Abstract
Objectives
This study examined the performance of serum glial fibrillary acidic protein (GFAP) in detecting traumatic intracranial lesions on computed tomography (CT) scan in children and youth with mild and moderate traumatic brain injury (TBI), and assessed its performance in trauma control patients without head trauma.
Methods
This prospective cohort study enrolled children and youth presenting to three Level I trauma centers following blunt head trauma with Glasgow Coma Scale (GCS) scores of 9 to 15, as well as trauma control patients with GCS scores of 15 who did not have blunt head trauma. The primary outcome measure was the presence of intracranial lesions on initial CT scan. Blood samples were obtained in all patients within six hours of injury and measured by ELISA for GFAP (ng/ml).
Results
A total of 257 children and youth were enrolled in the study and had serum samples drawn within 6 hours of injury for analysis: 197 had blunt head trauma and 60 were trauma controls. CT scan of the head was performed in 152 patients and traumatic intracranial lesions on CT scan were evident in 18 (11%), all of whom had GCS scores of 13 to 15. When serum levels of GFAP were compared in children and youth with traumatic intracranial lesions on CT scan to those without CT lesions, median GFAP levels were significantly higher in those with intracranial lesions (1.01, IQR 0.59 to 1.48) than those without lesions (0.18, IQR 0.06 to 0.47). The area under the receiver operating characteristic (ROC) curve (AUC) for GFAP in detecting children and youth with traumatic intracranial lesions on CT was 0.82 (95% CI = 0.71 to 0.93). In those presenting with GCS scores of 15, the AUC for detecting lesions was 0.80 (95% CI = 0.68 to 0.92). Similarly, in children under five years old the AUC was 0.83 (95% CI = 0.56 to 1.00). Performance for detecting intracranial lesions at a GFAP cutoff level of 0.15 ng/ml yielded a sensitivity of 94%, a specificity of 47%, and a negative predictive value of 98%.
Conclusions
In children and youth of all ages, GFAP measured within 6 hours of injury was associated with traumatic intracranial lesions on CT and with severity of TBI. Further study is required to validate these findings before clinical application.
Introduction
Conventionally, early risk stratification of brain injury is based on computed tomography (CT) scanning.1-3 According to recent estimates, over 4 million CT examinations are performed annually on children in the United States, and the risk of leukemia and brain cancer is highest from head CT scans for children younger than five years old.4 Children are considerably more sensitive to ionizing radiation than adults5,6 and their longer life expectancy provides greater opportunity for expressing damage from exposure.7 Some studies suggest that CT scans of the head may be among the largest contributors to radiation exposure, due to the frequency with which they are performed.8,9 This is apparent is organized sports where children and youth are at risk for repeated head trauma.10 The high rate of ordering CT scans for mild traumatic brain injury (mTBI; also known as concussion) is fostered by the nature of emergency medicine (EM) practice that includes high case volumes, brief physician-patient encounters, lack of follow-up, fear of missing catastrophic intracranial bleeding, and medicolegal action.11,12 The recognition that diagnostic imaging in children should be reduced has led to interest in alternative diagnostic strategies. Determining injury severity and identifying children and youth with intracranial lesions on CT following head trauma through a blood test could reduce the need for such neuroimaging.
For over a decade there has been a mounting body of research on TBI biomarkers.13,14 A systematic review of the literature on pediatric TBI biomarkers found that 99 different biomarkers have been assessed in over 49 published studies in humans.13 Despite these efforts, there is still a lack of brain injury biomarkers for clinical use in children and youth. Some of the shortfalls of the current evidence include evaluation of biomarkers lacking in brain specificity, the use of small sample sizes, single-center studies, inadequate comparison groups, and outcome measures that do not address the acute evaluation of children in the emergency department (ED).13 Important properties that should be considered when evaluating a biomarker for clinical application include: 1) demonstrate a high sensitivity and specificity for brain injury, 2) stratify patients by severity of injury, 3) have a rapid appearance in accessible biological fluid, 4) provide information about injury mechanisms, 5) have well-defined biokinetc properties, 6) monitor progress of disease and response to treatment, and 7) predict functional outcome.13,14
A number of recent studies have shown glial fibrillary acidic protein (GFAP) to be a promising brain-specific biomarker for mild and moderate TBI.15-18 GFAP is a monomeric intermediate protein found in the astroglial skeleton of white and gray matter and is released into serum following mild or moderate TBI within an hour of injury.15,19 In two recently published studies in adults, Papa et al. found that serum GFAP distinguished mild TBI patients from trauma patients without TBI and detected intracranial lesions on CT with a sensitivity of 97% to 100%.15,18 Moreover, GFAP out-performed S100β in the setting of multiple trauma when extracranial fractures were present.18,20 More studies evaluating its performance in children and youth in an acute trauma setting are needed.
This study examined whether GFAP was significantly elevated in the serum of children and youth with mild or moderate TBI compared to other trauma patients without TBI. Additionally, this study examined the relationship between GFAP levels and traumatic intracranial lesions on CT scan.
Methods
Study Design
This was a prospective controlled cohort study. The study was approved by the respective institutional review board of each institution. Informed consent was obtained from patients and/or their legal authorized representatives prior to enrollment, and assent was obtained for children between the ages of 7 to 18 years
Study Setting and Population
Study sites included the EDs of three Level I trauma centers: a pediatric Level I trauma center in Philadelphia, Pennsylvania, a pediatric Level I trauma center in Orlando, Florida, and an affiliated adult Level I trauma center in Orlando, Florida.
