Abstract
Ephrin-B2, a member of the Eph/ephrin family of cell signaling molecules, has been implicated in the guidance of cranial and trunk neural crest cells (NCC) and development of the branchial arches(BA), but detailed examination in mice has been hindered by embryonic lethality of Efnb2 null loss of function due to a requirement in angiogenic remodeling. To elucidate the developmental roles for Efnb2, we generated a conditional rescue knock-in allele that allows rescue of ephrin-B2 specifically in the vascular endothelium (VE), but is otherwise ephrin-B2 deficient. Restoration of ephrin-B2 expression specifically to the VE completely circumvents angiogenic phenotypes, indicating that the requirement of ephrin-B2 in angiogenesis is limited to the VE. Surprisingly, we find that expression of ephrin-B2 specifically in the VE is also sufficient for normal NCC migration and that conversely, embryos in which ephrin-B2 is absent specifically from the VE exhibit NCC migration and survival defects. Disruption of vascular development independent of loss of ephrin-B2 function also leads to defects in NCC and BA development. Together, these data indicate that direct ephrin-B2 signaling to NCCs is not required for NCC guidance, which instead depends on proper organization of the embryonic vasculature.
Keywords: Eph, ephrin, neural crest, branchial arches, craniofacial, palate, pharyngeal arches, angiogenesis, foregut, hindgut, reverse signaling, bidirectional signaling
Introduction
The neural crest (NC) is a population of cells unique to vertebrates which contributes to the development and final composition of numerous, diverse tissue types. Following their induction, NCCs delaminate from the ectoderm/neurectoderm boundary and migrate along highly stereotyped routes dependent on a milieu of guidance cues to reach their destinations (Bhatt et al., 2013; Bronner and LeDouarin, 2012; Kulesa and Gammill, 2010). Rostral to the otic placode, cranial NCCs (CNCCs) are induced prior to neural tube closure, undergo an epithelial to mesenchymal transition and acquire a migratory phenotype. In their dorsolateral route, CNCCs migrate in loosely connected streams that correspond to their ultimate destination and come into intimate contact with the surface ectoderm and mesodermal cell types such as the vascular endothelium (VE) as they home toward and ultimately invade the frontonasal mass and branchial arches (BA) (Kulesa et al., 2010). CNCCs give rise to facial skeleton, connective tissue, glia, Schwann cells, and ciliary and cranial sensory ganglia and pigment cells (Bronner and LeDouarin, 2012; Kulesa et al., 2010).
Trunk NCCs also have highly stereotyped migration pathways. An early intersomitic migrating population of NCC gives rise to the sympathetic ganglia. Later migrating ventral pathway cells are segmented by repulsive cues from the caudal somite to migrate through the rostral somite and also contribute to the sympathetic ganglia, whereas those that remain in the somite coalesce to form sensory neurons and glia of the dorsal root ganglia. Finally, NCCs migrating dorsolaterally give rise to melanocytes (Gammill and Roffers-Agarwal, 2010). The cardiac crest, which contributes to heart development, and the vagal and sacral NC, which give rise to the enteric ganglia of the gut, also exhibit highly regulated migration patterns. The highly stereotyped migration pattern of the NCCs is critical to their ultimate fate and function and numerous signaling molecules have been identified that can act as guidance cues in the cranial and trunk regions. Eph receptors and their ephrin ligands are membrane-bound signaling molecules that have been implicated in NCC guidance and have established roles in a variety of other developmental contexts including axon guidance, boundary formation, early morphogenesis, neurogenesis, and vascular development. Comprising the largest class of receptor tyrosine kinases, Eph receptors transduce a forward signal by catalytic activation of their intracellular kinase domains (Kullander and Klein, 2002). In addition to activating Eph receptor forward signaling, ephrins also transduce a reverse signal into the cell on which they are expressed (Bush and Soriano, 2012; Davy and Soriano, 2005). In the case of the transmembrane B-type ephrins this is thought to occur principally via tyrosine phosphorylation and PDZ-binding capacity of the intracellular domain, though other forms of reverse signaling have also been suggested (Bush and Soriano, 2012).
Important roles in cranial and trunk NCC guidance have been attributed to signaling by B-type ephrins in multiple organisms. Based on overexpression and dominant negative studies in Xenopus, ephrin-B2 expression in the NC and mesoderm of the second BA (BA2) was proposed to restrict intermingling of second and third arch NCCs into adjacent regions (Smith et al., 1997). In the trunk of rat and mouse embryos, ephrin-B2 was found to be expressed in the caudal half of the somitic sclerotome, and the EphB2 receptor was detected in NCCs migrating through the rostral half (Davy and Soriano, 2007; Wang and Anderson, 1997). A similar situation was reported in chick, though in this case it was ephrin-B1 that was expressed in the caudal sclerotome, complementary to EphB3 expression in the NCCs and the rostral sclerotome. In explants from rat and chick, NCCs avoided stripes of B-type ephrin, and when signaling was blocked, they inappropriately invaded ephrin-B-expressing territories. These studies were amongst the first to elucidate the repulsive nature of Eph/ephrin signaling and led to a model wherein repulsive ephrin-B was involved in the segmental migration of trunk NCCs through the rostral half of the scleretome (Krull, 1998; Krull et al., 1997; Wang and Anderson, 1997).
Roles for ephrin-B2 in CNCCs, BA2 morphogenesis and trunk NCC migration have been supported by phenotypes in mouse mutants (Adams et al., 2001; Davy and Soriano, 2007), but the requirement for ephrin-B2 in vascular angiogenesis has complicated detailed analysis of these and other putative Efnb2 loss of function phenotypes (Adams et al., 1999; Wang et al., 1998). Ephrin-B2/EphB4 signaling is a critical regulator of vascular morphogenesis, where it is required for angiogenic remodeling of the primitive capillary plexus into hierarchically branched vessels, and its complete loss leads to embryonic lethality by E9.5 due to angiogenic defects. Ephrin-B2 is highly expressed within the VE and its conditional loss in this cell type, mediated by Tie2-Cre, also resulted in angiogenic remodeling defects similar to those found in Efnb2 homozygous null embryos (Adams et al., 1999; Gerety et al., 1999; Wang et al., 1998). Ephrin-B2 is also expressed highly in the BA ectoderm and at lower levels in BA CNCCs (Adams et al., 2001, 1999; Davy and Soriano, 2007; Gerety et al., 1999; Wang et al., 1998). Whereas null mutation of Efnb2 led to defective CNCC migration and dysmorphic BA2, a carboxy-terminally truncated reverse signaling-dead form of ephrin-B2 was reported to exhibit normal CNCC migration and BA2 morphogenesis while exhibiting defective angiogenesis (Adams et al., 2001; Davy and Soriano, 2007). This truncated ephrin-B2 protein may not localize normally to the plasma membrane, however, and an allele of ephrin-B2 in which the intracellular domain was instead replaced with β-galactosidase (Efnb2LacZ) did not display defective angiogenesis, NCC or BA phenotypes, indicating these processes are most likely mediated by forward signaling.
