Abstract
Engraftment syndrome encompasses a continuum of peri-engraftment complications after autologous hematopoietic stem cell transplantation. ES may include non-infectious fever; skin rash; diarrhea; hepatic dysfunction; renal dysfunction; transient encephalopathy; and capillary leak features, such as non-cardiogenic pulmonary infiltrates, hypoxia, and weight gain with no alternative etiologic basis other than engraftment. Given its pleiotropic clinical presentation, the transplant field has struggled to clearly define ES and related syndromes. Here, we present a comprehensive review of ES in all documented disease settings. Furthermore, we discuss the proposed risk factors, etiology, and clinical relevance of ES. Finally, our current approach to ES is included along with a proposed treatment algorithm for the management of this complication.
Introduction
Engraftment syndrome (ES) was first defined by Lee et al. in a retrospective analysis of 248 patients with cancer undergoing autologous stem cell transplant (ASCT)1. Fifty-nine percent of patients developed a skin rash and non-infectious fever, with a median onset at 7 days post-ASCT. Capillary leak, pulmonary infiltration, and hypoxia were also commonly observed. ES has been documented in patients undergoing ASCT or syngeneic SCT for multiple myeloma, POEMS (Polyneuropathy, Organomegaly, Endocrinopathy, Monoclonal protein and Skin abnormalities) syndrome, light chain amyloidosis, lymphoma, breast cancer, and multiple sclerosis. ES is now considered a formidable complication following ASCT2-4. While most cases of ES are mild and resolve spontaneously or with corticosteroid therapy, ES can occasionally be fatal. Although ES has been documented in the transplant literature for more than 45 years, the etiology of this syndrome remains incompletely understood. Updated diagnostic criteria are necessary to standardize future research in ES. Here, we review the literature on ES and related syndromes and discuss potential risk factors, underlying mechanisms, and clinical relevance of ES. Furthermore, we include our current management approach for the diagnosis and treatment of ES.
Differential Diagnosis of Engraftment Syndrome
The clinical features of ES have been defined according to Spitzer and Maiolino diagnostic criteria5, 6. Spitzer criteria were developed from a broad literature review of ES like syndromes in patients that had undergone autologous, syngeneic, and allogeneic SCT. According to Spitzer, the major criteria for ES include fever (≥101° F), erythrodermatous rash over more than 25% of the body that cannot be linked to medication, and noncardiogenic pulmonary edema. Secondarily, ES is characterized by hepatic dysfunction (i.e., bilirubin ≥ 2 mg/dl or a 2-fold increase in transaminase over baseline), renal insufficiency (i.e., 2-fold increase in serum creatinine over baseline), weight gain (i.e., 2.5% increase), and unexplained transient encephalopathy. A diagnosis of ES requires the presence of all three major criteria or two major and one or more minor criteria within 96 hours of neutrophil engraftment (Table 1). Maiolino criteria were developed from a single-center review of 125 patients that had undergone autologous SCT. According to Maiolino, ES is defined as the development of a noninfectious fever in combination with diarrhea, rash, or pulmonary infiltrates within 24 hours of engraftment (Table 1).
Table 1.
Criteria for Diagnosis of Engraftment Syndrome
| Spitzer Criteria | Maiolino Criteria | |
|---|---|---|
| Requirements | 3 Major or 2 Major + 1 Minor | Major + 1 Minor |
| Major Criteria | Non-infectious Fever, Skin Rash, or Pulmonary Edema | Non-infectious Fever |
| Minor Criteria | Weight Gain, Hepatic Dysfunction, Renal Dysfunction, or Transient Encephalopathy | Skin Rash, Pulmonary Infiltrates, or Diarrhea |
| Timing of Symptoms Relative to Engraftment | 4 Days Within ANC 0.5 × 109/L | 1 Day Within Neutrophils Present |
Other studies have described more liberal timing for the development of ES. Capizzi et al. retrospectively assessed signs of ES in auto-SCT patients that developed peri-engraftment respiratory distress syndrome (PERDS) and found that symptoms occurred within 5 days before or 4 days after engraftment7. Dispenzieri et al. and Carerras et al. found that symptoms of ES were evident within 7 days of neutrophil engraftment2, 8. Our group has found that symptoms most commonly occur from 3 days prior to 7 days after engraftment9.
Although no definitive biomarkers of ES have been identified, elevated levels of CRP have been associated with ES2, 10. A recent case report suggested that expression of elafin, a protein secreted by epithelial cells in response to IL-1 and TNFα, may be an early biomarker of ES11. In more severe cases of ES, the diagnosis can be confirmed using a skin or colonic biopsy to assess histology and mononuclear cell infiltrate12-16.
Engraftment Syndrome – A Spectrum of Disease Severities
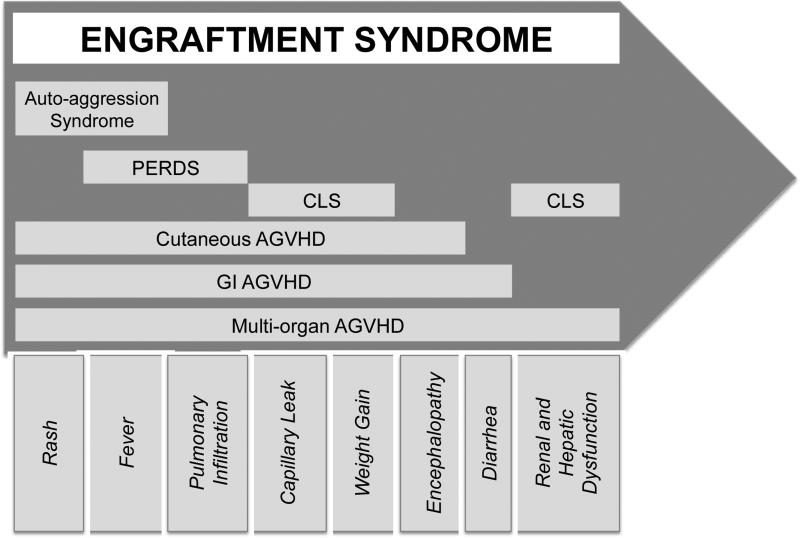
Use of multiple nomenclatures in the ES literature has complicated the field. ES has been used synonymously with capillary leak syndrome (CLS), auto-aggression syndrome, PERDS, and autologous graft versus host disease (AGVHD)7, 17-19. Each of these syndromes encompasses all, or a subset, of symptoms that have been attributed to ES (Figure 1). Although the ES nomenclature appears widely accepted, the use of the term “AGVHD” and the relationship between AGVHD and ES is still debated.
Figure 1. Engraftment syndrome encompasses a spectrum of autologous SCT-associated syndromes that vary in degrees of severity.
PERDS, peri-engraftment respiratory distress syndrome; CLS, capillary leak syndrome; AGVHD, autologous graft versus host disease; GI, gastrointestinal.
