Abstract
Introduction:
We evaluated the prognostic effects of hematologic parameters of preoperative leukocytosis and neutrophil-to-lymphocyte ratio (NLR) in patients who underwent radical cystectomy for bladder cancer.
Methods:
We retrospectively reviewed the medical records of 363 patients who underwent radical cystectomy for bladder cancer between January 1990 and June 2013. In total, 286 patients were included in the study. Age, gender, pathologic stage, lymph node involvement, preoperative hydronephrosis, histologic sub-type, surgical margin status, and lymphovascular invasion were recorded for each patient. Univariate and multivariate analysis were performed to determine the prognostic value of the preoperative clinical and laboratory parameters on disease-specific survival (DSS). Additionally, the correlation between leukocytosis and other factors were evaluated.
Results:
According to the univariate analysis preoperative leukocytosis and NLR were detected as negative prognostic factors on DSS. Preoperative leukocytosis, NLR, stage, lymph node involvement, histologic subtype, grade and age were independent prognostic factors for DSS, on multivariate analysis. Patients with leukocytosis had higher stage, grade and lymphovascular invasion.
Conclusions:
Inexpensive, reproducible, and readily available peripheral blood count components of white blood cell count and NLR were independent prognostic factors, which can stratify DSS risks in bladder cancer patients who underwent radical cystectomy.
Introduction
Patients with high-risk non-muscle invasive and muscle-invasive urothelial carcinoma of the bladder are treated with radical cystectomy.1 However about 50% of these patients will develop distant metastases, and 5-year survival of locally advanced disease ranges from 26% to 64%.2,3 These poor survival outcomes suggest the need for a new risk stratification. New preoperative predicting models based on systemic inflammatory models have used only preoperative factors to identify oncologic outcome.4
Tumours associated with indicators of the systemic inflammatory-immunological process play critical roles in the development and progression of various cancers.5 Neutrophil count, lymphocyte count or neutrophil-to-lymphocyte ratio (NLR) can be independent prognostic and predictive systematic inflammatory markers for unfavourable survival in patients with urinary tract malignancies.6–9 Although elevated NLR and poor overall and disease-specific survival (DSS) in muscle-invasive disease have been reported,10 to date, the prognostic significance of leukocytosis in patients with bladder carcinoma treated with radical cystectomy has not yet been determined.
Therefore, we evaluated the prognostic impact of pre-operative leukocytosis in patients with bladder carcinoma treated with radical cystectomy. We also evaluated the prognostic impact of possible hematologic factors, such as neutrophilia, lymphopenia and NLR, in predicting DSS.
Methods
Following institutional review board approval (IRB 15-572-13), we reviewed the records of 369 patients who underwent RC between January 1990 and June 2013 at our institution. The diagnosis of bladder cancer was histologically confirmed by transurethral resection of bladder tumour (TURBT) in each patient. A genitourinary pathologist reviewed all surgical specimens and the diagnosis of urothelial or nonurothelial carcinoma of the bladder was confirmed. The indications for radical cystectomy included muscle-invasive tumours without evidence of distant metastasis (cT2–4, NX, M0), recurrent multifocal superficial disease refractory to repeat transurethral resection with intravesical therapy, or Bacille Calmette-Guérin (BCG)-resistant carcinoma in situ. Tumours were graded according to the 1973 World Health Organization (WHO) grading system,11 and clinical T stage was determined according to the 2002 American Joint Committee on Cancer TNM staging system.12 We excluded patients who received neoadjuvant chemotherapy, radiation, with hematologic malignancies, without or unreachable preoperative complete blood count (CBC), with an active infection at the time of surgical intervention, and patients with prior blood transfusion or usage of drugs that may affect hematologic parameters.
A routine CBC test was part of the standard preoperative blood work and the analysis was performed close to the date of surgery. Patient characteristics included age, sex, preoperative white blood cell count (WBC), neutrophil and lymphocyte levels, NLR, preoperative hydronephrosis, clinical tumour stage, surgical margin status, pathologic tumour stages, tumour size, histology, presence of lymph node involvement, and lymphovascular invasion.
Categorical variables were presented as numbers and percentages, and metric variables as mean ± standard deviation (SD) or median (minimum-maximum). To compare two groups for categorical variables, we used the chi-squared test (Fishers exact test). For each group, DSS curves were estimated according to the Kaplan-Meier survival analysis. Survival estimates between groups were compared using the log-rank test. Univariate and multivariate Cox regression analyses were performed to identify independent prognostic factors for DSS. Multivariate logistic analysis of predictors included all possible prognostic factors, such as patient age, lymph node pathological stage, histologic stage, surgical margin, tumour grade at TURBT, lymphovascular invasion, hydronephrosis, leukocyte count, neutrophil count, and NLR size. Hazard ratio (HR) with 95% confidence intervals (CI) was given. All statistical analysis was performed using SPSS 11.5, p < 0.05 were deemed statistically significant. P values of 0.10 and 0.20 were used for the entry and removal criteria, respectively, in multivariate Cox regression analysis.