Efnb2LacZ/LacZ mice display a variety of defects including cardiac valve abnormalities, defects in urogenital and anorectal development, laryngotracheoesophageal cleft, cleft palate, anterior commissure axon guidance defects, and pulmonary hypoplasia (Bennett et al., 2013; Cowan et al., 2004; Dravis et al., 2004; Dravis and Henkemeyer, 2011). Some of these phenotypes, including hypospadias and cleft palate also occurred in Efnb2LacZ/+ heterozygous embryos, suggesting they may be attributable to gain of function modes of action (Dravis et al., 2004; Mäkinen et al., 2005). Indeed, it has been recently shown that Efnb2LacZ/LacZ embryos exhibit elevated phosphorylation of the EphB4 receptor, raising uncertainty as to which of the reported Efnb2LacZ phenotypes are attributable to loss of reverse signaling, and which are attributable to hyperactivation of forward signaling (Zhang et al., 2015).
To clarify the developmental roles for ephrin-B2 in non-vascular cell types, and particularly in NCC guidance and BA formation, we have generated a novel Efnb2 conditional rescue mouse model that maintains the endogenous expression of ephrin-B2 in the VE, but ablates it in all other tissues. This approach allows the study of ephrin-B2 loss of function throughout development without the complications of defective angiogenesis and embryonic lethality. Surprisingly, we find that ephrin-B2 expression in the VE is sufficient to rescue NCC guidance and branchial arch formation, but is not sufficient for normal tracheoesophageal, urogenital, or thymus development. Interestingly, conditional loss of ephrin-B2 specifically in the VE also leads to NCC and branchial arch phenotypes associated with reduced survival of the NCCs. These data clarify the roles of ephrin-B2 during development and suggest that the circulatory function conferred by proper angiogenic remodeling and possibly signaling from the VE is critical for normal NCC migration and branchial arch development.
Materials and Methods
Mice
The Efnb2CR targeting construct included in 5′-to-3′ order: a 1,910bp EcoRV/XbaI 5′-homology arm including the first exon of Efnb2, a LoxP-flanked adenoviral splice acceptor sequence-H2BCherry-triple polyA signal cassette, a FRT-flanked PGKNEO-positive selection cassette, a 4,018 bp XbaI 3′ homology arm and a pGKDTAbpA-negative selection cassette (Figure S1A). This construct was electroporated into 129S4 AK7.1 ES cells and targeting was initially screened with a forward PCR primer located upstream of the 5′ arm with sequence 5′-CCAAACCAATTCGTAGTTGCAG-3′ and a reverse primer corresponding to the 5′ end of the genetrap cassette with sequence 5′-GACCGCGAAGAGTTTGTCCTC-3′ which amplified a band of 2304bp from targeted clones. PCR-positive ES cell clones were screened by Southern hybridization with 5′ and 3′ external genomic and Neo internal probes to ensure single targeted insertion. The 5′ probe was a 397 bp sequence amplified by PCR with a sense primer 5′-CATTATTGCCCTGAGAGATGCC-3′ and reverse primer 5′CTTTAACAGATAGGGCTGAGGG-3′ used to screen ES cell DNA digested with Asp718 and HindIII. The 3′ probe was a 315 bp sequence amplified by PCR with a forward primer 5-GGCTGTTTGGTTATGTGAGAGC-3′ and reverse primer 5′GCCCAAATAGATGAAGGCAAGG-3′ used to screen ES cell DNA digested with Asp718 and EcoRV. Screening 132 ES cell clones resulted in 10 strong PCR positive correctly targeted ES cell clones five of which were injected into C57Bl/6J blastocysts resulting in nine high-percentage male chimeras. These chimeras were bred to wild-type or ROSA26FlpO/FlpO females (Raymond and Soriano, 2007) in the 129S4 co-isogenic background to remove the Frt-flanked NEO cassette and confirmed by PCR. Two mouse lines from independent ES cell clones were found to be phenotypically indistinguishable. Mice were then crossed 5 or more generations to the C57Bl/6J strain prior to analysis.
Genotyping was performed using standard PCR protocols on yolk sacs of embryos up through E12.0 and tail snips thereafter. Detection of Efnb2CR and recombined Efnb2CRΔ was achieved by PCR with an annealing temperature of 58°C for 30 cycles pairing primers 5′-GGCCTTGGACCCTGTTTCTAG-3′ (forward) with 5′-GACCGCGAAGAGTTTGTCCTC-3′ (reverse) or 5′-CGGACATGTACAGAGCTCGAG-3′ (reverse), respectively yielding bands of 213bp and 120bp. Efnb2+, as distinct from Efnb2GFP, Efnb2CR and Efnb2CRΔ, was detected by PCR with an annealing temperature of 61°C for 35 cycles using primers 5′-TAAGTGGTGTCCACGAGCC-3′ (forward) and 5′-GAGCCTAATTAGAGTACCCTTGG-3′ (reverse), yielding a band of 206bp.
The following pre-existing lines were used in conducting this work: Actin-CreTg (MGI:2176050), Efnb2GFP (MGI:3526818), Efnb2lox (MGI:2176538), Ephb4ap, Ephb4tauLacZ (MGI:2183162), Ncx1lacZ (MGI:2384543) and Tie2-CreTg (MGI:3608912).
Histology, immunofluorescence and in situ hybridization
Embryos for histology were fixed in Bouin’s fixative for a minimum of three days, processed into and embedded in paraffin, cut at a thickness of 7μm and stained with hematoxylin and eosin following standard protocols. Embryos for whole mount protocols were fixed overnight in 4% PFA in PBS at 4°C, graded into methanol and stored at −20°C. Embryos for cryosectioning were fixed identically, graded into 25% sucrose in PBS, embedded in OCT, stored at −80°C, cut at a thickness of 10–12μm and stored with desiccant at −20°C.
Immunostaining was performed using antibodies recognizing cleaved Caspase3 (1:800, Cell Signaling #9664), CD31 (1:150, BD Pharmingen #553370), GFP (1:800, Abcam #ab13970), Neurofilament 2H3 (1:250, DSHB 2H3) and Sox10 (1:75, Santa Cruz Biotechnologies #sc-17342). Both section and whole mount immunostainings were performed according to standard protocols. Sections were imaged either by epifluorescence microscopy or by laser scanning confocal microscopy. Whole mount samples were optically cleared either by grading into 80% glycerol or according to the SeeDBp protocol as described (Ke et al., 2013). Imaging was subsequently performed by laser scanning confocal microscopy or by spinning disc confocal microscopy. Z-stacks obtained by confocal microscopy were condensed into 2-dimensional images in Fiji (ImageJ) using the standard deviation method within the Z projection function.
