Abstract
The mammalian arcuate nucleus (ARC) houses neurons critical for energy homeostasis and sexual maturation. Proopiomelanocortin (POMC) and Neuropeptide Y (NPY) neurons function to balance energy intake and Kisspeptin neurons are critical for the onset of puberty and reproductive function. While the physiological roles of these neurons have been well established, their development remains unclear. We have previously shown that Notch signaling plays an important role in cell fate within the ARC of mice. Active Notch signaling prevented neural progenitors from differentiating into feeding circuit neurons, whereas conditional loss of Notch signaling lead to a premature differentiation of these neurons. Presently, we hypothesized that Kisspeptin neurons would similarly be affected by Notch manipulation. To address this, we utilized mice with a conditional deletion of the Notch signaling co-factor Rbpj-κ (Rbpj cKO), or mice persistently expressing the Notch1 intracellular domain (NICD tg) within Nkx2.1 expressing cells of the developing hypothalamus. Interestingly, we found that in both models, a lack of Kisspeptin neurons are observed. This suggests that Notch signaling must be properly titrated for formation of Kisspeptin neurons. These results led us to hypothesize that Kisspeptin neurons of the ARC may arise from a different lineage of intermediate progenitors than NPY neurons and that Notch was responsible for the fate choice between these neurons. To determine if Kisspeptin neurons of the ARC differentiate similarly through a Pomc intermediate, we utilized a genetic model expressing the tdTomato fluorescent protein in all cells that have ever expressed Pomc. We observed some Kisspeptin expressing neurons labeled with the Pomc reporter similar to NPY neurons, suggesting that these distinct neurons can arise from a common progenitor. Finally, we hypothesized that temporal differences leading to premature depletion of progenitors in cKO mice lead to our observed phenotype. Using a BrdU birthdating paradigm, we determined the percentage of NPY and Kisspeptin neurons born on embryonic days 11.5, 12.5, and 13.5. We found no difference in the timing of differentiation of either neuronal subtype, with a majority occurring at e11.5. Taken together, our findings suggest that active Notch signaling is an important molecular switch involved in instructing subpopulations of progenitor cells to differentiate into Kisspeptin neurons.
Keywords: Notch; Kisspeptin; Arcuate; Neurogenesis; Pomc, Rbpj
INTRODUCTION
The hypothalamus is a key mediator of many homeostatic functions in mammalian physiology (Brooks. 1988). In humans and rodents, it has been linked to regulation of water balance, energy balance, thermal regulation, reproductive function, body size, stress and others (Epelbaum. 1992; McCann, et al. 1994; Rothwell. 1994; Becu-Villalobos and Libertun. 1995; Stratakis and Chrousos. 1995; Bing, et al. 1996). In order to perform such a wide array of functions, this region of the brain is separated into subsets of neurons, called nuclei, which signal through distinct neuropeptides produced by specialized neuronal subtypes (Swaab, et al. 1993). One nucleus, the arcuate nucleus (ARC), has classically been characterized as the “feeding center” of the brain (Swaab, et al. 1993). This function is carried out by the antagonistic actions of anorexigenic Proopiomelanocortin (POMC) and orexigenic Neuropeptide-Y (NPY)/Agouti-related Peptide (AgRP) neurons (Cowley, et al. 2001; Luquet, et al. 2005). These neuronal subsets interact not only with each other, but send projections to other areas of the brain (Wang, et al. 2015). Aside from regulating energy homeostasis, the ARC also has a population of Kisspeptin expressing neurons (Gottsch, et al. 2004). Kisspeptin neurons have been suggested to play a role in puberty onset and reproductive function by communicating with Gonadotropin-releasing hormone (GnRH) neurons and acting as a pulse generator for the release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) (Irwig, et al. 2004; Li, et al. 2009). There are at least two other neuron populations found within the ARC as well, one expressing Growth Hormone Releasing Hormone (GHRH) and the other Tyrosine Hydroxylase (TH) (Balan, et al. 1996; Romero and Phelps. 1997). Despite the differences in peptide content and function, ARC neurons likely all arise from a common pool of progenitor cells in the developing diencephalon (Yee, et al. 2009). The exact molecular pathways that allow a progenitor to create these neurons of distinct fates are still largely unknown. Importantly, genetic disorders that affect early development of the hypothalamus, can result in life-long deficiencies that persist into adulthood. For example, many of the phenotypes observed in Prader-Willi patients are regulated by neurons found within the ARC (Ge, et al. 2002). Namely, hyperphagy may be modulated by the POMC and NPY expressing population of neurons within the ARC, whereas the Kisspeptins of the ARC may be contributing to the observed infertility and hypogonadism. This example highlights the importance of understanding neural development and the genes responsible for differentiation of each neuronal subtype within the hypothalamus.
Neurogenesis and development of the ARC begins as early as embryonic day 10.5 (e10.5) (Shimada and Nakamura. 1973; McNay, et al. 2006). Previous reports have shown that in the mouse a great deal of the neurogenesis specifically within the ARC occurs in a very narrow window, and by e16.5 all the cellular subtypes within the ARC are present (Alvarez-Bolado, et al. 2012; Ishii and Bouret. 2012). Progenitor cells contributing to the ARC are housed within the hypothalamic ventricular zone (HVZ) where they receive cues to rapidly proliferate and remain undifferentiated (Ye, et al. 2009). When progenitors are cued to differentiate, they will become post-mitotic and migrate out into the peripheral ARC where they will then begin expressing their unique neuropeptides (McClellan, et al. 2008). A number of early patterning genes have been defined in the developing anterior diencephalon. (Shimogori, et al. 2010). Specifically, Nkx2.1 is expressed broadly throughout the ventral border of the anterior hypothalamus corresponding with the tissue of the presumptive ARC (Nakamura, et al. 2001). Following pattern specification, progenitors will begin differentiating into their respective mature cellular subtypes. It is becoming increasingly clear that each neuronal population is dependent on a distinct set of developmental factors. For instance, studies have shown the necessity for Ngn3 in the development of both POMC and NPY neurons (Pelling, et al. 2011). Similarly, Gsh1 is critical for the formation of GHRH neurons (Li, et al. 1996). Although few early markers of the developing ARC have been uncovered (Kimura, et al. 1996; Wang and Lufkin. 2000; Cremona, et al. 2004), the factors involved in the selection of a Kisspeptin fate remain largely unknown. Similarly, the lineage pathways shared between each neuronal subtype involved in maintaining energy balance and other homeostatic functions regulated by the ARC have yet to be uncovered.
The Notch signaling pathway is an evolutionarily conserved signaling pathway found throughout the developing embryo (Kortschak, et al. 2001; Schroder and Gossler. 2002). In general, Notch signaling is a cell-to-cell dependent pathway by which a cell expressing one of the several Notch receptors receives a signal from a neighboring cell presenting a Notch ligand (Shimizu, et al. 2000). Upon activation, the Notch intracellular domain (NICD) undergoes a series of cleavage events and will eventually translocate to the nucleus, where it will complex with a number of cofactors to initiate transcription (Struhl and Adachi. 1998). The most important factor is RBPJ-κ (Drosophila CBF1 (Olave, et al. 1998)), which allows the activated complex to bind DNA and promote gene transcription (Nam, et al. 2003). Notch signaling is implicated in two major steps in the progression of differentiation. First, as seen in the brain, liver, and other tissues, active Notch signaling is important in the decision of a progenitor cell to remain in a progenitor-like state or to exit the cell cycle and undergo differentiation (Qu, et al. 2013; Bhat. 2014; Wang, et al. 2014). However, Notch signaling is also important in selecting fates of cells which have begun differentiation. As seen in the immune system, active Notch signaling instructs intermediate hematopoietic stem cells to differentiate into immature T-lymphocytes and inhibits formation of B-lymphocytes (De Obaldia, et al. 2013; Ayllon, et al. 2015). Indeed, work from our lab and others has shown that Notch components as well as active downstream components of signaling are present in the presumptive hypothalamus (Casarosa, et al. 1999; Aujla, et al. 2013). We have previously shown that RBPJ-κ mediated Notch signaling is important for maintenance of ARC progenitors in the HVZ and plays a role in repressing the canonical pro-neural gene Mash1 expressed within the ARC (Aujla, et al. 2013). Conversely, loss of Rbpj-κ mediated Notch signaling leads to a premature burst of differentiation during early development, resulting in greater numbers of POMC and NPY neurons at e18.5.
The overall goal of this study is to provide further insight into how different neuronal subtypes of the ARC are specified from a common progenitor pool of the HVZ. Specifically, we hypothesized that the Notch signaling pathway would be involved in differentiation of Kisspeptin neurons of the ARC, and this neuronal subtype would arise from a common Pomc expressing intermediate progenitor cell. Utilizing both loss- and gain-of-function models, we have conditionally knocked out the RBPJ-κ component of the active Notch signaling complex (Rbpj cKO) or persistently expressed the constitutively active Notch1 intracellular domain (ICD) (NICD tg) within Nkx2.1 expressing cells in the developing ventral hypothalamus (Murtaugh, et al. 2003; Tanigaki, et al. 2004). In both models by which Notch signaling has been manipulated, a drastic reduction in Kisspeptin neurons occurs within the ARC. This suggests that persistent Notch signaling prevents neurogenesis irrespective of neuronal subtype; however, active Notch signaling is also important to instruct an immature neuron to adopt a Kisspeptin fate. This is not due to a differential timing of Kisspeptin neuron birth with respect to NPY neurons, and thus is not the result of progenitor depletion prior to Kisspeptin neurogenesis. Further, lineage tracing experiments have uncovered that a subset of Kisspeptin neurons of the ARC do indeed develop through a Pomc expressing intermediate progenitor, similar to NPY neurons. These findings taken together would suggest that Rbpj-κ dependent Notch signaling may be an important molecular switch to instruct early and/or intermediate progenitor cells to adopt the Kisspeptin identity.
