Abstract
The inclusion of anti-thymocyte globulin (ATG) in cord blood transplantation is controversial. We evaluated outcomes according to ATG inclusion in 297 children and adolescents with acute lymphoblastic leukemia (ALL) who received myeloablative total body irradiation-based conditioning and either single (74%) or double-unit (26%) grafts. Ninety-two patients (31%) received ATG and 205 (69%) did not. ATG recipients were more likely to be cytomegalovirus seronegative. The incidence of day-100 grade II-IV acute GVHD (30% versus 54%, p = 0.0002), and chronic GVHD (22% versus 43%, p = 0.0008) were lower with ATG compared to non-ATG regimens. However, day 100 grade III-IV acute GVHD was comparable (11% versus 17%, p = 0.15). The 3-year incidences of transplant-related mortality (16% versus 17%, p = 0.98) and relapse (17% versus 27%, p = 0.12), and leukemia-free survival (66% versus 55%, p = 0.23) in ATG and non-ATG recipients were similar. There were no differences in viral reactivation between treatment groups (60% versus 58%, p=0.83). Therefore, the data suggest that incorporation of ATG with myeloablative conditioning regimens may be useful in reducing the risk of acute and chronic GVHD without any deleterious effect on transplant-related mortality, relapse or leukemia-free survival in children and adolescents with ALL.
Keywords: Acute lymphoblastic leukemia, myeloablative conditioning, in vivo T-cell depletion
Introduction
Acute lymphoblastic leukemia (ALL) is the most common indication for allogeneic transplantation in children and adolescents. In the absence of a human leukocyte antigen (HLA)-matched sibling or adult unrelated donor, cord blood is frequently used as an alternative source of hematopoietic stem cells for allogeneic transplantation. In cord blood transplantation (CBT) for pediatric ALL, however, the addition of anti-thymocyte globulin (ATG) to transplant conditioning is controversial. ATG is a potent immunosuppressive agent that induces T-cell clearance from the circulation and suppression of T-cell activation, homing and cytotoxic activities1. ATG may also cause induction of apoptosis in B-cell lineages2, and interference with dendritic cell function2-4. Formulations of ATG that are generated by immunization of horse or rabbit with human thymocytes also have direct effects on the thymus, impairing thymic output, particularly of CD4+ T cells including regulatory T cells6. The resultant in vivo T-cell depletion has been used to prevent graft rejection and abrogate graft-versus-host disease (GVHD), although ATG is known to cause profound T-cell deficiency and repertoire restriction after allogeneic transplantation5 which may also increase the risk of mortality due to serious opportunistic infections and leukemia recurrence6-10.
In myeloablative adult unrelated donor transplantation for hematologic malignancy, ATG has been associated with a lower incidence and severity of chronic GVHD and reduced late mortality risk11,12. In contrast, in reduced intensity or non-myeloablative conditioning for hematologic malignancy, a higher relapse risk and lower leukemia-free survival have been observed with the use of ATG10. In myeloablative unrelated adult donor transplantation for ALL in children and adolescents, Veys and colleagues using data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) demonstrated comparable transplant-related mortality, relapse, and leukemia-free survival regardless of whether ATG or alemtuzumab were included in transplantation regimens13. In that report all transplant conditioning regimens included total body irradiation (TBI) dose of 1000 cGy or higher. There are reports in children and adolescents undergoing CBT that support the concept that inclusion of ATG to conditioning regimens is not optimal with respect to immune reconstitution or survival. 14,15 Lindemans and colleagues reported the results of ATG inclusion in recipients of myeloablative or non-myeloablative conditioning for malignant and non-malignant diseases and found that, while the probability of severe acute GVHD were lower in the ATG-containing group, immune reconstitution was delayed and viral reactivations was higher in this group14. However, the survival was similar among recipients of ATG-containing and non-ATG containing regimens14. Pascal and colleagues from the Eurocord reported lower GVHD, higher transplant-related mortality and lower survival with ATG-containing (rabbit derived) compared non ATG-containing regimens after myeloablative CBT for acute leukemia, myelodysplastic/myeloproliferative syndromes and lymphoma.15 As ATG is routinely used, in the current analyses, we sought to investigate the risks and benefits of including ATG to a myeloablative TBI-containing conditioning regimen for ALL in a recent cohort of children and adolescents who received CBT and reported to the CIBMTR. Our hypothesis was that inclusion of ATG would lead to lower leukemia-free survival.
