Highlights
-
•
Teens’ neural response to reward during risk taking was assessed twice over 1.5 years.
-
•
Teens’ positive parent–child relationships and risk taking were also measured.
-
•
Increases in positive parent–child relationships related to declines in risk taking.
-
•
This is mediated by longitudinal decreases in ventral striatum activation.
-
•
Results highlight the key role of neural reactivity to rewards in this process.
Keywords: Adolescence, Parent–child relationships, Risk taking, fMRI
Abstract
Adolescence is marked by a steep increase in risk-taking behavior. The serious consequences of such heightened risk taking raise the importance of identifying protective factors. Despite its dynamic change during adolescence, family relationships remain a key source of influence for teenagers. Using a longitudinal fMRI approach, we scanned 23 adolescents twice across a 1.5-year period to examine how changes in parent–child relationships contribute to changes in adolescent risk taking over time via changes in adolescents’ neural reactivity to rewards. Results indicate that although parent–child relationships are not associated with adolescent risk taking concurrently, increases in positive parent–child relationships contribute to declines in adolescent risk taking. This process is mediated by longitudinal decreases in ventral striatum activation to rewards during risk taking. Findings highlight the neural pathways through which improvements in positive parent–child relationships serve to buffer longitudinal increases in adolescent risk taking.
Adolescence is a time of dramatic changes in brain, behavioral, and psychological functioning. A key change during this phase of development is a steep rise in risk taking. Compared with younger children, adolescents tend to engage in a variety of risky behaviors, such as reckless driving, substance use, and unprotected sexual activity (Arnett, 1992, Karriker-Jaffe et al., 2008), resulting in significant increases in morbidity and mortality rates in an otherwise healthy developmental period (e.g., National Vital Statistics Report, 2011). It is therefore crucial to identify protective factors that can prevent these upward trajectories of risk-taking behavior. Given the important role of family relationships in adolescents’ adjustment (Collins and Steinberg, 2006, Smetana et al., 2006), the current study aimed to examine how changes in family relationships during the teen years influence trajectories of adolescent risk taking through changes in neural reactivity.
During adolescence, youth tend to individuate from their family and become more oriented toward peers (Collins and Steinberg, 2006, Nelson et al., 2005). Indeed, dramatic changes in parent–child relationships are well-documented (Keijsers and Poulin, 2013, Laursen et al., 1998, Loeber et al., 2000, McGue et al., 2005). While normative patterns may characterize family relationships as increasing in negativity during adolescence (Laursen et al., 1998, Tsai et al., 2013), this is also a time during which some adolescents experience improvements in the quality of their family relationships, including more positive interactions, greater feelings of cohesion, and a heightened sense of family identity (e.g., Steinberg, 2001). The changing nature of parent–child relationships may serve an important protective role for adolescents’ psychological well-being. Indeed, positive parent–child relationships are a key factor related to reduced adolescent risk taking. For example, greater parental support and child disclosure to parents are associated with adolescents’ lower problem behavior, such as drinking, delinquency, and drug use (Jessor et al., 2003, Stattin and Kerr, 2000), and lower rates of parent–child conflict are associated with less externalizing symptoms, conduct problems, and antisocial behavior (Burt et al., 2006, Klahr et al., 2011a, Klahr et al., 2011b). Thus, higher quality family relationships serve to buffer adolescents from engaging in risk-taking behavior.
Theories and empirical studies on adolescent brain development suggest that increases in risk taking during adolescence may occur, in part, due to increased activation in reward-related regions. The development of the reward system, and the ventral striatum in particular, develops relatively early, peaking in neural reactivity around adolescence (Casey et al., 2008, Steinberg, 2008). Emerging evidence has shown that the social environment, such as parents and peers may play a role in adolescent brain function during risk taking. For example, prior research shows that negative environmental factors, such as in the presence of peers, amplify adolescents’ ventral striatum activation, leading to greater risk-taking behavior (Chein et al., 2011). In contrast, the presence of mothers serves as a protective factor by reducing adolescents’ ventral striatum activation during risk taking (Telzer et al., in press). However, little is known about how the quality of parent–child relationships or how changes in relationship quality contribute to longitudinal changes in adolescents’ neural sensitivity to risk taking.
Several prior studies have demonstrated that early family relationships impact children's brain development. For example, early maternal deprivation and severe parental neglect or abuse in young children is associated with altered ventral striatum activity during adolescence (Goff et al., 2013, Hart and Rubia, 2012, Mehta et al., 2010). Thus, early parent–child relationships may influence adolescent brain development in the long run. However, no prior studies have carefully examined how changes in parent–child relationships during later development affect adolescents’ neural reactivity. Given that family relationships change substantially during adolescence, a time when the brain is highly sensitive to sociocultural processing (Blakemore and Mills, 2014), it is key to understand how the changing nature of these relationships may affect adolescents’ risk taking and neural sensitivity over time.
We sought to examine how changes in positive parent–child relationships are associated with changes in adolescents’ neural sensitivity to rewards and changes in their risk-taking behavior over time. To this end, we used a longitudinal design in which adolescents completed an fMRI scan twice, approximately 1.5 years apart. We focus on middle to late adolescence, because this is a time when adolescents are given more autonomy for decision making (Wray-Lake et al., 2010), and therefore have more opportunities to engage in risky behaviors. Moreover, their family relationships may also witness important changes. For example, prior studies have shown changes in the time adolescents spend with their parents, and adolescents’ perceptions of relationships with parents continue to change during this phase of development (De Goede et al., 2009; Lam et al., 2012). In particular, adolescents report significant declines in their sense of family cohesion and family identity declines that tend to taper off at 12th grade (Tsai et al., 2013). Moreover, although many studies have demonstrated a rise in risk-taking behavior as children enter early adolescence, risk taking and reward seeking also show large changes from middle to late adolescence (Steinberg, 2010). Thus, it is important to examine individual differences in such changes and the neural correlates associated with such changes.
In the current study, we examined whether changes in parent–child relationships contribute to changes in adolescent risk taking. We hypothesized that greater increases in positive parent–child relationships from Time 1 (T1) to Time 2 (T2) would be associated with greater declines in adolescent risk-taking behavior during this same period. Second, we investigated how changes in positive parent–child relationships are associated with longitudinal changes in adolescents’ neural reactivity to rewards during risk taking. Given the important role of the ventral striatum in reward processing, we hypothesized that greater increases in positive parent–child relationships from T1 to T2 would be associated with declines in ventral striatum activation during risk taking from T1 to T2. Finally, we conducted mediation analyses to test whether longitudinal changes in neural activation explain the link between changes in positive parent–child relationships and changes in adolescent risk taking. We hypothesized that greater increases in positive parent–child relationships would contribute to greater declines in adolescent risk-taking behavior through changes in neural reactivity to rewards over time.