NLR was analyzed as dichotomous variable according to an approximate “optimal” cut point of 2.5 obtained by a validated web-based software.13–16 Neutrophil and lymphocyte counts were defined as 1800–7700 cells/microL and 1500–4000 cells/microL, respectively, according to WHO global standards. Leukocytosis was defined as WBC >11 000 cells/microL.
Results
Of the 363 patients, we excluded 77 patients who did not meet our inclusion criteria. In the end, we had 286 patients in our study: 256 males and 30 females. The mean (± SD) patient age was 60.7 ± 9.42 years (range: 29–83). The median follow-up period was 28 months (range: 0–144). Clinicopathologic characteristics for patients with and without preoperative leukocytosis did not differ in parameters of age, gender, lymph node invasion, histological type, surgical margin, carcinoma in-situ or hydronephrosis (p > 0.05 for all) (Table 1). The median time from preoperative leukocytosis and NLR to radical cystectomy was 7 days (range: 1–13). Patients with WBC >11 000 had more T4 category tumours (p = 0.048), G3 tumours (p = 0.004), and positive lymphovascular invasion (p = 0.005) (Table 1).
Table 1.
Clinicopathologic characteristics of the study cohort stratified by WBC
| Parameters | Total (n = 286) | WBC ≤11 000 (n = 252) | WBC >11 000 (n = 34) | p values |
|---|---|---|---|---|
| N | 286 | 252 | 34 | |
| Age | ||||
| <70 | 240 (83.9%) | 212 (84.1%) | 28 (82.4%) | 0.792 |
| ≥70 | 46 (16.1%) | 40 (15.9%) | 6 (17.6%) | |
| Gender | ||||
| Male | 256 (89.5%) | 225 (89.3%) | 31 (91.2%) | 1.000 |
| Female | 30 (10.5%) | 27 (10.7%) | 3 (8.8%) | |
| Lymph node invasion | ||||
| Negative | 244 (85.3%) | 215 (85.3%) | 29 (85.3%) | 1.000 |
| Positive | 42 (14.7%) | 37 (14.7%) | 5 (14.7%) | |
| Tumour stage | ||||
| T0 | 32 (11.2%) | 32 (12.7%) | 0 (0%) | 0.048 |
| T1 | 49 (17.1%) | 44 (17.5%) | 5 (14.6%) | |
| T2 | 81 (28.3%) | 70 (27.8%) | 11 (32.4%) | |
| T3 | 73 (25.5%) | 66 (26.2%) | 7 (20.6%) | |
| T4 | 51 (17.8%) | 40 (15.8%) | 11 (32.4%) | |
| Histological type | ||||
| Urothelial carcinoma | 236 (82.5%) | 209 (82.9%) | 27 (79.4%) | 0.611 |
| Non-urothelial carcinoma | 50 (17.5%) | 43 (17.1%) | 7 (20.6%) | |
| Surgical margin | ||||
| Positive | 18 (6.3%) | 14 (5.6%) | 4 (9.4%) | 0.247 |
| Negative | 268 (93.7%) | 238 (94.4%) | 30 (90.6%) | |
| Grade | ||||
| 3 | 190 (66.4%) | 160 (63.4%) | 30 (83.3%) | 0.004; |
| ≤2 | 96 (33.6%) | 92 (36.6%) | 4 (6.7%) | |
| Lymphovascular invasion | ||||
| Positive | 51 (17.8%) | 39 (15.5%) | 12 (35.3%) | 0.005 |
| Negative | 235 (82.2%) | 213 (84.5%) | 22 (64.7%) | |
| Carcinoma in-situ | ||||
| Positive | 19 (6.6%) | 17 (6.7%) | 2 (5.9%) | 1.000 |
| Negative | 267 (93.4%) | 235 (93.3%) | 32 (94.1%) | |
| Preoperative hydronephrosis | ||||
| Positive | 66 (23.1%) | 56 (22.2%) | 10 (29.4%) | 0.350 |
| Negative | 220 (76.9%) | 196 (77.8%) | 24 (70.6%) |
WBC: white blood cell count.