Eligibility for the study was determined by the treating physician based on the history of blunt head trauma presenting to the ED within 6 hours of injury with an initial GCS score of 9 to 15. Eligibility was also prospectively verified by the research team prior to enrollment. Head trauma patients were categorized into children with and without TBI symptoms (loss of consciousness, amnesia, disorientation, or change in behavior) based on the American Congress of Rehabilitation Medicine definition.21 Head CT scans were performed at the discretion of the treating physicians. Patients were excluded if they 1) had syncope or seizure prior to the head trauma; 2) had known chronic psychosis, neurological disorder, or active central nervous system pathology; 3) were pregnant; 4) were incarcerated; 5) had spinal cord injury; or 6) had hemodynamic instability.
The trauma control patients included patients with GCS scores of 15 presenting to the ED with traumatic mechanisms of injury who did not have blunt head trauma and had normal mental status since injury (as verified by the research team), and had no evidence of acute brain injury or hemodynamic instability. These patients were carefully screened to ensure they had no loss of consciousness, no amnesia, and no alteration in sensorium or behavior at any time after injury. Mechanisms of injury included falls, motor vehicle collisions, and sports injuries. Trauma controls were enrolled during the same period as head trauma patients. The purpose of including trauma controls was to examine biomarker levels in patients who were exposed to traumatic forces without direct blunt head trauma.
Study Protocol
All initial patient assessments were made by emergency physicians (EPs) board certified in either pediatric or adult EM, and trained by a formal one-hour session on evaluating patient eligibility. At the time of enrollment, the study team carefully reassessed every patient to ensure each patient met inclusion and exclusion criteria. A single blood sample was obtained from each head trauma and non-head injured trauma control patient shortly after arrival to the ED and within 6 hours of the reported time of injury. A blood sample of 2.5 ml to 5 ml (based on weight) was placed in a serum separator tube and allowed to clot at room temperature before being centrifuged. Volume was based on recommendations of the National Institutes of Health pediatric TBI biospecimens and biomarkers workgroup.22 The serum was placed in bar-coded aliquot containers and stored at -70 °C until transport to a central laboratory where samples were analyzed in batches using sandwich enzyme-linked immunosorbent assays (ELISA) for GFAP. Lab personnel conducting the ELISA assays were blinded to the clinical data.
After assessment and treatment in the ED, patients were either discharged home or admitted to hospital based on severity of their injuries and patient management was not altered by the study. Patients underwent standard CT scan of the head according to the judgment of the treating physicians. CT examinations were interpreted by board-certified radiologists who recorded location, and extent and type of brain injury. Radiologists were blinded to the study protocol but had the usual clinical information. All physicians, investigators, and research personnel were blinded to the serum biomarker results.
Outcome Measurements
The primary outcome measure was the presence of intracranial lesions on initial CT scan. Only children who had actual CTs performed were included in this analysis; no surrogate measures were used. Intracranial lesions on CT included any acute traumatic intracranial lesions visualized on CT scan, including intracranial hemorrhage (epidural, subdural, subarachnoid hemorrhage) or contusion, cerebral edema, diffuse axonal injury, midline shift of intracranial contents or signs of brain herniation, or pneumocephalus.23 Isolated skull fractures were assessed separately as the force required to injure the skull may be enough to release biomarkers into the circulation. The secondary outcome measure included the performance of the biomarkers in trauma controls (without head trauma) versus head trauma patients.
Earlier biomarker studies of myelin basic protein, neuron specific enolase, and S100β in children have shown differential expression of these markers by age,24 so we evaluated the performance GFAP in children of different ages by subdividing them into blocks of five years: birth to 5 years (early childhood); 5 to 10 years (late childhood); 10 to 15 years (early adolescence); and 15 to 21 years (late adolescence/early adulthood).
Biomarker Analysis
Serum GFAP levels were measured in duplicate for each sample using a validated ELISA platform (Banyan Biomakers Inc., Alachua Florida). The lower limit of quantification (LLOQ) for this assay is 0.030 ng/ml and upper limit of quantification (ULOQ) is 50 ng/ml. The limit of detection (LoD) is 0.008 pg/mL. Any sample yielding a signal over the quantification or calibrator range was diluted and re-assayed.
Data Analysis
Descriptive statistics with means and proportions were used to describe the data. For statistical analysis, biomarker levels were treated as continuous data, measured in ng/ml, and expressed as means (95% CI). Data were assessed for equality of variance and distribution. Logarithmic transformations were conducted on non-normally distributed data. Group comparisons for different trauma groups were performed using analysis of variance with multiple comparisons using the Games-Howell post-hoc test. Receiver operating characteristics (ROC) curves were created to detect intracranial lesions on CT scan. Estimates of the area under these curves (AUC) were obtained (AUC = 0.5 indicates no discrimination and AUC = 1.0 indicates a perfect diagnostic test). GFAP cutpoints were selected based on the ROC curve to maximize the sensitivity and correctly identify as many patients with CT lesions as possible. Performance was also assessed by sensitivity, specificity, and positive and negative predictive values with 95% confidence intervals (CI). All analyses were performed using the statistical software package PASW version 17.0.
A feasibility study conducted in children at the participating sites provided preliminary data to calculate a sample size for distinguishing children with positive versus negative CT scans.25 A sample of 15 from the positive CT group and 15 from the negative CT group achieves 80% power to detect a difference of 0.22 between the area under the ROC curve AUC0 (best studied biomarker) under the null hypothesis of 0.65 and an AUC1 (GFAP) under the alternative hypothesis of 0.87 using a two-sided z-test at a significance level of 0.05.