1. Method
1.1. Participants
A community sample of 24 adolescents1 completed two fMRI scans, approximately 1.5 years apart, a developmental window characterized by significant changes in brain function (Van den Bulk et al., 2013). One participant was excluded from analyses due to excessive head movement (i.e., >2.5 mm). All participants were recruited from one public high school and were in the 10th or 11th grade at T1 and in the 11th or 12th grade at T2. Our final sample comprised 23 adolescents (15 girls; Mage T1 = 15.78 years, range = 15.34–17.13 years, SD = .60; Mage T2 = 17.13 years, range = 16.43–18.42 years, SD = .70). Participants were not currently taking any medications and did not report being diagnosed with any mood disorders. Most participants were from low-SES families. A majority of fathers (87%) and mothers (91%) had high school diploma or less, with an average annual family income of $26,000 (range = $10,000–53,200). Participants completed written consent and assent in accordance with the University's Institutional Review Board.
1.2. Positive parent–child relationships
To obtain a full scope of family relationship quality, at both T1 and T2 adolescents reported on their sense of parental support, disclosure to parents, and conflict with parents, three key aspects that reflect parent–child relationships in daily life (Smetana et al., 2006, Steinberg, 2001). Adolescents’ sense of parental support was assessed by 9 items of the Inventory of Parent and Peer Attachment (IPPA) (Armsden and Greenberg, 1987). Adolescents rated the degree to which each item (e.g., “My parents respected my feelings.” and “My parents helped me to talk about my difficulties.”) was true for them in the past month on a 5-point scale ranging from “almost never or never true” to “almost always or always true”. α = .94 at T1 and .95 at T2. Adolescents reported on their spontaneous disclosure to parents in the past month using 5 items (Stattin and Kerr, 2000; e.g., “Did you spontaneously tell your parents about your friends?” and “Did you hide a lot from your parents about what you do during nights and weekends?”) on a 5-point scale ranging from “almost never” to “almost always”. α = .74 at T1 and .81 at T2. Parent–child conflict was assessed by 10 items (Ruiz et al., 1998, Telzer et al., 2014b; e.g., “You and your parents had a serious argument or fight.” and “You and your parents yelled or raised your voices at each other.”). Adolescents reported how true each item was for them in the past month on a 5-point scale ranging from “almost never” to “almost always”. α = .91 at T1 and .85 at T2. The mean of parent–child conflict was taken, with higher scores indicating greater parent–child conflict.
The scores of parental support, child disclosure, and parent–child conflict were correlated: greater parental support was associated with greater child disclosure (T1: r = .44, p < .05; T2: r = .51, p = .01) and lower family conflict (T1: r = −.50, p < .05; T2: r = −.59, p < .01). Child disclosure was not associated with parent–child conflict (T1: r = −.09, p = .68; T2: r = −.32, p = .14). To capture a comprehensive measure of family relationships, we created a composite score of positive parent–child relationships by taking the average of parental support, child disclosure, and reverse-scored parent–child conflict, with higher scores indicating more positive parent–child relationships. This composite score can provide us a clearer variable by creating a more cohesive measure using these 3 dimensions. Because all three questionnaires are 5-point scales, no further transformation was involved. To examine longitudinal changes in positive parent–child relationships, we computed a difference score representing T2 − T1. The average difference in the current sample was −.06, ranging from −1.13 (reflecting declines in positive parent–child relationships) to .85 (reflecting increases in positive parent–child relationships).
1.3. fMRI Task
To examine longitudinal changes in neural sensitivity to rewards during risk taking, adolescents underwent an fMRI scan while completing the Balloon Analog Risk Task (BART) at both T1 and T2. Behavioral performance on the BART is associated with a variety of actual risky behaviors such as adolescent smoking, addiction, and drug use (Aklin et al., 2005, Lejuez et al., 2003, Lejuez et al., 2007), suggesting that this task is an ecologically valid measure of real-life risk taking. Furthermore, the BART is widely used in neuroimaging studies to examine adolescents’ neural responses to risk taking (Chiu et al., 2012, Galván et al., 2013, Telzer et al., 2014a).
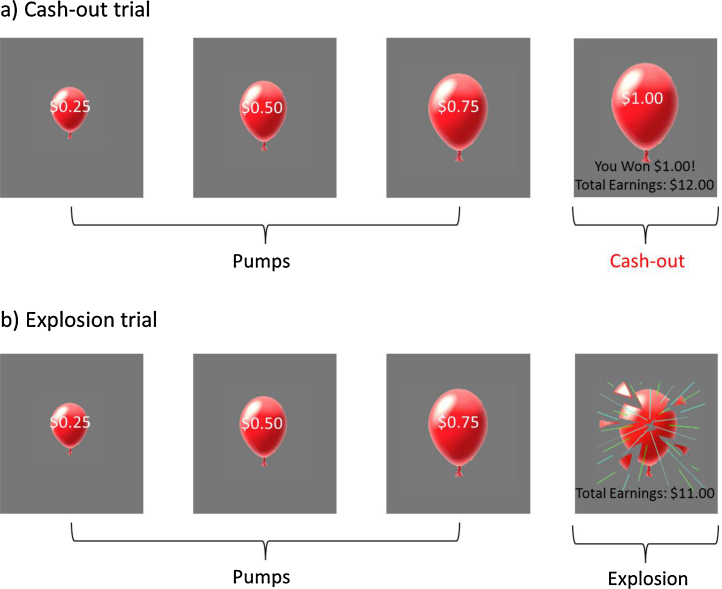
Participants completed the BART during one 9 min self-paced run. At the beginning of each trial, participants are presented with a virtual red-colored balloon. By pressing corresponding buttons, participants can choose either a risky option (i.e., pump the balloon), which results in bigger monetary rewards but a greater probability of getting no rewards (i.e., explosion of the balloon), or a safe option (cash out current rewards). For each successful pump without explosion, participants receive 25 cents. However, if the balloon explodes before cashing out, participants receive no payoff for that trial. The number of inflations before explosion is varied probabilistically according to a Poisson distribution, which models the unpredictable rewards and punishments of real-world risky behaviors. As number of pumps increases during a trial, explosion probability increases exponentially. The explosion point of each balloon was drawn from a uniform distribution from 1 to 12 pumps. After each pump, the balloon image disappeared for a jittered interval of 1–3 s before the outcome, either a larger balloon or an exploded one. There was an interstimulus interval of variable (jittered) length ranging from 1 to 12 s (M = 4 s) after the end of each balloon (i.e., after explosion or cash-out). The payoff for each trial is accumulated, and participants receive the total payoff at the end of the task. To assess risk-taking behavior on the BART, following previous studies (e.g., Lejuez et al., 2003), we examined the number of pumps before cash-outs, with a greater number of pumps before cash-outs indicating greater risk-taking behavior. In addition to red-colored balloons, participants were presented with white-colored balloons which were not associated with a reward or possible explosion. White balloons did not explode but inflated according to the same distribution as the red balloons and therefore were not associated with risk (Fig. 1).