Rappeport et al. first described evidence of an acute cutaneous graft versus host-like disorder following syngeneic bone marrow transplants between identical twins for leukemia19. Hood et al. reported a similar response in patients undergoing either autologous or syngeneic bone marrow transplant for leukemia or lymphoma20. Skin biopsies, taken 6 to 62 days post-transplant, showed histological signs of GVHD with features of: altered polarity, dyskeratosis, and basal vacuolization, with or without mononuclear cell infiltration19-21. While AGVHD cases involving only the skin are typically self-limiting or managed with corticosteroids, more severe cases of AGVHD may affect the gastrointestinal (GI) tract and liver12, 13, 20, 22-24. In one study, GI AGVHD occurred in 13% of ASCT patients and was defined by persistent symptoms, mucosal abnormalities, and the presence of apoptotic crypt cells, with or without lymphoid infiltrates, upon histological examination23. In a subset of these studies, rash and/or fever were noted in conjunction with GI and liver involvement13, 22, 24.
Despite overlapping symptoms observed in ES and AGVHD, some investigators view AGVHD and ES as distinct syndromes. For example, in a recent review of ES in the context of both autologous and allogeneic transplantation, Spitzer suggested that ES be defined as a non-infectious fever, capillary leak, and rash in the absence of histological evidence of GVHD25. The diagnosis of ES, rather than AGVHD, in past literature may be due to the lack of a tissue biopsy confirming classic GVHD pathology. In an attempt to unify the field, we propose that all forms of AGVHD (i.e., cutaneous, GI, and liver) be included under the umbrella of “engraftment syndrome” (Figure 1). We encourage investigators and clinicians, henceforth, to note the severity of ES based on clinical symptoms and histological evidence of immune involvement.
Engraftment Syndrome in Multiple Myeloma
In a retrospective analysis by Katzel et al. of 90 patients with multiple myeloma that received ASCT after melphalan therapy, 10% of patients developed ES according to the Spitzer or Maiolino criteria26. All patients developed a non-infectious fever and 8/9 had diarrhea. A skin rash was evident in 4 patients, while pulmonary infiltrates were seen in 6 patients. Three of 4 patients that developed hypoxia responded to high-dose steroids, while the remaining patient died of multisystem failure. This patient did not receive steroid therapy until day 17. The incidence of ES reached 29% (13/45) in Maiolino's cohort of patients with myeloma5.
Giralt et al. reported 14 patients with myeloma who developed varied degrees of ES/AGVHD after ASCT and cyclosporine (CsA) treatment27. Specifically, 10 patients developed mucositis, 8 patients developed kidney or liver complications, and 4 developed cardiac problems. Histologic signs of acute GVHD were evident in 7 patients. One patient was diagnosed with clinically and histologically evident GVHD and was responsive to steroid therapy. In our retrospective analysis of 421 patients with myeloma, the incidence of ES reached 21%9. The most common symptoms were fever, diarrhea, and rash. Symptoms resolved spontaneously or with corticosteroid treatment in the majority of patients (95%).
In contrast to most studies that document mild, treatable cases of ASCT-associated ES, our group reported the development of severe ES/AGVHD in patients undergoing ASCT13. Severe ES occurred in 5 of 223 (2%) patients with myeloma. Three of the 5 patients that developed complications were undergoing a second autologous SCT. Histologic signs of GVHD were seen in the skin, liver, and/or the GI tract. Lymphocytes, mononuclear cells, and neutrophils were reported in liver and GI biopsies. Histological GVHD occurred on average within the first 2 weeks and commonly followed signs of ES. Patients were only partially responsive to steroid therapy, and 4 died due to complications of ES/AGVHD or to tumor relapse. In agreement with this study, Goddard et al. reported a fatal case of multi-organ (i.e., skin, GI, liver), steroid-resistant AGVHD in a single myeloma patient undergoing secondary ASCT. Liver and digestive tract biopsies after day 41 were indicative of Grade 4 GVHD and showed evidence of immune infiltration by lymphocytes and neutrophils22. In a review of 388 ASCT patients, Cogbill et al. reported the development of ES/AGVHD in 17 patients (4%), 16 of which were patients with myeloma. Grade I-IV GVHD was diagnosed in biopsies from skin, liver, small intestine, and most commonly, colon. Six of 16 patients died due to sepsis (n=4), CNS hemorrhage (n=1), or relapse of disease (n=1)12. Collectively, these data highlight the potential for serious complications in patients that develop ES and encourage early detection and treatment.
POEMS syndrome and Engraftment Syndrome
In a retrospective analysis of 30 POEMS patients that underwent ASCT, fever, diarrhea, weight gain, and rash was observed in 93%, 77%, 53%, 43%, respectively. ES was found to occur in 27-47% of patients depending on the criteria used8. The onset of ES symptoms occurred at a median of 9 days. When the required time to engraftment, based on Maiolino and Spitzer criteria, was broadened, the rate of ES surpassed 50%. The authors suggested that these patients might be highly susceptible to ES due to a preexisting aberrant cytokine milieu (VEGF, TNFα, IL-1β) that is exaggerated by ASCT.
Jimenez-Zepeda et al. reported the outcome of 8 patients with POEMS that had undergone ASCT28. In contrast to the previous study, only 37.5%, 12.5%, 25%, and 50% of patients developed fever, diarrhea, weight gain, and rash, respectively. Here, none of the patients developed ES, as strictly defined by Spitzer and Maiolino criteria. Importantly, prednisone was given to all patients prior to ASCT in this study. In the previous analysis, only 43% of the patients received corticosteroid therapy. Taken together, these studies emphasize the importance of monitoring for ES in patients undergoing ASCT therapy for POEMS and suggest that early treatment with corticosteroids may be beneficial.
Engraftment Syndrome in Light Chain Amyloidosis
Irazabal et al. reported ES in 29 of 377 (8%) patients who underwent ASCT for amyloidosis. Acute kidney injury, edema, fever, diarrhea, rash, and transient encephalopathy were observed in 93%, 93%, 83%, 69%, 48%, and 17% of patients, respectively29. Carerras et al. reported a high incidence (12/25; 48%) of ES in patients with amyloidosis2. In a prospective study, Sanchorawala et al. reported evidence of AGVHD in 2 of 30 patients with amyloidosis following ASCT with high-dose melphalan plus bortezomib as conditioning30.
Engraftment Syndrome in Other Disease Settings
Moreb et al. reported, an “auto-aggression syndrome” in patients with breast cancer or lymphoma that had undergone ASCT18. This syndrome was defined by the development of a skin rash that presented 5 to 13 days post-transplant. This ASCT-related skin rash was more frequently observed in patients with breast cancer (20/30; 67%) compared to patients with lymphoma (3/12; 25%). Fever was evident in 18 patients and was more prevalent in the breast cancer cohort (57% vs. 8%). Six of 10 patients with showed evidence of Grade I-II GVHD by skin biopsy18.
In a large retrospectively analysis of 452 lymphoma patients, Keung et al. found evidence of ES or AGVHD in 40 of 452 (9%) following ASCT31. We observed a frequency of 10% in our retrospective analysis of 170 patients with lymphoma9. ES has also been documented in patients undergoing ASCT for multiple sclerosis. Carerras et al. noted the development of ES, characterized by non-infectious fever, skin rash, and weight gain, in 3 of 15 (20%) patients undergoing ASCT for multiple sclerosis32. ES symptoms were readily resolved with corticosteroid therapy in these patients. While ASCT is commonly used in cases of autoimmune disease, corticosteroids are regularly used as a maintenance therapy in these patients and may prophylactically reduce the risk of ES.