Results
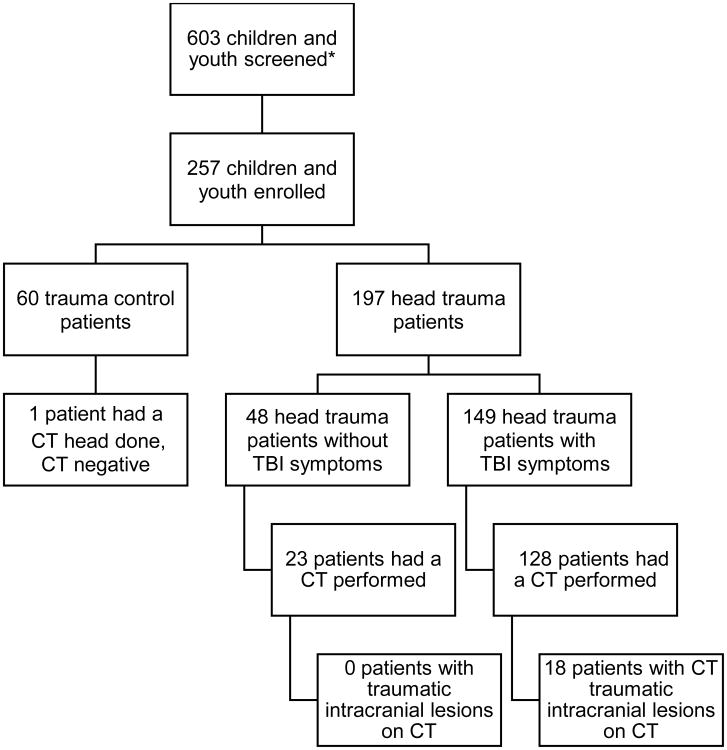
A total of 257 children and youth were enrolled in the study and had serum samples drawn within 6 hours of injury for analysis; 197 had blunt head trauma and 60 were trauma controls. Of the 197 patients with blunt head trauma, 149 had TBI symptoms and 48 did not. CT scans of the head waere performed in 152 patients, and traumatic intracranial lesions on CT scan were evident in 18 (11%), all of whom had GCS scores of 13 to 15. CT scans of the head were performed in 86% of head trauma patients with TBI symptoms and in 48% head trauma patients without TBI symptoms. A CT scan was also performed in one trauma control patient, despite the lack of head trauma, and it was negative. The flow diagram in Figure 1 describes the distribution of enrolled patients. The mean age of enrolled patients was 12 years, with a range from 2 weeks to 21 years, and 66% were male. The distribution of clinical characteristics in each group is presented in Table 1. There were no statistically significant differences in the age, sex, race, or admission rate between head trauma patients and trauma controls. Furthermore, the demographic characteristics of those who were eligible but refused to participate were similar to those who were enrolled with a mean age of 10 years (SD ±7 years) and 62% were male.
Figure 1. Flowchart of enrolled children and youth.
*Screening data only available for 2 of the 3 enrolling sites.
Table 1. Characteristics of Enrolled Children and Youth.
| Characteristics | Head Trauma With TBI Symptoms n=149 | Head Trauma Without TBI Symptoms n=48 | Trauma Controls n=60 | Total N=257 |
|---|---|---|---|---|
| Mean age, yrs (±SD) (range) | 12 (±7) (0.1-21) | 10 (±7) (0.6-21) | 12 (±6) (0.1-21) | 12 (±7) (0.1-21) |
| Age groups | ||||
| Birth – 5 years | 34 (23) | 16 (33) | 7 (12) | 57 (22) |
| 5.1 - 10 years | 16 (11) | 13 (27) | 19 (32) | 48 (19) |
| 10.1 – 15 years | 40 (27) | 3 (6) | 17 (28) | 60 (23) |
| 15.1 – 21 years | 59 (40) | 16 (33) | 17 (28) | 92 (36) |
| Sex: male | 101 (68) | 30 (63) | 39 (65) | 170 (66) |
| Race | ||||
| Asian | 2 (1) | 1 (2) | 0 (0) | 3 (1) |
| Black | 45 (30) | 8 (17) | 26 (43) | 79 (31) |
| Hispanic | 25 (17) | 17 (35) | 9 (15) | 51 (20) |
| White | 74 (50) | 22 (46) | 23 (38) | 119 (46) |
| Other | 3 (2) | 0 (0) | 2 (3) | 5 (2) |
| GCS score in ED | ||||
| GCS 9-12 | 3 (2) | 0 (0) | 0 (0) | 3 (1) |
| GCS 13 | 1 (1) | 0 (0) | 0 (0) | 1 (1) |
| GCS 14 | 13 (7) | 0 (0) | 0 (0) | 13 (5) |
| GCS 15 | 132 (89) | 48 (100) | 60 (100) | 240 (93) |
| Mechanism of injury | ||||
| Motor vehicle crash | 27 (18) | 7 (15) | 11 (18) | 45 (18) |
| Fall | 54 (36) | 19 (40) | 37 (62) | 110 (43) |
| Motorcycle/motorized bicycle | 5 (3) | 4 (8) | 0 (0) | 9 (4) |
| Pedestrian struck | 10 (7) | 3 (6) | 1 (2) | 14 (5) |
| Bicycle | 11 (7) | 5 (10) | 0 (0) | 16 (6) |
| Assault | 6 (4) | 0 (0) | 0 (0) | 6 (2) |
| Sports | 25 (17) | 3 (6) | 8 (13) | 36 (14) |
| Other | 11 (7) | 7 (15) | 3 (5) | 21 (8) |
| Admitted to hospital | 46 (32) | 11 (23) | 14 (23) | 71 (28) |
Data reported as n (%) unless otherwise noted.
