Summary
Background
Immune tolerance induction (ITI) in patients with congenital hemophilia A is successful in up to 70%. Although there is growing understanding of predictors of response to ITI, the probability and predictors of inhibitor recurrence following successful ITI are not well understood.
Objectives
To determine the association of clinical characteristics, particularly adherence to FVIII prophylaxis following ITI, with inhibitor recurrence in patients with hemophilia A who were considered tolerant following ITI.
Methods
In this multicenter retrospective cohort study, 64 subjects with FVIII level <2% who were considered successfully tolerant following ITI were analyzed to estimate the cumulative probability of inhibitor recurrence using the Kaplan-Meier method. The association of clinical characteristics with inhibitor recurrence was assessed using logistic regression.
Results
A recurrent inhibitor titer ≥ 0.6 BU/ml occurred at least once in 19 (29.7%) and more than once in 12 (18.8%). The probability of any recurrent inhibitor at 1 and 5 years was 12.8% and 32.5% respectively. Having a recurrent inhibitor was associated with having received immune modulation during ITI (OR 3.8, 95% CI: 1.2-22.4) and FVIII recovery of <85% at the end of ITI (OR 2.6, 95% CI: 1.3-5.9), but was not associated with adherence to post-ITI prophylactic FVIII infusion (OR=0.5, 95% CI: 0.06-4.3).
Conclusions
The use of immune modulation therapy during ITI and lower FVIII recovery at the end of ITI appear to be associated with an increased risk of inhibitor recurrence following successful ITI. Adherence to post-ITI prophylactic FVIII infusions is not a major determinant of recurrence.
Keywords: immune tolerance, hemophilia, incidence, recurrence, prophylaxis
Introduction
Formation of anti-factor VIII (FVIII) inhibitory antibodies (inhibitor) is the major complication of treatment of hemophilia A. Currently, immune tolerance induction (ITI) via regular infusion of FVIII is the most effective method to eradicate the inhibitor and is successful in about 70% of patients [1, 2]. ITI outcomes are influenced by both host and treatment regimen-related factors. Characteristics that are considered to be predictors of successful ITI include starting ITI at a young age, short duration between the onset of inhibitor and start of ITI, lower historical peak inhibitor titer (<200 BU/ml), lower inhibitor titer just prior to the start of ITI (<10 BU/ml), lower peak inhibitor titer on ITI (<100 BU/ml) and low risk F8 gene mutations such as small insertions, small deletions, and missense mutations [1-3]. Once tolerance is achieved, little is known about the probability of inhibitor recurrence or the clinical or treatment-related characteristics that are influential in maintaining tolerance. In clinical practice, continued regular exposure to FVIII (prophylactic treatment) is thought to be imperative for maintaining tolerance, but this has not been formally evaluated.
The overall purpose of this study is to estimate predictors of inhibitor recurrence after successful ITI and to determine the impact of poor adherence to prophylactic FVIII treatment following ITI and other clinical characteristics on inhibitor recurrence.
Methods and patients
Participants
After Institutional Review Board approval at 12 US Comprehensive Hemophilia Treatment centers, potential subjects were identified by review of patient databases. Patients with hemophilia A and a history of an inhibitor who successfully completed a course of ITI using a locally prescribed regimen between 1/1/1998 and 8/15/2010 were enrolled in the study. For this analysis, only subjects with negative inhibitor titer and normalized FVIII recovery (>66%) and/or FVIII half-life (>6 hours) to document successful tolerance were included.
Study Variables
Data for clinical demographic variables was obtained through retrospective review of the medical record and recorded on a standardized case report form. The primary outcome was FVIII inhibitor recurrence defined as any inhibitor titer ≥0.6 BU/ml following successful ITI. A secondary definition of inhibitor recurrence was 2 or more elevated inhibitor titers (≥0.6 BU/ml). The primary independent variable of interest was adherence during the 6 months prior to inhibitor recurrence or the last negative inhibitor titer. Adherence was determined by comparing the actual treatment regimen (as determined by infusion logs/calendars and/or pharmacy information) with the prescribed treatment regimen [(# actual infusions/#prescribed infusions) × 100]. Subjects were considered adherent to post-ITI prophylaxis if they had received > 80% of their prescribed infusions [4]. Other variables collected included: subject demographics (year of birth, race, ethnicity, family history of hemophilia and inhibitor), hemophilia history (FVIII level, F8 mutation: high risk mutations were those including intron 22, and intron 1 inversion, large deletions [>50 base pairs] and nonsense mutations) [5, 6], inhibitor characteristics (initial inhibitor titer, peak inhibitor titer prior to the start of ITI [historical peak], inhibitor titer at the start of ITI), characteristics of ITI regimen (product used, whether the reported successful course of ITI was the first course of ITI, dose category, frequency of infusion, duration, use of concomitant immune modulating agents, and FVIII recovery and half-life at the end of ITI), seropositivity for HIV or HCV at the time tolerance was achieved.
Statistical Analysis
Descriptive statistics were calculated for all variables. To determine associations between clinical variables and inhibitor recurrence, all variables were initially considered using univariate analysis. For each variable of interest, Chi-square test, Fisher's exact test or Wilcoxon-signed rank tests were used, as appropriate, to examine the relationship between inhibitor recurrence and the potential risk factor.