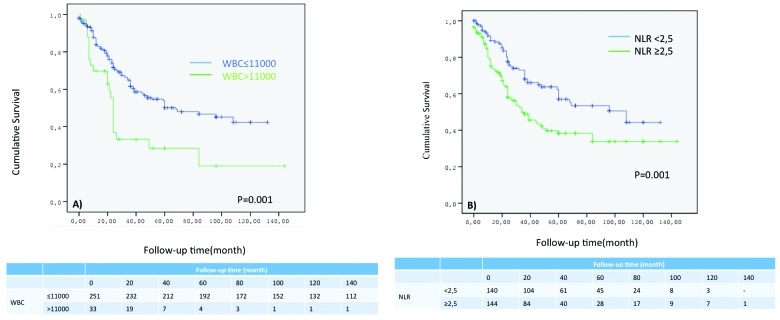
According to Kaplan-Meier analysis, patients with the following clinicopathological characteristics had shorter survival periods: patient age (≥70), p = 0.028; lymph node invasion (positive), p < 0.001; clinical T stage (T2>), p < 0.001; histological type (non-transitional cell carcinoma [TCC]), p < 0.001; surgical margin (positive), p = 0.026; tumour grade (high), p < 0.001; lymphovascular invasion (positive), p < 0.001; carcinoma in-situ (negative), p = 0.125; hydronephrosis (positive), p < 0.001; leukocyte count (>11000), p < 0.002; neutrophil count (≥7700), p = 0.035; and NLR (≥2.5), p < 0.001. DSS rates at 3 years were 49.8% and 73% for patients with a NLR ≥2.5 and NLR <2.5, respectively; and 36.9% and 64.9% for patients with preoperative WBC ≤11 000 and WBC >11 000, respectively (Fig. 1). After univariate Cox regression analysis, age ≥70 (HR 1.630, p = 0.042), positive lymph node (HR 1.517, p = 0.030), clinical T2> stage tumours (HR 5.463, p < 0.001), non-TCC bladder cancers (HR 1.533, p = 0.040), high-grade tumours (HR 3.337, p < 0.001), preoperative leukocytosis (HR 1.773, p = 0.020), and NLR ≥ 2.5 (HR 1.965, p = 0.022) predicted DSS in bladder cancer patients treated with radical cystectomy according to multivariate Cox regression analysis (Table 2).
Fig. 1.
A: Disease-specific survival (DSS) rates according to white blood cell (WBC) count; (B) DSS rates according to neutrophil-to-lymphocyte ratio.
Table 2.
Univariate and multivariate Cox regression analyses of prognostic factors on disease-specific survival of 286 patients who underwent cystectomy for bladder cancer
| Parameters | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% Cl | p value | HR | 95% Cl | p value | |
| Age | ||||||
| <70* | ||||||
| ≥70 | 1.648 | 1.047–2.594 | 0.028 | 1.630 | 1.092–2.608 | 0.042 |
| Gender | ||||||
| Female | ||||||
| Male | 1.322 | 0.832–2.026 | 0,736 | |||
| Lymph node invasion | ||||||
| Negative* | ||||||
| Positive | 3.940 | 2.623–5.919 | <0.001 | 1.517 | 1.097–2.370 | 0.030 |
| Pathological stage | ||||||
| T2≤* | ||||||
| T2> | 7.114 | 4.716–10.730 | <0.001 | 5.463 | 3.469–8.606 | <0.001 |
| Histologic type | ||||||
| Urothelial carcinoma | ||||||
| Non-urothelial carcinoma | 2.116 | 1.428–3.136 | <0.001 | 1.533 | 1.020–2.305 | 0.040 |
| Surgical margin | ||||||
| Negative* | ||||||
| Positive | 1.852 | 1.062–3.231 | 0,026 | |||
| Grade | ||||||
| Low* | ||||||
| High | 4.741 | 2.864–7.848 | <0.001 | 3.337 | 1.993–5.587 | <0.001 |
| Lymphovascular invasion | ||||||
| Negative* | ||||||
| Positive | 2.690 | 1.840–3.934 | <0.001 | |||
| Carcinoma in-situ | ||||||
| Positive* | ||||||
| Negative | 1.164 | 0.920–1.487 | 0,125 | |||
| Preoperative hydronephrosis | ||||||
| Negative* | ||||||
| Positive | 2.359 | 1.637–3.398 | <0.001 | |||
| Leukocyte | ||||||
| ≤11 000* | ||||||
| >11 000 | 2.086 | 1.314–3.310 | 0.002 | 1.773 | 1.096–2.687 | 0.020 |
| Neutrophil | ||||||
| <7700* | ||||||
| ≥7700 | 1.528 | 1.023–2.283 | 0.035 | |||
| Lymphocyte >1500 | ||||||
| Lymphocyte ≤1500 | 1.435 | 0.816–2.532 | 0.691 | |||
| NLR | ||||||
| <2.5* | ||||||
| ≥2.5 | 1.798 | 1.260–2.567 | 0.001 | 1.965 | 1.042–3.586 | 0.022 |
CI: Confidence interval; HR: hazard ratio;
Reference category; NLR: neutrophil-to-lymphocyte ratio.