Section and whole mount in situ hybridization were performed according to standard protocols. The Efnb2 in situ probe hybridizes to a region spanning from an XbaI site (position 538) to a PstI site (position 1056) on the Efnb2 mRNA (RefSeq NM_010111.5); this region, spanning exons 2–5, would be absent from the splice-trapped Efnb2CR mRNA. The Sema3F in situ probe hybridizes to a region spanning from an ApaI site (position 186) to a PstI site (position 1219) on the Sema3F mRNA (RefSeq NM_011349). The Sox10 in situ probe has been previously described (Kuhlbrodt et al., 1998).
Results
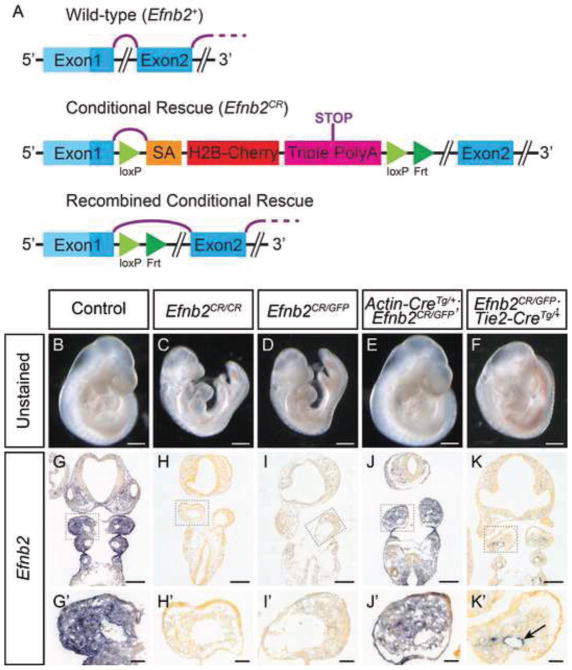
In order to study the developmental roles of ephrin-B2 outside of the VE, we generated a conditional rescue allele of Efnb2 (Efnb2CR) which expresses fully-functional ephrin-B2 only upon Cre-mediated recombination. Briefly, this allele was a gene targeted insertion into the first intron of Efnb2 with a cassette containing a strong splice acceptor and a triple polyadenylation signal, all flanked by LoxP sites (Fig. 1A, Supp. Fig. 1). To verify that the Efnb2CR allele functioned as a null loss of function allele in the absence of Cre, we compared it to a previously-generated Efnb2GFP null allele that expresses a fusion of the histone 2B and GFP proteins from the endogenous Efnb2 locus (Davy and Soriano, 2007). Efnb2CR/CR E9.75 homozygous embryos completely lacked Efnb2 mRNA expression and were alive with beating hearts, though they were growth retarded and displayed enlarged pericardia and dysmorphic branchial arches and frontonasal mass. These were embryonic lethal at midgestation, indistinguishable from Efnb2CR/GFP embryos and identical to what has been previously reported for Efnb2GFP/GFP (Fig. 1C-D,H–I,H′-I′)(Davy and Soriano, 2007). To verify Cre-dependent rescue in this allele, we utilized the ubiquitously expressing β-Actin-Cre allele to generate embryos with global rescue of ephrin-B2 (Lewandoski et al., 1997). As expected, E9.75 Efnb2CR/GFP; β-Actin-CreTg/+ embryos displayed restoration of Efnb2 mRNA expression in all contexts as well as complete phenotypic rescue, indistinguishable from controls (Fig. 1B,E,G,G′,J,J′). We next reasoned that since conditional loss of ephrin-B2 in the VE results in angiogenic phenotypes similar to those in Efnb2null embryos, expression of ephrin-B2 solely in the VE may also be sufficient for normal vascular angiogenesis (Gerety and Anderson, 2002). To test this hypothesis, we utilized Tie2-Cre transgenic mice, in which Cre-recombinase is expressed specifically in the VE, to generate Efnb2CR/GFP; Tie2-CreTg/+ embryos (Braren et al., 2006). As intended, these embryos exhibited rescued expression of Efnb2 mRNA specifically within the VE, and lacked expression in other cell types in which it would normally be expressed (Fig. 1G,K; Supp. Fig. 2), including branchial arch ectoderm, endoderm, mesoderm and NCCs (Fig. 1G′,K′). These embryos appeared overtly normal at E9.5, without signs of angiogenic defects (Fig.1F,K,K′). To verify that the endothelial rescue of ephrin-B2 restored normal angiogenesis, we performed whole-mount immunostaining for CD31 on Efnb2CR/GFP; Tie2-CreTg/+ mutants at E9.5. Whereas Efnb2CR/GFP embryos failed to undergo proper angiogenesis, the remodeling of the primitive vascular plexus into hierarchically branched vessels was similar in Efnb2CR/GFP; Tie2-CreTg/+embryos compared to wild-type control (Fig. 2A–C; Supp. Fig. 5A,B,J,K). Together, these data indicate the Efnb2CR allele faithfully allows the tissue-specific, Cre-conditional rescue of ephrin-B2 expression under its endogenous promoter allowing us to assess loss of function phenotypes outside of vascular development.
Figure 1. The Efnb2CR allele facilitates tissue-specific Cre-dependent maintenance of ephrin-B2 expression.
(A) Schematic illustrating the structure of the Efnb2CR splice trap before and after Cre-mediated recombination compared to the wild-type Efnb2. Purple lines represent mRNA splicing. (B–F) Unstained embryos at E9.75 of indicated genotypes. Embryos with global (E) and endothelial (F) rescued ephrin-B2 expression and controls (B) develop normally whereas Efnb2CR/CR (C) or Efnb2CR/GFP (D) mutants do not. (G–K) In situ hybridization against Efnb2 on cryosections of E9.75 embryos of indicated genotypes with insets of the mandibular component of the first branchial arch. Embryos with globally rescued ephrin-B2 (J) show a normal Efnb2 expression pattern when compared to positive (G) and negative (H–I) controls, whereas endothelial rescued embryos (K) show Efnb2 expression restricted to the endothelium, indicated by the black arrow. Scale bars: B–F 500μm, G–K 200μm, G′–K′ 50μm.