Patients and Methods
Data Source
The CIBMTR is a voluntary working group of transplant centers worldwide that contribute data consecutive on allogeneic and autologous transplants performed at their centers. Patients are followed longitudinally. Legal guardians and/or patients provided informed consent for research. The Institutional Review Boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
Eligibility Criteria
Patients had a diagnosis of ALL, were aged less than 21 years, in complete remission (CR) at transplantation, received a myeloablative TBI-conditioning regimen (TBI dose ≥1000 cGy) with chemotherapeutic agents and transplanted in the United States. Single-unit grafts had a total nucleated cell (TNC) dose of at least 2.5 × 107 per kilogram (kg) patient body weight prior to cryopreservation whereas in double-unit grafts each unit had a TNC of at least 1.5 × 107/kg and therefore a total cryopreserved TNC of at least 3 × 107/kg. Excluded were patients in morphologic relapse or refractory disease or reduced intensity conditioning regimens.
Endpoints
Time to neutrophil recovery was defined as the first of 3 consecutive days with a sustained absolute neutrophil count ≥ 0.5 × 109/l and time to platelet recovery, the first of 3 consecutive days at ≥20 × 109/l and at least 7 days without platelet transfusion support. Grading of acute GVHD and diagnosis of chronic GVHD were based on standard criteria.16,17 Relapse was defined as morphological or cytogenetic recurrence. Death in CR was considered as transplant-related mortality. Treatment failure was defined as relapse or death from any cause (inverse of leukemia-free survival). Surviving patients were censored at last follow-up.
Statistical Analysis
Patient, disease, and transplant-related factors were compared between transplantations that included ATG in the conditioning regimen and non-ATG regimens with the Chi-square test for categorical variables and the Wilcoxon two-sample test for continuous variables. The probabilities of leukemia-free and overall survival were calculated using the Kaplan-Meier estimator18. The probabilities of hematopoietic recovery, acute and chronic GVHD, transplant-related mortality and relapse were calculated using the cumulative incidence estimator19.
Cox regression models were built to study the effect of ATG-containing compared to non-ATG regimens for GVHD, transplant-related mortality, relapse, treatment failure and overall mortality. Logistic regression models were built for neutrophil and platelet recovery. Variables tested included: the inclusion of ATG versus none to conditioning regimens, age (≤5 versus 6-10 versus 11-20 years), sex (male versus female), performance score (90, 100 versus <90), race (Caucasian versus non-Caucasian), disease status (CR1 versus CR2/CR3), recipient cytomegalovirus serostatus (positive versus negative), TNC (<3.0 versus 3.0-5.0 versus ≥5.0 × 107/kg), GVHD prophylaxis (calcineurin inhibitor [CNI] + mycophenolate versus CNI + methotrexate versus CNI alone and transplant period (2007-2008 versus 2009-2011). A stepwise model selection approach was used to identify all significant risk factors. Variables that attained a significance level of 0.05 or less were held in the final model. The chronic GVHD model was stratified by HLA-match and transplant-related mortality model by TNC due to nonproportionality. There were no significant interactions between variables held in the final model and the inclusion of ATG versus none in transplant conditioning regimens. All p-values are two-sided and analyses were performed using SAS version 9.3 (Cary, NC).
Results
Patient and Transplant Characteristics
Table 1 summarizes patient, disease and transplant characteristics. Transplants were performed between 2007 and 2011 in the United States at 71 centers. Transplant conditioning regimens at 14 centers included ATG-containing and non-ATG containing regimens, 17 centers used only ATG-containing regimens and 40 centers, non-ATG containing regimens. Patients had similar distributions of gender and race, but ATG recipients were younger and were less likely to have performance scores of 90-100 or be cytomegalovirus seropositive. Disease status was similar in the two groups with approximately two-thirds of all patients transplanted in second or third CR. ATG recipients were more likely to receive a single cord blood unit. Approximately 60% of recipients of single unit and double CBT received TNC greater than 5 × 107/kg. Donor-recipient HLA-match was similar across the two treatment groups. Cord blood units were 4-6/6 HLA-matched to the recipient at HLA-A and -B (antigen-level) and HLA-DRB1 (allele-level). For double-unit grafts, the transplantation was assigned the worst HLA-match between the recipient and cord blood unit; e.g., if one unit was 6/6 HLA-matched and the other unit, 4/6 HLA-matched to the recipient, the transplantation was assigned 4/6 HLA-matched. The most common ATG source was horse derived (n=58, 63%); the remaining patients received rabbit derived ATG (n=33; 37%). In non-ATG recipients, CNI with mycophenolate was the predominant GVHD prophylaxis regimen and in the ATG group, CNI alone was most likely to be used. The median follow-up of both groups is 3 years.