Risk Factors for Engraftment Syndrome
Elucidation of the risk factors for ES has been hindered by variations in patient populations, subject number, pretreatment regimens, and lack of clearly defined disease criteria. Numerous risk factors for ES have been suggested in the literature. A correlation with female gender has been suggested but has not been consistent in all reports2, 33. In their initial definition of ES, Lee et al. noted a positive correlation between post-transplant granulocyte colony stimulation factor (G-CSF) therapy and development of ES; however, this association has not been confirmed in more recent studies1, 18. Akasheh et al. found that ES was more common in breast cancer patients that received GM-CSF (8/10) compared with G-CSF (4/9)34. Similarly, a recent study in patients undergoing ASCT for myeloma suggested that GM-CSF treatment was associated with a higher risk of ES (28% vs. 3%, OR = 12.5, p = 0.001)35. Conflicting reports have been published assessing the role of CD34+ cell number and engraftment rate in development of ES9, 33, 34, 36. Of note, a recent comparison of CD34+ donor cells from patients that developed ES/AGVHD (n=9) versus those that did not (n=42) revealed increased expression of GATA-2 and CD130, and decreased expression of CXCR4, suggesting that the phenotype of the donor cells may play a role37.
A number of studies have attempted to link the development of ES with various pre-treatment regimens. While Ravoet et al. showed a correlation with the use of busulfan, Gonzelez-Vincent et al. found the opposite to be true28, 36. Jimenez-Zepeda et al. suggested that cyclophosphamide pre-treatment was responsible for the reduced incidence of ES in patients with POEMS. The interpretation of these results was complicated by the fact that all patients also received prednisone prior to ASCT28. However, in support of their findings, our assessment of pre-treatment regimens in patients with myeloma or lymphoma revealed that cyclophosphamide exposure was associated with reduced risk for ES9. Of interest, Carreras et al. showed, in a broad spectrum of patients, that a less aggressive history of chemotherapy was associated with a higher risk of ES2. In line with this theory, Moreb et al. found that patients undergoing ASCT for breast cancer were more likely to develop ES if they had previously undergone only a single round of chemotherapy or radiotherapy18. Furthermore, in patients with myeloma, previous exposure to bortezomib or lenalidomide, rather than broad cytotoxic chemotherapies, was linked to a higher risk of ES9. Future large, multicenter studies using the current criteria are necessary to more accurately define the risk factors for ES across the broad spectrum of patients undergoing ASCT.
Mechanisms of Engraftment Syndrome
The mechanisms responsible for ES/AGVHD development are poorly understood. Although a number of studies suggest that ES can be reversed by corticosteroid therapy, controlled trials have not been performed to directly assess the benefit of corticosteroids38. Nevertheless, a number of studies in animal models and humans suggest that the immune system plays a role in the development of ES, despite the absence of HLA and minor histocompatibility antigen mismatch.
Early work in the Lewis rat model showed that a GVHD-like syndrome occurred after transplantation of syngeneic bone marrow into irradiated recipients that received CsA post-transplant. Specifically, irradiated rats received a bone marrow graft and were treated with CsA for 20-40 days post-transplant after engraftment was complete. Syngeneic GVHD (SGVHD) developed 12-40 days after the last CsA treatment39-41. In this model, SGVHD was delayed in the absence of CD4+ T cells and completely inhibited in the absence of CD8+ T cells42, 43. Furthermore, the authors showed that MHCII-specific antibodies significantly delayed the development of SGVHD43. Analysis of the TCR repertoire in this model revealed that Vβ8.5+ T cells were predominant in SGVHD lesions and more frequent in rats treated with CsA, suggesting that T cell selection is skewed in the presence of CsA44. In support of this hypothesis, CsA is known to alter T cell development and selection in the thymus45. Induction of SGVHD required that the recipient undergo high dose irradiation and CsA treatment, and transfer of the disease required an irradiated recipient. Of interest, SGVHD was abrogated if the disease-promoting T cells were transferred with CD4+ T cells from untreated normal littermates46. These studies suggest that a radiation-sensitive CD4+ T cell is necessary for controlling the development of SGVHD.
A number of clinical trials were designed based on the knowledge gained from the Lewis rat model. In an early study by Jones et al., CsA appeared to induce cutaneous GVHD in 5 patients after autologous transplant47. Of interest, peripheral blood lymphocytes isolated post-transplant displayed cytotoxic capacity against autologous lymphocytes that were recovered from peripheral blood prior to the start of treatment. This autoreactivity was not observed in lymphocytes isolated from peripheral blood following resolution of AGVHD or in patients that were not induced with CsA. Blocking antibodies specific for MHC II and the invariant chain (CLIP) inhibited T cell cytotoxicity ex vivo47, 48. Thus, in agreement with the rat model, CsA-induced AGVHD is dependent upon recognition of MHC II, presumably by T cells that hold a relatively high affinity for self-MHC and have escaped negative selection. To further investigate the role of the T cell response in AGVHD, Massumoto et al. compared the incidence of AGVHD in patients before or after treatment with IL-2. IL-2 treatment was given for 5 consecutive days 25-58 days post-transplant. The incidence of AGVHD increased from 30% (3/10) at baseline to 79% (11/14) following treatment with IL-2. In this scenario, IL-2 may have enhanced an ensuing auto-reactive T cell response or encouraged a break in tolerance. Importantly, in the absence of a control group, it is difficult to determine whether this delayed presentation of AGVHD was mediated by IL-249. While CsA-induction studies of SGVHD/AGVHD provided valuable insight into the mechanism of this disorder, the penetrance of CsA-induced AGVHD is highly variable in humans, suggesting that genetic variability and disease history in patients play an important role.
In line with the Lewis rat model, Rappeport et al. hypothesized that suppressive T cells, perhaps analogous to regulatory T cells (Tregs), would be eliminated by irradiation and prior chemotherapy in patients undergoing syngeneic or autologous SCT. In the absence of these suppressive T cells, auto-reactive T cells would be free to target “self” in the immune-ablated graft recipient 19, 50. Of interest, mobilization of stem cells with cyclophosphamide is known to enrich for regulatory T cells (Tregs) while lenalidomide has been shown to inhibit Treg proliferation and suppressive function in vitro51, 52, The immune-modulatory effects of these pre-treatment regimens may explain the respective negative and positive associations with risk of ES. A detailed analysis of in vivo immune modulatory effects of broad cytotoxic regimens and more targeted therapies (i.e., bortezomib and lenalidomide) may shed light on the role of immune ablation in ES.
Engraftment of neutrophils and other myeloid derived cells generally occurs within 2 weeks while T cell engraftment is not complete until 2 months after autologous transplant with CD34+ peripheral blood stem cells53. ES is defined, in part, by early presentation of symptoms that coincide with neutrophil engraftment. Surprisingly, the presence of neutrophils and other innate immune cells in skin grafts has rarely been assessed. Kennedy et al. noted that AGVHD was evident in their CsA-treated patients prior to the appearance of leukocytes in the blood54. This is in sharp contrast to the rat model of SGVHD where T cells are fully reconstituted prior to CsA treatment. Lymphocytic infiltrates have been noted in biopsies from patients with more aggressive AGVHD. In the study by Goddard et al., the biopsy was taken 41 days post-transplant when T cells reconstitution would be nearly complete22. Our group has also noted lymphocytic infiltrates at 26 days post-transplant13. Lee et al. performed immunohistochemistry to characterize infiltrating immune cells in skin biopsies from patients that developed ES following bone marrow or peripheral stem cell transplants. Perivascular mononuclear cells infiltrates were composed primarily of cells expressing CD2 (NK and T cells), CD4 and CD3 (T cells), and CD5 (B and T cells), and showed low levels of CD8 (T, NK, and dendritic cells), CD16 (neutrophils and NK cells), and CD56 (NK cells). Importantly, the presence of contaminating lymphocytes in the transplant was not assessed and the time of biopsy acquisition was not reported1. A prospective, detailed analysis of the cellular infiltrate in skin biopsies following ASCT with purified CD34+ cells would shed light on the role of both the innate and adaptive immune response in ES development and progression.