Note: Due to rounding, percentages may not add up to 100
GCS = Glasgow Coma Scale; GFAP = glial fibrillary acidic protein; TBI = traumatic brain injury
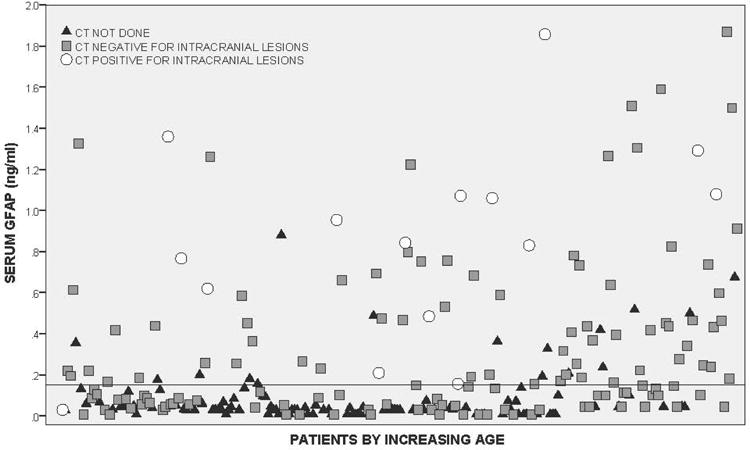
Both the head trauma and trauma control patients had serum samples drawn within 6 hours of injury with the average time from injury to serum sample collection at 3.5 hours (95% CI = 3.3 to 3.7 hours). The average time to serum collection for head trauma patients was 3.3 hours (95% CI = 3.1 to 3.5 hours) and for non-head injured trauma controls it was 4.1 hours (95% CI = 3.7 to 4.5 hours). GFAP was detectible within an hour of injury. The distribution of GFAP levels in children and youth with head trauma within six hours post-injury is shown in Figure 2 (and Data Supplement 1).
Figure 2.
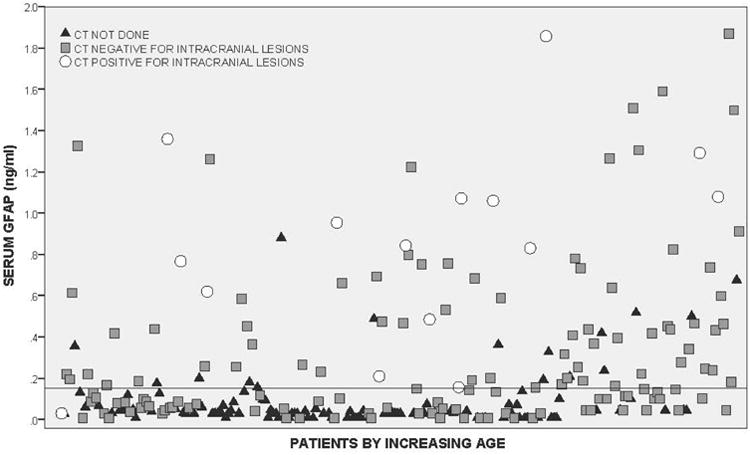
In Figure 3 levels of GFAP are compared between four groups of participants: 1) trauma controls, 2) head trauma patients without TBI symptoms, 3) head trauma patients with TBI symptoms, and 4) patients with intracranial lesions. After adjusting for multiple comparisons, there were statistically significant differences between each of the groups relative to the trauma controls and relative to CT lesions (p < 0.001). Median GFAP levels increased incrementally from trauma controls (0.03, IQR 0.01 to 0.05), to head trauma without TBI symptoms (0.09, IQR 0.04 to 0.20), to head trauma patients with TBI symptoms (0.15, IQR 0.05 to 0.45), and were highest in those with intracranial lesions (1.01, IQR 0.59 to 1.48).
Figure 3.
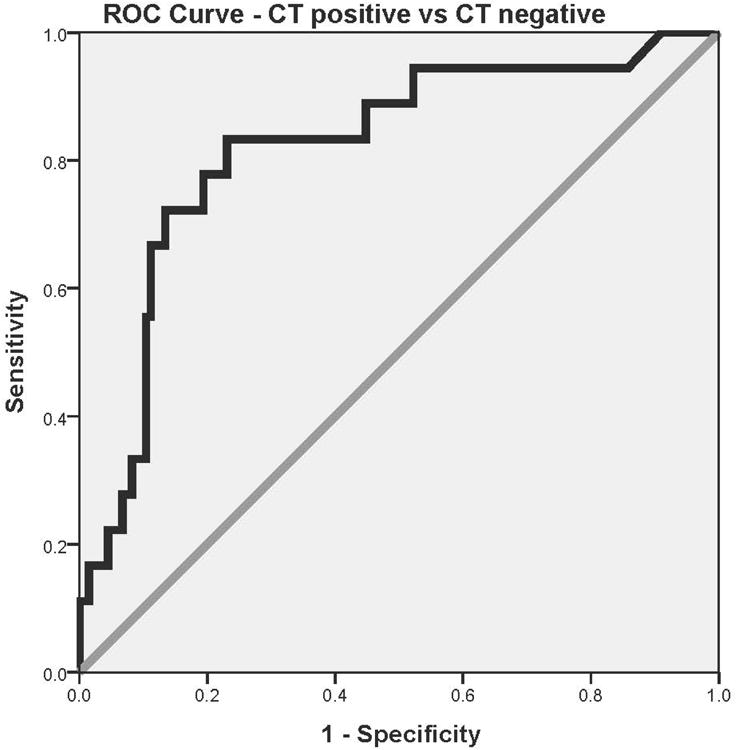
When serum levels of GFAP were compared in children and youth with traumatic intracranial lesions on CT scan (n = 18) to those without CT lesions (n = 134), median levels were significantly higher in those with intracranial lesions (1.01, IQR 0.59 to 1.48) than those without lesions (0.18, IQR 0.06 to 0.47) among all children who had CTs performed (p < 0.001). The AUC was calculated from the ROC curves constructed to assess the performance of early GFAP levels (within 6 hours of injury) in predicting traumatic intracranial lesions on CT. The AUC for discriminating between CT scan positive and CT scan negative was 0.82 (95% CI = 0.71 to 0.93)(Figure 4a). When patients with GCS scores of 15 were assessed independently, median GFAP levels were significantly higher in those with CT scan lesions (0.84, IQR 0.49 to 1.36, n=15) than those without (0.15, IQR 0.06 to 0.46, n=120); p < 0.001. The AUC for detecting intracranial lesions in those with GCS 15 was 0.80 (95% CI = 0.68 to 0.92)(Figure 4b). Moreover, when isolated skull fractures were combined with intracranial lesions for the analysis, the AUC was 0.79 (95% CI = 0.69 to 0.89).