The cumulative probability of inhibitor recurrence was determined using Kaplan Meier method. The time of follow-up was from confirmation of successful tolerance induction as defined by a negative inhibitor titer and FVIII recovery >66% and/or FVIII half-life > 6 hours and continued until the last recorded negative inhibitor titer (<0.6 BU/ml) or inhibitor recurrence (inhibitor titer ≥0.6 BU/ml). Smoothed survival curves were constructed based on parametric probability estimates derived from the HAZARD procedure in SAS (http://www.clevelandclinic.org/heartcenter/hazard). This procedure uses maximum likelihood estimates to resolve risk distribution of time to event in up to 3 phases of risk (early, constant and late).
The association of adherence and other variables with the outcome of interest, recurrent FVIII inhibitor, was analyzed using a logistic regression model. Cox proportional hazards model was also performed, but since relevant variables violated the Cox proportional hazards assumptions, the logistic model is presented here. In the first model, adherence was the predictor of interest and included in the model. In this model, propensity scoring was used to adjust for variables that were associated with adherence on bivariable analysis and therefore potentially confounding the association between adherence and inhibitor recurrence. Frequency of FVIII infusions during ITI (once or twice daily vs. three to four times a week), duration of ITI and historical peak inhibitor titer were variables included in the propensity score. The propensity score had a receiver operating curve (ROC) of 0.8. A second model was built to evaluate other predictors of inhibitor recurrence by including variables significant on bivariable analysis at the 0.10 level or known to be associated with successful ITI (historical peak titer, pre-ITI titer, peak titer during ITI [< or ≥ 100 BU/ml], time to ITI start [< or ≥ 2 years]). For this model additional dichotomized variables were, FVIII recovery, < or ≥ 85%, after assessing the sensitivity and specificity of multiple different cut points (70-90%) and dose of FVIII used for ITI, < or ≥ than 100 IU/kg. Variables were kept in the model if they were associated with inhibitor recurrence (p<0.10) or confounded the association between another exposure variable and the outcome, inhibitor recurrence. Variables that were not included based on their bivariable association or known association with ITI success were individually evaluated in the model. For multivariable logistic modeling, exact odds ratios are reported with mid-p correct exact p-values. The McFadden R2 was used to measure fit of the logistic regression models. Values between 0.2 and 0.4 indicated good model fit (Louviere et al. 2000, Stated Choice Methods: Analysis and Applications, Cambridge University Press). SAS version 9.3 (Cary, NC) was used to perform all analyses.
Results
One hundred eighteen patients that completed ITI between 1/1/1998 and 8/15/2010 were reviewed to determine if ITI was considered clinically successful or not. Ninety-one patients (77%) were considered by their treating center to have successfully completed ITI based on the local institutional criteria during the 12 year and 8 months time span. Of the latter 91 who were considered to have successfully completed ITI, 7 were excluded due to lack of available documentation of a negative inhibitor titer at the end of ITI, resulting in a total of 84 patients were enrolled on study (Fig.1). Neither FVIII recovery nor FVIII half-life was available in 17 subjects. The population for this analyses was further limited to include only subjects with a FVIII level <2% (n=64), since the biology of disease may be different for those with milder hemophilia and the population was not adequately represented (n=3) to allow for adjustment or comparison. Sixty four patients with FVIII activity <2% who had completed ITI were included in the analysis.
Figure 1. Subject flow chart.
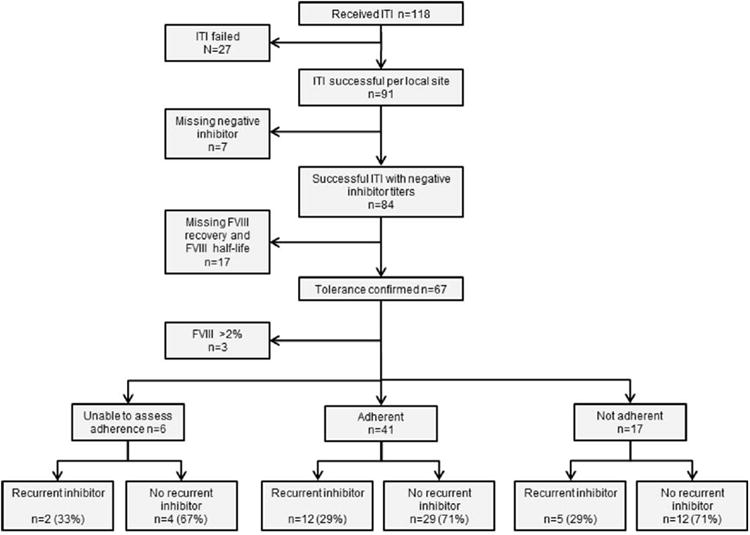
