Abstract
This multicenter prospective phase II study examines the activity and tolerability of brentuximab vedotin as second-line therapy in patients with Hodgkin lymphoma that was relapsed or refractory after induction therapy. Brentuximab vedotin (1.8 mg/kg) was administered intravenously on day 1 of a 21-day cycle for a total of 4 cycles. Patients then proceeded to autologous hematopoietic cell transplantation (AHCT), if eligible, with or without additional salvage therapy, based on remission status post brentuximab vedotin. The primary endpoint was overall response rate (ORR). Secondary endpoints were safety, stem cell mobilization/collection, AHCT outcomes and association of CD68+ with outcomes. Of 37 patients, the ORR was 68% (13 complete remission, 12 partial remission). The regimen was well tolerated with few grade 3/4 adverse events including lymphopenia (1), neutropenia (3), rash (2), and hyperuricemia (1). Thirty-three (89%) patients were able to proceed to AHCT, with 24 (65%) in CR at time of AHCT. Thirteen patients in CR, 4 in PR and 1 in SD (49%) received AHCT without salvage combination chemotherapy. CD 68 expression did not correlate with response to brentuximab vedotin. The median number of stem cells mobilized was 6.0 × 106 (2.6–34) and median number of days to obtain minimum collection (2 × 106) was 2 (1–6). Brentuximab vedotin as second-line therapy is active, well tolerated, and allows adequate stem cell collection and engraftment. For Hodgkin lymphoma patients with relapsed/refractory disease post-induction therapy, second-line brentuximab vedotin, followed by combination chemotherapy for residual disease, can effectively bridge patients to AHCT.
Introduction
Up to 30% of patients with Hodgkin lymphoma (HL) will relapse or are refractory to primary induction chemotherapy1,2. Standard second-line therapies for these patients include combination chemotherapy regimens such as ICE (ifosfamide, carboplatin, etoposide), DHAP (dexamethasone, high dose cytarabine, cisplatin), or GDP (gemcitabine, dexamethasone, cisplatin) which typically yield responses rates of ~60–80%, but can have significant myelosuppression including: grade 3/4 thrombocytopenia, febrile neutropenia, grade 3/4 anemia. Up to 60% of patients may require packed red blood cell transfusion and 30% may require platelet transfusion. Combination chemotherapies may also impair the ability to successfully mobilize stem cells (14%) for autologous hematopoietic stem cell transplantation (AHCT)3–7. Alternative salvage strategies with fewer hematological adverse effects would be advantageous to patients destined for AHCT.
Brentuximab vedotin is an antibody-drug conjugate linking an anti-CD30 antibody to the microtubule-disrupting agent, monomethyl auristatin E8. It selectively induces apoptosis in CD 30-expressing cells while sparing toxicity to off-target tissues9. Based on a phase II trial demonstrating an overall response rate of 75% with a tolerable safety profile, the United States Food and Drug Administration approved brentuximab vedotin for use in patients with Hodgkin who have received at least two prior lines of therapy10,11. We expect that brentuxmiab vedotin as second-line treatment will also be efficacious and tolerable. Previously we demonstrated that brentuximab vedotin can act as an effective bridge to allogeneic transplantation12,13. We now hypothesize that brentuximab vedotin as second-line treatment can be an effective bridge to autologous transplantation with no deleterious effects on stem cell mobilization or engraftment.
Patients and Methods
This is a multicenter, investigator-initiated phase II clinical trial performed at City of Hope and Weill Cornell Medical College. This trial was registered with clinicaltrials.gov (NCT01393717), approved by both Institutional Review Boards, and an assurance was filed with the Department of Health and Human Services. Informed consent was obtained for all study participants in compliance with the Declaration of Helsinki.
Patient Eligibility
Patients over the age of 10 years with histologically confirmed CD 30+ relapsed/refractory classical HL were eligible. All patients had biopsy proven relapsed/refractory HL post induction therapy with ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine), BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone), or a combination +/− consolidative radiotherapy. Pediatric patients may have received ABVE-PC (doxorubicin, bleomycin, vinblastine, etoposide, prednisone, cyclophosphamide). Patients were ineligible if they had received any second-line therapy chemotherapy. Patients were required to show radiographically measureable disease and standard organ functions.