Figure 4.
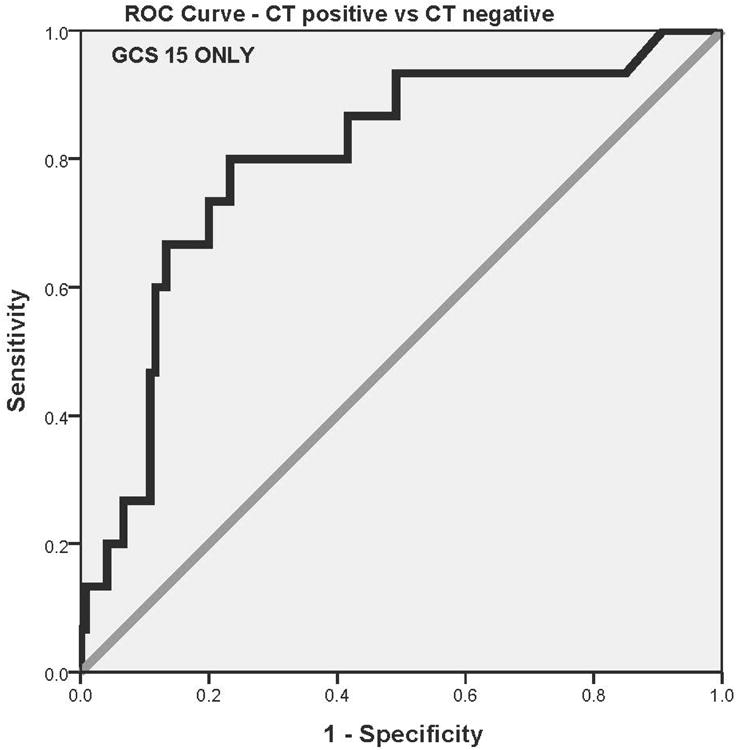
Children and youths were subdivided to assess the performance of GFAP for detecting intracranial lesions on CT by age. Among patients with head trauma, the highest proportion of CT scans (85%) were performed in the 15 to 21 year age group. However, 74% of children from birth to 5 years also had CT scans performed following head trauma (Table 2). The AUCs for predicting intracranial lesions in each age category spanned from 0.78 to 0.91 and are shown in Table 2. For children younger than 5 years old, the AUC was 0.83 (95% CI 0.56 to 1.00).
Table 2. Performance of GFAP in detecting intracranial lesions on CT among different age categories.
| Age Categories | Proportion of CTs done in patients with head trauma | Proportion of CTs with traumatic intracranial lesions | Area Under the ROC Curve (95% CI) |
|---|---|---|---|
| Birth – 5 years | 37/50 (74%) | 6/37 (16%) | 0.83 (0.56-1.00) |
| 5.1 - 10 years | 16/29 (55%) | 1/16 (6%) | 0.87 (0.70-1.00) |
| 10.1 – 15 years | 35/43 (81%) | 6/35 (17%) | 0.78 (0.60-0.95) |
| 15.1 – 21 years | 64/75 (85%) | 5/64 (8%) | 0.91 (0.83-0.99) |
CT = computed tomography; GFAP = glial fibrillary acidic protein; ROC = receiver operating characteristic
Cutoff points for GFAP were derived from the ROC curves for detecting intracranial lesions on CT scan to maximize the sensitivity and to correctly classify all traumatic intracranial lesions. Classification performance for detecting intracranial lesions on CT at a GFAP cutoff level of 0.15 ng/ml yielded a sensitivity of 94%, a specificity of 47%, and a negative predictive value of 98% (Table 3a). When isolated skull fractures were considered together with intracranial lesions, the performance was almost identical, with a sensitivity of 95%, a specificity of 48%, and a negative predictive value of 98% (Table 3b). GFAP's performance in children and youth with an unaltered mental status (GCS 15) remained notably consistent in predicting intracranial lesions, with a sensitivity of 93% and a specificity of 50%.
Table 3.