Table 1.
Patient, disease and transplant characteristics
| ATG-containing | Non ATG-containing | P-value | |
|---|---|---|---|
| TBI + cyclophosphamidea | TBI + cyclophosphamideb | ||
| Number | 92 | 205 | |
| Age at transplant | 0.20 | ||
| ≤ 5 years | 29 (32) | 52 (25) | |
| 6-10 years | 36 (39) | 71 (35) | |
| 11-20 years | 27 (29) | 82 (40) | |
| Gender | 0.15 | ||
| Male | 52 (57) | 134 (65) | |
| Performance score | 0.003 | ||
| < 90% | 13 (14) | 26 (13) | |
| ≥ 90% | 69 (75) | 175 (85) | |
| Not reported | 10 (11) | 4 (2) | |
| Race | 0.30 | ||
| Caucasian | 77 (84) | 161 (79) | |
| Non-Caucasian | 15 (16) | 44 (21) | |
| Recipient cytomegalovirus serostatus | 0.02 | ||
| Positive | 39 (42) | 114 (56) | |
| Negative | 51 (55) | 91 (44) | |
| Not reported | 2 (2) | __ | |
| Disease status | 0.57 | ||
| First complete remission | 35 (38) | 71 (35) | |
| Second/third complete remission | 57 (62) | 134 (65) | |
| GVHD prophylaxis | < 0.001 | ||
| Tacrolimus/cyclosporine + mycophenolate | 28 (30) | 143 (70) | |
| Tacrolimus/ cyclosporine + methotrexate | 12 (13) | 48 (23) | |
| Tacrolimus/ cyclosporine alone | 52 (57) | 14 (7) | |
| Number of cord blood units infused | < 0.001 | ||
| One | 82 (89) | 138 (67) | |
| Two | 10 (11) | 67 (33) | |
| Median infused TNC dose (range) × 107/kg | 5 (2-31) | 5 (2-20) | 0.90 |
| TNC dose (× 107/kg) | 0.53 | ||
| < 3 | 13 (14) | 21 (10) | |
| 3-5 | 25 (27) | 65 (32) | |
| ≥ 5 | 54 (59) | 119 (58) | |
| Donor-recipient HLA-match | 0.38 | ||
| 4/6 | 30 (33) | 72 (35) | |
| 5/6 | 42 (46) | 102 (50) | |
| 6/6 | 20 (22) | 31 (15) | |
| Transplant period | 0.75 | ||
| 2007-2008 | 35 (38) | 82 (40) | |
| 2009-2011 | 57 (62) | 123 (60) |
Abbreviations: ATG = anti-thymocyte globulin; TBI = total body irradiation; Cy = cyclophosphamide; GVHD = graft-versus-host disease; TNC = total nucleated cell; HLA = human leukocyte antigen.
TBI + Cy + ATG: TBI + Cy + fludarabine (n=6), TBI + Cy only (n=49), TBI + Cy + thiotepa (n=19), TBI + Cy + etoposide (n=15), TBI + Cy + cytosine arabinoside (n=2), TBI + Cy + clofarabine (n=1).
TBI + Cy (non-ATG) cohort: TBI + Cy + fludarabine (n=135), TBI + Cy only (n=11), TBI + Cy + thiotepa (n=42), TBI + Cy + etoposide (n=9), TBI + Cy + cytosine arabinoside (n=8).
Neutrophil and Platelet Recovery
The median time to neutrophil recovery was not different, 21 days (range 10-58) in ATG and 22 days (range 12-77) in non-ATG recipients, respectively. There was no difference in the incidence of neutrophil recovery at day-28, 72% (95%CI: 62-80) in ATG versus 67% (95%: 61-74) in non-ATG recipients, p=0.15. Additionally, the incidence of neutrophil recovery at day-45 was also similar between the two groups, 93% (95%CI: 88-98) and 90% (95%CI: 86-94) in ATG and non-ATG recipients, respectively (p=0.15). However, in multivariate analysis, the likelihood of recovery at day-28 was higher with ATG compared to non-ATG containing regimens (OR 1.82, 95% CI 0.99-3.34, p=0.05) but the observed effect was only marginally significant for the threshold set for this analysis. None of the other factors tested for including disease status at transplantation, HLA-match and TNC were associated with neutrophil recovery. Secondary graft failure low and occurred in 1 ATG recipient and 4 non-ATG recipients, p=0.59.