Tolerance was characterized by a negative inhibitor titer and FVIII recovery >66% in 36 (56.3%) patients, a negative inhibitor titer and FVIII half-life without FVIII recovery in 1 (1.6%), and a negative inhibitor titer, FVIII recovery >66%, and FVIII half-life >6 hours in 27 (42.2%). The median age at inhibitor diagnosis was 1.6 years (Q1, Q3: 1, 3.3) and the median age ITI was initiated was 3.3 years (Q1, Q3: 1.5, 8.5) (Table 1). Seventy-two percent of patients (n=46) were White and 18.7% (n=12) were Black race; 25% (n=16) were Hispanic ethnicity. Nearly all patients, 62 (96.9%) had severe FVIII deficiency. Sixty percent (n=39) had a positive family history of hemophilia, 33.3% (n=13) of whom had a sibling, uncle or cousin with a history of an inhibitor. Of the 31 with known F8 mutations; 25 had high risk mutations (18 intron 22 inversion, 1 intron 1 inversion, 2 large deletions, and 4 nonsense mutations) and 6 had low risk mutations (2 small deletion/insertion, 2 splice site and 2 missense mutations). Few patients were HIV (4.7%) or hepatitis C antibody positive (9.4%). A recombinant product was used during ITI in 57 (89%) patients. The daily dose of FVIII used during ITI varied widely and included 2 (3.1%) patients who received <50 IU/kg/day, 13 (20.3%) who received 51-99 IU/kg/day, 42 (65.6%) who received ≥100-199 IU/kg/day and 7 (10.9%) patients who received ≥200 IU/kg/day. The majority of patients, 59 (92.2%), were treated with FVIII infusions alone whereas 5 (7.8%) received at least one immune modulatory therapy in conjunction with ITI (Table 2). None of these 5 treated with immune modulating therapy during the reported successful course of ITI had previously failed a prior attempted course of ITI. In the overall cohort, only 3 subjects had previously received ITI unsuccessfully. Demographic and clinical characteristics of the 20 subjects enrolled but not included in the analysis cohort were not different from the 64 included (data not shown).
Table 1. Characteristics of study cohort.
| Characteristic | Overall Cohort N=64 | Recurrent Inhibitor N=19 | No Recurrent Inhibitor N=45 | |
|---|---|---|---|---|
|
|
||||
| Median (Q1,Q3) N (%) |
Median (Q1,Q3) N (%) |
Median (Q1,Q3) N (%) |
p-value | |
| Demographics | ||||
| Age at inhibitor onset, y | 1.6 (1,3.3) | 1.2 (0.7,2.9) | 1.8 (1.1,3.5) | 0.20 |
| Age at start ITI, y | 3.3 (1.5,8.5) | 3.2 (1.1,6.8) | 3.3 (1.5,11.4) | 0.55 |
| Race | 0.74 | |||
| White | 46 (71.9) | 15 (78.9) | 31 (68.9) | |
| Black | 12 (18.8) | 3 (15.8) | 9 (20.0) | |
| Other | 6 (9.3) | 1 (5.3) | 5 (11.1) | |
| Hispanic ethnicity | 16 (25.0) | 6 (31.6) | 10 (22.2) | 0.53 |
| Hemophilia and health history | ||||
| Severe Hemophilia | 62 (96.9) | 18 (94.7) | 44 (97.8) | 1.0 |
| Family History of inhibitor | 13 (20.3) | 4 (21.1) | 9 (20.0) | 0.92 |
| F8 genotype (n=31) | 0.95 | |||
| High-risk | 25 (80.6) | 8 (80.0) | 17 (80.9) | |
| Low-risk | 6 (19.4) | 2 (20.0) | 4 (19.0) | |
| HIV positive | 3 (4.7) | 0 | 3 (6.7) | |
| Hepatitis C antibody positive | 6 (9.4) | 2 (10.5) | 4 (8.9) | 1.0 |
| Inhibitor characteristics | ||||
| Historical peak titer (BU/ml) | 9.9 (3.8,34) | 11.2 (3,38) | 8.5 (4.5,30) | 0.77 |
| Historical peak titer > 5 BU/ml | 43 (67.2) | 12 (63.1) | 31 (68.9) | 0.77 |
| Historical peak titer > 200 BU/ml | 3 (4.7) | 2 (10.5) | 1 (2.2) | 0.21 |
| Pre-ITI titer (BU/ml) | 2.1 (1,5.5) | 2.0 (1,4.5) | 2.5 (1,5.5) | 0.71 |
| Pre-ITI titer > 10 BU/ml | 5 (7.8) | 2 (10.5) | 3 (6.7) | 0.63 |
| Peak titer during ITI (BU/ml) | 2.8 (0.5,33) | 10 (0.6,40) | 2.7 (0.5,24) | 0.36 |
| Peak titer during ITI > 100 BU/ml | 6 (9.4) | 3 (15.8) | 3 (6.7) | 0.35 |
| Characteristics of ITI | ||||
| Time from inhibitor onset to start ITI, y | 0.75 (0.2,2.1) | 1.1 (0.4,2.4) | 0.6 (0.2,3.7) | 0.67 |
| ITI started 2 years after inhibitor onset | 14 (21.9) | 5 (26.3) | 9 (20.0) | 0.74 |
| First course of ITI | 61 (95.3) | 18 (94.7) | 43 (95.6) | 1.0 |
| ITI with recombinant FVIII product | 57 (89.1) | 17 (89.5) | 40 (88.9) | 0.95 |
| ITI regimen, FVIII infusions ≥ daily | 16 (84.2) | 34 (75.6) | 0.53 | |
| Dose of FVIII use for ITI < 100 IU/kg/day | 15 (23.4) | 3 (15.8) | 12 (26.7) | 0.52 |