Fig. 1.
Illustration of the Balloon Analog Risk Task. Examples of trials on the BART: (a) risk-taking trial with a cash-out outcome, and (b) risk-taking trial with an explosion outcome. Given that our key interest is neural reactivity to rewards, we focus on the cash-out events for analyses.
1.4. fMRI data acquisition
Imaging data were collected using a 3.0 Tesla Siemens Trio MRI scanner. The BART consisted of T2*-weighted echoplanar images (EPI) [slice thickness, 4 mm; 34 slices; TR = 2000 ms; TE = 30 ms; flip angle = 90°; matrix = 64 × 64; FOV = 200 mm; voxel size 3 mm × 3 mm × 4 mm]. A T2*weighted, matched-bandwidth (MBW), high-resolution, anatomical scan and magnetization-prepared rapid-acquisition gradient echo (MPRAGE) scan were acquired for registration purposes (TR: 2.3; TE: 2.1; FOV: 256; matrix: 192 × 192; sagittal plane; slice thickness: 1 mm; 160 slices). The orientation for the MBW and EPI scans was oblique axial to maximize brain coverage.
1.5. fMRI data preprocessing and analysis
Analyses were performed using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Preprocessing for each participant's images included spatial realignment to correct for head motion (no participant exceeded 2 mm of maximum image-to-image motion in any direction). For each participant, the realigned functional data at T1 and T2 were coregistered to the corresponding T1 and T2 high resolution MPRAGE, which was then segmented into cerebrospinal fluid, gray matter, and white matter. The normalization transformation matrix from the segmentation step was then applied to the functional and T2 structural images, thus transforming them into standard stereotactic space as defined by the Montreal Neurological Institute and the International Consortium for Brain Mapping. The normalized functional data were smoothed using an 8 mm Gaussian kernel, full-width-at-half maximum, to increase the signal-to-noise ratio.
Statistical analyses were performed using the general linear model in SPM8. Each trial was convolved with the canonical hemodynamic response function. High-pass temporal filtering with a cutoff of 128 s was applied to remove low-frequency drift in the time series. Serial autocorrelations were estimated with a restricted maximum likelihood algorithm with an autoregressive model order of 1. In the first level-model, the preprocessed data from T1 and T2 were concatenated, and multiple regressors were applied to separate different events: risk taking (i.e., pumps for red balloons), receipt of rewards (i.e., cash outs), receipt of negative outcome (i.e., explosions), and control balloons (i.e., pumps on white balloons) for T1 and T2. Following prior studies (Lejuez et al., 2002), for the risk-taking event, we used pumps on balloons that did not explode (i.e., pumps on each balloon prior to cash-out) as the index of risk-taking behavior, because pumps on the explosion trials were necessarily constrained.
Because our key interest is adolescents’ neural reactivity to rewards, we focus our analyses on cash-out trials (i.e., adolescents’ decision to keep monetary rewards). Following previous studies (e.g., Telzer et al., 2013a, Telzer et al., 2013b), cash outs were modeled with a parametric regressor to test the linear relationship between brain activation and the magnitude of reward. We used pump number as a parametric modulator, with each pump in a trial mean centered within the individual. On cash-out trials, this number represented how many pumps occurred before the cash out. Thus, brain activation reflects adolescents’ neural reactivity when they receive increasing monetary rewards. By parametrically modulating the level of pumps prior to cashing out, we were able to examine whether the ventral striatum shows increasing activation as the level of reward-value increases. Null events, consisting of the jittered intertrial intervals, were not explicitly modeled and therefore constituted an implicit baseline.
To examine longitudinal changes in adolescents’ neural reactivity when they receive increasing rewards, contrasts between T1 and T2 were computed at the individual level. The contrast that we focus on is longitudinal change in neural reactivity to rewards (i.e., T2 cash out minus T1 cash out). These individual contrast images were then used in all group-level analyses. To test how changes in positive parent–child relationships are associated with changes in neural reactivity, we conducted whole-brain regression analyses in which we examined how changes in positive parent–child relationships from T1 to T2 are related to the longitudinal difference in neural activity to rewards (i.e., T2 cash out minus T1 cash out). Thus, findings from these whole-brain regressions reflect the regions of the brain showing significant association between changes in positive parent–child relationships from T1 to T2 and changes in neural activity during T2 − T1.
To correct for multiple comparisons, we conducted a Monte Carlo simulation implemented using 3dClustSim in the software package AFNI (http://afni.nimh.nih.gov/afni/), which takes into account the size of the search space and the estimates smoothness of the data. Results of the simulation indicated a voxel-wise threshold of p < .005 combined with a minimum cluster size of 63 voxels. This joint voxelwise and cluster-size threshold corresponds to a false-positive discovery rate of 5% across the whole brain. Given the small structure of the ventral striatum, activity in this region is not expected to survive a volume correction of 63 voxels. Therefore, following previous studies (e.g., Giuliani and Pfeifer, 2015), to investigate task-related activity in the ventral striatum, we relaxed the cluster threshold to 20. We used the MarsBaR toolbox to extract parameter estimates for significant clusters in the group-level analyses. Parameter estimates of signal intensity were extracted from the entire cluster of activation. For visualization, statistical maps of all analyses were projected onto a T2 template. In order to take into account potential differences driven by gender, adolescents’ gender were controlled for in all behavioral and fMRI analyses. However, behavioral and neuroimaging analyses without controlling for gender yield identical results.
2. Results
2.1. Positive parent–child relationships and adolescent risk taking at T1 and T2
We first investigated adolescent risk taking at each time point. To this end, we examined behavioral performance on the BART, such that greater average pumps indicate greater risk taking. The mean and standard deviation for positive parent–child relationships and adolescent risk taking are presented in Table 1. We examined mean difference and individual variability in positive parent–child relationships and adolescent risk taking from T1 to T2 using the repeated t test and intraclass correlation coefficient (ICC).
Table 1.
Mean and standard deviation for variables at T1 and T2.
| T1 mean (SD) | T2 mean (SD) | Difference between T1 and T2 | ICC | |
|---|---|---|---|---|
| Positive parent–child relationships | 3.25 (.71) | 3.24 (.73) | t = .14, p = .89 | .62 |
| Mean pumps on BART | 3.44 (.97) | 3.15 (.90) | t = 1.76, p = .09 | .64 |
2.2. Increases in positive parent–child relationships relate to declines in adolescent risk taking
We examined the associations between parent–child relationships and risk taking at each time point. Greater positive parent–child relationships at T1 was marginally related to less adolescent risk taking on the BART at T1, r = −.38, p = .07, but the association between positive parent–child relationships and adolescent risk taking was not significant at T2, r = −.10, p > .05.