There were no differences in median time to platelet recovery by inclusion of ATG to conditioning regimens; 49 days (range 14-127) and 48 days (range 23-433) for ATG and non-ATG containing regimens, respectively. The day-100 incidence of platelet recovery was higher for ATG-containing regimens at 84% (95%CI: 76-91) compared to 73% (95%CI: 66-79) for non-ATG regimens (p=0.004). In multivariate analysis, the likelihood of platelet recovery at day-100 was higher with ATG-containing compared to non-ATG regimens (OR 4.36, 95% CI 1.55-12.24, p=0.005). Additionally, platelet recovery was lower in patients aged 11-20 years compared to younger recipients (OR 0.17, 95% CI 0.05-0.53, p=0.002), and those transplanted in second or third CR compared to those transplanted in first CR (OR 0.38, 95%CI: 0.16-0.89, p = 0.03).
Acute and Chronic GVHD
Recipients of ATG-containing regimens had significantly lower day-100 incidence of grade II-IV acute GVHD of 30% (95%CI: 21-40) compared to recipients of non-ATG-containing regimens at 54% (95% CI 47-61), p<0.001 (Figure 1A). Among recipients of non-ATG containing regimens, the day-100 incidence of grade II-IV acute GVHD after transplantation of one unit was 52% (95%CI: 44-61) compared to 58% (95%CI: 46-70) after transplantation of two units. However, the day-100 incidence of grade III-IV acute GVHD was not different between the two treatment groups; the corresponding incidence rates were 11% (95% CI 5-18) and 17% (95% CI 12-23), p=0.15 (Figure 1B). In multivariate analysis, ATG-containing regimens were associated with significantly lower risks of grade II-IV acute GVHD compared to non-ATG regimens (Table 2). There were no differences in grade II-IV acute GVHD risks by ATG type (rabbit versus horse; HR 0.99, 95% CI 0.47 – 2.09, p=0.9). Grade IIIV acute GVHD was lower in patients aged 11-20 years compared to those aged less than 5 years (HR 0.57, 95% CI 0.37-0.88, p = 0.01) and those aged 5-10 years (HR 0.56, 95% CI 0.38-0.84, p=0.005). The effect of age on acute GVHD risk was independent of inclusion of ATG to conditioning regimens. Results of multivariate analysis confirmed grade III-IV acute GVHD risks were not different between treatment groups (Table 2). Among patients with grade III-IV acute GVHD, gut involvement was more common with non-ATG containing regimens (p=0.04).
Figure 1A.
Cumulative incidence of grade II-IV acute graft-versus-host disease
Figure 1B.
Cumulative incidence of grade III-IV acute graft-versus-host disease
Table 2.
Risks associated with inclusion of ATG in transplant conditioning regimens compared to non-ATG containing regimens
| Outcome | Hazard Ratio (95% confidence interval) | P-value |
|---|---|---|
| Acute grade II-IV GVHD | ||
| Non-ATG containing | 1.00 | |
| ATG – containing | 0.44 (0.29 – 0.65) | <0.0001 |
| Acute grade III-IV GVHD | ||
| Non-ATG containing | 1.00 | |
| ATG – containing | 0.60 (0.30 – 1.22) | 0.16 |
| Chronic GVHD | ||
| Non-ATG containing | 1.00 | |
| ATG – containing | 0.43 (0.26 – 0.71) | 0.0009 |
| Relapse | ||
| Non-ATG containing | 1.00 | |
| ATG – containing | 0.85 (0.41 – 1.75) | 0.66 |
| Transplant-related mortality | ||
| Non-ATG containing | 1.00 | |
| ATG – containing | 1.02 (0.55 – 1.88) | 0.97 |
| Treatment failure | ||
| Non-ATG containing | 1.00 | |
| ATG – containing | 1.28 (0.85 – 1.93) | 0.24 |
| Overall mortality | ||
| Non-ATG containing | 1.00 | |
| ATG – containing | 0.96 (0.61 – 1.50) | 0.85 |
The 3-year cumulative incidence of chronic GVHD in recipients of ATG-containing conditioning regimen was lower at 22% (95% CI 14-31) compared to 43% (95% CI 36-50) in recipients of non ATG-containing regimens, p < 0.001, (Figure 2). Among recipients of non-ATG containing regimens, the 3-year incidence of chronic GVHD after transplantation of one unit was 42% (95%CI: 34-51) compared to 44% (95%CI: 32-56) after transplantation of two units. In multivariate analysis, chronic GVHD risks were lower with ATG-containing compared to non-ATG containing conditioning regimens when stratified for HLA-matching (Table 2). However, among those reported to have chronic GVHD there were no differences in the severity of GVHD (p=0.19). Ten of 19 (53%) patients who received ATG-containing reported mild chronic GVHD compared to 56 of 81 (69%) patients who received non ATG-conditioning regimens. The corresponding rates for moderate or severe chronic GVHD was 9 (47%) and 25 (31%). The median duration of immunosuppression for chronic GVHD treatment did not differ by treatment groups, 20 months for ATG recipients and 22 months, for non-ATG recipients. Additionally, there were no differences in chronic GVHD risks by rabbit versus horse-ATG (HR 0.99, 95% CI 0.47 – 2.09, p=0.9).