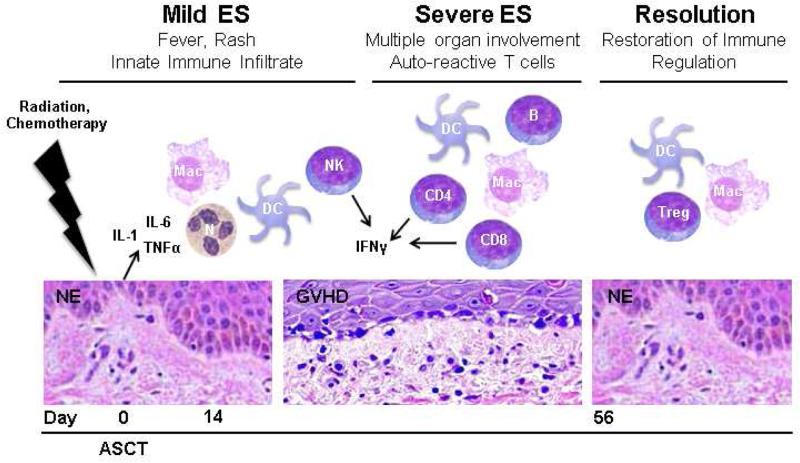
Overall, mechanistic studies of ES in humans have been sparse. We suggest that, even in the absence of HLA mismatch, the principles of allogeneic GVHD may be applicable to ES55 (Figure 2). Pro-inflammatory cytokines (i.e., IL-1, TNFα, and IFNγ) and innate immune cells likely play a role in the initial stages of ES6, 56. These cytokines predispose patients to heightened antigen presentation and T cell activation in the allogeneic setting and may contribute to a break in peripheral tolerance in the autologous setting. Spontaneous resolution in mild cases of ES likely coincides with tissue repair and completion of engraftment. More aggressive presentations of ES may occur in patients that have a predisposition to self-reactivity as the lymphocytes are reconstituted. In these patients, T cells that recognize self-MHC and self-peptide may evade central tolerance and become cytotoxic in the absence of effective peripheral regulation. At later stages of engraftment, peripheral regulatory mechanisms would be restored, allowing spontaneous remission of symptoms. Those patients that succumb to ES may fail to regenerate these regulatory mechanisms. Of note, efficient Treg reconstitution has been associated with reduced risk of allogeneic GVHD57. Future characterization of the cytokines milieu, immune infiltrate, and Tregs in patients with ES is necessary to validate this hypothetical model.
Figure 2. Hypothetical mechanisms of engraftment syndrome.
Proposed stages and severities of ES following ASCT are modeled. Predicted cytokine and leukocyte involvement is shown for each stage. Neutrophil and T cell engraftments are complete by days 14 and 56, respectively. N, neutrophil; DC, dendritic cell, Mac, macrophage; NK, NK cell; CD4, helper T cell; CD8, cytotoxic T cell; B, B cell; Treg, regulatory T cell; NE, normal epithelium; GVHD, graft versus host disease histology.
Clinical Implications of Engraftment Syndrome
It has been postulated that an ES-associated graft-versus-tumor effect may ensue in patients undergoing ASCT, contributing to improved survival in cancer patients58. Survival outcomes are rarely reported in the ES literature. In a single case report, Byrne et al. reported a possible graft-versus-myeloma effect, as evidenced by a reduction in Bence-Jones protein excretion to 0.08 g/dl and less than 2% plasma cell infiltration in a bone marrow aspirate59. In sharp contrast to this case, a review of 461 lymphoma patients by Keung et al. failed to show improved survival in patients that developed ES, and, instead, revealed an association between ES/AGVHD and the development of a secondary myelodysplastic syndrome31. Similarly, Khan et al. studied the association between disease outcome and ES in 85 patients with breast cancer and reported that disease-related mortality was higher in relapsed patients that developed ES during ASCT60. Our review of 591 patients undergoing ASCT for myeloma or lymphoma found no association between development of ES and mortality, relapse, or survival9.
Early studies, performed shortly after the discovery of autologous and syngeneic GVHD, attempted to augment this phenomenon in order to generate an anti-tumor effect47. As seen in the rat models of CsA-induced AGVHD, Yeager et al. reported that 15/19 (79%) patients undergoing SCT for CML developed ES/AGVHD following post-transplant treatment with 1 mg/kg CsA61. Kennedy et al. found that CsA induced ES/AGVHD in a dose-dependent manner in patients with breast cancer. One of 7 (14%), 21 of 31 (68%), and 12 of 13 (92%) patients developed ES/AGVHD when treated with 1 mg/kg, 2.5 mg/kg, or 3.75 mg/kg, respectively54. While the rate of ES/AGVHD induction with 2.5 mg/kg CsA was not increased with the addition of IFNγ (56%), a larger proportion of patients developed a more severe rash (Grades 2 and 3)62. A small study (n=17) by Ratanatharathorn et al. suggested that IFNα treatment could enhance CsA-induced ES/AGVHD. Specifically, the incidence of grade II/III GVHD increased from 50% (2/4) in CsA-induced patients to 100% (8/8) in those receiving CsA in combination with IFNα. Of note, 4/4 patients that received IFNα alone also developed grade II/III GVHD63. Bolanos et al. reported the first controlled trial comparing untreated or CsA-induced patients64. Fifty-one patients with lymphoma were evaluated in a prospective trial for any benefit of CsA-induced AGVHD. Unfortunately, only 4 of the 24 patients that received CsA developed AGVHD, and no conclusions could be drawn from such a small population.
Controversy over the Existence of Engraftment Syndrome
Investigators who question the premise that ES is a true clinical entity have introduced additional controversy in the field. The major arguments for this stance are that ES is an artifact of non-transplant related complications (i.e., infection and pre-treatment toxicity), that the features of ES and AGVHD overlap, and that the incidence of ES is not uniform among the disease subtypes65. While it is possible that some cases of ES may be explained by post-transplant complications, in the most stereotypical cases of ES, the onset of fever, rash, and diarrhea invariably occurs during or after neutrophil engraftment when the risk of infection is actually decreased. In our experience, many of these patients failed to exhibit evidence of infection prior to or concurrent with the development of ES. Furthermore, ES does not occur during bone marrow recovery in a non-transplant setting (i.e., chemotherapy), suggesting that stem cell transplant is required. Secondly, in those patients for whom biopsies are performed for evaluation of diarrhea, the pathological findings are typically indistinguishable from what is observed with allogeneic GVHD. As discussed above, we believe that ES encompasses a continuum of disease severities, including more severe, sometimes fatal, cases with histological evidence of GVHD. We suggest that the overlap of symptoms with AGVHD, on the contrary, proves the existence of ES. This controversy is rooted in semantics of nomenclature rather than in evidence of clinical relevance. Thirdly, the lack of a uniform incidence across disease subtypes can be explained by differences in the underlying disease, prior therapy, and inherent patient susceptibility. We would concur with others that the pathophysiology of ES requires further elucidation. Our view remains that ES is a complication that occurs in a significant percentage of autologous stem cell transplant recipients. Appropriate awareness and management of this problem is essential to reduce patient morbidity and, in some cases, mortality.