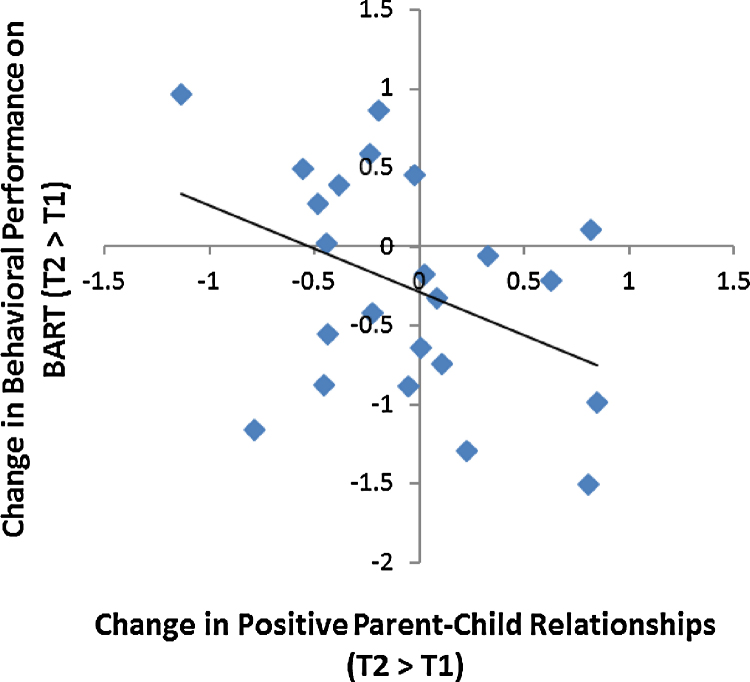
Next, we investigated whether changes in positive parent–child relationships are associated with changes in adolescent risk-taking behavior over time. To this end, we computed a difference score for behavioral risk taking (average pumps at T2 − T1). In line with our hypothesis, greater increases in positive parent–child relationships from T1 to T2 were associated with greater decreases in risk-taking behavior over time as demonstrated by less risky behavior on the BART (i.e., decreases in the average number of pumps across time; r = −.45, p < .05; Fig. 2). We also examined whether this association was influenced by the starting values of adolescents’ risk taking and parent–child relationships. We ran partial correlations controlling for T1 parent–child relationships and T1 risk taking. The association between changes in parent–child relationships and changes in risk taking remained significant (r = −.44, p < .05), suggesting that regardless of their starting points, greater increases in positive parent–child relationships were associated with greater declines in adolescent risk taking.
Fig. 2.
Adolescents who reported greater increases in positive parent–child relationships showed decreased risk-taking behavior on the BART over time.
2.3. Changes in parent–child relationships and changes in neural reactivity
For our fMRI analyses, we first tested the association between parent–child relationships and adolescents’ neural reactivity to rewards at each time point. To this end, we ran whole-brain regression analyses in which parent–child relationships at T1 (or T2) were regressed on the contrast of cash-outs at T1 (or T2). At both T1 and T2, parent–child relationships were not associated with activation in any brain regions.
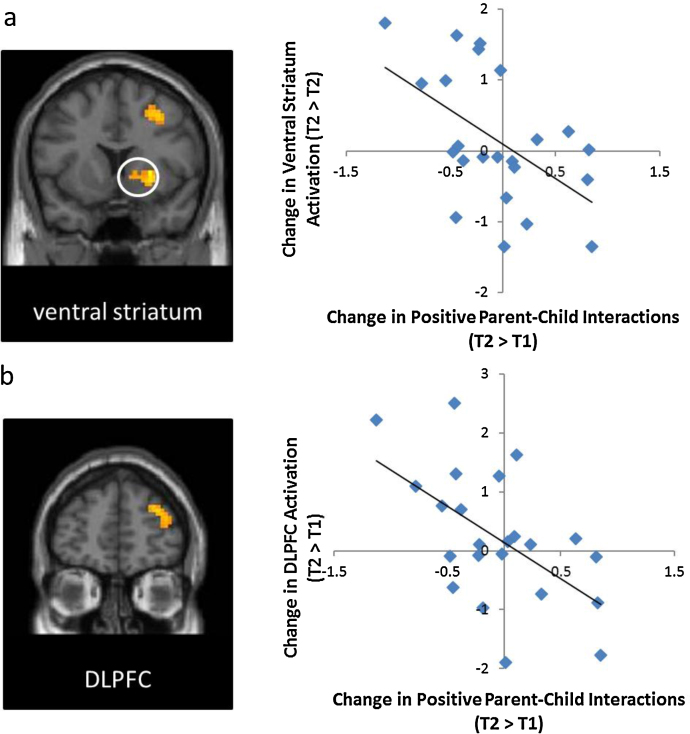
Next, we examined the association between changes in parent–child relationships from T1 to T2 and changes in adolescents’ neural reactivity to rewards. To this end, we computed whole brain regression analyses with changes in parent–child relationships (T2 − T1) regressed onto changes in neural activation (T2 − T1). As shown in Fig. 3a, greater increases in positive parent–child relationships were related to greater longitudinal decreases in ventral striatum activation to rewards over time, suggesting that more positive parent–child relationships are associated with greater decreases in sensitivity to rewards during risk taking. In addition to the ventral striatum, adolescents who reported increases in positive parent–child relationships from T1 to T2 showed longitudinal decreases in the DLPFC to rewards during risk taking (Fig. 3b; Table 2).
Fig. 3.
Adolescents who reported greater increases in positive parent–child relationships showed greater decreases in (a) the ventral striatum and (b) the DLPFC activation to rewards over time.
Table 2.
Correlation between longitudinal change in positive parent–child relationships and longitudinal change in brain activity when receiving rewards.
| Anatomical Region | BA | x | y | z | t | k |
|---|---|---|---|---|---|---|
| Right DLPFC | 10/46 | 30 | 56 | 25 | 3.80 | 117 |
| Right VS | 12 | 11 | −2 | 3.10 | 40 | |
| Right cuneus | 18 | −73 | 19 | 3.31 | 100 |
Note: BA refers to putative Broadman's areas. x, y, and z refer to MNI coordinates; t refers to the t-score at those coordinates (local maxima); k refers to the number of voxels in each significant cluster. DLPFC = dorsolateral prefrontal cortex. VS = ventral striatum.
Because participants had a different number of cash-out trials available at T1 and T2, we controlled for the number of cash out trials available at T1 and T2. With these covariates in the model, increases in positive parent–child relationships continue to predict declines in risk-taking behavior over time (r = −.45, p < .05) and decreases in ventral striatum activation to rewards (r = −.56, p < .01), suggesting that our findings are not driven by differences in the number of cashed out trials at T1 and T2.