Figure 2.
Cumulative incidence of chronic graft-versus-host disease
Transplant-related Mortality and Relapse
The 3-year cumulative incidences of transplant-related mortality were not different between treatment groups; 16% (95% CI 10-25) after ATG-containing regimens and 17% (95% CI 13-23) after non-ATG regimens, p=0.98. In multivariate analysis, transplant-related mortality risks were not different after ATG-containing and non-ATG containing regimens (Table 2) stratified for total nucleated cell dose of cord blood unit(s). However, risks were higher for patients who were cytomegalovirus sero-positive compared to those who were sero-negative (HR 1.85, 95% CI 1.03-3.32, p = 0.04).
Data on infections within the first 100 days after transplantation was available for 90 of 92 recipients of ATG-containing and 202 of 205 recipients of non ATG-containing regimens. We did not observe differences in viral, bacterial or fungal infections by treatment group. Fifty-five of 92 (60%) recipients reported viral infections with ATG-containing regimens compared to 118 of 205 (58%) non ATG-containing regimens (p=0.83). The corresponding rates for bacterial and fungal infections were 53 of 92; 58% versus 125 of 205; 61% (p=0.88) and 9 of 92; 10% versus 19 of 205; 9% (p=0.90), respectively. Only two patients were reported to have Epstein-Barr virus post-transplant lymphoproliferative disease (EBV-PTLD) (1 in the ATG group and 1 in the non-ATG group), and no deaths were directly attributed to EBV-PTLD.
The 3-year cumulative incidences of relapse were not different in the two treatment groups; 17% (95% CI 10-26) after ATG-containing and 27% (95% CI 21-34), p=0.12, after non-ATG containing regimens. In multivariate analysis, relapse risks after ATG-containing and non ATG-containing regimens were not different (Table 2). Relapse risks were higher for patients transplanted in second or subsequent remission compared to first remission (HR 2.18, 95% CI 1.22-3.89, p=0.008) and this effect was independent of whether ATG was included in the condition regimen.
Overall Survival and Leukemia-Free Survival
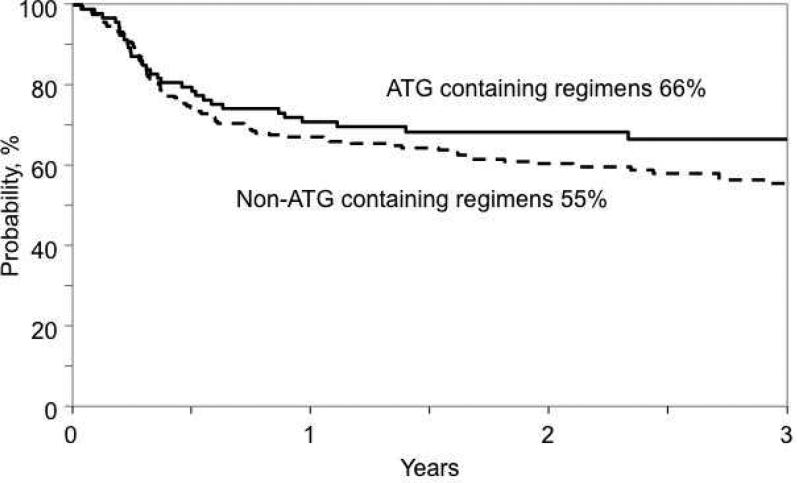
There were no significant differences in overall and leukemia-free survival between the treatment groups. The 3-year probability of overall survival after ATG-containing and non-ATG containing regimens were 67% (95% CI 57-77) and 65% (95% CI 58-72), respectively, p = 0.76. The corresponding leukemia-free survival rates were 66% (95% CI 56-76) and 55% (95% CI 48-62), p = 0.23 (Figure 3). In multivariate analysis, there were no significant differences in overall mortality or treatment failure after ATG-containing compared to non-ATG containing conditioning regimens (Table 2). Transplantation in second or subsequent remission was associated with higher mortality (HR 2.01, 95%CI: 1.25-3.24, p=0.004) and treatment failure (HR 2.01, 95%CI: 1.31-3.08, p=0.001). Additionally, patients who were cytomegalovirus sero-positive were at higher risk for mortality compared to those who were sero-negative (HR 1.85, 95%CI: 1.22-2.86, p=0.004).