MATERIALS AND METHODS
Mice
Rbpj-κ conditional knock-out (cKO) mice or Notch1 intracellular domain constitutively active (NICD tg) mice were generated using established genetic mouse models. Rbpjtm1Hon (Rbpj-κ floxed) mice provided by Dr. Tasuku Honjo (Kyoto University, Japan) (Tanigaki, et al. 2004) or Gt(ROSA)26Sortm1(Notch1)Dam/J (RosaNotch1ICD floxed mice) (Murtaugh, et al. 2003) purchased from Jackson Laboratories (Bar Harbor, ME, USA) were bred to C57BL/6J-Tg(Nkx2-1-cre)2Sand/J (Nkx2.1-cre) mice (Xu, et al. 2008) purchased from Jackson Laboratories to generate Rbpj cKO and NICD tg mice, respectively. Lineage-tracing experiments to determine the origin of NPY and Kisspeptin neurons were performed by mating Tg(Pomc1-cre)16Lowl/J (Pomc-cre) mice purchased from Jackson Laboratories (Bar Harbor, ME, USA) (Balthasar, et al. 2004) to B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J (RosatdTomato floxed) mice (Madisen, et al. 2010) purchased from Jackson Laboratories. Prior to pairings for experiments, Pomc-cre mice were back-crossed one generation onto a CD-1 background (Charles River Laboratories, USA). For birthdating studies, timed pregnant CD-1 mice were generated. Unless otherwise noted, both sexes of mice were used for data analysis. To genotype, tail biopsies were collected and DNA was extracted either via salt-out or HotSHOT method (Miller, et al. 1988; Truett, et al. 2000). PCR was performed on DNA utilizing primer sets previously described or from Jackson Laboratory’s online database (Tanigaki, et al. 2004; Goldberg, et al. 2011). Breeding colonies were maintained in a facility with a 12-hour light/dark cycle at the University of Illinois at Urbana Champaign (UIUC). All protocols were approved by the UIUC Institutional Animal Care and Use Committee.
Tissue Collection
Rbpjfl/fl; Nkx2.1-cre+/+ (Rbpj control), Rbpjfl/fl; Nkx2.1-cre+/cre (Rbpj cKO), RosaNotch1ICD/+; Nkx2.1-cre+/+ (NICD control), RosaNotch1ICD/+; Nkx2.1-cre+/cre (NICD tg) and CD-1 mice were collected on the day of birth (p0) and fixed overnight at 4° C in 3.7% formaldehyde solution (Sigma-Aldrich, USA) diluted in phosphate-buffered saline (PBS). Tissue was then dehydrated through graded ethanols, methyl salicylate, and embedded in paraffin wax. 6 μm serial coronal sections were collected and three sequential sections were mounted on charged slides for histological analysis. Pomc-cre+/cre; RosatdTomato/+ pups were collected at p0, fixed overnight at 4°C in 4% paraformaldehyde (PFA) (Fisher Scientific, USA) dissolved in PBS, cryoprotected at 4°C in 30% sucrose solution in PBS and then snap frozen in O.C.T compound (TissueTek, CA, USA). 10 μm serial coronal sections were collected and mounted on charged slides for histological analysis. Rbpj control and cKO brains collected at p35 were collected from mice perfused with 4% PFA and frozen in an identical fashion as described above.
BrdU Injections for Birthdating
CD-1 female and male mice were bred together and plugs were checked every morning at 0900 h. 1200 h on the day a plug was observed was considered e0.5. Pregnant dams were injected once intraperitoneally with 5-Bromo-2-deoxyuridine (BrdU) (Sigma-Aldrich, MO, USA), 0.1 mg/g body weight, on either e11.5, e12.5, or e13.5 at 1030 h. Cages were then checked daily at 1200 h and pups were collected on the day of birth for histological analysis.
Immunohistochemistry
Immunohistochemistry was performed as previously described by our lab (Aujla, et al. 2013). Briefly, sections were deparaffinized, washed, and boiled in citrate solution (10 μM, pH 6.0). Following antigen retrieval, slides were blocked in blocking solution (5% normal donkey serum, 3% bovine serum albumen and 0.5% Triton-X100 diluted in sterile PBS). For peptides detected utilizing tyramide signal amplification (TSA) (Perkin Elmer, MA, USA) (Wang, et al. 1999), slides were instead blocked in TNB blocking solution composed of 0.1 M Tris-HCl, 0.15 M NaCl, and 0.5% TSA Blocking Reagent diluted in sterile ddH2O (pH 7.5). Frozen sections were thawed, fixed for 10 minutes in 4% PFA, washed in PBS, and blocked with identical solutions. Following blocking, antibody was incubated on tissue sections overnight at 4° C diluted in appropriate blocking solution. Primary antibodies used were raised against the following peptides: Neuropeptide Y (1:5000; Peninsula Labs, CA, USA), Tyrosine Hydroxylase (AB152 1:1500; Millipore, MA, USA) and BrdU (1:150; BD Biosciences, CA, USA), Kisspeptin was detected by either with either antibody AC053 (1:1500, a kind gift from Dr. Isabelle Franceschini, (Franceschini, et al. 2013)) or Kp10 (AB9754 1:500; Millipore, MA, USA). Slides were then incubated with biotin-conjugated rabbit (NPY, TH, Kp10), mouse (BrdU), or sheep (AC053) secondary antibody diluted in appropriate blocking solution for one hour at room temperature. Slides were washed and incubated with either streptavidin conjugated Cy3 or Cy2. Secondary and tertiary antibodies were purchased from Jackson ImmunoResearch (PA, USA) and all were used at a concentration of 1:200. Following biotinylation, slides used to detect Kisspeptin using AC053 were conjugated to streptavidin-HRP (1:100) and then incubated with Cyanine-3 within the manufacturer’s TSA kit (Perkin Elmer, MA, USA) according to the supplied TSA protocol. Slides were mounted with mounting media containing 4’,6-diamidino-2-phenylindole (DAPI, 1:1000; Sigma-Aldrich, MO, USA). Slides were then visualized at either 100× or 200× using a Leica DM2560 microscope or at 400× using a Leica DMI4000B confocal microscope. Images were taken using a Retiga 2000R color camera and acquired using Q-Capture software (Q-Imaging, Surrey, Canada). Images were processed using Adobe Photoshop CS6 (Adobe, CA, USA) and cells counts were quantified using ImageJ software (NIH, MA, USA).
In situ hybridization
In situ hybridization was also performed as previously described by our lab (Aujla, et al. 2015). Briefly, slides were deparaffinized, washed, permeabilized, digested with Proteinase-K, acetylated, and incubated in hybridization solution (at 64° C for Pomc or 55° C for Kiss1). Probes for either Pomc (1:200; a kind gift from Dr. Malcom Low (Japon, et al. 1994)) or Kiss1 (1:100; a kind gift from Dr. Alexander Kauffman (Gottsch, et al. 2004)) were linearized with appropriate enzymes and transcribed with polymerase in the presence of digoxigenin-labeled nucleotides. Labeled probes were denatured for 3 minutes and incubated overnight at hybridization temperature. Slides were then washed in 50% 0.5× formamide solution, washed in 0.5× sodium citrate, and blocked in ISH blocking solution (10% heat-inactivated sheep serum, 2% bovine serum albumen and 0.1% Triton-X100 in Tris-buffered saline). Following blocking, slides were incubated with anti-digoxigenin (1:500, Roche, IN, USA) antibody diluted in ISH block. Slides were then washed in Tris-buffered saline (pH 7.5, then pH 9.5) and incubated for 12-36 hours in NBT/BCIP developing solution (1:50; Roche, IN, USA). Samples were visualized identically to immunohistochemical stains and processed using the same software.
Identification of Arcuate Nucleus of the Hypothalamus at p0 and p35 for Cell Counts
The location of the ARC at p0 and p35 in cKO mice was determined by the presence of NPY- or Pomc-positive neurons. At p0, 4 slides separated by roughly 50 μm spanning 250 μm of the ARC were stained with either NPY or Pomc. At p35, the ARC was identified in a similar fashion, however 4 slides separated by roughly 120 μm, spanning 500 μm were stained. Slides were chosen according to Bregma −2.15.
Kiss1 positive neurons of the ARC in female mice at p0 were quantified by staining slides adjacent to those used for identification. Four slides spanning the ARC were hybridized with Kiss1 riboprobe. Each section was individually quantified and sections of the same slide were averaged together. Averages of each slide were summated for each animal to determine a representative amount of Kiss1-neurons spanning the rostrocaudal extent of the ARC. Control littermates for Rbpj cKO (n=4) and NICD tg mice (n=3) were compared in order to remove the possibility of genetic background difference as a confounding factor. Total Kiss1 counts between animals were then averaged and the standard error of the mean was calculated in Microsoft Excel. A Student’s 2-tailed t-test was performed in Microsoft Excel with an alpha of 0.05 set to determine significance.
Cell Counts for Lineage Tracing and Birthdating Studies
Lineage tracing studies were quantified similarly as described above: 4 slides separated by roughly 90 μm containing 3 serial sections spanning the rostrocaudal extent of the ARC of female mice were stained and quantified. Due to the inherent properties of the fluorescent reporter, only staining for NPY or Kisspeptin was necessary. Kisspeptin slides were stained first, and then the adjacent slide to each was stained with NPY. Pups were collected across multiple litters and females were used due to higher levels of Kisspeptin at p0.
Birthdating studies were quantified identically to Kiss1 counts in female mice as described above. However, slides were co-stained with Kisspeptin/BrdU, and the subsequent slide from the same animal was co-stained with NPY/BrdU. We defined the ARC as a roughly 0.018 mm3 volume lateral to both sides of the third ventricle (3V) encompassing nearly all Kisspeptin and NPY neurons. Both sides lateral to the 3V were quantified and summated, then averaged with other sections present on the same slide. Four slides from each animal were summated and averaged with other animals within each age group.
Identification of the Rostral Periventricular Area of the Third Ventricle/Anteroventral Periventricular Nucleus of the Hypothalamus at p35 in Rbpj cKO Mice
The location of the RP3V/AVPV in p35 cKO mice was determined by the presence of the optic chiasm and TH-positive neurons. For RP3V/AVPV identification, 5 slides separated by roughly 120 μm spanning approximately 630 μm were stained with TH or Kisspeptin to ensure both the RP3V and AVPV were represented for analysis. Slides were chosen according to −0.23 of Bregma.