Our Management Approach to Engraftment Syndrome
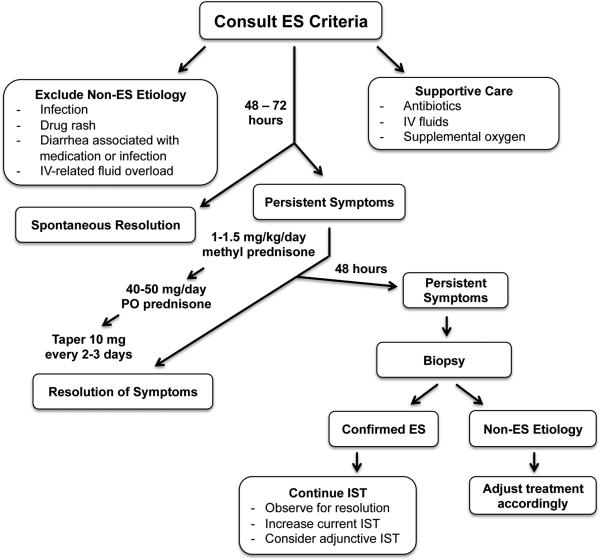
The most important aspect in management of ES is early recognition of the defined clinical features according to the Spitzer and Maiolino criteria (Table 1). It is important to recognize that a subset of patients, including those with POEMS, may have ES up to 7 days prior to engraftment may not adhere to these criteria8. Also, in patients that present with more severe symptoms, early signs of ES at the time of neutrophil engraftment may have been overlooked. In these cases, a diagnosis of ES should be suspected based on the clinical criteria regardless of the timing of symptoms relative to engraftment. In our study, approximately 95% of patients developed symptoms consistent with ES between 3 days prior and 7 days following engraftment.
Our first step in management of suspected ES is to exclude alternative causes (Figure 3). Patients should be treated with broad-spectrum antibiotics while ruling out infection. In addition, the potential etiology for drug-induced rash should be assessed. Causes for medicationand infection-induced diarrhea should be evaluated. Features of capillary leak and fluid overload should be confirmed to be independent of IV fluid administration. Brain-natriuretic peptide (BNP) assessment can be helpful to evaluate for possible cardiac etiology. In many cases, clinical features of ES will resolve spontaneously. If symptoms are not improved after 48 to 72 hours a diagnosis of ES should be considered. The decision to treat is based upon the severity of symptoms and judgment of the treatment provider that other etiologies are excluded and treatment is warranted. When treatment is indicated, we suggest initiation of methylprednisolone 1-1.5 mg/kg/day until the symptoms are resolving; which typically occurs within 2 to 3 days, followed by a reduction to 40-50mg PO prednisone/day for 2 to 3 days. PO prednisone can then be tapered 10 mg every 2 to 3 days as long as symptoms continue to resolve. Alternatively, methylprednisolone may be reduced to 0.5 mg/kg/day, followed by a slower taper of 10mg/day. We find that early intervention with corticosteroids is sufficient to mitigate progression to more severe manifestations9. If clinical features have not improved 72 hours after initiation of corticosteroids, further evaluation is warranted. In corticosteroid-refractory cases, we recommend biopsy of the site of end-organ damage: skin biopsy for skin rash, colon biopsy for severe diarrhea and/or liver biopsy for LFT abnormalities. If the diagnosis of ES is confirmed histologically, additional immune suppressants should be administered until symptoms subside. If an alternative etiology is found, patients should be managed accordingly.
Figure 3. Our Management Approach to Engraftment Syndrome.
IST; immune suppressive therapy.
Future directions
Future studies are required to better characterize the risk factors and underlying mechanisms in ES. Development of a grading system, such as that for NIH criteria for acute and chronic GVHD, would be a useful guide for determination of management decisions for ES. Basic immunohistochemistry studies are necessary to identify the specific leukocyte populations (e.g., myeloid lineage, NK cells, Tregs, CD4+ T cells, and CD8+ T cells) contributing to early and late stages of ES. Larger prospective analyses of elafin and inflammatory cytokines in peripheral blood may confirm the role of such biomarkers in predicting ES. New biomarkers may be identified through molecular profiling of archived HSC from ES+ and ES− patients. Collectively, these studies will improve diagnosis and treatment of ES.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the assistance of Jena D. F. Baertschi for review of the manuscript.
This manuscript was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 8UL1TR000055.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
R.F.C. performed the literature review and wrote the manuscript; P.H. and W.D. substantially contributed to manuscript development and review.
CONFLICT OF INTEREST
The authors declare no competing financial conflicts of interest.
This manuscript or any of its parts have not been presented elsewhere.