Figure 3.
Probability of leukemia-free survival
Causes of Death
The primary causes of death by treatment group are shown in Table 3. A similar proportion of patients died in the ATG group (29/92, 32%) when compared to the non-ATG group (69/205, 34%), p=0.22. The most common cause of death in both groups was leukemia recurrence accounting for about half of all deaths in each treatment group. Other common causes of mortality in the ATG group were GVHD and infection accounting for a third of deaths. Other common causes of mortality in the non-ATG group included graft failure, GVHD and infection accounting for 40% of deaths.
Table 3.
Causes of death
| ATG | Non-ATG | |
|---|---|---|
| Number | 29 | 69 |
| Relapse | 13 (45%) | 34 (49%) |
| Graft failure | 2 (7%) | 8 (12%) |
| Graft vs. Host Disease | 7 (24%) | 6 (9%) |
| Infection | 3 (10%) | 13 (19%) |
| Organ failure | 1 (3%) | 4 (6%) |
| Idiopathic pneumonia syndrome | 2 (7%) | 4 (6%) |
| Hemorrhage | 1 (7%) | ____ |
ATG=anti-thymocyte globulin; GVHD=graft-versus-host disease.
Discussion
In this study, we examined the inclusion of ATG to transplant conditioning regimens for children and adolescents with ALL undergoing CBT and received myeloablative TBI-containing regimens. Our study demonstrated that the inclusion of ATG to the transplant conditioning regimen resulted in lower rates of grade II-IV acute GVHD and chronic GVHD but not grade 3-4 acute GVHD. Despite lower GVHD risks, transplant-related mortality, relapse, leukemia-free and overall survival were not different between the treatment groups. We did not observe an effect of GVHD prophylaxis regimens on acute or chronic GVHD risks. These findings are consistent with the report of Veys et al in a similar population of patients after unrelated adult donor transplantation. In that report, in vivo T-cell depletion had no effect on transplant-related mortality, relapse, survival and leukemia-free survival despite lower acute and chronic GVHD13. However, our findings differ from the report by Pascal et al were mortality was higher with rabbit derived ATG in the setting of myeloablative CBT for acute leukemia, lymphoma and myelodysplastic/myeloproliferative syndrome.15 Myeloablative TBI-containing regimen is immunosuppressive and the only regimen in the current analysis. In this setting, it is plausible the addition of ATG may not have introduced marked differences in immune reconstitution in recipients of ATG-containing and non ATG-containing regimens.
Our findings have a number of important implications for CBT. ATG was originally included in CBT conditioning regimens to prevent graft rejection and GVHD. Both complications were initially a major concern given the low progenitor cell dose and the high degree of HLA-mismatch inherent in CB grafts.20,21 To date, however, the benefit of ATG in relation to neutrophil engraftment is unproven with the exception of non-myeloablative regimens in the setting of CBT for recipients who have not received prior therapy with immunosuppressive properties22. The current analyses showed an early margin advantage with ATG for neutrophil recovery, and a significant advantage for platelet recovery. Inferior platelet recovery with non-ATG containing regimens may in part be explained by the higher rates of acute GVHD in and the effects of additional immune suppression for GVHD treatment. Low cell dose is no longer a concern as cell doses in excess of 3 × 107/kg the accepted minimum for engraftment was delivered with either a single cord blood unit or by infusing two units. In fact over half of transplants in the current analysis received cell doses greater than 5 × 107/kg.
From the stand-point of grade II-IV acute and chronic GVHD, the fact that ATG-containing conditioning regimens were associated with a lower incidence of acute GVHD and chronic GVHD is consistent with multiple other adult donor allograft series10-13 and CBT series.8,14,23-26 Moreover, partial abrogation of GVHD risk was not associated with a demonstrable reduction in transplant-related mortality or higher leukemia relapse. However, chronic GVHD is debilitating and others have reported on the deleterious effects of chronic GVHD on health-related quality of life.27 Data on immune reconstitution were not available in this analysis, and unlike other CBT series.6,7,9,26,28 In vivo T-cell depletion may induce slower immune reconstitution that may offset any benefit of GVHD reduction. However, we did not observe differences in rates of viral reactivation, bacterial and fungal infections between the treatment groups. If viral reactivation were to be used as a surrogate for early immune reconstitution the current analysis suggests there were no significant differences between ATG-containing and non ATG-containing regimens. The current analysis utilized data reported to an observational registry and it is challenging to collect data on immune reconstitution systematically and consistently in the setting of practice variation between transplant centers.