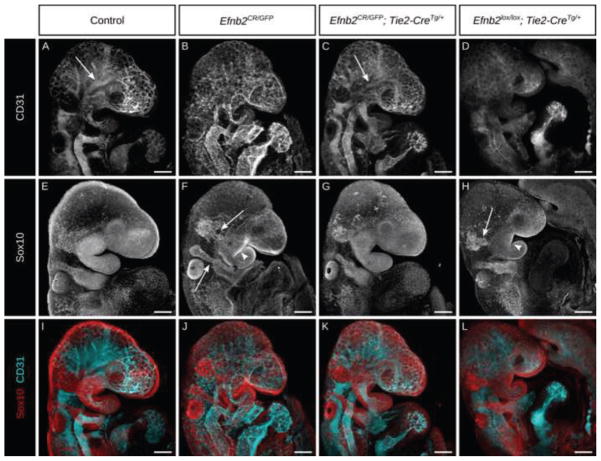
Figure 2. Ephrin-B2 expression in the vascular endothelium is necessary and sufficient to allow normal CNCC migration and BA morphogenesis.
(A–H) Whole mount co-immunostain against the endothelial marker CD31 (A–D) and a marker of migratory neural crest Sox10 (E–H) with channels merged (I–L) in E9.5 embryos of the indicated genotypes. Embryos with endothelial rescue of ephrin-B2 (C) undergo angiogenic remodeling, hierarchical branching shown by arrows, similarly to controls (A), whereas embryos with endothelial deletion of ephrin-B2 (D) and Efnb2null embryos (B) fail to do so. Conversely, endothelial ablation of ephrin-B2 (H) and Efnb2null (F) embryos exhibit defects in neural crest migration, shown by white arrows, and dysmorphic branchial arches, shown by white arrowheads, in contrast to endothelial rescue of ephrin-B2 (G) and control (E) embryos which display normal neural crest migration and branchial arch morphogenesis. Scale bars: 200μm.
Efnb2CR/GFP; Tie2-CreTg/+ embryos survived to birth, but died perinatally. Previous studies have suggested roles for Efnb2 in secondary palate development, tracheoesophageal and urorectal septation, and normal positioning of the thymus (Dravis et al., 2004; Dravis and Henkemeyer, 2011; Foster et al., 2010). Examination of these structures in E15.5 histological sections revealed that indeed Efnb2CR/GFP; Tie2-CreTg/+ mutants exhibit tracheoesophageal and urorectal fistulae as well as an incorrectly positioned thymus (Table 1; Supp. Fig. 3C–J). Other bilateral structures such as the lungs, vena cava and clavicles were comparable between control and Efnb2CR/GFP; Tie2-CreTg/+ mutant embryos and examination of adjacent sections confirmed that thymus mispositioning was not attributable to variable or asymmetric planes of section. These phenotypes are consistent with what has been previously reported in other Efnb2 mutant mouse models (Dravis et al., 2004; Dravis and Henkemeyer, 2011; Foster et al., 2010). We did not, however, observe cleft palate in Efnb2CR/GFP; Tie2-CreTg/+ embryos (0/7 embryos) (Table 1, Supp. Fig. 3A–B), despite complete loss of Efnb2 from the palatal shelves (Supp. Fig. 2A–B).
Table 1.
Summary of Efnb2CR/GFP; Tie2-CreTg/+ phenotypes.
| Phenotype | Incidence in Efnb2CR/GFP; Tie2-CreTg/+ embryos |
|---|---|
| Defective NCC migration | 0/11 |
| Branchial arch dysmorphogenesis | 1/35 |
| Tracheoesophageal fistula | 12/12 |
| Urorectal fistula | 3/3 |
| Thymus mislocalization | 5/5 |
| Cleft palate | 0/7 |
We next sought to utilize this tool to investigate the roles of ephrin-B2 in NCC guidance and BA morphogenesis without complications from angiogenic phenotypes. We and others have observed that Efnb2null embryos exhibit hypoplastic mandibular and second branchial arches (Fig. 1C,D; Fig. 2E,F,I,J)(Adams et al., 2001; Davy and Soriano, 2007). Based on immunostaining for Sox10, CNCC migration was perturbed in Efnb2CR/GFP null E9.5 embryos, with reduced and disorganized trigeminal NCC streams that failed to properly enter BA1 (Fig. 2E,F, white arrows). In general, BA2 NCC streams were less dramatically affected than the trigeminal stream with the shape of the stream typically appearing thinner but intact (Fig. 2E,F,I,J white arrows). Sox10 expression is progressively restricted to the condensing cranial ganglia and indeed, immunostaining of Efnb2CR/GFP null embryos with the anti-neurofilament (2H3) antibody confirmed defects in these cells (Supp. Fig. 4 A-B′). To determine if other NCC populations were also affected, we examined the expression of pan-NCC marker Crabp1 by in situ hybridization in E9.5 Efnb2CR/GFP null embryos and found reduced trigeminal and BA2 NCC streams as well as less abundant Crabp1 positive NCCs populating the first and second branchial arches (Supp. Fig 4C,D). Immunostaining of E9.0 (12ss) Efnb2CR/GFP null embryos for Sox10 revealed that initial CNCC emigration and guidance were overtly normal, but by E9.25 (16ss), Efnb2CR/GFP null embryos began to exhibit a thinner trigeminal NCC stream (Supp. Fig. 5). Notably, the appearance of this phenotype correlated with the initial appearance of defects in vascular patterning. These data are consistent with what has been published previously for Efnb2null embryos, and demonstrate that global loss of Efnb2 has substantial consequences for BA and CNCC development.
It has been reported that loss of ephrin-B2 also leads to disruption, but not complete loss, of segmental migration of trunk NCCs that parallels defects in somite polarity and perturbed somitic expression of Sema3F, a gene that is required for NCC guidance (Davy and Soriano, 2007; Gammill et al., 2006). In situ hybridization for Sox10 in E9.5 (23–25ss) Efnb2CR/GFP null embryos revealed reduced and disorganized TNCC migratory streams with variably perturbed interspacing when compared with controls; these streams were often associated with smaller and disorganized somites, possibly contributing to apparent TNCC phenotypes (Fig. 3A–B,A′-B′; Fig. 4E,F; Fig. 5J,L). TNCC streams in the more posterior embryo could not be identified at all in Efnb2CR/GFP null embryos at this stage, however, we observed a delay in the initial intersomitic migration of TNCCs at this position (Supp. Fig. 6A,B). In addition, immunostaining for neurofilament revealed that initiation of the dorsal root ganglia was disrupted in Efnb2CR/GFP null mutants (Fig. 4A,B). However, somite polarity appeared unperturbed in Efnb2CR/GFP null embryos as assessed by analysis of ephrin-B2-GFP, which exhibited elevated expression in the caudal halves of somites that was comparable to control (Fig. 4E,F). We did not observe differences in Sema3F expression, in Efnb2CR/GFP null embryos, suggesting this may not be the cause of TNCC defects (Fig. 4I,J).