References
- 1.Lee CK, Gingrich RD, Hohl RJ, Ajram KA. Engraftment syndrome in autologous bone marrow and peripheral stem cell transplantation. Bone marrow transplantation. 1995;16(1):175–182. [PubMed] [Google Scholar]
- 2.Carreras E, Fernandez-Aviles F, Silva L, Guerrero M, Fernandez de Larrea C, Martinez C. Engraftment syndrome after auto-SCT: analysis of diagnostic criteria and risk factors in a large series from a single center. Bone marrow transplantation. 2010;45(9):1417–1422. doi: 10.1038/bmt.2009.363. doi: 10.1038/bmt.2009.363. [DOI] [PubMed] [Google Scholar]
- 3.Foncillas MA, Diaz MA, Sevilla J, Gonzalez Vicent M, Fernandez-Plaza S, Perez A, et al. Engraftment syndrome emerges as the main cause of transplant-related mortality in pediatric patients receiving autologous peripheral blood progenitor cell transplantation. Journal of pediatric hematology/oncology. 2004;26(8):492–496. doi: 10.1097/01.mph.0000130217.41531.fb. [DOI] [PubMed] [Google Scholar]
- 4.Lopes da Silva R, Costa F, Ferreira G, de Sousa AB. Post-autologous hematopoietic SCT engraftment syndrome: a single center experience. Bone marrow transplantation. 2012;47(3):456–457. doi: 10.1038/bmt.2011.92. doi: 10.1038/bmt.2011.92; 10.1038/bmt.2011.92. [DOI] [PubMed] [Google Scholar]
- 5.Maiolino A, Biasoli I, Lima J, Portugal AC, Pulcheri W, Nucci M. Engraftment syndrome following autologous hematopoietic stem cell transplantation: definition of diagnostic criteria. Bone marrow transplantation. 2003;31(5):393–397. doi: 10.1038/sj.bmt.1703855. doi: 10.1038/sj.bmt.1703855. [DOI] [PubMed] [Google Scholar]
- 6.Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone marrow transplantation. 2001;27(9):893–898. doi: 10.1038/sj.bmt.1703015. doi: 10.1038/sj.bmt.1703015. [DOI] [PubMed] [Google Scholar]
- 7.Capizzi SA, Kumar S, Huneke NE, Gertz MA, Inwards DJ, Litzow MR, et al. Periengraftment respiratory distress syndrome during autologous hematopoietic stem cell transplantation. Bone marrow transplantation. 2001;27(12):1299–1303. doi: 10.1038/sj.bmt.1703075. doi: 10.1038/sj.bmt.1703075. [DOI] [PubMed] [Google Scholar]
- 8.Dispenzieri A, Lacy MQ, Hayman SR, Kumar SK, Buadi F, Dingli D, et al. Peripheral blood stem cell transplant for POEMS syndrome is associated with high rates of engraftment syndrome. European journal of haematology. 2008;80(5):397–406. doi: 10.1111/j.1600-0609.2008.01037.x. doi: 10.1111/j.1600-0609.2008.01037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornell RF, Hari P, Zhang MJ, Zhong X, Thompson J, Fenske TS, et al. Divergent effects of novel immunomodulatory agents and cyclophosphamide on the risk of engraftment syndrome after autologous peripheral blood stem cell transplantation for multiple myeloma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19(9):1368–1373. doi: 10.1016/j.bbmt.2013.06.017. doi: 10.1016/j.bbmt.2013.06.017; 10.1016/j.bbmt.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Fassas AB, Miceli MH, Grazzlutti M, Dong L, Barlogie B, Anaissie E. Serial measurement of serum C-reactive protein levels can identify patients at risk for severe complications following autologous stem cell transplantation. Leukemia & lymphoma. 2005;46(8):1159–1161. doi: 10.1080/10428190500086121. doi: 10.1080/10428190500086121. [DOI] [PubMed] [Google Scholar]
- 11.Chacon AH, Farooq U, Shiman MI, Elgart GW. Elafin: a possible new biomarker and immunohistochemical stain for pre-engraftment syndrome. J Am Acad Dermatol. 2013;69(2):e102–103. doi: 10.1016/j.jaad.2012.11.024. e-pub ahead of print 2013/07/23; doi: 10.1016/j.jaad.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Cogbill CH, Drobyski WR, Komorowski RA. Gastrointestinal pathology of autologous graft-versus-host disease following hematopoietic stem cell transplantation: a clinicopathological study of 17 cases. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24(1):117–125. doi: 10.1038/modpathol.2010.163. doi: 10.1038/modpathol.2010.163. [DOI] [PubMed] [Google Scholar]
- 13.Drobyski WR, Hari P, Keever-Taylor C, Komorowski R, Grossman W. Severe autologous GVHD after hematopoietic progenitor cell transplantation for multiple myeloma. Bone marrow transplantation. 2009;43(2):169–177. doi: 10.1038/bmt.2008.295. doi: 10.1038/bmt.2008.295. [DOI] [PubMed] [Google Scholar]
- 14.Esteban JM, Somlo G. Skin biopsy in allogeneic and autologous bone marrow transplant patients: a histologic and immunohistochemical study and review of the literature. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1995;8(1):59–64. [PubMed] [Google Scholar]
- 15.Ross WA, Ghosh S, Dekovich AA, Liu S, Ayers GD, Cleary KR, et al. Endoscopic biopsy diagnosis of acute gastrointestinal graft-versus-host disease: rectosigmoid biopsies are more sensitive than upper gastrointestinal biopsies. The American Journal of Gastroenterology. 2008;103(4):982–989. doi: 10.1111/j.1572-0241.2007.01639.x. doi: 10.1111/j.1572-0241.2007.01639.x. [DOI] [PubMed] [Google Scholar]
- 16.Washington K, Jagasia M. Pathology of graft-versus-host disease in the gastrointestinal tract. Hum Pathol. 2009;40(7):909–917. doi: 10.1016/j.humpath.2009.04.001. e-pub ahead of print 2009/06/16; doi: 10.1016/j.humpath.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Cahill RA, Spitzer TR, Mazumder A. Marrow engraftment and clinical manifestations of capillary leak syndrome. Bone marrow transplantation. 1996;18(1):177–184. [PubMed] [Google Scholar]
- 18.Moreb JS, Kubilis PS, Mullins DL, Myers L, Youngblood M, Hutcheson C. Increased frequency of autoaggression syndrome associated with autologous stem cell transplantation in breast cancer patients. Bone marrow transplantation. 1997;19(2):101–106. doi: 10.1038/sj.bmt.1700615. doi: 10.1038/sj.bmt.1700615. [DOI] [PubMed] [Google Scholar]
- 19.Rappeport J, Mihm M, Reinherz E, Lopansri S, Parkman R. Acute graft-versus-host disease in recipients of bone-marrow transplants from identical twin donors. Lancet. 1979;2(8145):717–720. doi: 10.1016/s0140-6736(79)90644-5. e-pub ahead of print 1979/10/06. [DOI] [PubMed] [Google Scholar]
- 20.Hood AF, Vogelsang GB, Black LP, Farmer ER, Santos GW. Acute graft-vs-host disease. Development following autologous and syngeneic bone marrow transplantation. Archives of Dermatology. 1987;123(6):745–750. doi: 10.1001/archderm.123.6.745. [DOI] [PubMed] [Google Scholar]
- 21.Slavin RE, Santos GW. The graft versus host reaction in man after bone marrow transplantation: pathology, pathogenesis, clinical features, and implication. Clinical immunology and immunopathology. 1973;1(4):472–498. doi: 10.1016/0090-1229(73)90005-6. e-pub ahead of print 1973/07/01. [DOI] [PubMed] [Google Scholar]
- 22.Goddard DS, Ruben BS, Mathes ED, Nixon M, Wolf J, Fox LP. A case of severe cutaneous, GI and liver GVHD in a patient with multiple myeloma, status-post-second auto-SCT. Bone marrow transplantation. 2010;45(2):409–411. doi: 10.1038/bmt.2009.157. doi: 10.1038/bmt.2009.157. [DOI] [PubMed] [Google Scholar]