Complicating the assessment of the risks and benefits of pre-transplant ATG is the possibility that the impact of ATG inclusion may vary according to the dose of ATG, the formulation used, and the timing of administration in relation to the infusion of the graft. A subset analysis by ATG type (rabbit versus horse) in the current analysis failed to show significant differences in acute and chronic GVHD. In a study of 127 pediatric CBT recipients with malignant and non malignant hematologic diseases, Lindemans et al reported that those transplanted without ATG had more GVHD, better immune reconstitution, and lower rates of viral reactivation compared to those who received ATG immediately prior to CB infusion, although survival was similar in both groups14. In another report, Admiraal et al evaluated ATG pharmacokinetics in 280 pediatric allograft recipients with malignant and non-malignant hematologic diseases28. They found that, while high post-transplantation ATG exposure was associated with a lower incidence of grade II-IV acute GVHD, worse T-cell recovery and lower survival. These findings suggest that individualized ATG dosing guided by pharmacokinetic analyses may optimize exposure and potentially clinical outcomes. A limitation of the Lindeman and Admiraal reports is the heterogeneity of the population with respect to diseases studied, conditioning regimen intensity and donor/graft types. Therefore, it remains to be seen if their observations would hold true in a homogenous population with ALL who received myeloablative TBI-containing conditioning regimen. Almost a decade ago Parkman and colleagues reported lower relapse risks in children with acute leukemia and successful immune reconstitution after single unit myeloablative CBT.29 In that report, all patients received myeloablative-conditioning regimens and is more comparable to the population in the current analysis. As there were no differences in relapse risks in patients who received ATG and those who did not in the current analysis, it is plausible immune reconstitution in recipients of ATG-containing and non ATG-containing regimens were not significantly different when myeloablative dose of TBI was included in the regimen.
The omission of ATG from myeloablative TBI-containing regimens did not improve leukemia-free survival as hypothesized. Conditioning regimen strategies including use of ATG, its dose and timing are best studied in the setting of randomized trials for specific populations (diseases and conditioning regimens) with well defined clinical and laboratory endpoints including immune reconstitution. In the absence of such trials data from transplant registries may be used to guide clinical care. The data from the current analyses support the inclusion of ATG to myeloablative TBI-containing conditioning regimen for ALL in children and adolescents is associated with lower grade II-IV acute and chronic GVHD compared to non-ATG regimens. Leukemia relapse and survival are not compromised with ATG inclusion and lowering GVHD is likely to lower the burden of morbidity in children.
Highlights.
Higher rates of acute and chronic GVHD with non-ATG regimens
No differences in leukemia-free survival in recipients of ATG and non-ATG regimens
No differences in viral reactivation in recipients of ATG and non-ATG regimens
Acknowledgements
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children's Foundation; Kiadis Pharma; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick's Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: There are no relevant conflicts of interest to disclose.
References
- 1.Haidinger M, Geyeregger R, Poglitsch M, et al. Antithymocyte globulin impairs T-cell/antigen-presenting cell interaction: disruption of immunological synapse and conjugate formation. Transplantation. 2007;84(1):117–121. doi: 10.1097/01.tp.0000266677.45428.80. [DOI] [PubMed] [Google Scholar]
- 2.Monti P, Allavena P, Di Carlo V, Piemonti L. Effects of anti-lymphocytes and anti-thymocytes globulin on human dendritic cells. Int Immunopharmacol. 2003;3(2):189–196. doi: 10.1016/s1567-5769(02)00253-9. [DOI] [PubMed] [Google Scholar]
- 3.Gillet-Hladky S, de Carvalho CM, Bernaud J, Bendahou C, Bloy C, Rigal D. Rabbit antithymocyte globulin inhibits monocyte-derived dendritic cells maturation in vitro and polarizes monocyte-derived dendritic cells towards tolerogenic dendritic cells expressing indoleamine 2,3-dioxygenase. Transplantation. 2006;82(7):965–974. doi: 10.1097/01.tp.0000235549.47976.d0. [DOI] [PubMed] [Google Scholar]
- 4.Fang L, Fehse B, Engel M, Zander A, Kroger N. Antithymocyte globulin induces ex vivo and in vivo depletion of myeloid and plasmacytoid dendritic cells. Transplantation. 2005;79(3):369–371. doi: 10.1097/01.tp.0000150210.77543.1b. [DOI] [PubMed] [Google Scholar]