We next investigated the stability of neural changes over time. To this end, we extracted parameter estimates of signal intensity from the VS and DLPFC cluster that showed significant changes as a function of changes in parent–child relationships at each time point (T1 and T2). We examined individual variability in changes in the VS and DLPFC from T1 to T2 using the intraclass correlation coefficient (ICC). Consistent with prior research examining neural stability in prefrontal and subcortical regions during adolescence (Van den Bulk et al., 2013), both VS and DLPFC showed poor reliability: T1 and T2 VS were not correlated (r = .06, p = .78) and showed low reliability (ICC = .12), T1 and T2 DLPFC were not correlated (r = .21, p = .33) and showed low reliability (ICC = .34). This suggests substantial variability in change in neural activation over time among participants.
In addition to examining the cash-out trials, we conducted the same analyses with the decision period (i.e., pumps). To this end, we ran the whole brain regression analyses with changes in parent–child relationships (T2 − T1) regressed onto changes in neural activation during decision period (T2 pumps − T1 pumps). Changes in parent–child relationships were not associated with changes in the ventral striatum and PFC activation during the decision period, but were associated positively with activation in the corpus callosum and occipital lobe.
2.4. Change in neural reactivity and change in risk taking
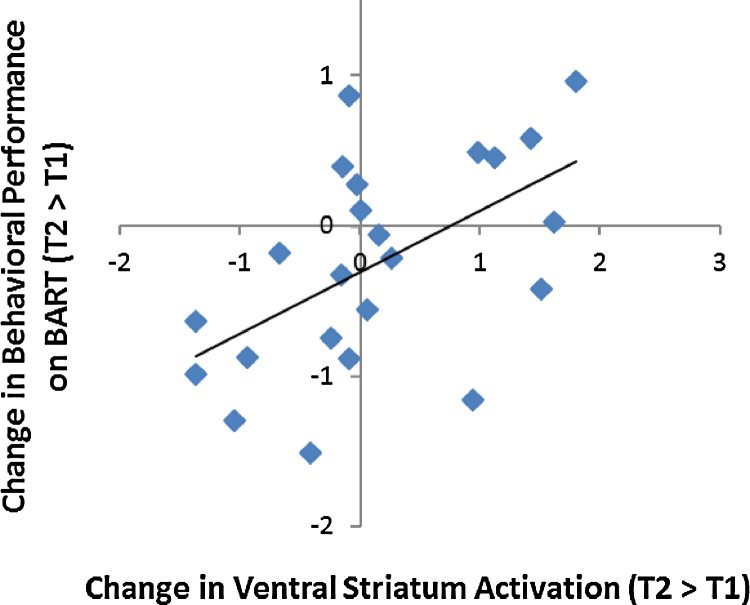
Next, we examined how changes in neural reactivity were related to changes in adolescents’ risk-taking behavior on the BART. We extracted parameter estimates of signal intensity from the VS cluster that showed significant changes as a function of changes in parent–child relationships and ran partial correlation analyses (controlling for adolescent gender) with this functional ROI in SPSS. Consistent with our hypothesis, decreases in the ventral striatum reactivity to rewards were associated with declines in risk-taking behavior on the BART (r = .56, p < .01; Fig. 4). To eliminate the possibility that this association was driven by adolescents’ initial level of risk taking, we further controlled for their risk-taking behavior at T1. The association between changes in ventral striatum activation and changes in risk taking remained significant (r = .54, p = .01).
Fig. 4.
Adolescents who showed greater longitudinal decrease in the ventral striatum were engaged less risky behavior on the BART over time.
2.5. Change in neural reactivity explains the link between parent–child relationships and adolescent risk taking
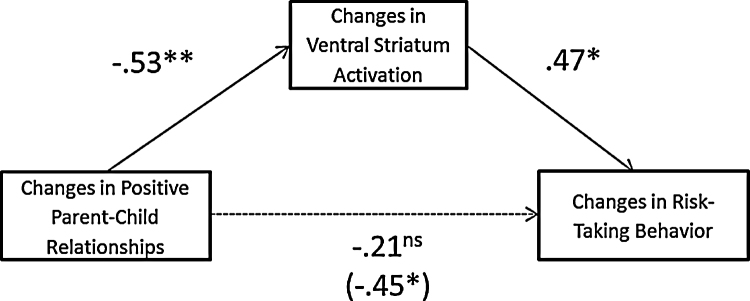
Finally, we tested if longitudinal changes in neural reactivity mediate the link between changes in positive parent–child relationships and changes in adolescent risk taking. To this end, we ran mediation analyses using bias-corrected bootstrapping resampling techniques (Preacher and Hayes, 2008). The independent variable was changes in positive parent–child relationships from T1 to T2, the dependent variable was changes in adolescent risk taking, and the mediator was longitudinal changes in the ventral striatum reactivity to rewards during risk taking. Based on 1000 bootstrap resamples, the indirect path from increases in positive parent–child relationships to decreases in ventral striatum activation to rewards over time to declines in adolescent risk-taking behavior was significant (Fig. 5), indirect effect = −.24, 95% CI: [−.58, −.06]. The link between changes in parent–child relationships and changes in adolescent risk taking was no longer significant after taking into account changes in adolescent ventral striatum activation, with a 53% reduction in the total effect. Thus, greater increases in positive parent–child relationships contribute to greater declines in adolescent risk-taking behavior through decreases in ventral striatum activation to rewards over time.
Fig. 5.
Changes in ventral striatum activation mediated the link between changes in positive parent–child relationships and changes in risk-taking behavior. *p < .05; **p < .01.
3. Discussion
Adolescence is a developmental period characterized by substantial increases in health-compromising risk-taking behaviors. Such increases in negative behaviors call for efforts to identify environmental factors that can prevent adolescents from heightened risk-taking behavior. As a key aspect of adolescents’ social relationships, parent–child relationships show substantial changes during the teen years and remain important in shaping adolescents’ psychological functioning (De Goede et al., 2009; Lam et al., 2012). Using a longitudinal neuroimaging approach, the current study identified the neural correlates through which positive parent–child relationships reduce adolescent risk taking. Increases in positive parent–child relationships contributed to declines in adolescent risk taking, which was mediated by longitudinal decreases in the ventral striatum to rewards during risk taking.