Treatment Plan
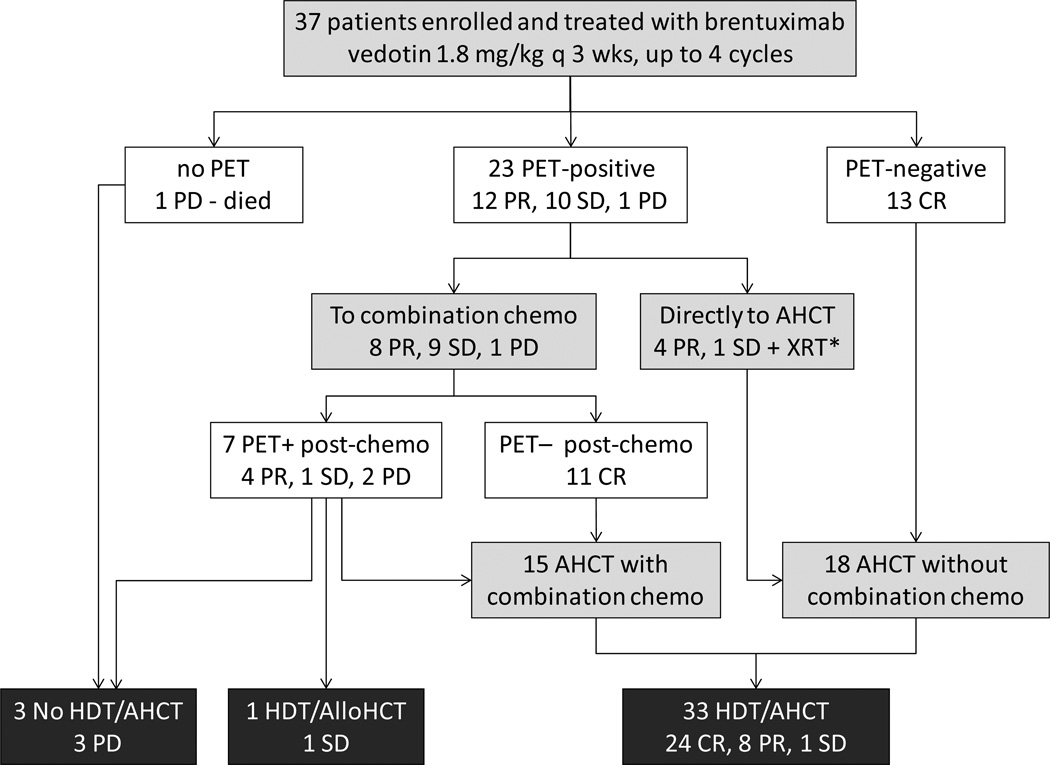
Treatment consisted of 1.8 mg/kg of brentuximab vedotin intravenously every 3 weeks for a maximum of 4 cycles. See Figure 1 for treatment schema. One cycle of therapy was defined as the 21 days following intravenous administration of brentuximab vedotin. Patients with complete remission, partial remission, or stable disease at the end of 2 cycles were permitted to continue brentuximab vedotin for two more cycles. Patients who achieved CR or PR were allowed to proceed to AHCT directly. Patients who achieved PR had the option of receiving additional combination chemotherapy prior to AHCT. Patients with stable or progressive disease were required to undergo salvage chemotherapy prior to AHCT. The choice of combination chemotherapy regimen was at the discretion of the treating physician. For stem cell mobilization the majority of patients received cyclophosphamide (1.5 gram/m2) and G-CSF (10ug/kg) as priming agents, but for slow collectors (<1.0 × 106 CD34+ cells first day), plerixafor was added per City of Hope standard operating procedures (SOP).
Figure 1. Study Schema.
Patients with relapsed/refractory HL were treated with brentuximab vedotin 1.8 mg/kg intravenously every 3 weeks for maximum of 4 cycles. Radiographic assessment was done at the end of cycle 2 with CT or CT/PET scan. Patients were allowed to continue study if they achieved CR/PR/SD after 2 cycles. Radiographic assessment at end of study was done with CT/PET scan. Patients who achieved CR went directly to AHCT. Patients who achieved PR had the option of going directly to AHCT or receiving other salvage chemotherapy by investigators choice.