- 5.Na IK, Wittenbecher F, Dziubianau M, et al. Rabbit antithymocyte globulin (Thymoglobulin(R)) impairs the thymic output of both conventional and regulatory CD4+ T cells after allogeneic hematopoietic stem cell transplantation in adult patients. Haematologica. 2013;98(1):23–30. doi: 10.3324/haematol.2012.067611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunstein CG, Weisdorf DJ, DeFor T, et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006;108(8):2874–2880. doi: 10.1182/blood-2006-03-011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaes AH, Cao Q, Wagner JE, Young JA, Weisdorf DJ, Brunstein CG. Monitoring and preemptive rituximab therapy for Epstein-Barr virus reactivation after antithymocyte globulin containing nonmyeloablative conditioning for umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2010;16(2):287–291. doi: 10.1016/j.bbmt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutler C, Stevenson K, Kim HT, et al. Double umbilical cord blood transplantation with reduced intensity conditioning and sirolimus-based GVHD prophylaxis. Bone Marrow Transplant. 2011;46(5):659–667. doi: 10.1038/bmt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YB, Aldridge J, Kim HT, et al. Reduced-intensity conditioning stem cell transplantation: comparison of double umbilical cord blood and unrelated donor grafts. Biol Blood Marrow Transplant. 2012;18(5):805–812. doi: 10.1016/j.bbmt.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacigalupo A, Lamparelli T, Barisione G, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006;12(5):560–565. doi: 10.1016/j.bbmt.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 12.Socie G, Schmoor C, Bethge WA, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117(23):6375–6382. doi: 10.1182/blood-2011-01-329821. [DOI] [PubMed] [Google Scholar]
- 13.Veys P, Wynn RF, Ahn KW, et al. Impact of immune modulation with in vivo T-cell depletion and myleoablative total body irradiation conditioning on outcomes after unrelated donor transplantation for childhood acute lymphoblastic leukemia. Blood. 2012;119(25):6155–6161. doi: 10.1182/blood-2012-01-405795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindemans CA, Chiesa R, Amrolia PJ, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood. 2014;123(1):126–132. doi: 10.1182/blood-2013-05-502385. [DOI] [PubMed] [Google Scholar]
- 15.Pascal L, Mohty M, Ruggeri A. Impact of rabbit ATG-containing myeloablative conditioning regimens on outcome of patients undergoing unrelated single-unit cord blood transplantation for hematological malignancies. Bone Marrow Transplant. 2015;50(1):45–50. doi: 10.1038/bmt.2014.216. [DOI] [PubMed] [Google Scholar]
- 16.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Klein JP, Zhang MJ. Survival Analysis. Epidemiology and Medical Statistics. 2008;27:281–320. [Google Scholar]
- 19.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337(6):373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 21.Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339(22):1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 22.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102(5):1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 23.Brunstein C, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplant outcomes in 110 adults with hematological disease. Blood. 2007;110(8):3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacMillan ML, Weisdorf DJ, Brunstein CG, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113(11):2410–2415. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanz J, Boluda JC, Martin C, et al. Single-unit umbilical cord blood transplantation from unrelated donors in patients with hematological malignancy using busulfan, thiotepa, fludarabine and ATG as myeloablative conditioning regimen. Bone Marrow Transplant. 2012;47(10):1287–1293. doi: 10.1038/bmt.2012.13. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson CA, Turki AT, McDonough SM, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(4):565–574. doi: 10.1016/j.bbmt.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraser CJ, Bhatia S, Ness K, et al. Impact of chronic graft-versus-host-disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood. 2006;108(8):2867–2873. doi: 10.1182/blood-2006-02-003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Admiraal R, van Kesteren C, Jol-van Der Zijde CM, et al. Population Pharmacokinetic Modeling of Thymoglobulin in Children Receiving Allogeneic-Hematopoietic Cell Transplantation (HCT): Towards Individualized Dosing to Improve Survival. Biology of Blood and Marrow Transplantation. 2014;20(2):S96–S98. doi: 10.1007/s40262-014-0214-6. [DOI] [PubMed] [Google Scholar]
- 29.Parkman R, Cohen G, Carter SL, et al. Successful immune reconstitution decreases leukemia relapse and improves survival in recipients of unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2006;12(9):919–927. doi: 10.1016/j.bbmt.2006.05.008. [DOI] [PubMed] [Google Scholar]