Previous studies have suggested the important role of positive parent–child relationships in reducing adolescent risk taking. For example, greater parental support and child disclosure are related to lower problem behavior and delinquency (Jessor et al., 2003, Stattin and Kerr, 2000). Similarly, lower parent–child conflict is associated with less externalizing symptoms, conduct problems, and antisocial behavior (Burt et al., 2006, Klahr et al., 2011a, Klahr et al., 2011b). In line with these studies, adolescents who reported increases in their positive parent–child relationships in the present study showed longitudinal decreases in their risk taking as indicated by less risky behavior on the BART over time. This finding contributes to the rich literature revealing that positive parent–child relationship quality benefits adolescents’ psychological adjustment. Importantly, during a time when family relationships tend to show declines in cohesion (Tsai et al., 2013), and adolescents tend to show increases in risk-taking behavior (Steinberg, 2004), improvements in the quality of family relationships may serve an important buffering role. These findings can contribute to interventions designed at decreasing adolescent risk taking. By improving adolescents’ perceptions of their family relationships, youth may find risk taking to be comparatively less rewarding and subsequently engage in less problem behavior.
A lot of attention has been paid to how early parent–child relationships affect adolescents’ neural reactivity. For example, previous research suggests that early maternal deprivation and severe parental neglect or abuse in early postnatal development is associated with altered ventral striatum activity during adolescence (Goff et al., 2013, Hart and Rubia, 2012, Mehta et al., 2010). However, we know little about how relatively normative changes in parent–child relationships during later development affect adolescents’ neural reactivity. This is an important limitation given that adolescence is a time of change in interpersonal relationships, neural development, and behavioral functioning. Although prior studies suggest that the presence of parents or peers modify adolescents’ reward-related reactivity during risk-taking settings (Chein et al., 2011, Telzer et al., in press), few studies have examined how the quality of adolescents’ interpersonal relationships influence such processes (for the effect of peer support, see Telzer et al., 2015). More importantly, no prior research has examined how adolescents’ social environment influences their neural development by using a longitudinal fMRI approach. To address this gap, the present study focuses on individual differences in parent–child relationship quality and how changes in relationship quality are associated with changes in adolescents’ neural reactivity to rewards during risk taking.
In the present study, the link between changes in positive parent–child relationships and trajectories of adolescent risk taking was mediated by changes in adolescents’ neural sensitivity to rewards during risk taking. Specifically, adolescents who reported increases in their positive parent–child relationships showed longitudinal decreases in ventral striatum activation over time, a neural region that codes for reward value (Delgado et al., 2000, Knutson et al., 2000). Moreover, decreases in ventral striatum sensitivity were associated with declines in risk-taking behavior. In contrast, adolescents who reported decreases in their positive parent–child relationships (as evidenced by the left panel of the scatterplot in Fig. 3a) showed longitudinal increases in ventral striatum activation over time, which were associated with increases in their risk-taking behavior. These findings suggest that increased negative family relationships may sensitize adolescents toward experiencing risk taking as subjectively more rewarding, resulting in greater engagement in risky decisions making. These effects are in line with prior studies showing that ventral striatum activation to reward anticipation and receipt is related to greater risk taking (e.g., Galván et al., 2007). Although we interpret ventral striatum activity to cash-outs as representing neural reactivity to reward value, it is also possible that this event represents risk avoidance given that participants decided not to engage in more risk taking. In this sense, this event may model the processing of threat of loss. However, we found that greater increases in ventral striatum activation during cash-outs were associated with greater increases in risk taking, suggesting that this event is sensitive to greater risk behavior rather than risk avoidance. Although ventral striatum is also involved in the processing of punishment (Delgado, 2007), our finding is consistent with prior studies showing that greater ventral striatum activation to reward anticipation and receipt is related to greater risk taking (e.g., Galván et al., 2007).
Our findings suggest that adolescents’ experience of positive parent–child relationships plays a key role in modulating their neural processing to rewards. Decreased positive parent–child relationships may expose adolescents to a less supportive environment in which adolescents receive less positive feedback from their parents. In order to compensate for the lack of social rewards at home, adolescents may try to seek out more rewards outside the family and therefore feel more rewarded when they engage in risk taking. On the other hand, increases in positive parent–child relationships provide adolescents with a supportive environment, which may dampen adolescents’ subjective sensitivity to rewards during risk taking. Adolescents in high quality homes may find risk taking to be comparatively less rewarding and therefore show declines in ventral striatum activation over time. In addition to capturing support and low conflict, our measure included adolescent's voluntary disclosure to their parents. Given that adolescents spend increasingly less time with their parents (Larson et al., 1996), adolescents’ more voluntary disclosure of their activities may provide opportunities for parents to give them advice and supervision, helping them minimize the rewarding nature of risk taking. Therefore, changes in parent–child relationships may affect adolescent risk taking via altering the reward system over time.
During the teen years, children experience a variety of changes in their brain development, behavior, and social relationships (Casey et al., 2008, Collins and Steinberg, 2006, Nelson et al., 2005). Risk taking and neural activity to rewards do not necessarily undergo a unified developmental trajectory. As we show in the present study, there are important individual differences in trajectories of adolescent risk-taking behavior and ventral striatum activity. Some adolescents show increases in their risk taking and ventral striatum activity over time, while others show decreases. Individual differences in such changes provide a window to understand different developmental pathways. Although cross-sectional studies provide rich insights into the concurrent links between adolescent brain, behavior, and their environment, such designs are unable to capture the dynamic nature of adolescence. Therefore, it is important to examine longitudinal changes in these processes in order to capture how relationships and neural development change and covary together. The current study used a longitudinal approach to measure changes in parent–child relationships, risk taking, and neural processing to rewards. By focusing on individual differences in developmental trajectories, the present study provides novel evidence into the neural correlates by which changes in parent–child relationships are associated with changes risk taking.
Parent–child relationships are only one of many environmental and contextual variables that may influence adolescent risk taking. Other factors, such as affiliation with antisocial peers and engagement in drugs may also play a role in affecting trajectories of adolescent risk taking over time (Monahan et al., 2009, Tapert et al., 2001). It is also possible that greater decreases in positive parent–child relationships may incur other negative consequences, such as affiliation with antisocial peers and engagement in drugs, which all contribute to greater adolescent risk taking (Brook et al., 1990, Kim et al., 1999). Guided by previous longitudinal studies, we hypothesized that changes in parent–child relationships contribute to changes in adolescent risk taking. However, it is also possible that an increase in adolescent risk-taking behavior results in impairments in parent–child relationships. Some other variables, such as adolescents’ maturational and pubertal changes, may also influence family relationships and they need to be taken into account in future research. In addition, although we used a comprehensive variable by combining three important aspects of parent–child relationships, future studies should also capture other aspects of parent–child relationships, such as attachment security and parental warmth, and examine how these factors play a role in adolescents’ neural development and risk-taking behavior. Moreover, our assessment of parent–child relationships is based on child report, which reflects children's perceptions of parent–child relationships. It is possible that changes in children's perceptions, but not changes in objective parent–child relationships, influence adolescents’ neural reactivity to rewards and their risk taking behavior. Parent report on parent–child relationships are needed in future studies to provide a more objective and nuanced assessment of the quality of youths’ family relationships. Finally, given that our participants were recruited from a low-SES community, they may have psychopathology despite not reporting it. Caution should be taken when interpreting the findings of the present study given the small sample size.