*XRT denotes local radiation therapy.
Study Design and Statistical Methods
The primary objective was to evaluate the anti-tumor activity of brentuximab vedotin as second-line therapy. The primary endpoint was the best overall response rate [complete response (CR) plus partial response (PR)] per 2007 Cheson criteria14, 15. Responses were assessed at end of cycle 2 and 4. All CR were confirmed by FDG-PET evaluation with the SUV value to be below the background of the mediastinal blood pool. Secondary endpoints were toxicity, stem cell mobilization, and engraftment. Based on historical data with standard chemotherapy regimens, we considered a response rate of at least 60% to be sufficient to warrant further evaluation. A Simon two-stage design, with a one-sided alpha of 5% and 80% power was used to assess ORR. In the first stage, 23 patients were enrolled, with the design specifying that if 12 or more patients achieved CR or PR, accrual would continue to a total of 37 patients, with 23 or more responses regarded as evidence of sufficient activity to warrant further investigation. Toxicity was assessed every cycle and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0314.
Immunohistochemistry
Immunohistochemical (IHC) staining was performed on pre-treatment tumor specimens for CD68. 1.5 mm duplicate cores of diagnostic biopsies of HL were obtained from representative areas containing HRS cells. The slides were independently scored by KM (Dr. Young Kim). For CD 68 staining, cells were scored in three representative high-powered fields and the relative percentage of CD68+ cells in relation to overall cellularity was reported as previously described in a prior publication15. Staining intensity was then assessed for relationship to response rate.
Results
Efficacy
A total of 37 patients were enrolled in the study between Aug 2011 and May 2014. All patients were evaluable for toxicity and efficacy. Table 1 shows baseline patient, disease and treatment characteristics. All 37 patients were evaluable for response. The median number of cycles received was 4 (range 1–4). Table 2 details the overall response rates. The ORR was 68% (25/37), with 13 patients attaining CR (35%), 12 patients attaining PR (32%), 10 patients attaining SD (stable disease, 27%), and 2 patient attaining PD (progressive disease, 5%). All patients who achieved CR did so after 2 cycles of therapy.
Table 1.
Patient, Disease and Treatment Characteristics
| Characteristics | N (%)or Median (Range) |
|---|---|
| Institution | |
| City of Hope | 31 (84%) |
| Weill Cornell | 6 (16%) |
| Gender | |
| Female | 17 (46%) |
| Male | 20 (54%) |
| Age | 34 (11–67) |
| Stage at Diagnosis | |
| I–II | 19 (51%) |
| III–IV | 18 (49%) |
| Prior radiation therapy | 9 (25%) |
| B symptoms (at diagnosis) | 23 (62%) |
| Bulky Disease (≥ 5 cm at diagnosis) | 32(86%) |
| Induction Chemotherapy | |
| ABVD | 34 |
| ABVD/BEACOPP | 2 |
| ABVE-PC | 1 |
| Prior XRT | 9 (24%) |
| Best Response to Induction | |
| Primary Refractory | 24 (65%) |
| Relapsed (median7 months) | 13 (35%) |
Table 2.
Response
| Best response to BV, N=37 |
Response to combination chemotherapy (ICE/DICE/IGEV/GND) post-BV, N=18 |
Disease Status at AHCT, N=33 |
|
|---|---|---|---|
| ORR | 25/37 (68%) | 16/18 (89%) | |
| CR | 13/37 (35%) | 11/18 (61%) | 24/33 (73%) |
| PR | 12/37 (32%) | 5/18 (28%) | 9/33 (27%) |
| SD | 10/37 (27%) | 1/18 (6%) | 1/33 (3%) |
| PD | 2/37 (5%) | 1/18 (6%) |
When stratified by stage at diagnosis, the ORR was 68% for stage I/II patients (13/19) and 67% for stage III/IV patients (12/18). When stratified by response to induction therapy, the ORR was 67% for primary refractory disease (16/24) and 69% for relapsed disease (9/13). Univariate analysis did not show significant differences in response rates in terms of age, gender, stage, and response to induction.