Breeding Studies and Vaginal Cytology for Female Cyclicity
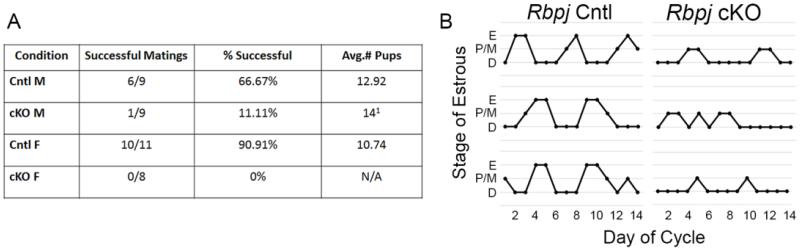
6-week old Rbpj control or cKO males or females were paired with age-matched CD-1 mates of proven reproductive success. Control and cKO mice were coded and blinded to researchers until the experiment was completed. Mice were paired for one week and then separated and housed individually for 3 weeks to allow gestation if a successful mating had occurred. After 3 weeks, litters and number of pups per litter were recorded and pups were removed from females. One week following removal of pups, mice were set up again with different counterpart mates and the breeding was repeated for a second trial. Results of the first mating are displayed in Figure 7. Following breeding studies, vaginal smears from control and cKO mice were collected for 14 consecutive days to assess cyclicity. Diestrus was defined as the presence of nearly all leukocytes, proestrus/metestrus was defined as the presence of a large mixture of leukoctyes and nucleated epithelial cells, and estrus was defined as the presence of nearly all cornified epithelial cells.
Fig. 7.
Female and male Rbpj cKO mice face severe reproductive challenges. (A) Results of a single mating challenge of Rbpj control of cKO males and females with age-matched CD-1 mice of proven reproductive success. Data are represented as a percentage of mice who successfully produced litters and average litter size has been reported. 1 Denotes the litter size of the single male Rbpj cKO who sired a litter. (B) Representative cyclicity of 3 Rbpj control (left) or cKO (right) females cycled for 14 consecutive days. Note that Rbpj cKO females entered the proestrus/metestrus phase, but never fully reached estrus. n=8-11 individuals.
RESULTS
Loss of Rbpjκ and persistent Notch1 expression both reduce Kisspeptin neuron number within the ARC
Previous work in our lab has shown that manipulation of Notch signaling was able to alter the number of Pomc and NPY expressing neurons of the ARC (Aujla, et al. 2013). Persistent expression of Notch signaling blocked neuronal differentiation of these two mature subtypes, whereas a loss of Rbpj-κ lead to a premature burst of differentiation. To determine the effect on Kisspeptin neurons derived from the same precursor pool of the HVZ, similar mouse models were utilized by which either Rbpj-κ was conditionally knocked out (Rbpj cKO) or the Notch1 intracellular domain was persistently expressed (NICD tg) in Nkx2.1-positive cells of the ventral hypothalamus. To ensure that the observations previously made at e13.5 and e18.5 persisted into the first postnatal day of life (p0), Pomc expression was visualized by in situ hybridization in both female and male mice. Consistent with previous findings, both female and male control mice (Fig. 1A, D respectively) showed robust Pomc expression in the ARC. Rbpj cKO mice showed a collapse in the HVZ paralleling observations made at e18.5 and showed random disbursement of Pomc neurons through this region in female and male mice (Fig. 1B, E respectively) as a result of this morphological disruption. Finally, the persistent NICD expressor model follows a similar trend observed at e18.5 such that Pomc expression appears absent in both females (Fig. 1C) and males (Fig. 1F).
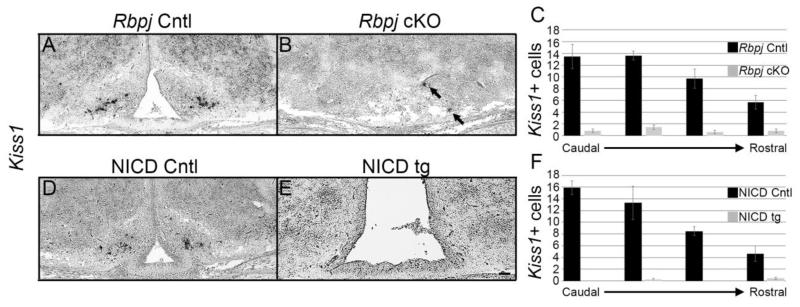
Fig. 1.
Both loss and persistent expression of Notch signaling results in a reduction in Kisspeptin expression. Coronal sections at p0 of both control female (A) and male (D) mice show robust Pomc expression lateral to the 3V dispersed through the ARC. Rbpj cKO female (B) and male (E) mice show a more disperse Pomc expression pattern and loss of the HVZ. NICD persistent expressor female (C) and male (F) mice show an expanded third ventricle (3V) and HVZ and block of neural differentiation. Kisspeptin expression for both female (G) and male (J) mice appears more modest and restricted more laterally than Pomc to the 3V. Both sexes show a visual reduction in Kisspeptin expression in both Rbpj cKO (H, K) and NICD tg (I, L) mice. Image magnification = 100×. n=3-4. Scale bar = 50 μm.
Other neuronal subtypes of the ARC explored previously followed a similar trend; however, Kisspeptin neurons were not examined. Since Kisspeptin neurons are similarly found within the ARC, we hypothesized that manipulating Notch signaling would also affect their fate. Compared to the robust Pomc expression in the ARC in both female and male mice, Kisspeptin peptide expression in females (Fig. 1G) and males (Fig. 1J) was much less pronounced and less dispersed throughout the ARC in control mice. Surprisingly, Rbpj cKO females (Fig. 1H) and males (Fig. 1K) both showed virtually no detectable Kisspeptin cell bodies nor labeled fibers within the ARC region containing Pomc neurons. Interestingly, Kisspeptin expression in NICD tg mice was also ablated in female (Fig. 1I) and male (Fig. 1L) mice at p0. In order to quantify the reduction of Kisspeptin expression in both of our Notch manipulations, cell bodies of Kiss1-positive neurons were detected utilizing in situ hybridization. Previous work has shown that Kiss1 expression within the ARC is downregulated by circulating sex steroids (Smith, et al. 2005). Given the prenatal testosterone surge experienced by male pups (Poling and Kauffman. 2012), females were exclusively quantified since peptide expression appeared nearly identical between the sexes (Fig. 1G, J). Paralleling Kisspeptin peptide expression, Kiss1 mRNA expression was far less robust than Pomc expression and less disperse throughout the ARC in control mice (Fig. 2A, D). A noticeable rostrocaudal gradient was also observed, consistent with previous reports (Knoll, et al. 2013) (Fig. 2C, F). Similar with Kisspeptin protein observation in Rbpj cKO mice, Kiss1 expression showed an obvious visual reduction throughout the entire rostrocaudal extent of the ARC, labeling very few Kiss1-positive cells (Fig. 2B, C). Also paralleling protein levels in NICD tg mice, virtually no Kiss1-positive cells were detected throughout the ARC (Fig. 2E, F). Upon quantification, we confirmed a reduction of Kiss1-positive neurons that exceeded 90% (42.5 ± 1.2 cells v. 3.6 ± 1.0 cells, P < 3.1×10−7, n=4) in Rbpj cKO mice and an even more drastic reduction in NICD tg mice, exceeding 98% (42.3 ± 0.3 cells v. 0.67 ± 0.4 cells, P < 1.1×10−7, n=3) compared to their respective littermate controls (Fig. 2C, F). Taken together, these results further confirm that active Notch signaling must be extinguished in order for all neuronal subtypes of the ARC to differentiate. Interestingly, these findings may also suggest that Notch signaling may be necessary to instruct a subset of progenitor cells towards a mature Kisspeptin fate.
Fig. 2.
Quantification of Kiss1-positive neurons of the ARC. (A) Kiss1 neurons detected by in situ hybridization in control female mice.. (B) Rbpj cKO mice show a drastic reduction in Kiss1 expression, however positive neurons were consistently observed (arrows). (C) Quantification of Kiss1 positive neurons shows a rostrocaudal gradient within control Rbpj mice, and very few cells are detected in Rbpj cKO mice. (D) Control littermates to NICD tg mice show a similar Kiss1 expression pattern to Rbpj control mice. (E) NICD tg mice show very few positive Kiss1 cells. (F) Similar to Rbpj mice, NICD control mice show a rostrocaudal gradient of Kiss1 expression and NICD tg mice show virtually no detectable cells.. Image magnification = 100×. n=3-4. Scale bar = 50 μm. * = P < 0.05. P-value = 3.1×10−7 for Rbpj cKO and 1.1×10−7 for NICD tg.
POMC, NPY, and Kisspeptin neurons share common lineages throughout development
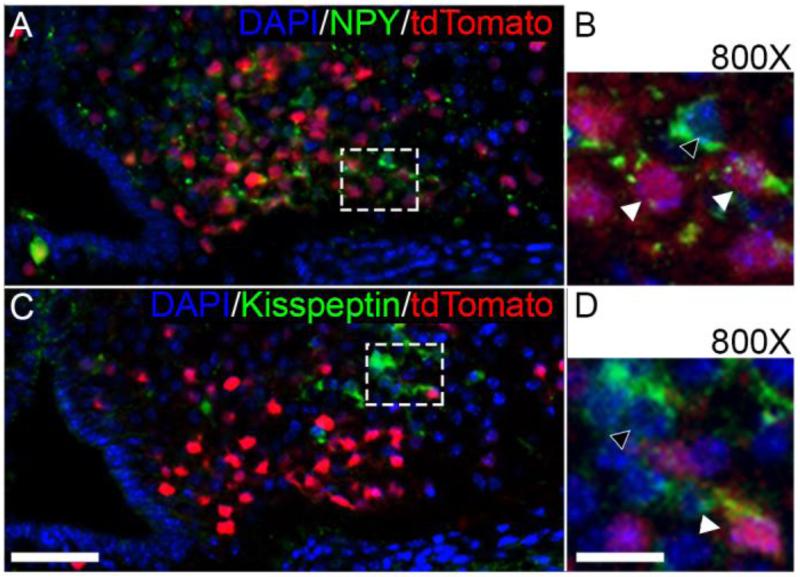
It has been well-established that an appreciable number of mature NPY neurons arise from a common intermediate progenitor cell which expressed Pomc at some point during its maturation (Padilla, et al. 2010). In Rbpj cKO mice, there is an increased number of Pomc and NPY expressing neurons of the ARC arising from this common intermediate lineage, however there is a near absence of Kisspeptin neurons (Aujla, et al. 2013) (Fig. 1). A possible explanation for the observed reduction of Kisspeptin/Kiss1 expression is that Notch signaling may act as a fate switch from a Pomc expressing lineage versus a Kisspeptin expressing lineage of neurons. In order to address this hypothesis, we bred Pomc-cre mice to RosatdTomato floxed to fluorescently label all cells of the ARC which expressed Pomc during some point of their development. This well characterized technique labels not only mature Pomc expressing neurons, but also any other neuronal subtype present within the ARC which had progressed through a Pomc expressing intermediate (Padilla, et al. 2012). Combining this inherently fluorescent label with immunohistochemistry, we were able to determine the proportion of mature NPY and Kisspeptin expressing neurons of the ARC. It is clear that many NPY neurons (55.8% ± 0.7%; n=3) double-label with our tdTomato reporter protein, suggesting that many neurons involved in energy homeostasis indeed arise from a common Pomc expressing immature progenitor cell, confirming what has been previously reported (Padilla, et al. 2012) (Fig. 3A). It is important to note that in our experiments while many NPY positive neurons co-express the tdTomato protein (Fig. 3B, white arrowheads), a number of NPY expressing neurons do not express the tdTomato fluorescent protein (Fig. 3B, black arrowheads), many of which in fact lie more distal from the third ventricle. Interestingly, we found Kisspeptin positive neurons (18.0% ± 1.3%; n = 3) within the ARC also double-labeled with the tdTomato fluorescent protein (Fig. 3C), indicating that an appreciable number of the Kisspeptin population indeed do arise from an immature Pomc progenitor. Similar to our observation for NPY neurons, some Kisspeptin positive cells retain the tdTomato protein (Fig 3D, white arrowheads), but many do not double-label with the tdTomato reporter (Fig. 3D, black arrowheads). Though fewer in number, certainly a significant number of Kisspeptin neurons seem to differentiate along a similar pathway as mature NPY neurons. These data would suggest that multiple neuron types found within the ARC can share a common Pomc expressing intermediate, and that Notch signaling is an important molecular switch involved in instructing progenitors of the HVZ and intermediate progenitors to differentiate into Kisspeptin neurons.