| Any immunosuppression used during ITI | 5 (7.8) | 4 (21.1) | 1 (2.2) | 0.007 |
| Duration of ITI, y | 1.2 (0.6,2.0) | 0.75 (0.4,2.2) | 1.2 (0.6,2) | 0.42 |
| Tolerance criteria met | 0.71 | |||
| Negative titer/recovery > 66% | 36 (56.3) | 12 (63.2) | 24 (53.3) | |
| Negative titer/half-life > 6h | 1 (1.6) | 0 | 1 (2.2) | |
| Negative titer/recovery/half-life | 27 (42.2) | 7 (36.8) | 20 (44.4) | |
| FVIII Recovery % (n=63) | 85 (71,98) | 79 (74,85) | 89 (70,106) | 0.03† |
| FVIII half-life, hours (n=28) | 8 (6,9.1) | 7 (6,8) | 8 (6.4,9.2) | 0.18 |
| After ITI | ||||
| Not adherent to post ITI FVIII prophylaxis | 17 (26.6) | 5 (26.3) | 12 (26.7) | 0.78 |
| Events during 6 months before recurrent inhibitor or last negative inhibitor titer | 0.53 | |||
| Surgery | 12 (18.8) | 6 (31.6) | 6 (13.3) | 0.09 |
| Vaccination | 3 (4.7) | 0 | 3 (6.7) | |
| Serious bleed | 1 (1.6) | 0 | 1 (2.2) | |
| Infection | 3 (4.7) | 3 (15.8) | 0 | |
| Hospitalization | 1 (1.6) | 0 | 1 (2.2) | |
Kolmogorov-Smirnov two sample test. Y indicates years; BU indicates Bethesda unit; ITI indicates Immune tolerance induction; FVIII indicates factor VIII; h indicates hours;
Table 2. Characteristics of subjects receiving immune modulation therapy.
| Subject | Peak inhibitor titer prior to ITI (BU/ml) | Peak inhibitor titer during ITI (BU/ml) | Age ITI started (years) | IM | Time from start of ITI to use of IM (years) | Inhibitor recurrence | Re-initiation of ITI | Tolerance criteria used |
|---|---|---|---|---|---|---|---|---|
| 1 | 23.4 | 11.3 | 1.5 | PE | 0 | no | no | Three |
| 2 | 1.9 | 28.4 | 1.1 | R+M | 1.0 | yes | yes-ongoing | Three |
| 3 | 25.9 | 175.4 | 0.8 | R+C+M | 2.4 | yes | yes-ongoing | Two |
| 4 | 27 | 15 | 20.1 | Ci+Mtx | 0 | yes | no | Three |
| 5 | 3 | 40 | 0.08 | R | 2.2 | yes | yes-ongoing | Three |
IM, immune modulation; PE, plasmapheresis; R, rituximab; C, cyclophosphamide; M, mycophenolate, Ci, cisplatin; MTX, methotrexate. Three: inhibitor titer < 0.6 BU/ml, recovery level >66% and ½ life >6 hours Two: inhibitor titer < 0.6 BU/ml, recovery level >66%
Adherence to post-ITI prophylaxis
Due to missing records adherence to post-ITI FVIII prophylaxis could not be assessed in 6 subjects (9.3%). Among the 58 of patients in whom adherence was assessed, 41 (70%) were adherent to post-ITI FVIII prophylaxis, infusing 80% or more of prescribed treatment during the 6 months prior to inhibitor recurrence or last follow up (Fig. 1). Of the 17 subjects who were not adherent to post-ITI FVIII prophylaxis, only 1 person had discontinued FVIII infusions altogether.
Inhibitor recurrence
The median duration of follow-up from confirmation of tolerance with FVIII recovery or FVIII half-life to inhibitor recurrence or last negative inhibitor titer was 3.4 years (Q1, Q3: 1.2, 7.0). The probability of any inhibitor recurrence at one, three and five years was 12.8 % (95% CI, 9.4-17.2), 26.6% (95% CI, 21.4-32.6) and 32.5% (95% CI, 27.7-39), respectively, as shown by the Kaplan-Meier inhibitor free survival curve (Fig. 2a). The probability of recurrence in those subjects with more than one inhibitor titer (N=12) at one, three and five years was 7.3% (95% CI, 4.9-10.7), 17.1% (95% CI, 12.7-22.5) and 22.5% (95% CI, 17.2-28.8), respectively (Fig. 2b). Of those with only 1 inhibitor titer as evidence of recurrence (n=7), 1 subject had a single high-titer inhibitor (>5 BU/ml) without a change in treatment, 4 subjects had a low-titer and no change in therapy, and 2 subjects with low-titer inhibitors and change in treatment, 1 restarted ITI and 1 received an increased dose of FVIII to manage bleeding without restarting ITI. Of those with more than one elevated inhibitor titer at the time of recurrence (n=12) 8 (66.7%) were high-titer, 10 (83.3%) restarted ITI, and 10 (83.3%) received a bypassing agent for treatment of bleeding. Of the 11 (1 after only 1 recurrent inhibitor titer and 10 after more than 1 recurrent inhibitor titer) that restarted ITI, 2 achieved tolerance for a second time, 3 did not, and 6 were still receiving ITI at the time of the study data collection.