Overall, treatment was well tolerated. Table 3 shows all the hematological adverse events, all Grade 3–4 adverse events, and all Grade 1–2 non-hematological adverse events occurring in ≥ 15% of the patients that were at least possibly related to drug. No patient required growth factor support, packed red blood cell, or platelet transfusions as a result BV. There were also no neutropenic fevers.
Table 3.
Adverse Events
| Adverse Events | Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
|---|---|---|---|---|
| Hematological AEs | ||||
| Anemia | 16% | 3% | ||
| Neutropenia | 11% | 5% | ||
| Thrombocytopenia | 8% | |||
| Lymphopenia | 3% | 3% | 3% | |
| Non-Hematological AEs | ||||
| Peripheral neuropathy | 49% | 3% | ||
| AST elevation | 32% | 5% | 3% | |
| ALT elevation | 27% | 11% | ||
| Rash (new) | 24% | 11% | 5% | |
| Muscle weakness | 24% | 5% | ||
| Hypoglycemia | 22% | |||
| Fatigue | 19% | 11% | ||
| Pruritis | 19% | 3% | 3% | |
| Nausea | 16% | 3% | ||
| Abdominal Pain | 11% | 5% | ||
| Creatinine elevated | 3% | |||
| Tumor lysis syndrome | 3% | |||
| Hyperuricemia | 3% | |||
Eighty-nine percent (33/37) of patients successfully proceeded to AHCT. Of the 4 not receiving AHCT, 1 went to alloHCT and 3 did not respond to second-salvage combination chemotherapy. See Figure 1 for a flow chart of patient treatment that includes disease response at each step. 17 out of 37 patients received only brentuximab vedotin (46%) prior to AHCT. Among the 13 CR patients, all proceeded to AHCT without additional chemotherapy. Among the 12 PR patients, 4 proceeded to AHCT without additional chemotherapy while 8 received additional salvage chemotherapy (ICE/DICE/IGEV/GND). All patients with SD/PD received additional salvage chemotherapy with the exception of 1 patient who received only local radiation therapy due to a single site of disease. This patient did not have repeat imaging assessment post radiation and thus is counted as a stable disease. At the time of AHCT, 24/37 (65%) were in CR, 8/37 (22%) were in PR, and 1/37 (3%) was in SD.
All 33 patients who underwent AHCT successfully mobilized stem cells. 22 patients were primed for stem cell mobilization with cyclophosphamide and G-CSF per institutional standard of care, and 2 patients received G-CSF only. Nine patients received plerixafor (per SOP). No patient required a second round of mobilization. The median cell dose collected was 6.0 × 106 CD34 cells (2.6–34). The median number of days required for collection was 2 (1–6). Patients received AHCT conditioning regimens by physician choice, including BEAM (20/33, BCNU, etoposide, cytarabine, melphalan), CBV (11/33, cyclophosphamide, BCNU, etoposide), or BEAM plus yttrium-90 labeled anti-CD25 (2/33). The median time to neutrophil engraftment (absolute neutrophil count ≥ 500 for 3 consecutive days) was 11 days (10–12) and platelet engraftment (≥ 20,000) was 13 days (9–23).
Correlative Assays
CD68 expression has been implicated as a poor prognostic factor in patients undergoing induction chemotherapy15 and has also been shown to predict for relapses post AHCT17. To determine whether CD68 expression is a poor prognostic marker for patients receiving BV, we performed IHC staining for CD68 in our patient samples (COH only) prior to BV treatment. Not surprisingly, all patients had an intensity score of at least 2 + since they were all either relapsed or refractory to induction chemotherapy. A total of 31% scored as 2+, 62% as 3+, and 6% as 4+ staining intensity for CD68. Fisher’s exact test was performed to test for an association between CD68 staining intensity and response to BV. In our small sample set, it does not appear that CD68 staining intensity negatively impacts response rates to BV (p=0.38). We will continue to follow these patients to determine whether CD68 might be a predictor of relapse post-AHCT in patients who achieved CR to BV as second-line therapy.