- 23.Holmberg L, Kikuchi K, Gooley TA, Adams KM, Hockenbery DM, Flowers ME, et al. Gastrointestinal graft-versus-host disease in recipients of autologous hematopoietic stem cells: incidence, risk factors, and outcome. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2006;12(2):226–234. doi: 10.1016/j.bbmt.2005.10.011. doi: 10.1016/j.bbmt.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Kline J, van Besien K, Nathanson J, Noffsinger A, Artz A. Severe intestinal graft-versus-host disease following autologous stem cell transplantation. Bone marrow transplantation. 2006;38(5):391–392. doi: 10.1038/sj.bmt.1705460. doi: 10.1038/sj.bmt.1705460. [DOI] [PubMed] [Google Scholar]
- 25.Spitzer TR. Engraftment syndrome: double-edged sword of hematopoietic cell transplants. Bone Marrow Transplant. 2015 doi: 10.1038/bmt.2014.296. e-pub ahead of print 2015/01/13; doi: 10.1038/bmt.2014.296. [DOI] [PubMed] [Google Scholar]
- 26.Katzel JA, Mazumder A, Jagannath S, Vesole DH. Engraftment syndrome after hematopoietic stem cell transplantation in multiple myeloma. Clinical lymphoma & myeloma. 2006;7(2):151. doi: 10.1016/S1557-9190(11)70311-0. doi: 10.1016/S1557-9190(11)70311-0. [DOI] [PubMed] [Google Scholar]
- 27.Giralt S, Weber D, Colome M, Dimopoulos M, Mehra R, Van Besien K, et al. Phase I trial of cyclosporine-induced autologous graft-versus-host disease in patients with multiple myeloma undergoing high-dose chemotherapy with autologous stem-cell rescue. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15(2):667–673. doi: 10.1200/JCO.1997.15.2.667. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez-Zepeda VH, Trudel S, Reece DE, Chen C, Rabea AM, Kukreti V. Cyclophosphamide and prednisone induction followed by cyclophosphamide mobilization effectively decreases the incidence of engraftment syndrome in patients with POEMS syndrome who undergo stem cell transplantation. American Journal of Hematology. 2011;86(10):873–875. doi: 10.1002/ajh.22115. doi: 10.1002/ajh.22115; 10.1002/ajh.22115. [DOI] [PubMed] [Google Scholar]
- 29.Irazabal MV, Eirin A, Gertz MA, Dispenzieri A, Kumar S, Buadi FK, et al. Acute kidney injury during leukocyte engraftment after autologous stem cell transplantation in patients with light-chain amyloidosis. American Journal of Hematology. 2012;87(1):51–54. doi: 10.1002/ajh.22202. doi: 10.1002/ajh.22202; 10.1002/ajh.22202. [DOI] [PubMed] [Google Scholar]
- 30.Sanchorawala V, Brauneis D, Shelton AC, Lo S, Sun F, Sloan JM, et al. Induction Therapy with Bortezomib Followed by Bortezomib-High Dose Melphalan and Stem Cell Transplantation for Light Chain Amyloidosis: Results of a Prospective Clinical Trial. Biol Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.04.001. e-pub ahead of print 2015/04/11; doi: 10.1016/j.bbmt.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Keung YK, Beaty MW, Pettenati M, Levitan D, Hurd DD. Possible role of engraftment syndrome and autologous graft-versus-host disease in myelodysplastic syndrome after autologous stem cell transplantations: retrospective analysis and review of the literature. Clinical lymphoma, myeloma & leukemia. 2010;10(2):129–133. doi: 10.3816/CLML.2010.n.018. doi: 10.3816/CLML.2010.n.018. [DOI] [PubMed] [Google Scholar]
- 32.Carreras E, Saiz A, Marin P, Martinez C, Rovira M, Villamor N, et al. CD34+ selected autologous peripheral blood stem cell transplantation for multiple sclerosis: report of toxicity and treatment results at one year of follow-up in 15 patients. Haematologica. 2003;88(3):306–314. [PubMed] [Google Scholar]
- 33.Edenfield WJ, Moores LK, Goodwin G, Lee N. An engraftment syndrome in autologous stem cell transplantation related to mononuclear cell dose. Bone marrow transplantation. 2000;25(4):405–409. doi: 10.1038/sj.bmt.1702155. doi: 10.1038/sj.bmt.1702155. [DOI] [PubMed] [Google Scholar]
- 34.Akasheh M, Eastwood D, Vesole DH. Engraftment syndrome after autologous hematopoietic stem cell transplant supported by granulocyte-colony-stimulating factor (G-CSF) versus granulocyte-macrophage colony-stimulating factor (GM-CSF). Bone Marrow Transplant. 2003;31(2):113–116. doi: 10.1038/sj.bmt.1703784. e-pub ahead of print 2003/03/07; doi: 10.1038/sj.bmt.1703784. [DOI] [PubMed] [Google Scholar]
- 35.Tuazon S, Daskalakis C, Mandala A, Saini N, O'Hara WJ, Alpdogan O, et al. Comparison of Engraftment Syndrome with G-CSF Versus GM-CSF after Autologous Hematopoietic Progenitor Cell Transplantation for Multiple Myeloma.. Poster presented at: American Society for Blood and Marrow Transplantation BMT Tandem Meetings; San Diego, CA. 2015. [Google Scholar]
- 36.Ravoet C, Feremans W, Husson B, Majois F, Kentos A, Lambermont M, et al. Clinical evidence for an engraftment syndrome associated with early and steep neutrophil recovery after autologous blood stem cell transplantation. Bone marrow transplantation. 1996;18(5):943–947. [PubMed] [Google Scholar]
- 37.Lazarus HM, Sommers SR, Arfons LM, Fu P, Ataergin SA, Kaye NM, et al. Spontaneous autologous graft-versus-host disease in plasma cell myeloma autograft recipients: flow cytometric analysis of hematopoietic progenitor cell grafts. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(7):970–978. doi: 10.1016/j.bbmt.2011.03.005. doi: 10.1016/j.bbmt.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Otegbeye F, Gale RP, Lazarus HM. Autologous GVHD? Bone Marrow Transplant. 2014;49(11):1349–1351. doi: 10.1038/bmt.2014.169. e-pub ahead of print 2014/08/05; doi: 10.1038/bmt.2014.169. [DOI] [PubMed] [Google Scholar]
- 39.Glazier A, Tutschka PJ, Farmer ER, Santos GW. Graft-versus-host disease in cyclosporin A-treated rats after syngeneic and autologous bone marrow reconstitution. J Exp Med. 1983;158(1):1–8. doi: 10.1084/jem.158.1.1. e-pub ahead of print 1983/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hess AD, Horwitz L, Beschorner WE, Santos GW. Development of graft-vs.-host disease-like syndrome in cyclosporine-treated rats after syngeneic bone marrow transplantation. I. Development of cytotoxic T lymphocytes with apparent polyclonal anti-Ia specificity, including autoreactivity. J Exp Med. 1985;161(4):718–730. doi: 10.1084/jem.161.4.718. e-pub ahead of print 1985/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tutschka PJ, Berkowitz SD, Tuttle S, Klein J. Graft-versus-leukemia in the rat--the antileukemic efficacy of syngeneic and allogeneic graft-versus-host disease. Transplantation proceedings. 1987;19(1 Pt 3):2668–2673. e-pub ahead of print 1987/02/01. [PubMed] [Google Scholar]
- 42.Hess AD, Fischer AC, Beschorner WE. Effector mechanisms in cyclosporine A-induced syngeneic graft-versus-host disease. Role of CD4+ and CD8+ T lymphocyte subsets. J Immunol. 1990;145(2):526–533. e-pub ahead of print 1990/07/15. [PubMed] [Google Scholar]