Fig. 3.
Both NPY and Kisspeptin neurons arise from a common Pomc lineage. (A) 200× magnification of the ARC. Many NPY-positive neurons of the ARC (green) also co-label with the fluorescent tdTomato reporter. (B) 800× confocal microscopy reveals that while many NPY-positive neurons label with the reporter protein (white arrowheads), a number of NPY-positive cells are not tdTomato positive (black arrowheads). (C) 200× magnification also reveals that a subset of Kisspeptin-positive neurons (green) label with the tdTomato reporter. (D) 800× confocal microscopy reveals that some Kisspeptin cells label with the reporter (white arrowheads), while many positive neurons do not (black arrowheads). n=3. Scale bar in C = 50 μm, D =25 μm.
Both NPY and Kisspeptin neurons differentiate as early as e11.5
Next, we determined if the reason we observed differences at p0 in Pomc versus Kisspeptin/Kiss1 expression in Rbpj cKO mice was that feeding circuit neurons differentiated earlier in development, followed by a second wave of Kisspeptin neurogenesis. We hypothesized that if neurogenesis occurred in a sequential manner, the progenitor pool of the HVZ would be completely depleted before Kisspeptin differentiation begins. Previous work by Ishii and Bouret (Ishii and Bouret. 2012) has suggested that the peak of neurogenesis within the ARC is e12.5. However, their studies birthdated neurons every other day of development and did not specifically look at unique markers of specific neuronal subtypes. While these previous studies have proven to be helpful in narrowing a window to determine when neurogenic events occur within the ARC, they failed to determine the timing of birth of the many subpopulations of neurons within the ARC.
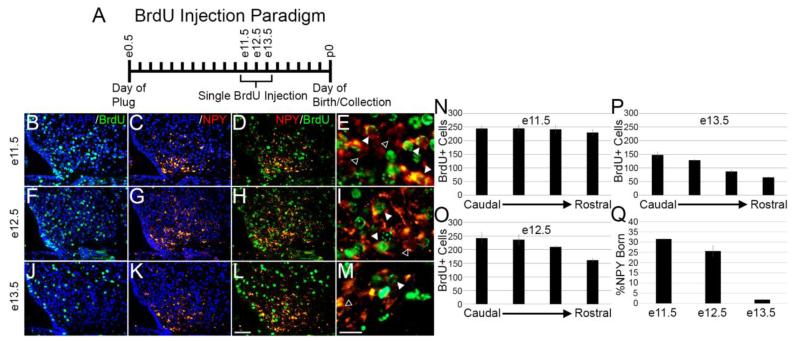
We first examined neurogenesis at e11.5, the earliest time point not explored during Ishii and Bouret’s birthdating experiments. Previous work by Padilla, et al. (Padilla, et al. 2010) reported that a subset of neurons indeed exit the cell cycle this early in development. We observed strong BrdU signal extending the entire rostrocaudal extant of the ARC as well as a relatively uniform distribution lateral to the third ventricle when examined at p0 (Fig. 4B, N). Visualization of NPY also revealed uniform expression through the rostrocaudal extant of the ARC, however neurons appeared mainly in the more medial and ventral regions of the ARC (Fig. 4C, G, K). Upon overlaying images, we observed many cells labeling with both BrdU and NPY (Fig. 4E, white arrowheads), and upon quantification we determined that 31.5% ± 0.2% (n=3) of NPY neurons double-labeled (Fig. 4Q).
Fig. 4.
NPY neurogenesis within the ARC occurs between e11.5 and e12.5. (A) Single BrdU injection paradigm on either e11.5, e12.5, or e13.5. Pups were collected on the day of birth (p0) and histology was performed. BrdU distribution when exposed at e11.5 (B) or e12.5 (F) appeared abundant and relatively evenly distributed throughout the ARC (N, O). When exposed at e13.5 (J), a rostrocaudal gradient was observed and far fewer cells retained the BrdU label (P). (C, G, K) NPY distribution was uniform throughout in the ventromedial region of the ARC at p0 in each of the days of embryonic exposure to BrdU. (D, H, L) A number of neurons visually double-labeled with BrdU and appeared to be born on e11.5 (D) and e12.5 (H), however few, if any, appeared to differentiate on e13.5 (L). (E, I, M) 800× magnification clearly shows several BrdU positive NPY neurons (white arrowheads) and some BrdU negative NPY neurons (black arrowheads) when injected at e11.5 (E) and e12.5 (I), but few at e13.5 (M). (Q) Quantification of double-labeled neurons show that most of neurogenesis occurs at e11.5 to e12.5 and sharply declines by e13.5. Image magnification for B-D, F-H, J-L = 200×, E, I, M = 800×. n=3. Scale bar in L = 50 μm, M = 10 μm.
We followed these observations up by determining the percentage of NPY neurons born at e12.5. Similar to the findings of Ishii and Bouret, we noted heavy labeling of BrdU throughout the whole extent of the ARC at p0 and an appreciable amount of NPY neurons double-labeled with BrdU (Fig. 4I, O). Quantification revealed that 25.6% ± 2.6% (n=3) of NPY neurons were born at e12.5 (Fig. 4Q), and combined with our findings from our e11.5 birthdating, we have accounted for a large number of NPY neurogenesis with our single-injection paradigm.
Since previous work has shown that by e14.5, neurogenesis in the ARC is nearly complete, we chose to check e13.5 as our final observation to determine if neurogenesis is still occurring. At this time point, we observed a rostrocaudal gradient of BrdU at p0, with many more BrdU-positive cells present in the more caudal regions of the ARC (Fig. 4J, P). Visually few, if any, NPY-positive neurons co-expressed BrdU (Fig. 4M, white arrowheads). Indeed, upon quantification we determined that 2.0% ± 1.2% (n=3) of NPY neurons are born at e13.5 (Fig. 4Q). Taken together, these findings have revealed that neurogenesis of NPY-positive cells begins at least by e11.5 and is tapering off by e13.5.
Using our previous findings of the timeline of NPY development within the ARC, we were then able to determine if Kisspeptin neurons developed along a similar progression. One possible explanation for loss of Kisspeptin/Kiss1 in Rbpj cKO mice is that the majority of these neurons are born at either e12.5 or e13.5, later than their NPY counterparts. If there is a subtle delay present, it is possible the premature burst of Pomc/NPY neurogenesis would have depleted the progenitor pool completely, leaving no precursors remaining to become Kisspeptin neurons. These experiments are equally interesting regardless, as the earliest reports of Kisspeptin or Kiss1 expression are e13.5 within the presumptive ARC of the mouse and the birthdate of Kisspeptin neurons have only been well-characterized in the rat (Desroziers, et al. 2012; Kumar, et al. 2014).
Given that the same animals were used to birthdate Kisspeptin neurogenesis, BrdU labeling patterns appeared identical to NPY double-stains (Fig. 5B, F, J, N, O, P). Paralleling our experimental design to birthdate NPY neurons of the ARC, we first examined Kisspeptin birth at e11.5. Similar to what was observed when characterizing the ARC of our Rbpj control and cKO mice (Fig. 2), we noticed a rostrocaudal gradient of Kisspeptin expression, showing more Kisspeptin-positive neurons in the caudal regions of the ARC. It is also important to note that Kisspeptin expression was mainly restricted more laterally and dorsally than NPY expression (Fig. 5C, G, K). When we explored dual-labeling of BrdU- and Kisspeptin-positive cells of the ARC, we saw a number of dual-labeled neurons (Fig. 5D, E). Upon quantification, we determined 33.6% ± 1.5% (n=3) of Kisspeptin neurons are born on e11.5, two days before we and others (Kumar, et al. 2014) are able to detect these neurons in the embryo (Fig. 5Q). Following this finding, we went on to determine the number of Kisspeptin neurons differentiating at e12.5. Interestingly, we visualized a number of double-labeled BrdU and Kisspeptin neurons (Fig. 5I). Quantification uncovered that, in fact, 19.7% ± 1.0% (n=3) of Kisspeptin neurons differentiated on e12.5 (Fig. 5Q), a very similar trend to what was observed for NPY neurons.
Fig. 5.
Kisspeptin neurogenesis also occurs between e11.5 and e12.5 within the ARC. (A) An identical injection paradigm was utilized to birthdate Kisspeptin neurogenesis within the ARC. Injections occurred on either e11.5, e12.5 or e13.5 and pups were collected on p0. (B, F, J) BrdU expression patterns mimicked what was observed when birthdating NPY neurons. (C, G, K) Kisspeptin expression appeared to be more localized more laterally and dorsally with respective to NPY neurons. (D, H, L) Similar to observations made when birthdating NPY neurons, a number of Kisspeptin neurons differentiated at e11.5 (D) and e12.5 (H), and again nearly none differentiated by e13.5 (L). (E, I, M) 800× magnification clearly shows several BrdU positive Kisspeptin neurons (white arrowheads) when injected at e11.5 (E) and e12.5 (I), but again very few at e13.5 (M). BrdU negative Kisspeptin neurons were also detected at all ages of injection (black arrowheads). (Q) Quantification revealed differentiation of Kisspeptin neurons, peaking between e11.5 and e12.5, and sharply declining by e13.5. Image magnification = 200× for B-D, F-H, J-L, E, I, M = 800×. n=3. Scale bar in L = 50 μm. M = 10 μm.