Discussion
The ORR of 68% and CR rate of 35% in this study are similar to the ORR of 75% and CR 34% achieved in the pivotal study of brentuximab vedotin for Hodgkin lymphoma10. The toxicity profile was mild and similar to that seen in the post-AHCT brentuximab vedotin trial, with two exceptions. Eight percent of patients on the pivotal trial developed Grade III peripheral neuropathy, while none of the patients on this trial developed grade III/IV peripheral neuropathy. This difference may be related to the brentuximab vedotin 4-dose maximum for patients on our trial, compared to 16 doses in the post-AHCT trial, suggesting that peripheral neuropathy from brentuximab vedotin is a cumulative toxicity. Unlike the pivotal post-AHCT trial, 40% of our patients developed rashes, with 2 patients developing grade 3 rashes. The appearance of this toxicity may be related to the fact that patients in the pivotal trial had received a median of 3.5 prior therapies, while patients in the current study had only received 1 induction regimen and were likely better able to mount an immune-mediated skin reaction to the antibody-drug conjugate.10 The results of our study suggest that brentuximab vedotin has similar activity and tolerability when given in the second-line setting as it has in the setting of post-AHCT failure.
Our results also suggest that brentuximab vedotin as second-line therapy can effectively bridge patients to AHCT. Patients in this study received BV as second-line therapy, and those who achieved a CR proceeded directly to AHCT. For patients who did not achieve CR, additional combination salvage chemotherapy such as ICE was then given to improve the depth of response prior to AHCT. This sequential strategy resulted in thirteen patients (35%) in CR immediately post BV and another 11 patients (30%) in CR post salvage chemotherapy, yielding a total of 24 patients (65%) in CR at the time of AHCT. This trial was not designed to replace traditional salvage chemotherapy with BV, but rather to demonstrate that BV as second-line therapy can be safely given prior to traditional salvage chemotherapy to bridge patients to AHCT. Granted, the CR rate of 35% achieved by brentuximab vedotin is lower than the 60% PET CR rate seen for ICE4. However, CR rate of multiagent chemotherapy given as third-line therapy is 61% (11/18), suggesting that delaying multiagent chemotherapy does not have a deleterious effect on efficacy.
It is also important to note that 18 patients (49%) proceeded directly to AHCT without multiagent salvage chemotherapy. The toxicity profile for brentuximab vedotin in this trial was milder than that seen for historical controls for salvage chemotherapy regimens. Toxicities such as neutropenia, thrombocytopenia, anemia, fever, nausea, vomiting, and mucositis are fairly common in multi-agent salvage chemotherapy3–5. The initial ICE study resulted in 13% grade IV neutropenia requiring hospitalization and a 14% stem cell mobilization failure rate6. It is important to note that none of the patients in this trial required growth factor support or blood product transfusions as a result of brentuximab vedotin administration. There were also no stem cell mobilization failures in our patients. Brentuximab vedotin did not decrease the median number of stem cells collected or prolong the median number of days required to reach the minimum collection target. The ANC and platelet engraftment times were also similar to historical controls. All but 4 patients were able to proceed to AHCT, demonstrating that delaying combination salvaging chemotherapy did not negatively impact bridging patients to AHCT.
This study was not designed to predict how this bridging strategy will affect long term outcomes post-AHCT. As secondary endpoints, we continue collecting toxicity profile data, PFS, OS, and relapse rate. However, follow-up is too short for us to draw any conclusions at this point. We do know from prior publications that the best predictor of outcomes for HL patients post-AHCT is CR status prior to AHCT.4 Moskowitz et al. recently published a similar trial using weekly brentuximab vedotin followed by augmented ICE prior to AHCT16. They showed that sequential salvage therapy with BV followed by augmented ICE resulted in a higher proportion of patients achieving CR at the time of AHCT than historically seen for ICE alone. They observed a 2-year event-free survival of 80% following the sequential strategy. Although longer follow up is needed to determine the survival outcomes of the patients who received AHCT following our sequential strategy, our current results are consistent with the Moskowitz study and support the use of sequential BV salvage therapy followed by combination chemotherapy prior to AHCT.