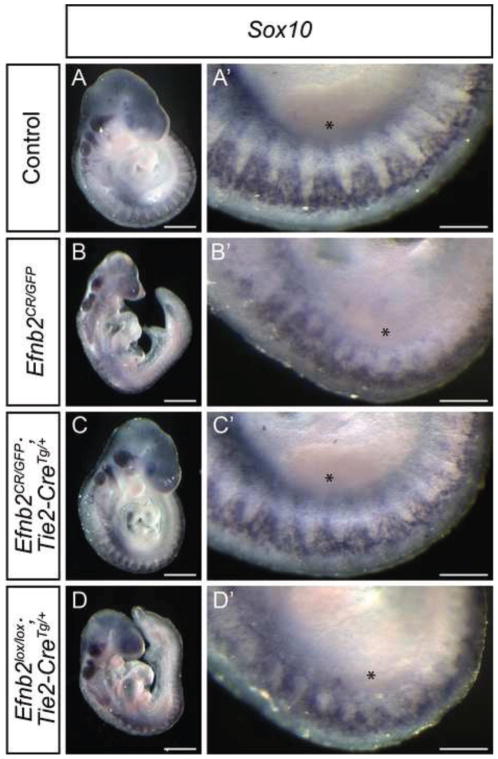
Figure 3. Ephrin-B2 expression in the vascular endothelium is necessary and sufficient to allow normal trunk NCC migration.
(A–D) Whole mount in situ hybridization against Sox10 in E9.75 embryos of the indicated genotypes with high magnification of the trunk on the contralateral side. Embryos with endothelial rescue of ephrin-B2 (C) exhibit a migratory neural crest pattern comparable to controls (A), whereas Efnb2null embryos (C) and Efnb2lox/lox; Tie2-CreTg/+ embryos with endothelial deletion of ephrin-B2 (D) and display defective cranial neural crest migration and a disorganization of the trunk neural crest. Asterisks indicate the position of the limb bud. Scale bars: A–D 500μm, A′–D′ 200μm.
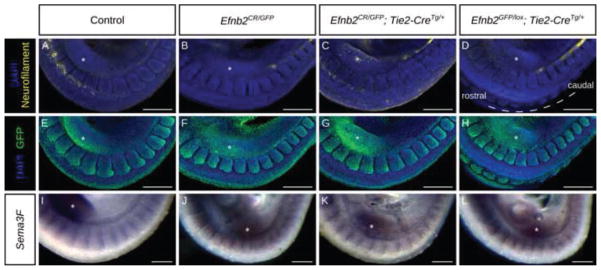
Figure 4. Loss of ephrin-B2 does not affect somite polarity.
(A–H) Whole mount co-immunostain against neurofilament (2H3) (A–D) and ephrin-B2-GFP (E–H) in E9.5 embryos of the indicated genotypes. Efnb2null embryos (B) and embryos with endothelial deletion of Efnb2 (D) fail to initiate DRG morphogenesis, in contrast to embryos with endothelial rescue of Efnb2 (C) and controls (A). (I–J) Whole mount in situ hybridization for Sema3F on E9.5 embryos of the indicated genotypes. Asterisks indicate the forelimb bud position. Scale bars: 200μm.
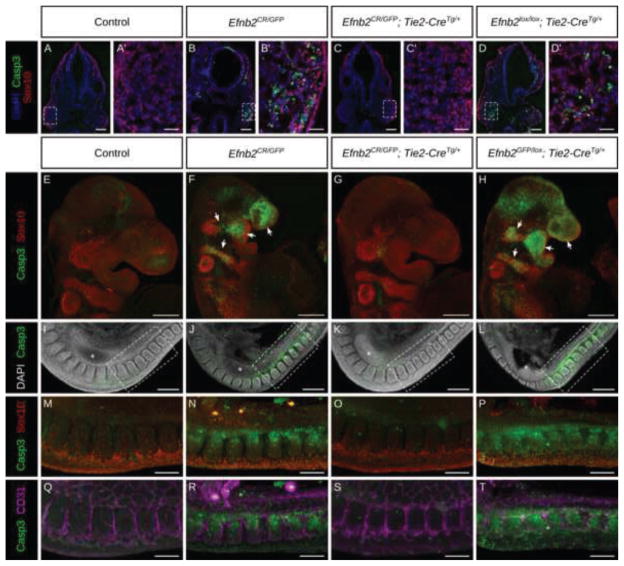
Figure 5. Elevated apoptosis in the CNCC in the absence of vascular endothelial ephrin-B2.
(A–D′) Immunostaining for cleaved Caspase3 and Sox10 on cryosections of E9.75 embryos of indicated genotypes and high magnification insets. Efnb2null embryos (B) and Efnb2GFP/lox; Tie2-CreTg/+ embryos with endothelial deletion of ephrin-B2 (D) exhibit large amounts of cell death localized to the migratory and post-migratory CNCCs compared with control (E) or Efnb2CR/GFP; Tie2-CreTg/+ embryos with expression of ephrin-B2 only within the VE (G). In the trunk region elevated apoptosis is also associated with, but not limited to migratory NCCs in Efnb2null embryos (J,N,R), or Efnb2GFP/lox; Tie2-CreTg/+with deletion of ephrin-B2 from the VE (L,P,T) compared to control (A) or Efnb2CR/GFP; Tie2-CreTg/+embryos with expression of ephrin-B2 only within the VE (G,K,O,S). Asterisks indicate forelimb bud. Scale bars: A–D 100μm, A′–D′ 25μm, E–L 200μm, M–T 100μm.
Surprisingly, Efnb2CR/GFP; Tie2-CreTg/+ embryos did not exhibit defects in BA morphogenesis or cranial or trunk neural crest development (Fig. 1F,K,K′; Fig. 2G,K; Fig. 3C,C′; Fig. 4C,G,K; Supp. Fig. 6C), indicating that expression of ephrin-B2 in the VE alone is sufficient to allow proper NCC development. We therefore next asked whether ephrin-B2 expression in the VE was necessary for normal NCC and branchial arch development. We generated Efnb2lox/lox; Tie2-CreTg/+ embryos, which are known to be embryonic lethal due to angiogenic failure (Fig 2D; Supp. Fig. 5C,L) (Gerety and Anderson, 2002), but have not been examined for NCC migration phenotypes. Interestingly we found that at E9.5, in addition to failed angiogenesis, these mutants displayed dysmorphic branchial arches and defective cranial and trunk NCC migration similar to Efnb2CR/GFP null embryos (Fig. 2D,H,L; Fig. 3D,D′; Fig. 4D; Supp. Fig. 5F,R; Supp. Fig. 6D). Also similar to Efnb2CR/GFP null embryos, loss of ephrin-B2 from the VE in Efnb2GFP/lox; Tie2-CreTg/+ did not result in defects in somite polarity or Sema3F expression (Fig. 4H,L). Together, these results indicate that expression of ephrin-B2 in the VE is necessary and sufficient to allow normal NCC migration and branchial arch morphogenesis.