Despite these limitations, our findings have important practical implications for intervention, highlighting the important role of positive parent–child relationships in affecting adolescents’ neural sensitivity to rewards and subsequent risk-taking behavior. During a time when adolescents’ perceptions of the quality of their family relationships tend to show declines (Tsai et al., 2013), clinicians, teachers, and families should focus on ways to provide adolescents with a more supportive and less hostile family environment in which adolescents are willing to disclose to their parents. By focusing on ways of promoting positive parent–child relationships, the substantial increase in adolescent risk taking may be minimized. Such an approach can help families experience high quality relationships and provide positive feedback to adolescents, which may protect them from seeking external rewards by engaging in risk-taking behavior.
In conclusion, the current study provides novel evidence on the important role of positive parent–child relationships on adolescents risk taking. Longitudinal increases in positive parent–child relationships over the teen years were associated with dampened ventral striatum sensitivity during risk taking, which contributed to their reduced risk-taking behavior over time. This finding highlights the key role of neural reactivity to rewards in the process through which parent–child relationships contributes to adolescent risk taking.
Conflict of interest statement
The authors declare no potential financial and personal conflict of interest.
Acknowledgments
Support for this study was provided by the NICHD (R01HD057164-S and R01HD057164, Fuligni), the NSF (SES 1023293, Fuligni and Telzer; BCS 1539651, Telzer), and NIDA (R01DA039923, Telzer).
Footnotes
This sample is a subsample of a larger study of 48 adolescents who completed a scan at T1. Prior data from the full sample at T1 with the BART data have been published (e.g., Telzer et al., 2013a, Telzer et al., 2013b, Telzer et al., 2015). Based on factor analyses, the measures used in the present study are distinct from measures in prior reports. Published work from this longitudinal data using the BART task and the same sample only focuses on the main effect of longitudinal changes in neural reactivity during risk taking (Qu et al., in press).
References
- Aklin W.M., Lejuez C.W., Zvolensky M.J., Kahler C.W., Gwadz M. Evaluation of behavioral measures of risk taking propensity with inner city adolescents. Behav. Res. Ther. 2005;43:215–228. doi: 10.1016/j.brat.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Armsden G.C., Greenberg M.T. The inventory of parent and peer attachment: individual differences and their relationship to psychological well-being in adolescence. J. Youth Adolesc. 1987;16:427–454. doi: 10.1007/BF02202939. [DOI] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: a developmental perspective. Dev. Rev. 1992;12:339–373. [Google Scholar]
- Blakemore S., Mills K.L. Is adolescence a sensitive period for sociocultural processing? Annu. Rev. Psychol. 2014;65:187–207. doi: 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- Brook J.S., Brook D.W., Gordon A.S., Whiteman M., Cohen P. The psychosocial etiology of adolescent drug use: a family interactional approach. Genet. Soc. Gen. Psychol. Monogr. 1990;116:111–267. [PubMed] [Google Scholar]
- Burt S.A., McGue M., Iacono W.G., Krueger R.F. Differential parent–child relationships and adolescent externalizing symptoms: cross-lagged analyses within a monozygotic twin differences design. Dev. Psychol. 2006;42:1289–1298. doi: 10.1037/0012-1649.42.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Getz S., Galvan A. The adolescent brain. Dev. Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J., Albert D., O’Brien L., Uckert K., Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Dev. Sci. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C.-.P., Tlustos S.J., Walz N.C., Holland S.K., Eliassen J.C., Bernard L., Wade S.L. Neural correlates of risky decision making in adolescents with and without traumatic brain injury using the balloon analog risk task. Dev. Neuropsychol. 2012;37:176–183. doi: 10.1080/87565641.2011.632796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins W.A., Steinberg L. Adolescent development in interpersonal context. In: Eisenberg N., editor. Handbook of Child Psychology. 6th ed. vol. 3. Wiley; Hoboken, NJ: 2006. pp. 1003–1067. (Social, Emotional, and Personality Development). [Google Scholar]
- De Goede I.H.A., Branje S.J.T., Meeus W.H.J. Developmental changes in adolescents' perceptions of relationships with their parents. J. Youth Adolesc. 2009;38(1):75–88. doi: 10.1007/s10964-008-9286-7. [DOI] [PubMed] [Google Scholar]
- Delgado M., Nystrom L., Fissell C., Noll D., Fiez J. Tracking the hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Delgado M. Reward-related responses in the human striatum. Ann. N. Y. Acad. Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Galván A., Hare T., Voss H., Glover G., Casey B.J. Risk-taking and the adolescent brain: Who is at risk? Dev. Sci. 2007;10(2):F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Galván A., Schonberg T., Mumford J., Kohno M., Poldrack R.A., London E.D. Greater risk sensitivity of dorsolateral prefrontal cortex in young smokers than in nonsmokers. Psychopharmacology. 2013;229:345–355. doi: 10.1007/s00213-013-3113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani N.R., Pfeifer J.H. Age-related changes in reappraisal of appetitive cravings during adolescence. NeuroImage. 2015;108:173–181. doi: 10.1016/j.neuroimage.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B., Gee D.G., Telzer E.H., Humphreys K.L., Gabard-Durnam L., Flannery J., Tottenham N. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–138. doi: 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H., Rubia K. Neuroimaging of child abuse: a critical review. Front. Hum. Neurosci. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessor R., Turbin M.S., Costa F.M., Dong Q., Zhang H., Wang C. Adolescent problem behavior in China and the United States: a cross-national study of psychosocial protective factors. J. Res. Adolesc. 2003;13:329–360. [Google Scholar]
- Karriker-Jaffe K.J., Foshee V.A., Ennett S.T., Suchindran C. The development of aggression during adolescence: sex differences in trajectories of physical and social aggression among youth in rural areas. J. Abnorm. Child Psychol. 2008;36:1227–1236. doi: 10.1007/s10802-008-9245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijsers L., Poulin F. Developmental changes in parent–child communication throughout adolescence. Dev. Psychol. 2013;49:2301–2308. doi: 10.1037/a0032217. [DOI] [PubMed] [Google Scholar]
- Kim J.E., Hetherington E.M., Reiss D. Associations among family relationships, antisocial peers, and adolescents’ externalizing behaviors: gender and family type differences. Child Dev. 1999;70:1209–1230. doi: 10.1111/1467-8624.00088. [DOI] [PubMed] [Google Scholar]