Discussion
We found that preoperative leukocytosis was associated with advanced pathologic stage at time of cystectomy, higher grade (G3) tumours, and positive lymphovascular invasion. Furthermore, both hematologic parameters of preoperative leukocytosis (WBC >11 000) and NLR ≥2.5 were significant predictors of DSS in patients with bladder carcinoma undergoing radical cystectomy.
Previous studies have investigated the prognostic value of pretreatment leukocytosis with gynaecologic cancers and an elevated NLR with adverse oncologic outcomes, including breast, colorectal, non-small cell lung, gastric and renal cell cancers.16–21 In general, tumours can affect hematopoietic parameters by significant tumour bleed, or by affecting hematopoiesis through infiltration into the bone marrow or by production of pro-inflammatory cytokines and free radicals that damage hematopoietic progenitor cells, as paraneoplastic syndromes.22
Recently, several studies reported an elevated NLR having similar prognostic significance in those with urothelial carcinoma. An elevated NLR before cystectomy has been associated with decreased overall and DSS.6–10 In our study, NLR was significantly associated with DSS similar to previous reports.6,10 In contrast, Demirtas and colleagues did not find a significant association between patients with an NLR >2.5 and overall survival.15
On the other hand, a hematopoietic parameter, leukocytosis, which may be a possible prognostic factor, has largely been limited to individual case reports of paraneoplastic syndromes within the published data on urothelial carcinoma.23,24 To the best of our knowledge, this is the first study to demonstrate the prognostic significance of preoperative leukocytosis in patients who underwent radical cystectomy for bladder cancer. In the present study, patients with leukocytosis had significantly shorter DSS than patients without leukocytosis. Moreover, multivariate analyses revealed that preoperative leukocytosis was independently associated with decreased DSS. Previous studies have demonstrated that hematopoietic cytokines may stimulate leukocytosis and treatment with exogenous G-CSF stimulates the growth of bladder cancer cells in vitro.25
Similar to our current study, non-hematologic parameters of our multivariate analyses variables, such as age, positive lymph node, clinical T2> stage tumours, non-TCC bladder and high-grade tumours, have been suggested as one of the significant preoperative prognostic factors.10,26,27
Our study has its limitations, including its retrospective, non-randomized nature, which may have led to a selection bias with a limited number of patients from a single institution. Additionally all possible factors were analyzed that resulted as too many variables in a multivariate analysis. A possible inflammatory condition that may have affected the hematopoietic system was not accounted for in our study. Future prospective, well-controlled clinical studies are needed to confirm the exact role of hematologic parameters and whether an abnormal hematologic profile is an end result of tumour growth or an underlying cause of mortality in this group of patients.
Conclusion
In the current study, preoperative leukocytosis was associated with T4 category, tumour grade of 3 and positive lymphovascular invasion in patients with bladder carcinoma treated with radical cystectomy. Furthermore, both peripheral blood count components of an elevated NLR >2.5 and preoperative leukocytosis were significant predictors of worsened DSS. Further prospective studies in multiple institutions with larger cohorts are required to validate these findings.