To confirm that Kisspeptin neurogenesis indeed appears to follow a nearly identical pattern of birth to that of NPY neurons of the ARC, we chose to look at e13.5. We hypothesized that Kisspeptin neurogenesis may occur over a more broad time course than NPY neurogenesis, potentially explaining our phenotype in Rbpj cKO mice. However, we noticed that almost no Kisspeptin neurons double-labeled with BrdU (Fig. 5L, M). Quantification revealed that a mere 1.5% ± 0.8% of Kisspeptin neurons were born at e13.5, again paralleling what was observed for NPY neurogenesis in the ARC (Fig. 5Q). Since the timing of differentiation of NPY and Kisspeptin neurons is not different, this lends further evidence that Notch signaling is necessary in a specific subset of Pomc intermediate cells as well as other precursors for development of Kisspeptin neurons of the ARC.
Loss of Kisspeptin persists in the ARC up 5 weeks
Kisspeptin signaling is essential for the onset of puberty and reproductive function (Navarro, et al. 2004; Han, et al. 2005; Clarkson and Herbison. 2006), so we next determined the presence of Kisspeptin neurons within the ARC closer to the age of mature reproductive function to determine if there was a persistent phenotype in the Rbpj cKO mice. Similar to observations embryonically and at p0 (Fig. 1), we observed a persistent loss of HVZ progenitors and collapse of the 3V continuing as far out as p35. Due to this observation, we first visualized NPY neurons to both determine if feeding circuit neurons similarly behaved the same as e18.5/p0 as well as to identify the ARC in Rbpj cKO mice. In control mice, NPY expression was robust and lateral to the 3V (Fig. 6A). Rbpj cKO mice showed NPY-positive neurons scattered throughout the entire ARC, with no lateral restriction due to the loss of the 3V (Fig. 6B). After identifying the ARC in cKO mice, we next determined if Kisspeptin neurons were present. Kisspeptin neurons and fibers were easily detected in control females, lying similarly lateral to the 3V (Fig. 6C). Interestingly, our findings in Rbpj cKO mice paralleled observations made at p0. In slides adjacent to those used to identify the ARC via NPY immunohistochemistry (Fig. 6B), we observed virtually no Kisspeptin positive neurons or fibers (Fig. 6D). These results are interesting, as they further suggest the importance for Notch signaling in the development of Kisspeptin neurons not only during early neurogenesis, but also into adulthood.
Fig. 6.
Conditional loss of Rbpj-κ in Nkx2.1 positive cells reduces Kisspeptin expression in the ARC as late as p35. (A) NPY-positive neurons and fibers extend lateral to the 3V throughout the ARC in control mice. (B) In cKO mice, the 3V is still absent in the ARC and NPY-positive neurons are scattered throughout the entire region. (C) Kisspeptin-positive neurons also lie lateral to the 3V and appear to have a similar rostrocaudal gradient as observed at p0. (D) cKO mice persistently exhibit a loss of Kisspeptin neurons within the ARC as far out as 5 weeks of age. (E) TH-positive neurons of the periventricular nucleus are present in the RP3V and extend into the AVPV in control mice. (F) TH-positive neurons are also present within the RP3V of Rbpj cKO mice and found throughout the defined region. (G) Kisspeptin-positive cell bodies and fibers are found just lateral to the rostral 3V in the RP3V/AVPV in control mice. (H) Similar to within the ARC, virtually no Kisspeptin-postitive cells or fibers are found throughout the RP3V/AVPV. Image magnification = 100×. n=4. Scale bar in H = 50 μm.
Kisspeptin neurons of the RP3V/AVPV are also affected in cKO mice
While it would appear that Notch signaling is important for development of Kisspeptin neurons of the ARC, it is unclear if this regulation is context specific within the ARC. Many groups have shown that another population of Kisspeptin-positive neurons exists clustered mainly in the rostral periventricular region of the third ventricle (RP3V) and anteroventral periventricular nucleus (AVPV) (Cravo, et al. 2011; Knoll, et al. 2013). Factors necessary for the development of this population of Kisspeptin neurons are less well known and it is unclear whether Notch signaling is playing a role in development of this region. Previous studies have shown that Nkx2.1 expression extends into the region of the presumptive RP3V/AVPV (Salvatierra, et al. 2014), making it likely that Notch signaling is also disrupted in this region in the Rbpj cKO mice. It would be interesting to determine if Kisspeptin neurons follow a similar phenotype in the RP3V/AVPV as in the ARC, as it would offer some support that all Kisspeptin neurons of the brain share some common developmental homology. The location of the RP3V/AVPV was confirmed by observing the presence of tyrosine hydroxylase (TH)-positive neurons, a commonly used marker of a different subset of neurons within the periventricular nucleus (Kauffman, et al. 2007). In control mice, TH-positive cell bodies appeared to hug the rostral 3V and extend through the region of interest (Fig. 6E). Similar to findings within the ARC, TH-positive neurons and fibers also appeared throughout the extent of the RP3V/AVPV of Rbpj cKO mice (Aujla, et al. 2013) (Fig. 6F). We detected a number of Kisspeptin-positive cell bodies and fibers throughout the extent of the RP3V/AVPV in control mice (Fig. 6G). Much to our surprise, we observed an identical phenotype to that of the ARC in Rbpj cKO mice: we observed virtually no Kisspeptin-positive cell bodies or fibers in the RP3V/AVPV (Fig. 6H). These findings suggest that active Notch signaling is necessary not only for early Kisspeptin differentiation within the ARC, but is also required for formation of Kisspeptin neurons in the RP3V/AVPV.
Rbpj cKO Mice Are Reproductively Challenged and Females Display Compromised Cyclicity
Given that we observed a loss of Kiss1/Kisspeptin in both brain regions of Rbpj cKO mice, we wanted to determine the reproductive consequences of this phenotype. To this end, 6-week old male and female Rbpj control or cKO mice were paired for one week with age matched counterparts. Reproductive output was determined by both the presence of a litter and the total number of pups per litter. We observed that male mice were strikingly subfertile when compared to control counterparts, with only one Rbpj cKO mouse ever siring a litter (Figure 7A). Female mice had an even more severe phenotype, as Rbpj cKO females were rendered completely infertile compared to their control littermates (Figure 7A). To ensure that Rbpj cKO mice were not simply reproductively immature compared to control littermates, an identical mating was performed on the same mice one week following the initial breeding study. These results were nearly identical to those reported in Figure 7A, with no Rbpj cKO males or female mice producing a litter. This phenomenon was not due to Rbpj cKO mice not attempting to copulate, as cKO males consistently plugged females and cKO females had detectable vaginal plugs after mating. We further sought to determine if cKO female mice had a regular estrous cycle. We observed that Rbpj control mice regularly cycled through estrus; however Rbpj cKO mice appeared to occasionally enter the prosestrus/metestrus phase, but an abundance of cornified epithelial cells, indicating estrus, were not detected (Figure 7B). It is unclear which level of the hypothalamic-pituitary-gonadal (HPG) axis is directly responsible for this reported compromised estrous cyclity, but it would likely explain the complete infertility observed in Rbpj cKO female mice.
DISCUSSION
Our studies provide evidence for the necessity of active Rbpj-κ dependent Notch signaling for the development of Kisspeptin neurons within the ARC. This is in contrast to previous work that has shown that loss of Rbpj-κ leads to an increased number of energy balance neurons, as well as a clear presence of other neuronal subtypes (Aujla, et al. 2013). We also have shown that persistent Notch signaling leads to a reduction in the number of Kisspeptin neurons within the ARC, suggesting that active Notch signaling is not only blocking neural differentiation of HVZ progenitors, but is also a critical component of the development of the Kisspeptin system within the ARC. There appears to be no difference in the timing of neurogenesis for different neuronal subtypes of the ARC, and it also appears that there is a great deal of homology of development between each neuronal subpopulation. Based on these observations, we propose that early Notch signaling completely blocks neural differentiation of progenitors within the HVZ. However, when progenitors escape the cell cycle and begin maturing, subsequent Notch signaling is then necessary for Kiss1 development and expression (Fig. 8). This finding is especially interesting, as it is clear that some, but not all, neural subtypes within the hypothalamus arising from a common progenitor rely on differential sets of signals to fully mature. This would lend evidence to the argument that early fate choices are still rather plastic and subject to dynamic change during critical windows of development.
Fig. 8.
Proposed model of neural differentiation of SOX2-positive neurons of the HVZ. Previous studies have suggested that nearly all cell-types found within the ARC arise from SOX2-positive progenitors of the HVZ. Many of these cells will differentiate into a Pomc expressing immature progenitor cell (Pomc Prog.), and can then further mature into at least 3 other neural subtypes. While a subset of Kiss1 neurons appear to arise from a Pomc lineage, a number may come from another progenitor pool. There is the formal possibility that all Kisspeptin and NPY neurons are derived from a Pomc expressing progenitor as well. Persistent Notch signaling appears to completely block differentiation of each neural subtype, but Rbpjκ-dependent Notch signaling appears necessary for development of Kisspeptin neurons.
Neurons develop from early progenitors lining the ventricular zone throughout the entire brain (Fishell, et al. 1993). Early markers such as Nkx2.1, Lhx1, and Lhx9 within the presumptive hypothalamus play critical roles in forming the borders that ultimately become the specific nuclei of the anterior hypothalamus (Shimogori, et al. 2010). Lhx2 has also recently been shown to be expressed within the HVZ of the presumptive ARC and has a great deal of overlap with Nkx2.1 (Salvatierra, et al. 2014). One of the earliest defining boundaries of the developing ARC is Pomc (Shimogori, et al. 2010), detectable as early as e10.5 (McNay, et al. 2006). We have shown that at least a subset of Kisspeptin expressing neurons of the ARC are indeed derived from a common progenitor population of early expressing Pomc cells. Though it is clear that a larger proportion of NPY neurons develop through this progression, it is important to consider that Kisspeptin neurons of the ARC are developmentally somewhat similar to POMC and NPY (and presumably other neurons of the ARC). It is likely that early differentiation events induce neurons to express Pomc, and subsequent signals further instruct their differentiation to their respective mature lineages, which may or may not include continued Pomc expression.