Contingency tables and classification performance of Serum GFAP in detecting traumatic intracranial lesions on head CT.
| a. Isolated skull fractures excluded from intracranial lesions. | ||
|---|---|---|
|
| ||
| Table 3a. | CT positive | CT negative |
| GFAP positive >0.15 ng/ml | 17 | 71 |
| GFAP negative ≤0.15 ng/ml | 1 | 63 |
| Sensitivity | 94% (71-100) | |
| Specificity | 47% (38-56) | |
| NPV | 98% (90-100) | |
| PPV | 19% (12-29) | |
|
| ||
| b. Isolated skull fractures included with intracranial lesions. | ||
|
| ||
| Table 3b. | CT positive | CT negative |
|
| ||
| GFAP positive>0.15 ng/ml | 20 | 68 |
| GFAP negative ≤0.15 ng/ml | 1 | 63 |
| Sensitivity | 95% (74-100) | |
| Specificity | 48% (39-56) | |
| NPV | 98% (90-100) | |
| PPV | 22% (15-33) | |
|
| ||
| c. Patients presenting with a GCS 15. | ||
|
| ||
| Table 3c. | CT positive | CT negative |
|
| ||
| GFAP positive >0.15 ng/ml | 14 | 60 |
| GFAP negative ≤0.15 ng/ml | 1 | 60 |
| Sensitivity | 93% (66-100) | |
| Specificity | 50% (41-59) | |
| NPV | 98% (90-100) | |
| PPV | 19% (11-30) | |
CT = computed tomography; GFAP = glial fibrillary acidic protein; NPV = negative predictive value; PPV = positive predictive value
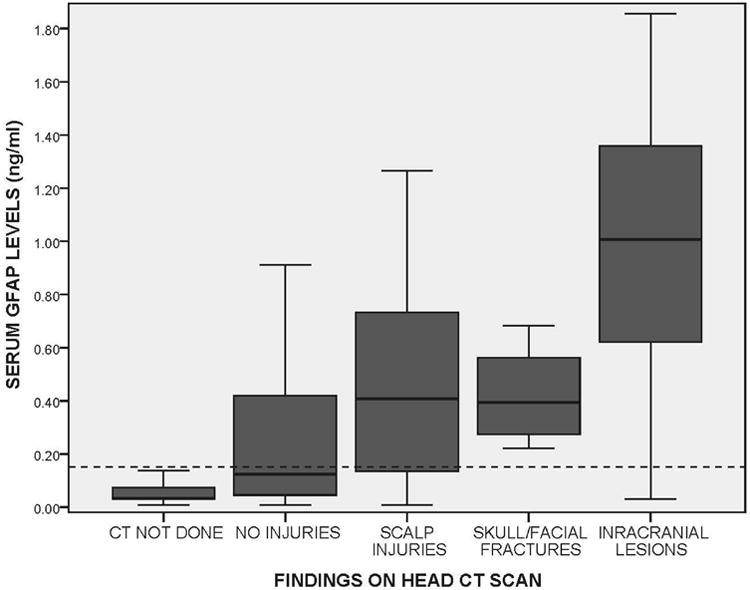
Patients were then distributed by severity of CT head lesions and compared to those who did not have head CT scans performed (Figure 5). Levels of GFAP increased with severity of CT lesions: no lesions (n = 98), scalp hematomas (n = 29), skull/facial fractures (n = 7), and intracranial lesions (n = 18); p < 0.001. Those who did not have CT scans performed had the lowest levels of GFAP compared to any other group (n = 105); p<0.001. The reference line in Figure 5 represents the GFAP threshold of 0.15 mg/ml selected to determine sensitivity and specificity for predicting the need for CT scan. Most of the patients who did not have CT scans performed were below the GFAP threshold of 0.15 ng/ml. Of the patients with intracranial lesions on CT, eight (44%) were admitted to ICU beds for observation and ten (66%) were admitted to either a step-down unit or a ward bed. In patients with isolated contusions (n = 3), the median GFAP level was 1.36 ng/ml (IQR 0.99 to 3.79 ng/ml), with an isolated subdural hematoma (n = 5) it was 0.95 ng/ml (IQR 0.21 to 1.06 ng/ml), and with an isolated epidural hematoma (n = 1) it was 1.07. When patients had two different types of lesions (n = 4) levels were 0.81 ng/ml (IQR 0.46 to 1.07 ng/ml) and three different lesions 1.08 ng/ml (IQR 0.83 to 1.86 ng/ml).
Figure 5.
Discussion
This prospective study assessed the performance of GFAP within 6 hours of trauma in a cohort of children and youth, with and without head trauma, presenting to two pediatric Level I trauma centers and one adult Level I trauma center. GFAP was significantly elevated in the serum of children and youth of all ages with head trauma compared to non-head injured trauma controls, thus supporting its brain-specific nature. Furthermore, GFAP was considerably higher in children and youth with traumatic intracranial lesions on head CT compared to those with no lesions, with a sensitivity of 94% and a specificity of 47%. When we isolated children and youth with an unaltered mental status (GCS 15) at presentation, GFAP still performed strongly in predicting intracranial lesions, with a sensitivity of 93% and a specificity of 50%. To the best of our knowledge this is among the largest published studies to date assessing GFAP in children and youth with mild and moderate TBI in an ED setting.
GFAP demonstrated consistent performance across all age groups for detecting intracranial lesions of CT. Most importantly, GFAP's performance in infants and toddlers 5 years old and younger yielded an AUC of 0.87. This finding has significant implications for the management of infants and toddlers with potential brain injury. Not only is head trauma very prevalent in this age group,26 but injury severity can be particularly difficult to assess clinically because many infants and toddlers are either non-verbal or unable to provide accurate histories. The CT ordering rate was 74% in this age group. Therefore, alternate diagnostic strategies to reduce ionizing radiation from head CTs, such as blood-based biomarkers, are crucial to improve care.