Although the results of this trial support the Memorial study in terms of bridging patients to AHCT, there are several differences. First, our brentuxiamb vedotin dosing schedule was 1.8 mg/kg once every 3 weeks, whereas Moskowitz et al. used 1.2 mg/kg once weekly dosing. Second, we let the individual physician choose the multiagent salvage chemotherapy regimen, given that this was a multicenter study and there is no data on superiority of any one particular muliagent chemotherapy regimen. Third, we allowed patients who were in PR after BV to proceed directly to AHCT without additional therapy, and 4 patients in our trial were transplanted in PR. Although there is evidence showing improved post AHCT outcome for patients in CR at the time of AHCT, there is no guarantee that CR can be achieved with additional therapy prior to AHCT. Last, but not the least, we performed rapid restaging after the first 2 doses of BV. All patients who achieved CR did so by cycle 2, and further dosing with 2 additional cycles did not convert any patients from PR to CR. In the initial pivotal trial, many patients who achieved PR eventually developed progressive disease while still on therapy. Interestingly, 3 of our patients achieved PR by cycle 2 and developed progressive disease later, suggesting that resistance to brentuximab vedotin can develop even after a short exposure. Based on the results of the study presented here, we would recommend giving additional multi-agent chemotherapy for patients not in CR after 2 cycles of BV.
CD68 expression has been implicated as a poor prognostic factor in patients undergoing induction chemotherapy15 and is also shown to predict for relapse post AHCT17. Although our sample size is small, our data suggests that intensity of CD 68 expression did not negatively impact response rates to brentuxiamb vedotin, at least in the context of a cohort where all patients exhibit at least 2+ staining for CD 68. We are currently assessing the expression of drug exporters in our tumor samples, since this class of molecules has been associated with resistance to anti-microtubule agents in other tumor types17–19.
This study demonstrated that brentuximab vedotin as second-line therapy for patients with Hodgkin lymphoma is active, well tolerated, and does not hinder stem cell collection or engraftment. Eighty-nine percent of the patients were effectively bridged to AHCT, 49% of which were spared multi-agent salvage chemotherapy. This toxicity sparing is particularly important for a patient population that is younger and has many years to live with their treatment toxicities. For Hodgkin lymphoma patients with relapsed/refractory disease after induction chemotherapy, brentuximab vedotin as second-line therapy followed by combination chemotherapy prior to AHCT can be considered a viable approach.
Acknowledgments
The authors would like to thank Dr. Eileen Smith, Dr. Chatchada Karanes, Dr. Ryotaro Nakamura, Dr. Pablo Parker, Dr. Margaret O’Donnell, Dr. Amrita Krishnan, Dr. Ricardo Spielberger, and Dr. Samer Khaled, for their dedication to treating these complex patients. We also would like to thank Michell Mott, Tanya Paris, and Bernie Pulone, for their assistance with collecting and managing the study data.
This work was supported directly by research grant from Seattle Genetics. This work was also indirectly supported by P30 CA33572, and the Tim Nesvig Lymphoma Research Fund. RC is supported by the National Cancer Institute of the National Institutes of Health under award number K12CA001727 and CCITLA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health
Financial Disclosure Statement
Robert Chen is a consultant for Seattle Genetics and has received research funding from Seattle Genetics.