In this study we found that many, but not all, NPY or Kisspeptin neurons appear to be derived from a Pomc expressing intermediate progenitor. A number of likely scenarios may explain this observed phenomenon. First, it is possible that the expression of the tdTomato is not fully robust in all cell types of the brain. This would lead to a subset of NPY- or Kisspeptin-expressing neurons which did in fact arise from a Pomc expressing progenitor, but do not label with the tdTomato protein. Another possibility is that cre recombinase may not be fully penetrant in Pomc expressing cells; however, it has been characterized to have high penetrance and specificity (upwards of 95%) (Balthasar, et al. 2004). A very recent report has performed similar lineage tracing experiments of Kisspeptin expressing neurons of the ARC utilizing two different Pomc-cre mouse strains (Pomc-cre(BAC1) (Balthasar, et al. 2004) or Pomc-cre(BAC2) (McHugh, et al. 2007)) by means of a fluorescent ribosomal labeling of Pomc fated cells (Sanz, et al. 2015). Interestingly, their findings utilizing the identical Pomc-cre strain (Pomc-cre(BAC1) (Balthasar, et al. 2004) with a different reporter technique suggest that approximately 27% of Kisspeptin neurons arise from Pomc expressing progenitors. These data are comparable to our findings utilizing the tdTomato protein to label Pomc cells and suggest that a proportion of Kisspeptin neurons are either derived from another undefined early intermediate subpopulation of cells or directly from Sox2 expressing progenitors of the HVZ. However, using the Pomc-cre(BAC2) model, Sanz, et al. detect a much higher percentage of Kisspeptin neurons fated through a Pomc lineage. Therefore, it is still possible that the majority of mature Kisspeptin neurons are indeed derived from an immature Pomc expressing intermediate.
Throughout the brain and within the hypothalamus, development of each different cellular subtype occurs at very distinct, well-defined time points throughout neurogenesis (Altman and Bayer. 1978; Altman and Bayer. 1990). It has been long-known that development of neurons of the hypothalamus occurs over a relatively narrow window during gestation in the mouse and rat; however, the techniques used, such as labeling with [3H] thymidine, do not allow for one to identify which specific neurons are being labeled. Ishii and Bouret characterized the birth of leptin responsive neurons within the ARC; however, these could be POMC, NPY or Kisspeptin neurons (Korner, et al. 2001; Balthasar, et al. 2004; Smith, et al. 2006). We hypothesized that if there was a subtle delay in neurogenesis between NPY and Kisspeptin neurons, the progenitor pool within the HVZ could be depleted by the time Kisspeptin neurons would be born in the Rbpj cKO mice. However, our data would suggest otherwise: differentiation of NPY and Kisspeptin neurons occur at nearly identical times. To our knowledge, this is the first study to birthdate Kisspeptin neurons in the ARC of the mouse. Desroziers, et al. performed similar BrdU birthdating of ARC Kisspeptin neurons within the rat and report that neurogenesis occurs over a more broad window, beginning at e12.5, peaking and e15.5, and not completely ending by e17.5 (Desroziers, et al. 2012). While our studies have only explored the wave of neurogenesis between e11.5 and e13.5, we find it very interesting that a large majority of Kisspeptin neurogenesis within the mouse occurs within this narrower timeframe. This does not preclude the possibility of a second wave of Kisspeptin neurogenesis later in embryonic development, however. Combining the lineage tracing data and these observations, we propose that Notch signaling is indeed playing two roles during neurogenesis: first to maintain progenitors within the HVZ, but also to induce differentiation of Kisspeptin neurons.
As discussed previously, it would appear that distinct neuronal subtypes of the ARC rely on specific factors to instruct their differentiation towards the numerous lineages of neurons present. A number of transcription factors, namely Mash1 and Ngn3, are critical in promoting neurogenesis and differentiation of both POMC and NPY neurons (McNay, et al. 2006; Pelling, et al. 2011). To date, very little data exists describing genes important in development of ARC Kisspeptin neurons; however, we propose that Rbpj-κ may be one candidate. Another potential factor which has been suggested to play a role in Kisspeptin neuron number within the ARC is the Bax gene (Semaan, et al. 2010). Semaan, et al. have shown that Bax knockout mice possess significantly more Kisspeptin neurons, suggesting that apoptosis during embryonic development and perinatally may play a role in the number of Kisspeptin cells of the ARC. Interestingly, in other tissues of the body Bax expression is regulated by Notch signaling (Ye, et al. 2012). Given that we have previously reported cell death via TUNEL staining in the HVZ of Rbpj cKO mice (Aujla, et al. 2013), it is entirely possible that Bax expression is dysregulated, resulting in apoptosis of specific progenitors giving rise to Kisspeptin neurons.
Another way Notch signaling may be regulating Kisspeptin neuron fate could be direct control of Kiss1 transcription. A recent study has revealed a critical ARC specific enhancer element upstream of the Kiss1 transcriptional start site (Goto, et al. 2015). Goto, et al. define a subset of transcription factors that have putative binding sites on the Kiss1 enhancer; however, did not describe Notch signaling as a potential regulator. We performed promoter and enhancer analysis of the Kiss1 gene, which revealed a number of potential Rbpj-κ binding sites both within the proximal promoter as well as the ARC-specific enhancer as defined in Goto et al. Within the context of C2C12 cells, Rbpj-κ frequently bound to DNA and upon complexing with NICD/MAML regulated gene expression at very distal sites from the transcriptional start site in addition to sites in proximal promoters (Castel, et al. 2013). Taken together, one could postulate that Rbpj-κ may be acting in a similar manner on the Kiss1 gene such that activation of Notch signaling would induce gene expression, which may explain the necessity for Notch signaling for Kisspeptin neurons of the ARC and AVPV.
Not only do intrinsic signals of the developing hypothalamus play a key role in pattern specification and cellular fate choice decisions, but external stimuli can influence development of tissue as well. One source of such environmental signaling may come from a pregnant mother’s amniotic environment. It is becoming increasingly clear that hormones such as leptin and insulin, which activate ARC neurons in adulthood, may play a role in the neuronal profile that is set up during gestation. In vitro data by Desai et al. have shown that high levels of leptin and insulin can prevent differentiation of hypothalamic progenitor cells which may be mediated by an increase in Notch signaling (Desai, et al. 2011). Given that we hypothesize that Notch signaling is necessary for development of Kisspeptin neurons, inappropriate expression of Notch signaling and downstream targets during critical windows of development may be able to bias intermediate progenitors towards a Kisspeptin fate. It is also possible that this bias may be at the expense of neurons critical to balancing energy homeostasis. These findings have major implications for human health, as many obese mothers are typically hyperinsulinemic and hyperleptinemic (Lesseur, et al. 2014; Shapiro, et al. 2015). Taken all together, it is clear that the narrow window of neurogenesis may be negatively impacted by the hormonal profile of an obese mother and that this dysregulation may be the result of an improper titration of Notch signaling, in turn putting the fetus at higher risk of developing obesity or altered reproductive development and function later in life.
Kisspeptin neurons have become increasingly interesting within the field of reproductive biology. Undoubtedly, these neurons play an important role in reproductive function, as global knockouts of Kiss1 or loss of its receptor, Gpr54, lead to infertility (Funes, et al. 2003; Lapatto, et al. 2007). Interestingly, while our mice do not completely lack expression of these neurons, there is a clear and obvious severe reduction in neuron number that persists in both brain regions which house Kisspeptin-positive neurons. There has been some dispute as to the importance of number of Kisspeptin neurons on reproductive function, although it is clear that even extraordinarily few neurons can result in normal (or suboptimal) reproductive output (Popa, et al. 2013). We report that loss of Rbpj-κ within Nkx2.1 expressing cells results in near complete infertility. Given their similar phenotype to a global Kiss1 knockout model, this further offers support for the importance of Notch signaling in development of this neuronal subtype.
HIGHLIGHTS.