Footnotes
Competing interests: The authors declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Faba OR, Palou J, Breda A, et al. High-risk non-muscle-invasive bladder cancer: Update for a better identification and treatment. World J Urol. 2012;30:833–40. doi: 10.1007/s00345-012-0967-1. [DOI] [PubMed] [Google Scholar]
- 2.Malkowicz SB, van Poppel H, Mickisch G, et al. Muscle-invasive urothelial carcinoma of the bladder. Urology. 2007;69:3–16. doi: 10.1016/j.urology.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 3.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 4.Wei Y, Jiang YZ, Qian WH. Prognostic role of NLR in urinary cancers: A meta-analysis. PLoS One. 2014;9:e92079. doi: 10.1371/journal.pone.0092079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 6.Gondo T, Nakashima J, Ohno Y, et al. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology. 2012;79:1085–91. doi: 10.1016/j.urology.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 7.Mano R, Baniel J, Shoshany O, et al. Neutrophil-to-lymphocyte ratio predicts progression and recurrence of non-muscle-invasive bladder cancer. Urol Oncol. 2015;33:67.e1–7. doi: 10.1016/j.urolonc.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Potretzke A, Hillman L, Wong K, et al. NLR is predictive of upstaging at the time of radical cystectomy for patients with urothelial carcinoma of the bladder. Urol Oncol. 2014;32:631–6. doi: 10.1016/j.urolonc.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Kaynar M, Yıldırım ME, Badem H, et al. Bladder cancer invasion predictability based on preoperative neutrophil-lymphocyte ratio. Tumor Biol. 2014;35:6601–5. doi: 10.1007/s13277-014-1889-x. [DOI] [PubMed] [Google Scholar]
- 10.Viers BR, Boorjian SA, Frank I, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol. 2014;66:1157–64. doi: 10.1016/j.eururo.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 11.Epstein JI, Amin MB, Reuter VR, et al. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22:1435–48. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Sobin LH, Wittekind C. TNM: Classification of Malignant Tumors. New York, NY: 2002. International Union against Cancer; p. xxiii. (239p). [Google Scholar]
- 13.Budczies J, Klauschen F, Sinn BV, et al. Cutoff Finder: A comprehensive and straight forward Web application enabling rapid biomarker cut off optimization. PLoS One. 2012;7:51862. doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atzpodien J, Royston P, Wandert T, et al. Metastatic renal carcinoma comprehensive prognostic system. Br J Cancer. 2003;88:348–53. doi: 10.1038/sj.bjc.6600768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demirtaş A, Sabur V, Akınsal EC, et al. Can neutrophil-lymphocyte ratio and lymph node density be used as prognostic factors in patients undergoing radical cystectomy? ScientificWorldJournal. 2013;31:703579. doi: 10.1155/2013/703579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krane LS, Richards KA, Kader AK, et al. Preoperative neutrophil/lymphocyte ratio predicts overall survival and extravesical disease in patients undergoing radical cystectomy. J Endourol. 2013;27:1046–50. doi: 10.1089/end.2012.0606. [DOI] [PubMed] [Google Scholar]
- 17.So KA, Hong JH, Jin HM, et al. The prognostic significance of preoperative leukocytosis in epithelial ovarian carcinoma: A retrospective cohort study. Gynecol Oncol. 2014;132:551–5. doi: 10.1016/j.ygyno.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Azab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil-to lymphocyte ratio in predicting short-and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217–24. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 19.Ishizuka M, Nagata H, Takagi K, et al. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer. 2013;109:401–7. doi: 10.1038/bjc.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarraf KM, Belcher E, Raevsky E, et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:425–8. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 21.Yamanaka T, Matsumoto S, Teramukai S, et al. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215–20. doi: 10.1159/000127412. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Zhang L, Liu WX, et al. Prognostic significance of preoperative anemia, leukocytosis and thrombocytosis in Chinese women with epithelial ovarian cancer. Asian Pac J Cancer Prev. 2015;16:933–9. doi: 10.7314/APJCP.2015.16.3.933. [DOI] [PubMed] [Google Scholar]
- 23.Dukes JW, Tierney LM., Jr Paraneoplastic leukemoid reaction as marker for transitional cell carcinoma recurrence. Urology. 2009;73:17–9. doi: 10.1016/j.urology.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Izard JP, Gore JL, Mostaghel EA, et al. Persistent, unexplained leukocytosis is a paraneoplastic syndrome associated with a poor prognosis in patients with urothelial carcinoma. Clin Genitourin Cancer. 2015;13:e253–8. doi: 10.1016/j.clgc.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Shameem IA, Kurisu H, Matsuyama H, et al. Direct and indirect effects of recombinant human granulocyte-colony stimulating factor on in vitro colony formation of human bladder cancer cells. Cancer Immunol Immunother. 1994;38:353–7. doi: 10.1007/BF01517203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girgin C, Sezer A, Uc R, et al. Outcome of the treatment of invasive non-transitional cell carcinoma. Int J Urol. 2003;10:525–9. doi: 10.1046/j.1442-2042.2003.00679.x. [DOI] [PubMed] [Google Scholar]
- 27.Thrasher JB, Frazier HA, Robertson JE. Clinical variables which serve as predictors of cancer-specific survival among patients treated with radical cystectomy for transitional cell carcinoma of the bladder and prostate. Cancer. 1994;73:1708–15. doi: 10.1002/1097-0142(19940315)73:6<1708::AIDCNCR2820730626>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]