We next asked whether loss of ephrin-B2 from the VE affected cell survival, either generally throughout the embryo or specifically within the NCCs. Immunostaining for Sox10 and cleaved Caspase3 in frontal sections of Efnb2CR/GFP null and Efnb2lox/lox; Tie2-CreTg/+ embryos showed elevated cell death in the dorsal neural tube and migratory NCCs compared to control (Fig. 5A,B,D,A′,B′,D′). Whole mount immunostaining of Efnb2CR/GFP null embryos and Efnb2GFP/lox; Tie2-CreTg/+ embryos revealed striking elevation of cleaved Caspase3 within the migratory streams of the CNCCs indicating that CNCCs are sensitized to loss of ephrin-B2 from the VE (Fig. 5E,F, H). Additionally, the trunk displayed widespread cell death which was associated with, but not restricted to, TNCCs and somites (Fig. 5I,J,L,M,N,P). Apoptosis in the trunk was largely excluded from the VE, however, indicating that effects on cell survival were not autonomous to the vasculature (Fig. 5 Q,R,T). Again, Efnb2CR/GFP; Tie2-CreTg/+ embryos did not present any of these anomalies (Fig. 5C,C′,G,K,O,S), indicating that ephrin-B2 expression in the VE alone is necessary and sufficient to allow normal NCC survival.
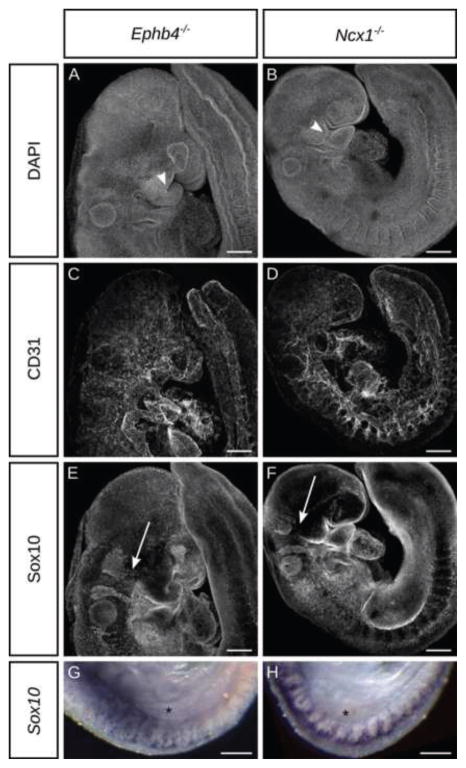
The delimited requirement for ephrin-B2 in the VE for NCC and BA development could be attributable to a requirement of the vasculature to deliver oxygen or other nutrients and signaling molecules to the embryo, or could suggest active signaling from ephrin-B2 in the VE to the NCCs. Homozygous null mutation of Ephb4 results in angiogenic remodeling phenotypes very similar to those found in Efnb2null embryos (Adams et al., 1999; Gerety et al., 1999) (Fig. 6C) Examining Ephb4−/− embryos also revealed branchial arch hypoplasia and reduced cranial and trunk NCC migration very similar to Efnb2CR/GFP embryos indicating that EphB4 was relevant to these phenotypes (Fig. 6A,E,G). Because it has been previously reported that EphB4 expression is highly restricted to the VE and is not expressed in the NCCs (Gerety et al., 1999; Kim et al., 2008), we therefore surmise that ephrin-B2/EphB4 signaling from the VE to the NCCs is not likely to underly these phenotypes. To determine whether NCC and BA defects are general features of angiogenic failure, we turned to an independent regulator of angiogenesis. The Ncx1 gene encodes a calcium transport channel expressed solely in the heart at E9.5, which is necessary for heart contraction and therefore for proper blood flow. In Ncx1 knockout mouse embryos, a primitive vascular plexus is formed, but does not undergo remodeling (Fig. 6D), though other aspects of development, such as limb morphogenesis appear normal until death of the embryo (Koushik et al., 2001; Wakimoto et al., 2000). Notably, Ncx1−/− embryos exhibited defective BA and NCC development and NCC survival phenotypes that were again similar to Efnb2CR/GFP homozygous loss of function (Fig. 6B,F,H; Supp. Fig. 7), indicating that proper angiogenesis is particularly required for NCC and BA development.
Figure 6. Ephrin-B2-independent perturbation of vascular function results in failed neural crest development.
(A–F) Whole mount immunostaining against CD31 and Sox10 in E9.5 embryos of indicated genotypes reveals dysmorphic branchial arches (white arrowheads) and greatly diminished trigeminal NCC streams (white arrows). (G,H) Whole mount in situ hybridization for Sox10 reveals reduced and disorganized segmental TNCC migration in Ephb4null (G) and Ncx1null (H) embryos. Asterisk marks the forelimb bud. Scale bars: 200μm.
Discussion
Here we clarify the extravascular developmental roles for Efnb2 by the generation of a novel conditional rescue mouse line. Though we find no evidence for a requirement for ephrin-B2 in secondary palate development, we do find that loss of function of ephrin-B2 results in defective tracheoesophageal and urorectal septation as reported for the Efnb2LacZ intracellular substitution allele (Cowan et al., 2004; Dravis et al., 2004; Dravis and Henkemeyer, 2011). Interestingly, whereas 16% of Efnb2LacZ/+ embryos exhibited urorectal defects, 71% of ephrin-B2LacZ/ΔV mutants had this phenotype, suggesting that the PDZ-domain binding capacity of ephrin-B2 was involved in this context (Dravis and Henkemeyer, 2011). All of the Efnb2CR/GFP; Tie2-CreTg/+ mutants we examined exhibited tracheoesophageal (12/12) and urorectal (3/3) phenotypes, supporting the notion that these Efnb2LacZ phenotypes are consequences of bonafide loss of function of reverse signaling. Together with the absence of such phenotypes in Efnb26YFΔV/6YFΔV or Efnb2ΔV/ΔV mutant embryos, these data support the notion of phosphorylation and PDZ-independent modes of reverse signaling (Bush and Soriano, 2012; Dravis and Henkemeyer, 2011; Mäkinen et al., 2005; Thakar et al., 2011).