Deletion or overexpression of Notch signaling results in drastic ARC Kiss1 reduction
A subset of Kisspeptin neurons are derived from Pomc progenitors
Both NPY and Kisspeptin neurons are born as early as e11.5
Conditional loss of Rbpj-κ results in persistent loss of ARC and AVPV Kisspeptin
Rbpj cKO mice face severe reproductive challenges
ACKNOWLEDGEMENTS
We thank Dr. Paven Aujla (Raetzman Lab, University of Illinois at Urbana Champaign) for technical help, experimental advice, and assistance. We also thank Dr. Alexander Kauffman and Alison Lawrence at the University of California, San Diego for providing us with the Kiss1 plasmid used to create the Kiss1 in situ hybridization riboprobe. We are grateful to Dr. Isabelle Franceschini (Physiologie de la Reproduction et des Comportements, Institut National de Recherche Agronomique, Nouzilly, France) for providing us with an anti-Kisspeptin antibody. We acknowledge Yvonne Ziegler (Nardulli Lab, University of Illinois at Urbana Champaign) for training and use of equipment to generate confocal microscopy images. This research was funded by National Institute of Health Grants R01 DK076647 (LTR) and T32 ES007326 (MJB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman J, Bayer SA. Migration and Distribution of Two Populations of Hippocampal Granule Cell Precursors during the Perinatal and Postnatal Periods. J Comp Neurol. 1990;301:365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the Diencephalon in the Rat. III. Ontogeny of the Specialized Ventricular Linings of the Hypothalamic Third Ventricle. J Comp Neurol. 1978;182:995–1015. doi: 10.1002/cne.901820513. [DOI] [PubMed] [Google Scholar]
- Alvarez-Bolado G, Paul FA, Blaess S. Sonic Hedgehog Lineage in the Mouse Hypothalamus: From Progenitor Domains to Hypothalamic Regions. Neural Dev. 2012;7 doi: 10.1186/1749-8104-7-4. 4-8104-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla PK, Bogdanovic V, Naratadam GT, Raetzman LT. Persistent Expression of Activated Notch in the Developing Hypothalamus Affects Survival of Pituitary Progenitors and Alters Pituitary Structure. Dev Dyn. 2015 doi: 10.1002/dvdy.24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla PK, Naratadam GT, Xu L, Raetzman LT. Notch/Rbpjkappa Signaling Regulates Progenitor Maintenance and Differentiation of Hypothalamic Arcuate Neurons. Development. 2013;140:3511–3521. doi: 10.1242/dev.098681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayllon V, Bueno C, Ramos-Mejia V, Navarro-Montero O, Prieto C, Real PJ, Romero T, Garcia-Leon MJ, Toribio ML, Bigas A, Menendez P. The Notch Ligand DLL4 Specifically Marks Human Hematoendothelial Progenitors and Regulates their Hematopoietic Fate. Leukemia. 2015 doi: 10.1038/leu.2015.74. [DOI] [PubMed] [Google Scholar]
- Balan IS, Ugrumov MV, Borisova NA, Calas A, Pilgrim C, Reisert I, Thibault J. Birthdates of the Tyrosine Hydroxylase Immunoreactive Neurons in the Hypothalamus of Male and Female Rats. Neuroendocrinology. 1996;64:405–411. doi: 10.1159/000127145. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin Receptor Signaling in POMC Neurons is Required for Normal Body Weight Homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Becu-Villalobos D, Libertun C. Development of Gonadotropin-Releasing Hormone (GnRH) Neuron Regulation in the Female Rat. Cell Mol Neurobiol. 1995;15:165–176. doi: 10.1007/BF02069564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KM. Notch Signaling Acts before Cell Division to Promote Asymmetric Cleavage and Cell Fate of Neural Precursor Cells. Sci Signal. 2014;7:ra101. doi: 10.1126/scisignal.2005317. [DOI] [PubMed] [Google Scholar]
- Bing C, Wang W, Pickavance L, Williams G. The Central Regulation of Energy Homeostasis: Roles of Neuropeptide Y and Other Brain Peptides. Biochem Soc Trans. 1996;24:559–565. doi: 10.1042/bst0240559. [DOI] [PubMed] [Google Scholar]
- Brooks CM. The History of Thought Concerning the Hypothalamus and its Functions. Brain Res Bull. 1988;20:657–667. doi: 10.1016/0361-9230(88)90075-5. [DOI] [PubMed] [Google Scholar]
- Casarosa S, Fode C, Guillemot F. Mash1 Regulates Neurogenesis in the Ventral Telencephalon. Development. 1999;126:525–534. doi: 10.1242/dev.126.3.525. [DOI] [PubMed] [Google Scholar]
- Castel D, Mourikis P, Bartels SJ, Brinkman AB, Tajbakhsh S, Stunnenberg HG. Dynamic Binding of RBPJ is Determined by Notch Signaling Status. Genes Dev. 2013;27:1059–1071. doi: 10.1101/gad.211912.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal Development of Kisspeptin Neurons in Mouse Hypothalamus; Sexual Dimorphism and Projections to Gonadotropin-Releasing Hormone Neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin Activates Anorexigenic POMC Neurons through a Neural Network in the Arcuate Nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr, Atkin S, Bookout AL, Rovinsky S, Frazao R, Lee CE, Gautron L, Zigman JM, Elias CF. Characterization of Kiss1 Neurons using Transgenic Mouse Models. Neuroscience. 2011;173:37–56. doi: 10.1016/j.neuroscience.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona M, Colombo E, Andreazzoli M, Cossu G, Broccoli V. Bsx, an Evolutionary Conserved Brain Specific homeoboX Gene Expressed in the Septum, Epiphysis, Mammillary Bodies and Arcuate Nucleus. Gene Expr Patterns. 2004;4:47–51. doi: 10.1016/s1567-133x(03)00151-0. [DOI] [PubMed] [Google Scholar]
- De Obaldia ME, Bell JJ, Wang X, Harly C, Yashiro-Ohtani Y, DeLong JH, Zlotoff DA, Sultana DA, Pear WS, Bhandoola A. T Cell Development Requires Constraint of the Myeloid Regulator C/EBP-Alpha by the Notch Target and Transcriptional Repressor Hes1. Nat Immunol. 2013;14:1277–1284. doi: 10.1038/ni.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, Li T, Ross MG. Hypothalamic Neurosphere Progenitor Cells in Low Birth-Weight Rat Newborns: Neurotrophic Effects of Leptin and Insulin. Brain Res. 2011;1378:29–42. doi: 10.1016/j.brainres.2010.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desroziers E, Droguerre M, Bentsen AH, Robert V, Mikkelsen JD, Caraty A, Tillet Y, Duittoz A, Franceschini I. Embryonic Development of Kisspeptin Neurones in Rat. J Neuroendocrinol. 2012;24:1284–1295. doi: 10.1111/j.1365-2826.2012.02333.x. [DOI] [PubMed] [Google Scholar]
- Epelbaum J. Intrahypothalamic Neurohormonal Interactions in the Control of Growth Hormone Secretion. Ciba Found Symp. 1992;168:54–64. doi: 10.1002/9780470514283.ch5. discussion 64-8. [DOI] [PubMed] [Google Scholar]
- Fishell G, Mason CA, Hatten ME. Dispersion of Neural Progenitors within the Germinal Zones of the Forebrain. Nature. 1993;362:636–638. doi: 10.1038/362636a0. [DOI] [PubMed] [Google Scholar]
- Franceschini I, Yeo SH, Beltramo M, Desroziers E, Okamura H, Herbison AE, Caraty A. Immunohistochemical Evidence for the Presence of various Kisspeptin Isoforms in the Mammalian Brain. J Neuroendocrinol. 2013;25:839–851. doi: 10.1111/jne.12069. [DOI] [PubMed] [Google Scholar]
- Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 Receptor GPR54 is Essential for the Development of the Murine Reproductive System. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Ge Y, Ohta T, Driscoll DJ, Nicholls RD, Kalra SP. Anorexigenic Melanocortin Signaling in the Hypothalamus is Augmented in Association with Failure-to-Thrive in a Transgenic Mouse Model for Prader-Willi Syndrome. Brain Res. 2002;957:42–45. doi: 10.1016/s0006-8993(02)03583-7. [DOI] [PubMed] [Google Scholar]
- Goldberg LB, Aujla PK, Raetzman LT. Persistent Expression of Activated Notch Inhibits Corticotrope and Melanotrope Differentiation and Results in Dysfunction of the HPA Axis. Dev Biol. 2011;358:23–32. doi: 10.1016/j.ydbio.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T, Tomikawa J, Ikegami K, Minabe S, Abe H, Fukanuma T, Imamura T, Takase K, Sanbo M, Tomita K, Hirabayashi M, Maeda K, Tsukamura H, Uenoyama Y. Identification of Hypothalamic Arcuate Nucleus-Specific Enhancer Region of Kiss1 Gene in Mice. Mol Endocrinol. 2015;29:121–129. doi: 10.1210/me.2014-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A Role for Kisspeptins in the Regulation of Gonadotropin Secretion in the Mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of Gonadotropin-Releasing Hormone Neurons by Kisspeptin as a Neuroendocrine Switch for the Onset of Puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin Activation of Gonadotropin Releasing Hormone Neurons and Regulation of KiSS-1 mRNA in the Male Rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Bouret SG. Embryonic Birthdate of Hypothalamic Leptin-Activated Neurons in Mice. Endocrinology. 2012;153:3657–3667. doi: 10.1210/en.2012-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japon MA, Rubinstein M, Low MJ. In Situ Hybridization Analysis of Anterior Pituitary Hormone Gene Expression during Fetal Mouse Development. J Histochem Cytochem. 1994;42:1117–1125. doi: 10.1177/42.8.8027530. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual Differentiation of Kiss1 Gene Expression in the Brain of the Rat. Endocrinology. 2007;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp Null Mouse: Thyroid-Specific Enhancer-Binding Protein is Essential for the Organogenesis of the Thyroid, Lung, Ventral Forebrain, and Pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- Knoll JG, Clay CM, Bouma GJ, Henion TR, Schwarting GA, Millar RP, Tobet SA. Developmental Profile and Sexually Dimorphic Expression of kiss1 and kiss1r in the Fetal Mouse Brain. Front Endocrinol (Lausanne) 2013;4:140. doi: 10.3389/fendo.2013.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner J, Savontaus E, Chua SC, Jr, Leibel RL, Wardlaw SL. Leptin Regulation of Agrp and Npy mRNA in the Rat Hypothalamus. J Neuroendocrinol. 2001;13:959–966. doi: 10.1046/j.1365-2826.2001.00716.x. [DOI] [PubMed] [Google Scholar]
- Kortschak RD, Tamme R, Lardelli M. Evolutionary Analysis of Vertebrate Notch Genes. Dev Genes Evol. 2001;211:350–354. doi: 10.1007/s004270100159. [DOI] [PubMed] [Google Scholar]
- Kumar D, Freese M, Drexler D, Hermans-Borgmeyer I, Marquardt A, Boehm U. Murine Arcuate Nucleus Kisspeptin Neurons Communicate with GnRH Neurons in Utero. J Neurosci. 2014;34:3756–3766. doi: 10.1523/JNEUROSCI.5123-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− Mice Exhibit More Variable Hypogonadism than Gpr54−/− Mice. Endocrinology. 2007;148:4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- Lesseur C, Armstrong DA, Paquette AG, Li Z, Padbury JF, Marsit CJ. Maternal Obesity and Gestational Diabetes are Associated with Placental Leptin DNA Methylation. Am J Obstet Gynecol. 2014;211:654.e1–654.e9. doi: 10.1016/j.ajog.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zeitler PS, Valerius MT, Small K, Potter SS. Gsh-1, an Orphan Hox Gene, is Required for Normal Pituitary Development. EMBO J. 1996;15:714–724. [PMC free article] [PubMed] [Google Scholar]