Figure 2.
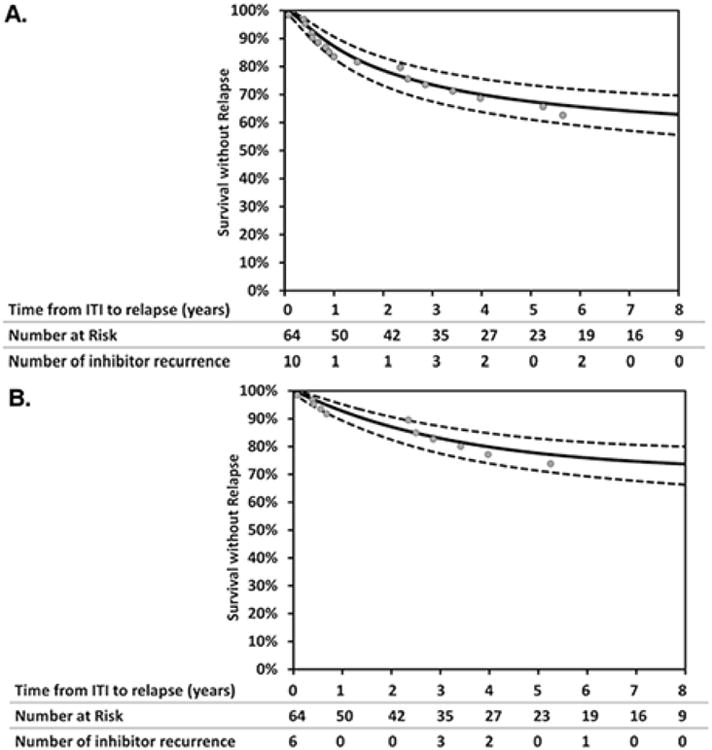
Kaplan Meier survival curves to estimate probability of any inhibitor recurrence, n=19 (A) and more than one inhibitor recurrence, n=12 (B). Dashed lines represent 95% confidence intervals.
Predictors of inhibitor recurrence
A recurrent FVIII inhibitor was detected in 12 (29%) of those adherent to post-ITI prophylaxis, 5 (29%) in those not adherent, and 2 (33%) for whom adherence could not be assessed (Fig. 1).
When comparing subjects with (n=19) and without inhibitor recurrence (n=45), there was no statistically significant differences in the median age at the time of inhibitor onset (1.2 vs. 1.8 years) and no difference in the age when ITI was initiated (3.2 vs. 3.3 years) (Table 1). There was no difference in inhibitor recurrence by race (p=0.74), by presence of high-risk mutations (p=0.95), nor by historical peak inhibitor titer, pre-ITI titer or peak titer during ITI (p >0.2 for each).
There were no differences in the ITI-regimen-related characteristics among subjects with and without a recurrent inhibitor, including whether it was the first course of ITI, the FVIII product used for ITI (recombinant vs. non-recombinant), frequency of FVIII infusions during ITI (once or twice daily vs. three-four times a week), dose of FVIII used for ITI (> or < 100 IU/kg/day) or duration of ITI in years. Among the 5 patients who received immune modulatory therapy as part of their ITI regimen, 4 (80%) experienced an inhibitor recurrence (p <0.01), while the single patient (20%) who received plasmapheresis did not (Table 2). To assess for confounding by indication, characteristics that might lead to the use of immunosuppression were compared between those that did and did not receive immunosuppression (Table 3). The duration of ITI was slightly longer in those who received immunosuppression [2.2 years (Q1, Q3: 1.2, 2.4)] compared with those that did not [1.1 years (Q1, Q3: 0.5, 2)] and the proportion of subjects who had a peak titer >100 BU/ml during ITI was greater (40%) in those who received immunosuppression than those that did not (6.8%).
Table 3. Characteristics based on receipt of immunosuppression.