- Klahr A.M., McGue M., Iacono W.G., Burt S.A. The association between parent–child conflict and adolescent conduct problems over time: results from a longitudinal adoption study. J. Abnorm. Psychol. 2011;120:46–56. doi: 10.1037/a0021350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahr A.M., Rueter M.A., McGue M., Iacono W.G., Burt S.A. The relationship between parent–child conflict and adolescent antisocial behavior: confirming shared environmental mediation. J. Abnorm. Child Psychol. 2011;39:683–694. doi: 10.1007/s10802-011-9505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Westdorp A., Kaiser E., Hommer D. fMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Lam C.B., McHale S.M., Crouter A.C. Parent–child shared time from middle childhood to late adolescence: Developmental course and adjustment correlates. Child Dev. 2012;83(6):2089–2103. doi: 10.1111/j.1467-8624.2012.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson R.W., Richards M.H., Moneta G., Holmbeck G., Duckett E. Changes in adolescents’ daily interactions with their families from ages 10 to 18: disengagement and transformation. Dev. Psychol. 1996;32(4):744–754. [Google Scholar]
- Laursen B., Coy K.C., Collins W.A. Reconsidering changes in parent–child conflict across adolescence: a meta-analysis. Child Dev. 1998;69:817–832. [PMC free article] [PubMed] [Google Scholar]
- Lejuez C.W., Aklin W.M., Zvolensky M.J., Pedulla C.M. Evaluation of the balloon analogue risk task (BART) as a predictor of adolescent real-world risk-taking behaviours. J. Adolesc. 2003;26:475–479. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Lejuez C.W., Aklin W., Daughters S., Zvolensky M., Kahler C., Gwadz M. Reliability and validity of the youth version of the balloon analogue risk task (BART-Y) in the assessment of risk-taking behavior among inner-city adolescents. J. Clin. Child Adolesc. Psychol. 2007;36:106–111. doi: 10.1080/15374410709336573. [DOI] [PubMed] [Google Scholar]
- Lejuez C.W., Read J.P., Kahler C.W., Richards J.B., Ramsey S.E., Stuart G.L., Brown R.A. Evaluation of a behavioral measure of risk taking: the balloon analogue risk task (BART) J. Exp. Psychol.: Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Loeber R., Drinkwater M., Yin Y., Anderson S.J., Schmidt L.C., Crawford A. Stability of family interaction from ages 6 to 18. J. Abnorm. Child Psychol. 2000;28:353–369. doi: 10.1023/a:1005169026208. [DOI] [PubMed] [Google Scholar]
- McGue M., Elkins I., Walden B., Iacono W.G. Perceptions of the parent–adolescent relationship: a longitudinal investigation. Dev. Psychol. 2005;41:971–984. doi: 10.1037/0012-1649.41.6.971. [DOI] [PubMed] [Google Scholar]
- Mehta M., Gore-Langton E., Golembo N., Colvert E., Williams S., Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. J. Cogn. Neurosci. 2010;22:2316–2325. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- Monahan K.C., Steinberg L., Cauffman E. Affiliation with antisocial peers, susceptibility to peer influence, and antisocial behavior during the transition to adulthood. Dev. Psychol. 2009;45:1520–1530. doi: 10.1037/a0017417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Vital Statistics Reports . 2011, October. Death Rates Per 100,000 Population in United States, 2009. vol. 60, No. 3 and vol. 61, No. 7. [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E., Pine D.S. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Preacher K.J., Hayes A.F. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Qu Y., Galván A., Fuligni A.J., Lieberman M.D., Telzer E.H. Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk taking. J. Neurosci. 2015 doi: 10.1523/JNEUROSCI.1553-15.2015. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz S.Y., Gonzales N.A., Formoso D. Multicultural, multidimensional assessment of parent–adolescent conflict. Poster session presented at the Seventh Biennial Meeting of the Society for Research on Adolescence; San Diego, CA; 1998. [Google Scholar]
- Smetana J.G., Campione-Barr N., Metzger A. Adolescent development in interpersonal and societal contexts. Annu. Rev. Psychol. 2006;57:255–284. doi: 10.1146/annurev.psych.57.102904.190124. [DOI] [PubMed] [Google Scholar]
- Stattin H., Kerr M. Parental monitoring: a reinterpretation. Child Dev. 2000;71:1072–1085. doi: 10.1111/1467-8624.00210. [DOI] [PubMed] [Google Scholar]
- Steinberg L. We know some things: parent–adolescent relationships in retrospect and prospect. J. Res. Adolesc. 2001;11:1–19. [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Ann. N. Y. Acad. Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Dev. Psychobiol. 2010;52(3):216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Tapert S.F., Aarons G.A., Sedlar G.R., Brown S.A. Adolescent substance use and sexual risk-taking behavior. J. Adolesc. Health. 2001;28(3):181–189. doi: 10.1016/s1054-139x(00)00169-5. [DOI] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Gálvan A. Meaningful family relationships: neurocognitive buffers of adolescent risk taking. J. Cogn. Neurosci. 2013;25:374–387. doi: 10.1162/jocn_a_00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Gálvan A. The effects of poor quality sleep on brain function during risk taking in adolescence. NeuroImage. 2013;71:275–283. doi: 10.1016/j.neuroimage.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Galván A. Neural sensitivity to eudaimonic and hedonic rewards differentially predict adolescent depressive symptoms over time. Proc. Natl. Acad. Sci. U. S. A. 2014;111:6600–6605. doi: 10.1073/pnas.1323014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Miernicki M., Gálvan A. The quality of adolescents’ peer relationships modulates neural sensitivity to risk taking. Soc. Cogn. Affect. Neurosci. 2015;10:389–398. doi: 10.1093/scan/nsu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Gonzales N., Fuligni A.J. Family obligation values and family assistance behaviors: protective and risk factors for adolescent substance use. J. Youth Adolesc. 2014;43(2):270–283. doi: 10.1007/s10964-013-9941-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Ichien N.I., Qu Y. Mothers know best: redirecting adolescent reward sensitivity to promote safe behavior during risk taking. Soc. Cogn. Affect. Neurosci. 2015 doi: 10.1093/scan/nsv026. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai K.M., Telzer E.H., Fuligni A.J. Continuity and discontinuity in perceptions of family relationships from adolescence to young adulthood. Child Dev. 2013;84:471–484. doi: 10.1111/j.1467-8624.2012.01858.x. [DOI] [PubMed] [Google Scholar]
- Van den Bulk B.G., Koolschijn P.C., Meens P.H.F., van Lang N.D., van der Wee N.J., Rombouts S.A.R.B., Crone E.A. How stable is activation in the amygdala and prefrontal cortex in adolescence? A study of emotional face processing across three measurements. Dev. Cogn. Neurosci. 2013;4:65–76. doi: 10.1016/j.dcn.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray-Lake L., Crouter A.C., McHale S.M. Developmental patterns in decision-making autonomy across middle childhood and adolescence: European American parents perspectives. Child Dev. 2010;81(2):636–651. doi: 10.1111/j.1467-8624.2009.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]