- 43.Hess AD, Horwitz LR, Laulis MK, Fuchs E. Cyclosporine-induced syngeneic graft-vs-host disease: prevention of autoaggression by treatment with monoclonal antibodies to T lymphocyte cell surface determinants and to MHC class II antigens. Clinical immunology and immunopathology. 1993;69(3):341–350. doi: 10.1006/clin.1993.1190. e-pub ahead of print 1993/12/01. [DOI] [PubMed] [Google Scholar]
- 44.Fischer AC, Ruvolo PP, Burt R, Horwitz LR, Bright EC, Hess JM, et al. Characterization of the autoreactive T cell repertoire in cyclosporin-induced syngeneic graft-versus-host disease. A highly conserved repertoire mediates autoaggression. Journal of immunology (Baltimore, Md.: 1950) 1995;154(8):3713–3725. [PubMed] [Google Scholar]
- 45.Jenkins MK, Schwartz RH, Pardoll DM. Effects of cyclosporine A on T cell development and clonal deletion. Science. 1988;241(4873):1655–1658. doi: 10.1126/science.241.4873.1655. e-pub ahead of print 1988/09/23. [DOI] [PubMed] [Google Scholar]
- 46.Fischer AC, Laulis MK, Horwitz L, Beschorner WE, Hess A. Host resistance to cyclosporine induced syngeneic graft-versus-host disease. Requirement for two distinct lymphocyte subsets. J Immunol. 1989;143(3):827–832. e-pub ahead of print 1989/08/01. [PubMed] [Google Scholar]
- 47.Jones RJ, Vogelsang GB, Hess AD, Farmer ER, Mann RB, Geller RB, et al. Induction of graft-versus-host disease after autologous bone marrow transplantation. Lancet. 1989;1(8641):754–757. doi: 10.1016/s0140-6736(89)92575-0. [DOI] [PubMed] [Google Scholar]
- 48.Hess AD, Bright EC, Thoburn C, Vogelsang GB, Jones RJ, Kennedy MJ. Specificity of effector T lymphocytes in autologous graft-versus-host disease: role of the major histocompatibility complex class II invariant chain peptide. Blood. 1997;89(6):2203–2209. e-pub ahead of print 1997/03/15. [PubMed] [Google Scholar]
- 49.Massumoto C, Benyunes MC, Sale G, Beauchamp M, York A, Thompson JA, et al. Close simulation of acute graft-versus-host disease by interleukin-2 administered after autologous bone marrow transplantation for hematologic malignancy. Bone Marrow Transplant. 1996;17(3):351–356. e-pub ahead of print 1996/03/01. [PubMed] [Google Scholar]
- 50.Reinherz EL, Rubinstein A, Geha RS, Strelkauskas AJ, Rosen FS, Schlossman SF. Abnormalities of immunoregulatory T cells in disorders of immune function. N Engl J Med. 1979;301(19):1018–1022. doi: 10.1056/NEJM197911083011902. e-pub ahead of print 1979/11/08; doi: 10.1056/NEJM197911083011902. [DOI] [PubMed] [Google Scholar]
- 51.Condomines M, Quittet P, Lu ZY, Nadal L, Latry P, Lopez E, et al. Functional regulatory T cells are collected in stem cell autografts by mobilization with high-dose cyclophosphamide and granulocyte colony-stimulating factor. Journal of immunology (Baltimore, Md.: 1950) 2006;176(11):6631–6639. doi: 10.4049/jimmunol.176.11.6631. [DOI] [PubMed] [Google Scholar]
- 52.Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer immunology, immunotherapy : CII. 2009;58(7):1033–1045. doi: 10.1007/s00262-008-0620-4. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steingrimsdottir H, Gruber A, Bjorkholm M, Svensson A, Hansson M. Immune reconstitution after autologous hematopoietic stem cell transplantation in relation to underlying disease, type of high-dose therapy and infectious complications. Haematologica. 2000;85(8):832–838. e-pub ahead of print 2000/08/16. [PubMed] [Google Scholar]
- 54.Kennedy MJ, Vogelsang GB, Beveridge RA, Farmer ER, Altomonte V, Huelskamp AM. Phase I trial of intravenous cyclosporine to induce graft-versus-host disease in women undergoing autologous bone marrow transplantation for breast cancer. J Clin Oncol. 1993;11(3):478–484. doi: 10.1200/JCO.1993.11.3.478. e-pub ahead of print 1993/03/01. [DOI] [PubMed] [Google Scholar]
- 55.Socie G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124(3):374–384. doi: 10.1182/blood-2014-01-514752. e-pub ahead of print 2014/06/11; doi: 10.1182/blood-2014-01-514752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jadus MR, Wepsic HT. The role of cytokines in graft-versus-host reactions and disease. Bone Marrow Transplant. 1992;10(1):1–14. e-pub ahead of print 1992/07/01. [PubMed] [Google Scholar]
- 57.Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108(4):1291–1297. doi: 10.1182/blood-2006-02-003996. e-pub ahead of print 2006/04/22; doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santos GW. Autologous graft-versus-host disease. Leukemia. 1992;6(Suppl 4):110–111. e-pub ahead of print 1992/11/01. [PubMed] [Google Scholar]
- 59.Byrne JL, Carter GI, Ellis I, Haynes AP, Russell NH. Autologous GVHD following PBSCT, with evidence for a graft-versus-myeloma effect. Bone marrow transplantation. 1997;20(6):517–520. doi: 10.1038/sj.bmt.1700922. doi: 10.1038/sj.bmt.1700922. [DOI] [PubMed] [Google Scholar]
- 60.Khan SA, Gaa B, Pollock BH, Shea B, Reddy V, Wingard JR, et al. Engraftment syndrome in breast cancer patients after stem cell transplantation is associated with poor long-term survival. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2001;7(8):433–438. doi: 10.1016/s1083-8791(01)80010-0. [DOI] [PubMed] [Google Scholar]
- 61.Yeager AM, Vogelsang GB, Jones RJ, Farmer ER, Hess AD, Santos GW. Cyclosporine-induced graft-versus-host disease after autologous bone marrow transplantation for acute myeloid leukemia. Leuk Lymphoma. 1993;11(3-4):215–220. doi: 10.3109/10428199309086998. e-pub ahead of print 1993/10/01; doi: 10.3109/10428199309086998. [DOI] [PubMed] [Google Scholar]
- 62.Kennedy MJ, Vogelsang GB, Jones RJ, Farmer ER, Hess AD, Altomonte V, et al. Phase I trial of interferon gamma to potentiate cyclosporine-induced graft-versus-host disease in women undergoing autologous bone marrow transplantation for breast cancer. J Clin Oncol. 1994;12(2):249–257. doi: 10.1200/JCO.1994.12.2.249. e-pub ahead of print 1994/02/01. [DOI] [PubMed] [Google Scholar]
- 63.Ratanatharathorn V, Uberti J, Karanes C, Lum LG, Abella E, Dan ME, et al. Phase I study of alpha-interferon augmentation of cyclosporine-induced graft versus host disease in recipients of autologous bone marrow transplantation. Bone marrow transplantation. 1994;13(5):625–630. [PubMed] [Google Scholar]
- 64.Bolanos-Meade J, Garrett-Mayer E, Luznik L, Anders V, Webb J, Fuchs EJ, et al. Induction of autologous graft-versus-host disease: results of a randomized prospective clinical trial in patients with poor risk lymphoma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13(10):1185–1191. doi: 10.1016/j.bbmt.2007.06.011. doi: 10.1016/j.bbmt.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gale RP, Lazarus HM. Engraftment syndrome, the Emperor's new clothes and the artist formerly known as prince. Bone Marrow Transplant. 2015 doi: 10.1038/bmt.2014.317. e-pub ahead of print 2015/02/03; doi: 10.1038/bmt.2014.317. [DOI] [PubMed] [Google Scholar]