Footnotes
Clinicaltrials.gov identifier: NCT01393717
References
- 1.American Cancer Society. Cancer Facts and Figures. 2014 http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/index. [Google Scholar]
- 2.Connors JM. State-of-the-art therapeutics: Hodgkin's lymphoma. J Clin Oncol. 2005;23(26):6400–6408. doi: 10.1200/JCO.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Josting A, Rudolph C, Mapara M, et al. Cologne High-Dose Sequential Chemotherapy in Relapsed and Refractory Hodgkin Lymphoma: Results of a Large Multicenter Study of the German Hodgkin Lymphoma Study Group (GHSG) AnnOncol. 2005;16(1):116–123. doi: 10.1093/annonc/mdi003. [DOI] [PubMed] [Google Scholar]
- 4.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97(3):616–623. doi: 10.1182/blood.v97.3.616. [DOI] [PubMed] [Google Scholar]
- 5.Kuruvilla J, Nagy T, Pintilie M, Tsang R, Keating A, Crump M. Similar response rates and superior early progression-free survival with gemcitabine, dexamethasone, and cisplatin salvage therapy compared with carmustine, etoposide, cytarabine, and melphalan salvage therapy prior to autologous stem cell transplantation for recurrent or refractory Hodgkin lymphoma. Cancer. 2006;106(2):353–360. doi: 10.1002/cncr.21587. [DOI] [PubMed] [Google Scholar]
- 6.Moskowitz CH, Bertino JR, Glassman JR, et al. Ifosfamide, carboplatin, and etoposide: a highly effective cytoreduction and peripheral-blood progenitor-cell mobilization regimen for transplant-eligible patients with non-Hodgkin's lymphoma. J Clin Oncol. 1999;17(12):3776–3785. doi: 10.1200/JCO.1999.17.12.3776. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett NL, Niedzwiecki D, Johnson JL, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol. 2007;18(6):1071–1079. doi: 10.1093/annonc/mdm090. [DOI] [PubMed] [Google Scholar]
- 8.Francisco JA, Cerveny CG, Meyer DL, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102(4):1458–1465. doi: 10.1182/blood-2003-01-0039. [DOI] [PubMed] [Google Scholar]
- 9.Sutherland MS, Sanderson RJ, Gordon KA, et al. Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J Biol Chem. 2006;281(15):10540–10547. doi: 10.1074/jbc.M510026200. [DOI] [PubMed] [Google Scholar]
- 10.Younes A, Gopal AK, Smith SE, et al. Results of a Pivotal Phase II Study of Brentuximab Vedotin for Patients With Relapsed or Refractory Hodgkin's Lymphoma. J Clin Oncol. 2012;30(18):2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Claro RA, McGinn K, Kwitkowski V, et al. U.S. Food and Drug Administration approval summary: brentuximab vedotin for the treatment of relapsed Hodgkin lymphoma or relapsed systemic anaplastic large-cell lymphoma. Clin Cancer Res. 2012;18(21):5845–5849. doi: 10.1158/1078-0432.CCR-12-1803. [DOI] [PubMed] [Google Scholar]
- 12.Chen R, Palmer JM, Thomas SH, et al. Brentuximab vedotin enables successful reduced-intensity allogeneic hematopoietic cell transplantation in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2012;119(26):6379–6381. doi: 10.1182/blood-2012-03-418673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen R, Palmer JM, Tsai NC, et al. Brentuximab Vedotin is Associated with Improved Progression-Free Survival after Allogeneic Transplantation for Hodgkin Lymphoma. Biol Blood Marrow Transplant. 2014 doi: 10.1016/j.bbmt.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancer Therapy Evaluation Program, National Cancer Institute, National Institues of Health. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. 2010 http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.
- 15.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362(10):875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moskowitz AJ, Schoder H, Gerecitano J, et al. 12th International Conference on Malignant Lymphoma. Lugano, Switzerland: Wiley; 2013. PET-adapted seequential therapy with brentuximab vedotin and augmented-ICE induces FDG-PET normalization in 92% of patients with relapsed and refractory Hodgkin lymphoma; p. 143. [Google Scholar]
- 17.O'Brien C, Cavet G, Pandita A, et al. Functional genomics identifies ABCC3 as a mediator of taxane resistance in HER2-amplified breast cancer. Cancer Res. 2008;68(13):5380–5389. doi: 10.1158/0008-5472.CAN-08-0234. [DOI] [PubMed] [Google Scholar]
- 18.Dumontet C, Duran GE, Steger KA, Beketic-Oreskovic L, Sikic BI. Resistance mechanisms in human sarcoma mutants derived by single-step exposure to paclitaxel (Taxol) Cancer Res. 1996;56(5):1091–1097. [PubMed] [Google Scholar]
- 19.Greaves W, Xiao L, Sanchez-Espiridion B, et al. Detection of ABCC1 expression in classical Hodgkin lymphoma is associated with increased risk of treatment failure using standard chemotherapy protocols. J Hematol Oncol. 2012;5:47. doi: 10.1186/1756-8722-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]