Surprisingly, ephrin-B2 expression in the branchial arch ectoderm or somites is not required for proper NCC migration. It is possible that NCC guidance could be provided by redundant signaling from ephrin-B1. Though ephrin-B1 is not highly expressed in the branchial arch ectoderm in the E9.5 head, it is expressed in caudal half somites and could potentially provide a repulsive signal redundant to ephrin-B2 (Wang and Anderson, 1997). Loss of function of ephrin-B1 alone within the neural crest resulted in defects in migration of BA3 and BA4 NCCs, but no defects in TNCCs and compound Efnb1+/−; Efnb2+/GFP double heterozygous embryos did not exhibit defects in trunk NCC migration or somite development, though loss of ephrin-B1 and ephrin-B2 in the somite has not yet been reported. (Davy et al., 2004; Davy and Soriano, 2007). Such compensation is apparently not at play for the BA or CNCC phenotypes that arise from loss of ephrin-B2 alone in Efnb2−/− embryos, however. Complete loss of segmental migration of trunk NCCs in mutant mice with disrupted Neuropillin2/Semaphorin3F signaling indicates that this pathway may be sufficient to provide repulsive guidance from the caudal scleretome (Gammill et al., 2006; Gammill and Roffers-Agarwal, 2010), and might explain why repulsive signaling from ephrin-B2 is dispensable. On the other hand, it is also possible that reducing Neuropilin/Semaphorin signaling, for example in a Sema3F+/−; Efnb2+/− trans-heterozygote, may sensitize the embryo and reveal requirements for ephrin-B2 in this process. We do not find any evidence of perturbed Sema3F expression in any of our Efnb2 mutant genotypes, suggesting that this is not the cause of the trunk NCC defects that we observe. Whereas, segmental TNCC migration defects and Sema3F expression changes were variable in severity in a previous report, in our study, embryos with expression of ephrin-B2 in the VE alone never exhibit defects in TNCCs. Taken together, these data indicate expression of ephrin-B2 in the somite is not responsible, directly or indirectly, for the proper pattern of neural crest migration.
Instead, we provide evidence indicating endothelial ephrin-B2 is both necessary and sufficient to allow normal NCC migration and branchial arch development. Other recent studies have suggested that VE-NCC signaling might be important for NCC morphogenesis. Knockout of Flk1 in the VE led to CNCC migration defects and death in the absence of generalized hypoxia, possibly attributable to disrupted Tgfβ signaling (Milgrom-Hoffman et al., 2014). This study suggested that in addition to roles in perfusion of the embryo, the VE had a differential requirement for maintaining CNCCs but not pharyngeal mesoderm markers. Further, explant cultures were performed to minimize the impact of loss of circulation and hypoxia; under these conditions, disrupted angiogenesis and reduced endothelial cells still resulted in failed CNCC migration. Our data is consistent with this view, as CNCCs in Efnb2CR/GFP; Tie2-CreTg/+ embryos showed reduced survival compared with other cell types in the embryo, even when the heart was still beating. Why CNCCs exhibit elevated apoptosis relative to other cell types under these conditions is a question for further study, but previous work in a mouse model of the neurocristopathy Treacher Collins found that the neuroepithelium and early neural crest are highly sensitive to cellular stress possibly due to the high metabolic requirement of these cells (Jones et al., 2008). It is possible, therefore, that angiogenic defects deprive NCCs of nutrition required to satisfy their metabolic requirements.
In Efnb2 mutant embryos, the trigeminal stream consistently exhibited more severe defects than BA2 NCCs; this could be correlated with the effective stage of NCC migration because this occurs in a rostral-caudal progression. Indeed, defects in trigeminal NCCs were not apparent at 12ss but were by 16ss, whereas BA2 defects were not apparent at 16ss but manifested by 20ss. We do not expect that BA dysmorphogenesis and disrupted NCC migration phenotypes are caused by defective ephrin-B2/EphB4 signaling between VE and CNCCs because previous studies have indicated that EphB4 expression is restricted to the VE at these stages and is not expressed in the NCCs (Gerety et al., 1999; Kim et al., 2008). Rather, we conclude that failed angiogenesis in ephrin-B2 mutant embryos leads to perturbed NCC migration and survival either by depriving NCCs of oxygen and other nutrients or possibly by disrupted signaling from the VE through other pathways.
Supplementary Material
Highlights.
A novel conditional rescue allele clarifies Efnb2 loss of function phenotypes.
Extravascular Efnb2 loss affects foregut, hindgut, thymus, not secondary palate.
Efnb2 is not required in NCCs, BA ectoderm, or somites for normal NCC development.
Efnb2 is required only within the vascular endothelium for normal NCC survival.
NCC survival exhibits a heightened requirement for proper vascular development.