| Clinical Characteristics | Immunosuppression Therapy | ||
|---|---|---|---|
|
|
|||
| Yes N=5 | No N=59 | p-value | |
| Race, White, n (%) | 5 (100) | 41(69.5) | 0.34 |
| Age at start of ITI, years, median (Q1,Q3) | 1.1 (0.8,1.8) | 3.4 (1.5, 10.3) | 0.12 |
| Time from inhibitor onset to start ITI, years, median (Q1,Q3) | 1.1 (0.1, 15.6) | 0.7 (0.2, 2.7) | 0.80 |
| ≥ 2 years, n (%) | 1 (20.0) | 13 (22.0) | 1.0 |
| Historical peak titer, BU/ml, median (Q1,Q3) | 25.9 (3,27) | 9.8 (4,34) | 0.79 |
| ≥ 200 BU/ml, n (%) | 0 | 3 (5.1) | - |
| Titer at start ITI, BU/ml, median (Q1,Q3) | 2.0 (1.9,4) | 2.1 (1,5.5) | 0.59 |
| Duration of ITI, years, median (Q1,Q3) | 2.2 (1.2, 2.4) | 1.1 (0,5.2) | 0.39 |
| Peak titer during ITI ≥ 100 BU/ml, n (%) | 2 (40) | 4 (6.8) | 0.07 |
| FVIII recovery level, %, median (Q1,Q3) | 79 (75,85) | 85 (71,98) | 0.63 |
| Tolerance Criteria Met | 0.23 | ||
| Titer and FVIII recovery only | 1 (20) | 34 (57.6) | |
| Titer, FVIII recovery, and half-life | 4 (80) | 24 (40.7) | |
| Time to relapse, years, median (Q1,Q3) | 0.42 (0.39,0.56) | 3.8 (1.8,7.0) | 0.001 |
Among those with and without a recurrent inhibitor titer, tolerance was documented by a FVIII recovery level only in 63% (n=12) and 53% (n=24) and both FVIII recovery level and FVIII half-life in 37% (n=7) and 44% (n=20), respectively. Tolerance was documented by FVIII half-life without FVIII recovery level in 2.2% (n=1) subject without a recurrent inhibitor. A sensitivity analysis showed that FVIII recovery of less than 85% had a sensitivity of 79% and specificity of 59% (p <0.01) for predicting inhibitor recurrence. The median FVIII recovery was 79% (Q1, Q3: 74, 85) in those with and 89% (Q1, Q3: 70, 106) in those without inhibitor recurrence (p=0.03). Half-life was reported in a similar proportion of subjects with FVIII recovery level > or < 85% (50% and 42% respectively). Among the 28 subjects with a reported FVIII half-life, there was no difference in half-life between those with and without inhibitor recurrence (p=0.18).
During the 6 months prior to inhibitor recurrence or the last negative inhibitor titer, 12 subjects underwent surgery; 6 developed a recurrent inhibitor and 6 did not. The surgical procedures in those with a recurrent inhibitor included port placement, port removal, dental procedure, and creation of an arteriovenous fistula, and 3 of these were complicated by an infection. In those without a recurrent inhibitor, surgical procedures included port placement and removal, right total knee replacement and Broviac repair, and none were complicated by an infection.
Multivariable Analysis
After adjustment for age, race, and the propensity score, which included frequency of FVIII infusions during ITI, duration of ITI, and historical peak inhibitor titer, adherence to prophylactic FVIII infusions following ITI was not a predictor of having any recurrent FVIII inhibitor (adjusted OR=0.5 [95% CI: 0.06-4.3], p=0.53).
In the second model, after adjusting for a peak inhibitor titer of > 100 BU/ml after the initiation of ITI and the duration of ITI, having FVIII recovery of <85% at the end of ITI and use of concomitant immune modulatory therapy were shown to be independently associated with inhibitor recurrence (OR 2.6 [95% CI:1.3-5.9] and 3.8 [95%, CI 1.2-22.4] respectively) regardless of the end point used, (one or more than one recurrent inhibitor) (Table 4). A time lapse of over 2 years from inhibitor onset to the start of ITI trended towards a statistically significant association, OR of 2.0 (95% CI: 0.9-4.6), (p=0.09). No other variables in the model appeared to be associated or demonstrated confounding. A sensitivity analysis was performed by excluding the three subjects who previously had an unsuccessful course of ITI and similar results of multivariable models were obtained.
Table 4. Multivariable logistic regression model.
| Variables | OR | 95% CI | p-value |
|---|---|---|---|
| ≤ 85% FVIII Recovery | 2.6 | (1.3 – 5.9) | 0.007 |
| Immunosuppression therapy used in ITI | 3.8 | (1.2 – 22.4) | 0.02 |
| ≥ 2 years from inhibitor onset to start ITI | 2.0 | (0.9 – 4.6) | 0.09 |
Adjusted for peak inhibitor titer during ITI > 100 BU/ml and duration of ITI
ROC: 0.78 (0.67 – 0.90),
R2McF = 1 – ln (LM) / ln (L0) = 0.24
LR Test for Model Fit p = 0.001
Discussion
The analysis of this retrospective cohort demonstrates a significant risk of recurrent inhibitor following successful ITI that is associated with low FVIII recovery level and the use of immune modulatory therapy during ITI, but not with adherence to post-ITI FVIII prophylaxis.
The overall proportion of subjects who experienced a recurrent FVIII inhibitor reported in this study, 29.7%, is higher than the rates reported in the NAITR, IITR and PROFIT studies of 10%, 4.6% and 2.3%, respectively [1, 3, 7]. There are several potential reasons for these differences: first the stringency of the definition of tolerance varied among the studies. In the PROFIT study, subjects were not considered tolerant until one year following normalization of recovery and FVIII half-life; this may have excluded early relapses that occurred due to insufficient induction of tolerance. In the current study there was a higher, though not statistically significant, rate of inhibitor recurrence in those subjects without clear documentation of their half-life at the end of ITI. A similar observation was made in the NAITR study [1]. Secondly, the studies differed in their definition of inhibitor recurrence. In this study, the definition of recurrent inhibitor was any inhibitor titer ≥0.6 BU/ml after the end of ITI. When a more rigorous definition of 2 elevated inhibitor titers was used, the rate of recurrence is lower but still higher than previously published results (7.3% at 1 year and 22.5% at 5 years). Importantly, no inhibitor recurrence was detected after 5 or more years of completion of successful ITI, with 35.9% of the cohort being observed for at last 5 years. In the PROFIT and IITR study, a single recurrent inhibitor was detected at 7 and 15 years, respectively [3, 7], suggesting that the risk is present but low.