Using trauma patients without head trauma as a comparison group, instead of uninjured controls, allowed us to assess GFAP's brain specificity in a population that had been exposed to significant trauma. The forces that induced injuries in trauma control patients paralleled that of TBI patients, with the exception that trauma controls lacked both blunt head trauma and TBI symptoms. Indeed, GFAP was much higher in children and youth with head trauma than trauma controls who did not strike their heads. These findings are comparable to the results of GFAP in adult studies that have used similar control groups and have shown GFAP to detect TBI in the presence of extracranial injuries.15,18
In an ED setting physicians often evaluate head trauma patients within a few hours of injury. In this study, GFAP was detectable within an hour post-injury and was also detectable at 6 hours, suggesting that GFAP could eventually be used clinically in this timeframe. There may be a potential role for such biomarkers in children with conditions such as autism spectrum disorders who cannot express themselves, or in cases of child abuse where histories can be dubious and inconsistent. Accordingly, we elected to include both head trauma patients with and without TBI symptoms to explore the levels of GFAP in these groups. Despite a CT ordering rate of 48% in head trauma patients without TBI symptoms, there was not a single case with traumatic intracranial lesions on CT. All the positive CT cases were among head trauma patients with TBI symptoms.
We included both mild and moderate TBI because initial GCS scores in the ED in this population can be surprisingly deceptive, and definitions of TBI and concussion obscure.27 The classification of a TBI as a mild or a moderate can change based on neuroimaging results and the presence of factors altering mental status such as intoxication, medications, and other injuries. Although we studied TBI patients from GCS score 9 to 15, we included focused analyses of those with GCS scores of 15 carrying a diagnoses of “concussion.”
A number of clinical decision rules have been developed to help guide clinical decision-making for ordering head CT in children with head trauma.23,28,29 These rules have recently been compared in a separate cohort of children with head trauma, and have shown variability in their sensitivities and specificities for detecting brain injury.30 Some of the rules missed intracranial lesions and performed worse than clinical judgment. A blood test to detect intracranial lesions could add a layer of objectivity to clinical decision-making and perhaps become a useful adjunctive tool.
Of note, we also examined the performance of the biomarkers when CT was not performed, and when extracranial lesions were present on head CT without intracranial lesions. GFAP was not as elevated with extracranial lesions such as scalp hematomas or facial/skull fractures compared to when intracranial lesions were present. Therefore, the force required to injure the skull or fracture facial bones is enough to release biomarkers into the circulation. Those who did not have CT scans performed had the lowest levels of GFAP compared to any other group, reflecting that clinical suspicion of injury was very low in these patients.
If these findings can be validated, GFAP's association with the presence of intracranial lesions on CT scans could help EPs reduce the number of CTs performed, and it could be incorporated into guidelines for neuroimaging decisions and decisions to transfer patients to higher levels of care.
Limitations
While these data are encouraging, the authors recognize that there are limitations to this study. Patients were enrolled as a convenience sample because the research team could not be on duty 24/7, and the demographic characteristics of non-enrolled patients were not tracked on all potential patients. Despite this, patients were recruited consecutively when research assistants were on duty including on weekends and nights so a representative sample could be enrolled.
We did not determine the half-life or optimal timing after injury of GFAP in children and youth, and could only confirm its presence and its performance within six hours of injury. Although beyond the scope of this study, analyses of the biokinetics of GFAP are being conducted that will give insight into its pattern of release and characterize its temporal profile. Furthermore, this study addressed severity of injury in the acute care setting, and did not describe long-term outcomes in these patients.
The current study was performed in a substantial cohort of children and youth following trauma; however, the sample was inadequate to have a case requiring neurosurgical intervention. The rate of neurosurgical intervention in children is very low at 0.1%, and would require a much larger sample size to evaluate GFAP in these patients.23
Additionally, the CIs around the performance measures for detecting intracranial lesions were wide and reflect the relatively small number of children and youth with lesions on CT in the cohort.18 Again, a much larger number of children will be required to test the precision of the biomarker.
Although we cannot confirm that GFAP is entirely brain-specific, studies in the adult literature have shown that detection of intracranial lesions, amidst extracranial injuries on the head, body, and extremities, is still reliable.15,18 The findings (Figure 5) in this study further supports this.
Conclusions
This study introduces GFAP as a valuable candidate biomarker for detecting traumatic intracranial lesions on computed tomography in the setting of acute trauma in children and youth with suspected mild to moderate traumatic brain injury. Furthermore, the findings are consistent with, and a natural extension of, the work on GFAP in adults with mild to moderate traumatic brain injury. GFAP performed consistently across age groups including infants and toddlers 5 years old and younger, as well as in those with concussions with Glasgow Coma Scale scores of 15. This study has implications for reducing computed tomography use following head trauma in children and youth. A better understanding of the performance of this biomarker relative to severity of intracranial lesions and long term outcome in children and youth seem to be important next steps for this line of investigation. A larger multi-center study is required to validate these findings before clinical application.
Supplementary Material
Acknowledgments
Grant Support: This study was supported in part by Award Number R01NS057676 from the National Institute of Neurological Disorders and Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders And Stroke or the National Institutes of Health.
Footnotes
Disclosures: Dr. Papa is a scientific consultant for Banyan Biomarkers, Inc. but receives no stocks or royalties from the company and will not benefit financially from this publication.
