RFI_2015_-_CIBMTR_Disease_Cl.pdf.
- 24.Bearman SI, Anderson GL, Mori M, et al. Veno-occlusive disease of the liver: development of a model for predicting fatal outcome after marrow transplantation. J Clin Oncol. 1993 Sep;11(9):1729–1736. doi: 10.1200/JCO.1993.11.9.1729. [DOI] [PubMed] [Google Scholar]
- 25.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft vs. host disease in humans recipients of marrow from HLA-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 27.Lindley C, Shea T, McCune J, et al. Intraindividual variability in busulfan pharmacokinetics in patients undergoing a bone marrow transplant: assessment of a test dose and first dose strategy. Anticancer Drugs. 2004;15:453–459. doi: 10.1097/01.cad.0000127145.50172.51. [DOI] [PubMed] [Google Scholar]
- 28.Grochow LB, Jones RJ, Brundrett RB, et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol. 1989;25:55–61. doi: 10.1007/BF00694339. [DOI] [PubMed] [Google Scholar]
- 29.Slattery JT, Risler LJ. Therapeutic monitoring of busulfan in hematopoietic stem cell transplantation. Ther Drug Monit. 1998;20:543–549. doi: 10.1097/00007691-199810000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Chen TL, Grochow LB, Hurowitz LA, et al. Determination of busulfan in human plasma by gas chromatography with electron-capture detection. J Chromatogr. 1988;425:303–309. doi: 10.1016/0378-4347(88)80034-3. [DOI] [PubMed] [Google Scholar]
- 31.Ivanova A, Flournoy N, Chung Y. Cumulative cohort design for dose-finding. Journal of Statistical Planning and Inference. 2007;137:2316–2317. [Google Scholar]
- 32.Ivanova A, Qaqish BF, Schell MJ. Continuous toxicity monitoring in phase II trials in oncology. Biometrics. 2005;61:540–545. doi: 10.1111/j.1541-0420.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 33.Przepiorka D, Ippolii C, Khouri I, et al. Tacrolimus and minidose methotrexate for prevention of acute graft versus host disease after matched unrelated donor transplantation. Blood. 1996;88:4383–4389. [PubMed] [Google Scholar]
- 34.Andersson BS, Thall PF, Madden T, et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002;8:477–485. doi: 10.1053/bbmt.2002.v8.pm12374452. [DOI] [PubMed] [Google Scholar]
- 35.McCune JS, Gibbs JP, Slattery JT. Plasma concentration monitoring of busulfan: does it improve clinical outcome? Clin Pharmacokinet. 2000;39:155–165. doi: 10.2165/00003088-200039020-00005. [DOI] [PubMed] [Google Scholar]