In contrast to the general perception in clinical practice, lack of adherence to post-ITI FVIII prophylaxis was not predictive of inhibitor recurrence on both bivariable and multivariable analyses. Despite this, there are other compelling reasons for patients to be adherent to FVIII infusions such as prevention of joint bleeding and avoidance of subsequent disability. Unfortunately, in this small cohort, it was not possible to assess whether the influence of adherence to post-ITI FVIII prophylaxis on inhibitor recurrence changes over time. It is conceivable that post-ITI prophylaxis is more important early in the post-ITI course, becoming less important with time following ITI, but this remains unknown.
Factor VIII recovery was found to be associated with inhibitor recurrence: a FVIII recovery level less than 85% was associated with 3-fold greater odds for inhibitor recurrence than when a FVIII recovery level of greater than 85%. Currently, a level greater than 66% is used in clinical practice as indicative of emerging tolerance. It is important to note that in pivotal clinical trials of FVIII, the median recovery of children aged 1-6 years was 1.84 IU/dl per IU/kg which is 92% of predicted, and the lowest quartile of children had a recovery <1.64 IU/dl per IU/kg which is 82% of predicted [8]. In the IITI study, the 6 subjects with partial relapses during the first year after ITI [3] did not differ from the rest of the cohort in their FVIII recovery (92.5%) and FVIII half-life (7.0 hours) at the end of ITI. The lack of impact of FVIII recovery at the end of ITI in the IITI study may reflect that they were only partial relapses (negative inhibitor titer but reduced recovery) and the median recovery in the IITI study was 92.5%, indicating that the majority of subjects had a >85% FVIII recovery at the end of ITI. Ideally, this finding that a FVIII recovery <85% is predictive of inhibitor recurrence should be confirmed in other cohorts or on prospective investigation. Our findings could also reflect a lack of consistent washout prior to measurement of recovery. Given that this was a retrospective study, the duration of FVIII washout prior to measuring the FVIII recovery was not prescribed nor was this information collected. It is possible that in the absence of a sufficient FVIII washout, a recovery of 66-85% may represent an inadequate FVIII recovery that would likely be <66% following an adequate 48-72 hour FVIII washout. With either of these two explanations, these data, along with the IITI study median FVIII recovery of 92.5%, suggest that measurement of FVIII recovery at the end of ITI is an important marker of emerging tolerance and should be measured carefully.
There is no consensus about the use of immune modulation in conjunction with ITI as a first line therapy. In our study, those receiving immune modulatory therapy in conjunction with ITI showed no difference in historical peak inhibitor titer, inhibitor titer prior to the start of ITI, or peak inhibitor titer after ITI compared with those that did not receive immune modulatory therapy. The median duration of ITI appeared to be longer (2.2 years vs. 1.1 years) (Table 3), suggesting that immune modulation may have been started as the result of a clinical perception of lack of adequate response to ITI alone. Three of the 4 patients who relapsed received rituximab (Table 2). Although these worse outcomes could be the result of more refractory disease, it is also possible that rituximab reduced the inhibitor titer, but did not promote tolerance, leading to withdrawal of ITI following rituximab due to a false perception of tolerance. Notably, the time to relapse among patients that received immune modulation was markedly shorter compared to those who did not (0.42 years vs. 3.8 years, respectively; p=0.001). In the cohort study published by Collins et al, of 6 subjects receiving rituximab as part of ITI; 3 (50%) recurred, 2 remained tolerant after 3.5 and 2 years, and 1 had limited follow-up of only 16 weeks, suggesting that the tolerance induction with rituximab may be less effective and/or less durable, but this will require prospective study to resolve [9]. There are no reports on the impact of other immunosuppressive agents such as cyclophosphamide and mycophenolate on the durability of tolerance in hemophilia A. One may speculate that mycophenolate, in addition to suppressing effector T cells, may also suppress T regulatory cells thereby limiting the maintenance of a tolerant milieu.
There are several strengths and limitations of this study. One strength of the study is the multi-institutional design, thereby representing real world practice from a broad distribution of patients from 12 U.S. hemophilia treatment centers. The limitation of this study is its small sample size resulting in limited power and potential risk of overestimating the effect size. Additionally, as is the case with any observational study, there is a concern for unmeasured confounding. Lastly, despite an effort to include all potential subjects through review of clinical databases at each center, there is a possibility that some patients were omitted.