Category: Original Contribution
Supervising Editor: Michelle Macy, MD, MS
References
- 1.Jagoda AS, Bazarian JJ, Bruns JJ, Jr, et al. Clinical policy: neuroimaging and decision making in adult mild traumatic brain injury in the acute setting. J Emerg Nurs. 2009;35:e5–40. doi: 10.1016/j.jen.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Stein SC, Fabbri A, Servadei F, Glick HA. A critical comparison of clinical decision instruments for computed tomographic scanning in mild closed traumatic brain injury in adolescents and adults. Ann Emerg Med. 2009;53:180–8. doi: 10.1016/j.annemergmed.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher CN, Hutchinson PJ, Pickard JD. Neuroimaging in trauma. Curr Opin Neurol. 2007;20:403–9. doi: 10.1097/WCO.0b013e32821b987b. [DOI] [PubMed] [Google Scholar]
- 4.Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167:700–7. doi: 10.1001/jamapediatrics.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner DJ. Estimating cancer risks from pediatric CT: going from the qualitative to the quantitative. Pediatr Radiol. 2002;32:228–3. doi: 10.1007/s00247-002-0671-1. [DOI] [PubMed] [Google Scholar]
- 6.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 7.Linet MS, Slovis TL, Miller DL, et al. Cancer risks associated with external radiation from diagnostic imaging procedures. CA Cancer J Clin. 2012;62(2):75–100. doi: 10.3322/caac.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berrington de Gonzalez A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071–7. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner DJ. Slowing the increase in the population dose resulting from CT scans. Radiat Res. 2010;174(6):809–15. doi: 10.1667/RR1859.1. [DOI] [PubMed] [Google Scholar]
- 10.Papa L. Biomarkers for Concussion. In: Slobounov SM, Sebastianelli WJ, editors. Concussions in Athletics: From Brain to Behavior. New York, NY: Springer; 2014. pp. 235–71. [Google Scholar]
- 11.Long AE. Radiographic decision-making by the emergency physician. Emerg Med Clin North Am. 1985;3:437–46. [PubMed] [Google Scholar]
- 12.Nigrovic LE, Schunk JE, Foerster A, et al. The effect of observation on cranial computed tomography utilization for children after blunt head trauma. Pediatrics. 2011;127:1067–73. doi: 10.1542/peds.2010-3373. [DOI] [PubMed] [Google Scholar]
- 13.Papa L, Ramia MM, Kelly JM, Burks SS, Pawlowicz A, Berger RP. Systematic review of clinical research on biomarkers for pediatric traumatic brain injury. J Neurotrauma. 2013;30:324–38. doi: 10.1089/neu.2012.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papa L. Exploring the Role of Biomarkers for the Diagnosis and Management of Traumatic Brain Injury Patients. In: Man TK, Flores RJ, editors. Proteomics - Human Diseases and Protein Functions. Rijeka, Croatia: InTech; 2012. pp. 89–106. [Google Scholar]
- 15.Papa L, Lewis LM, Falk JL, et al. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann Emerg Med. 2012;59:471–83. doi: 10.1016/j.annemergmed.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metting Z, Wilczak N, Rodiger LA, Schaaf JM, van der Naalt J. GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology. 2012;78:1428–33. doi: 10.1212/WNL.0b013e318253d5c7. [DOI] [PubMed] [Google Scholar]
- 17.Diaz-Arrastia R, Wang KK, Papa L, et al. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J Neurotrauma. 2014;31:19–25. doi: 10.1089/neu.2013.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papa L, Silvestri S, Brophy GM, et al. GFAP out-performs S100beta in detecting traumatic intracranial lesions on computed tomography in trauma patients with mild traumatic brain injury and those with extracranial lesions. J Neurotrauma. 2014;31:1815–22. doi: 10.1089/neu.2013.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eng LF, Vanderhaeghen JJ, Bignami A, Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971;28:351–4. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- 20.Papa L, Mittal MK, Ramirez J, et al. In children and youth with mild and moderate traumatic brain injury GFAP out-performs S100beta in detecting traumatic intracranial lesions on computed tomography. J Neurotrauma. 2015 doi: 10.1089/neu.2015.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruff RM, Iverson GL, Barth JT, Bush SS, Broshek DK. Recommendations for diagnosing a mild traumatic brain injury: a National Academy of Neuropsychology education paper. Arch Clin Neuropsychol. 2009;24:3–10. doi: 10.1093/arclin/acp006. [DOI] [PubMed] [Google Scholar]
- 22.Berger RP, Beers SR, Papa L, Bell M. Common data elements for pediatric traumatic brain injury: recommendations from the biospecimens and biomarkers workgroup. J Neurotrauma. 2012;29:672–7. doi: 10.1089/neu.2011.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuppermann N, Holmes JF, Dayan PS, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet. 2009;374:1160–70. doi: 10.1016/S0140-6736(09)61558-0. [DOI] [PubMed] [Google Scholar]
- 24.Berger RP, Beers SR, Richichi R, Wiesman D, Adelson PD. Serum biomarker concentrations and outcome after pediatric traumatic brain injury. J Neurotrauma. 2007;24:1793–801. doi: 10.1089/neu.2007.0316. [DOI] [PubMed] [Google Scholar]
- 25.Papa L, Ramia M, Kirby S, et al. Serum GFAP out-performs S100B in detecting traumatic intracranial lesions on CT in children with suspected mild traumatic brain injury (Abstract) Acad Emerg Med. 2014;21:S315. [Google Scholar]
- 26.Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. [Accessed Aug 17, 2015]; Available at: http://www.cdc.gov/traumaticbraininjury/tbi_ed.html.
- 27.Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25:719–38. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osmond MH, Klassen TP, Wells GA, et al. CATCH: a clinical decision rule for the use of computed tomography in children with minor head injury. CMAJ. 2010;182:341–8. doi: 10.1503/cmaj.091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunning J, Daly JP, Lomas JP, Lecky F, Batchelor J, Mackway-Jones K. Derivation of the children's head injury algorithm for the prediction of important clinical events decision rule for head injury in children. Arch Dis Child. 2006;91:885–91. doi: 10.1136/adc.2005.083980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Easter JS, Bakes K, Dhaliwal J, Miller M, Caruso E, Haukoos JS. Comparison of PECARN, CATCH, and CHALICE rules for children with minor head injury: a prospective cohort study. Ann Emerg Med. 2014;64:145–52. doi: 10.1016/j.annemergmed.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.