- Li XF, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Millar RP, O’Byrne KT. Kisspeptin Signalling in the Hypothalamic Arcuate Nucleus Regulates GnRH Pulse Generator Frequency in the Rat. PLoS One. 2009;4:e8334. doi: 10.1371/journal.pone.0008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP Neurons are Essential for Feeding in Adult Mice but can be Ablated in Neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A Robust and High-Throughput Cre Reporting and Characterization System for the Whole Mouse Brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann SM, Gutkowska J, Franci CR, Favaretto AL, Antunes-Rodrigues J. Hypothalamic Control of Water and Salt Intake and Excretion. Braz J Med Biol Res. 1994;27:865–884. [PubMed] [Google Scholar]
- McClellan KM, Calver AR, Tobet SA. GABAB Receptors Role in Cell Migration and Positioning within the Ventromedial Nucleus of the Hypothalamus. Neuroscience. 2008;151:1119–1131. doi: 10.1016/j.neuroscience.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate Gyrus NMDA Receptors Mediate Rapid Pattern Separation in the Hippocampal Network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- McNay DE, Pelling M, Claxton S, Guillemot F, Ang SL. Mash1 is Required for Generic and Subtype Differentiation of Hypothalamic Neuroendocrine Cells. Mol Endocrinol. 2006;20:1623–1632. doi: 10.1210/me.2005-0518. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A Simple Salting Out Procedure for Extracting DNA from Human Nucleated Cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch Signaling Controls Multiple Steps of Pancreatic Differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kimura S, Yamazaki M, Kawaguchi A, Inoue K, Sakai T. Immunohistochemical Analyses of Thyroid-Specific Enhancer-Binding Protein in the Fetal and Adult Rat Hypothalami and Pituitary Glands. Brain Res Dev Brain Res. 2001;130:159–166. doi: 10.1016/s0165-3806(01)00226-7. [DOI] [PubMed] [Google Scholar]
- Nam Y, Weng AP, Aster JC, Blacklow SC. Structural Requirements for Assembly of the CSL.Intracellular Notch1.Mastermind-Like 1 Transcriptional Activation Complex. J Biol Chem. 2003;278:21232–21239. doi: 10.1074/jbc.M301567200. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and Hormonally Regulated Messenger Ribonucleic Acid Expression of KiSS-1 and its Putative Receptor, GPR54, in Rat Hypothalamus and Potent Luteinizing Hormone-Releasing Activity of KiSS-1 Peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Olave I, Reinberg D, Vales LD. The Mammalian Transcriptional Repressor RBP (CBF1) Targets TFIID and TFIIA to Prevent Activated Transcription. Genes Dev. 1998;12:1621–1637. doi: 10.1101/gad.12.11.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla SL, Reef D, Zeltser LM. Defining POMC Neurons using Transgenic Reagents: Impact of Transient Pomc Expression in Diverse Immature Neuronal Populations. Endocrinology. 2012;153:1219–1231. doi: 10.1210/en.2011-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla SL, Carmody JS, Zeltser LM. Pomc-Expressing Progenitors Give Rise to Antagonistic Neuronal Populations in Hypothalamic Feeding Circuits. Nat Med. 2010;16:403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelling M, Anthwal N, McNay D, Gradwohl G, Leiter AB, Guillemot F, Ang SL. Differential Requirements for Neurogenin 3 in the Development of POMC and NPY Neurons in the Hypothalamus. Dev Biol. 2011;349:406–416. doi: 10.1016/j.ydbio.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Poling MC, Kauffman AS. Sexually Dimorphic Testosterone Secretion in Prenatal and Neonatal Mice is Independent of Kisspeptin-Kiss1r and GnRH Signaling. Endocrinology. 2012;153:782–793. doi: 10.1210/en.2011-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa SM, Moriyama RM, Caligioni CS, Yang JJ, Cho CM, Concepcion TL, Oakley AE, Lee IH, Sanz E, Amieux PS, Caraty A, Palmiter RD, Navarro VM, Chan YM, Seminara SB, Clifton DK, Steiner RA. Redundancy in Kiss1 Expression Safeguards Reproduction in the Mouse. Endocrinology. 2013;154:2784–2794. doi: 10.1210/en.2013-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Afelik S, Jensen JN, Bukys MA, Kobberup S, Schmerr M, Xiao F, Nyeng P, Veronica Albertoni M, Grapin-Botton A, Jensen J. Notch-Mediated Post-Translational Control of Ngn3 Protein Stability Regulates Pancreatic Patterning and Cell Fate Commitment. Dev Biol. 2013;376:1–12. doi: 10.1016/j.ydbio.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Romero MI, Phelps CJ. Identification of Growth Hormone-Releasing Hormone and Somatostatin Neurons Projecting to the Median Eminence in Normal and Growth Hormone-Deficient Ames Dwarf Mice. Neuroendocrinology. 1997;65:107–116. doi: 10.1159/000127170. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ. CNS Regulation of Thermogenesis. Crit Rev Neurobiol. 1994;8:1–10. [PubMed] [Google Scholar]
- Salvatierra J, Lee DA, Zibetti C, Duran-Moreno M, Yoo S, Newman EA, Wang H, Bedont JL, de Melo J, Miranda-Angulo AL, Gil-Perotin S, Garcia-Verdugo JM, Blackshaw S. The LIM Homeodomain Factor Lhx2 is Required for Hypothalamic Tanycyte Specification and Differentiation. J Neurosci. 2014;34:16809–16820. doi: 10.1523/JNEUROSCI.1711-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz E, Quintana A, Deem JD, Steiner RA, Palmiter RD, McKnight GS. Fertility-Regulating kiss1 Neurons Arise from Hypothalamic Pomc-Expressing Progenitors. J Neurosci. 2015;35:5549–5556. doi: 10.1523/JNEUROSCI.3614-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder N, Gossler A. Expression of Notch Pathway Components in Fetal and Adult Mouse Small Intestine. Gene Expr Patterns. 2002;2:247–250. doi: 10.1016/s1567-133x(02)00060-1. [DOI] [PubMed] [Google Scholar]
- Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. BAX-Dependent and BAX-Independent Regulation of Kiss1 Neuron Development in Mice. Endocrinology. 2010;151:5807–5817. doi: 10.1210/en.2010-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AL, Schmiege SJ, Brinton JT, Glueck D, Crume TL, Friedman JE, Dabelea D. Testing the Fuel-Mediated Hypothesis: Maternal Insulin Resistance and Glucose Mediate the Association between Maternal and Neonatal Adiposity, the Healthy Start Study. Diabetologia. 2015;58:937–941. doi: 10.1007/s00125-015-3505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Nakamura T. Time of Neuron Origin in Mouse Hypothalamic Nuclei. Exp Neurol. 1973;41:163–173. doi: 10.1016/0014-4886(73)90187-8. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Chiba S, Saito T, Kumano K, Hirai H. Physical Interaction of Delta1, Jagged1, and Jagged2 with Notch1 and Notch3 Receptors. Biochem Biophys Res Commun. 2000;276:385–389. doi: 10.1006/bbrc.2000.3469. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Lee DA, Miranda-Angulo A, Yang Y, Wang H, Jiang L, Yoshida AC, Kataoka A, Mashiko H, Avetisyan M, Qi L, Qian J, Blackshaw S. A Genomic Atlas of Mouse Hypothalamic Development. Nat Neurosci. 2010;13:767–775. doi: 10.1038/nn.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 Neurones are Direct Targets for Leptin in the ob/ob Mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 Gene Expression in the Brain of the Female Mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Chrousos GP. Neuroendocrinology and Pathophysiology of the Stress System. Ann N Y Acad Sci. 1995;771:1–18. doi: 10.1111/j.1749-6632.1995.tb44666.x. [DOI] [PubMed] [Google Scholar]
- Struhl G, Adachi A. Nuclear Access and Action of Notch in Vivo. Cell. 1998;93:649–660. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Hofman MA, Lucassen PJ, Purba JS, Raadsheer FC, Van de Nes JA. Functional Neuroanatomy and Neuropathology of the Human Hypothalamus. Anat Embryol (Berl) 1993;187:317–330. doi: 10.1007/BF00185889. [DOI] [PubMed] [Google Scholar]
- Tanigaki K, Tsuji M, Yamamoto N, Han H, Tsukada J, Inoue H, Kubo M, Honjo T. Regulation of alphabeta/gammadelta T Cell Lineage Commitment and Peripheral T Cell Responses by Notch/RBP-J Signaling. Immunity. 2004;20:611–622. doi: 10.1016/s1074-7613(04)00109-8. [DOI] [PubMed] [Google Scholar]
- Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. Preparation of PCR-Quality Mouse Genomic DNA with Hot Sodium Hydroxide and Tris (HotSHOT) BioTechniques. 2000;29:52–54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- Wang D, He X, Zhao Z, Feng Q, Lin R, Sun Y, Ding T, Xu F, Luo M, Zhan C. Whole-Brain Mapping of the Direct Inputs and Axonal Projections of POMC and AgRP Neurons. Front Neuroanat. 2015;9:40. doi: 10.3389/fnana.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Achim CL, Hamilton RL, Wiley CA, Soontornniyomkij V. Tyramide Signal Amplification Method in Multiple-Label Immunofluorescence Confocal Microscopy. Methods. 1999;18:459–464. doi: 10.1006/meth.1999.0813. [DOI] [PubMed] [Google Scholar]
- Wang T, Chen T, Liang HY, Yan HT, Lin N, Liu LY, Luo H, Huang Z, Li NL, Liu WH, Tang LJ. Notch Inhibition Promotes Fetal Liver stem/progenitor Cells Differentiation into Hepatocytes Via the Inhibition of HNF-1beta. Cell Tissue Res. 2014;357:173–184. doi: 10.1007/s00441-014-1825-9. [DOI] [PubMed] [Google Scholar]
- Wang W, Lufkin T. The Murine Otp Homeobox Gene Plays an Essential Role in the Specification of Neuronal Cell Lineages in the Developing Hypothalamus. Dev Biol. 2000;227:432–449. doi: 10.1006/dbio.2000.9902. [DOI] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate Mapping Nkx2.1-Lineage Cells in the Mouse Telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Ye QF, Zhang YC, Peng XQ, Long Z, Ming YZ, He LY. Silencing Notch-1 Induces Apoptosis and Increases the Chemosensitivity of Prostate Cancer Cells to Docetaxel through Bcl-2 and Bax. Oncol Lett. 2012;3:879–884. doi: 10.3892/ol.2012.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Mairet-Coello G, Pasoreck E, Dicicco-Bloom E. Patterns of p57Kip2 Expression in Embryonic Rat Brain Suggest Roles in Progenitor Cell Cycle Exit and Neuronal Differentiation. Dev Neurobiol. 2009;69:1–21. doi: 10.1002/dneu.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee CL, Wang Y, Anderson S, Ekker M, Rubenstein JL. Arcuate Nucleus Expression of NKX2.1 and DLX and Lineages Expressing these Transcription Factors in Neuropeptide Y(+), Proopiomelanocortin(+), and Tyrosine Hydroxylase(+) Neurons in Neonatal and Adult Mice. J Comp Neurol. 2009;517:37–50. doi: 10.1002/cne.22132. [DOI] [PMC free article] [PubMed] [Google Scholar]