In summary, this study demonstrates that the probability of inhibitor recurrence is significant in the first five years after ITI, and is associated with a FVIII recovery of < 85% at the end of ITI, and the use of concomitant immune modulation therapy during ITI, but not adherence to post-ITI prophylaxis. These findings suggest that following ITI, patients should be carefully monitored for inhibitor recurrence, particularly those who have received immune modulation such as rituximab. Ideally the influence of recovery level and the use of immune modulation would be investigated in a larger prospective study to better elucidate the most effective practice.
Acknowledgments
This study was funded by an investigator-initiated grant from Novo Nordisk, Inc. (CLK) and by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454 (AA). Novo Nordisk, Inc. did not participate in the design, conduct, analysis or reporting of this study.
Footnotes
Disclosure of Conflict of Interests
C. L. Kempton reports personal fees from Biogen Idec, Baxter Healthcare, CSL Behring and Kedrion Biopharma, outside the submitted work. P. Monahan receives research support through the UNC from Asklepios and Novo Nordisk and has received research support in the past from Baxter Healthcare, Novo Nordisk, Pfizer, and Prolor. He holds patents which have been licensed by UNC to Asklepios, for which he receives royalties. He has received payment for consultation, services and speaking for Asklepios, Chatham LLC and Baxter Healthcare, and has, in addition, consulted for Bayer, CSL Behring, Novo Nordisk and Pfizer. M. Manco-Johnson has received honoraria from Novo Nordisk, Biogen Idec, Baxter Healthcare, CSL Behring and Bayer Healthcare. M. U. Callaghan has received honoraria from Baxter Healthcare, CSL Behring and Pfizer. S. L Carpenter has received research support from Pfizer and honoraria from Novo Nordisk. M. V. Ragni receives research support from Biogen Idec and Baxter Healthcare. J. A. Davis has received honoraria from Bayer Healthcare, CSL Behring and Novo Nordisk. R. Kruse-Jarres has received honoraria from Bayer Healthcare, Baxter Healthcare, Grifols, Novo Nordisk, Octapharma, Pfizer and Roche. C. L. Knoll has received honoraria from Baxter Healthcare, Biogen Idec, CSL Behring and Kedrion Biopharma. The other authors state that they have no conflict of interest.
Authorship: C. L. Kempton and P. Monahan designed the study. C. L. Kempton, P. Monahan, M. Manco-Johnson, M. U. Callaghan, M. Kanin, C. Knoll, S. L. Carpenter, J. A. Davis, M. F. Guerrera, R. Kruse-Jarres, M. V. Ragni and C. Witmer collected the data. C. L. Kempton, A. Antun and C. E. McCracken performed the analysis. A. Antun wrote the manuscript. All authors critically reviewed and significantly edited the manuscript.
References
- 1.DiMichele DM, Kroner BL. The North American Immune Tolerance Registry: practices, outcomes, outcome predictors. Thromb Haemost. 2002;87:52–7. [PubMed] [Google Scholar]
- 2.Hay CR, DiMichele DM. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood. 2012;119:1335–44. doi: 10.1182/blood-2011-08-369132. [DOI] [PubMed] [Google Scholar]
- 3.Coppola A, Margaglione M, Santagostino E, Rocino A, Grandone E, Mannucci PM, Di Minno G. Factor VIII gene (F8) mutations as predictors of outcome in immune tolerance induction of hemophilia A patients with high-responding inhibitors. J Thromb Haemost. 2009;7:1809–15. doi: 10.1111/j.1538-7836.2009.03615.x. [DOI] [PubMed] [Google Scholar]
- 4.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 5.Miller CH, Benson J, Ellingsen D, Driggers J, Payne A, Kelly FM, Soucie JM, Craig Hooper W. F8 and F9 mutations in US haemophilia patients: correlation with history of inhibitor and race/ethnicity. Haemophilia. 2012;18:375–82. doi: 10.1111/j.1365-2516.2011.02700.x. [DOI] [PubMed] [Google Scholar]
- 6.Gitschier J, Wood WI, Goralka TM, Wion KL, Chen EY, Eaton DH, Vehar GA, Capon DJ, Lawn RM. Characterization of the human factor VIII gene. Nature. 1984;312:326–30. doi: 10.1038/312326a0. [DOI] [PubMed] [Google Scholar]
- 7.Mariani G, Kroner B. Immune tolerance in hemophilia with factor VIII inhibitors: predictors of success. Haematologica. 2001;86:1186–93. [PubMed] [Google Scholar]
- 8.Bjorkman S, Oh M, Spotts G, Schroth P, Fritsch S, Ewenstein BM, Casey K, Fischer K, Blanchette VS, Collins PW. Population pharmacokinetics of recombinant factor VIII: the relationships of pharmacokinetics to age and body weight. Blood. 2012;119:612–8. doi: 10.1182/blood-2011-07-360594. [DOI] [PubMed] [Google Scholar]
- 9.Collins PW, Mathias M, Hanley J, Keeling D, Keenan R, Laffan M, Perry D, Liesner R. Rituximab and immune tolerance in severe hemophilia A: a consecutive national cohort. J Thromb Haemost. 2009;7:787–94. doi: 10.1111/j.1538-7836.2009.03332.x. [DOI] [PubMed] [Google Scholar]