Acknowledgments
We are grateful to our laboratory and UCSF colleagues for helpful advice, discussions and comments on the manuscript. The monoclonal antibody developed by Thomas M. Jessell and Jane Dodd was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. This work has been funded by R37HD25326 to P.S. and R00DE020855 and R01DE023337 from the National Institute of Dental and Craniofacial Research to J.O.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R. The Cytoplasmic Domain of the Ligand EphrinB2 Is Required for Vascular Morphogenesis but Not Cranial Neural Crest Migration. Cell. 2001;104:57–69. doi: 10.1016/S0092-8674(01)00191-X. [DOI] [PubMed] [Google Scholar]
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett KM, Afanador MD, Lal CV, Xu H, Persad E, Legan SK, Chenaux G, Dellinger M, Savani RC, Dravis C, Henkemeyer M, Schwarz MA. Ephrin-B2 Reverse Signaling Increases a5β1 Integrin–Mediated Fibronectin Deposition and Reduces Distal Lung Compliance. Am J Respir Cell Mol Biol. 2013;49:680–687. doi: 10.1165/rcmb.2013-0002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Diaz R, Trainor PA. Signals and Switches in Mammalian Neural Crest Cell Differentiation. Cold Spring Harb Perspect Biol. 2013;5:a008326. doi: 10.1101/cshperspect.a008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braren R, Hu H, Kim YH, Beggs HE, Reichardt LF, Wang R. Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J Cell Biol. 2006;172:151–162. doi: 10.1083/jcb.200506184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner ME, LeDouarin NM. Evolution and Development of the Neural Crest: An Overview. Dev Biol. 2012;366:2–9. doi: 10.1016/j.ydbio.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush JO, Soriano P. Eph/ephrin signaling: Genetic, phosphoproteomic, and transcriptomic approaches. Semin Cell Dev Biol, Signalling via Eph Receptors and Ephrins. 2012;23:26–34. doi: 10.1016/j.semcdb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CA, Yokoyama N, Saxena A, Chumley MJ, Silvany RE, Baker LA, Srivastava D, Henkemeyer M. Ephrin-B2 reverse signaling is required for axon pathfinding and cardiac valve formation but not early vascular development. Dev Biol. 2004;271:263–271. doi: 10.1016/j.ydbio.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Davy A, Aubin J, Soriano P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev. 2004;18:572–583. doi: 10.1101/gad.1171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Soriano P. Ephrin-B2 forward signaling regulates somite patterning and neural crest cell development. Dev Biol. 2007;304:182–193. doi: 10.1016/j.ydbio.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Soriano P. Ephrin signaling in vivo: look both ways. Dev Dyn Off Publ Am Assoc Anat. 2005;232:1–10. doi: 10.1002/dvdy.20200. [DOI] [PubMed] [Google Scholar]
- Dravis C, Henkemeyer M. Ephrin-B reverse signaling controls septation events at the embryonic midline through separate tyrosine phosphorylation-independent signaling avenues. Dev Biol. 2011;355:138–151. doi: 10.1016/j.ydbio.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, Shay J, Baker LA, Henkemeyer M. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271:272–290. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Foster KE, Gordon J, Cardenas K, Veiga-Fernandes H, Makinen T, Grigorieva E, Wilkinson DG, Blackburn CC, Richie E, Manley NR, Adams RH, Kioussis D, Coles MC. EphB–ephrin-B2 interactions are required for thymus migration during organogenesis. Proc Natl Acad Sci. 2010;107:13414–13419. doi: 10.1073/pnas.1003747107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammill LS, Gonzalez C, Gu C, Bronner-Fraser M. Guidance of trunk neural crest migration requires neuropilin 2/semaphorin 3F signaling. Development. 2006;133:99–106. doi: 10.1242/dev.02187. [DOI] [PubMed] [Google Scholar]
- Gammill LS, Roffers-Agarwal J. Division of labor during trunk neural crest development. Dev Biol. 2010;344:555–565. doi: 10.1016/j.ydbio.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerety SS, Anderson DJ. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Dev Camb Engl. 2002;129:1397–1410. doi: 10.1242/dev.129.6.1397. [DOI] [PubMed] [Google Scholar]
- Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4:403–414. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- Jones NC, Lynn ML, Gaudenz K, Sakai D, Aoto K, Rey JP, Glynn EF, Ellington L, Du C, Dixon J, Dixon MJ, Trainor PA. Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat Med. 2008;14:125–133. doi: 10.1038/nm1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke MT, Fujimoto S, Imai T. SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat Neurosci. 2013;16:1154–1161. doi: 10.1038/nn.3447. [DOI] [PubMed] [Google Scholar]
- Kim YH, Hu H, Guevara-Gallardo S, Lam MTY, Fong SY, Wang RA. Artery and vein size is balanced by Notch and ephrin-B2/EphB4 during angiogenesis. Dev Camb Engl. 2008;135:3755–3764. doi: 10.1242/dev.022475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koushik SV, Wang J, Rogers R, Moskophidis D, Lambert NA, Creazzo TL, Conway SJ. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J Off Publ Fed Am Soc Exp Biol. 2001;15:1209–1211. doi: 10.1096/fj.00-0696fje. [DOI] [PubMed] [Google Scholar]
- Krull CE. Inhibitory Interactions in the Patterning of Trunk Neural Crest Migration. Ann N Y Acad Sci. 1998;857:13–22. doi: 10.1111/j.1749-6632.1998.tb10103.x. [DOI] [PubMed] [Google Scholar]
- Krull CE, Lansford R, Gale NW, Collazo A, Marcelle C, Yancopoulos GD, Fraser SE, Bronner-Fraser M. Interactions of Eph-related receptors and ligands confer rostrocaudal pattern to trunk neural crest migration. Curr Biol. 1997;7:571–580. doi: 10.1016/S0960-9822(06)00256-9. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a Novel Transcriptional Modulator in Glial Cells. J Neurosci. 1998;18:237–250. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesa PM, Bailey CM, Kasemeier-Kulesa JC, McLennan R. Cranial neural crest migration: New rules for an old road. Dev Biol. 2010;344:543–554. doi: 10.1016/j.ydbio.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesa PM, Gammill LS. Neural Crest Migration: Patterns, Phases and Signals. Dev Biol. 2010;344:566–568. doi: 10.1016/j.ydbio.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Lewandoski M, Meyers EN, Martin GR. Analysis of Fgf8 gene function in vertebrate development. Cold Spring Harb Symp Quant Biol. 1997;62:159–168. [PubMed] [Google Scholar]
- Mäkinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom-Hoffman M, Michailovici I, Ferrara N, Zelzer E, Tzahor E. Endothelial cells regulate neural crest and second heart field morphogenesis. Biol Open. 2014;3:679–688. doi: 10.1242/bio.20148078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CS, Soriano P. High-efficiency FLP and PhiC31 site-specific recombination in mammalian cells. PloS One. 2007;2:e162. doi: 10.1371/journal.pone.0000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Robinson V, Patel K, Wilkinson DG. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Curr Biol CB. 1997;7:561–570. doi: 10.1016/s0960-9822(06)00255-7. [DOI] [PubMed] [Google Scholar]
- Thakar S, Chenaux G, Henkemeyer M. Critical roles for EphB and ephrin-B bidirectional signalling in retinocollicular mapping. Nat Commun. 2011;2:431. doi: 10.1038/ncomms1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakimoto K, Kobayashi K, Kuro-o M, Yao A, Iwamoto T, Yanaka N, Kita S, Nishida A, Azuma S, Toyoda Y, Omori K, Imahie H, Oka T, Kudoh S, Kohmoto O, Yazaki Y, Shigekawa M, Imai Y, Nabeshima Y, Komuro I. Targeted Disruption of Na+/Ca2+ Exchanger Gene Leads to Cardiomyocyte Apoptosis and Defects in Heartbeat. J Biol Chem. 2000;275:36991–36998. doi: 10.1074/jbc.M004035200. [DOI] [PubMed] [Google Scholar]
- Wang HU, Anderson DJ. Eph Family Transmembrane Ligands Can Mediate Repulsive Guidance of Trunk Neural Crest Migration and Motor Axon Outgrowth. Neuron. 1997;18:383–396. doi: 10.1016/S0896-6273(00)81240-4. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular Distinction and Angiogenic Interaction between Embryonic Arteries and Veins Revealed by ephrin-B2 and Its Receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/S0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Zhang G, Brady J, Liang W-C, Wu Y, Henkemeyer M, Yan M. EphB4 forward signalling regulates lymphatic valve development. Nat Commun. 2015;6 doi: 10.1038/ncomms7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.