Abstract
The epithelial to mesenchymal transition (EMT) is an essential process that occurs repeatedly during embryogenesis whereby stably adherent cells convert to an actively migrating state. While much is known about the factors and events that initiate the EMT, the steps that cells undergo to become directionally migratory are far less well understood. Zebrafish embryos lacking the transcription factors Tbx16/Spadetail and Mesogenin1 (Msgn1) are a valuable system for investigating the EMT. Mesodermal cells in these embryos are unable to perform the EMT necessary to leave the most posterior end of the body (the tailbud) and join the pre-somitic mesoderm, a process that is conserved in all vertebrates. It has previously been very difficult to study this EMT in vertebrates because of the multiple cell types in the tailbud and the morphogenetic changes the whole embryo undergoes. Here, we describe a novel tissue explant system for imaging the mesodermal cell EMT in vivo that allows us to investigate the requirements for cells to acquire migratory properties during the EMT with high spatio-temporal resolution. This method revealed that, despite the inability of tbx16;msgn1-deficient cells to leave the tailbud, actin-based protrusions form surprisingly normally in these cells and they become highly motile. However, tbx16;msgn1-deficient cells have specific cell-autonomous defects in the persistence and anterior direction of migration because the lamellipodia they form are not productive in driving anteriorward migration. Additionally, we show that mesoderm morphogenesis and differentiation are separable and that there is a migratory cue that directs mesodermal cell migration that is independent of Tbx16 and Msgn1. This work defines changes that cells undergo as they complete the EMT and provides new insight into the mechanisms required in vivo for cells to become mesenchymal.
Keywords: EMT, Tbx16, mesoderm, morphogenesis, zebrafish
Graphical Abstract
Introduction
Cells transition between adherent epithelial states and migratory mesenchymal states to shape tissues during embryogenesis and wound healing. These processes, called the epithelial to mesenchymal transition (EMT) and the mesenchymal to epithelial transition (MET), occur reiteratively throughout development in order for cells to reach a target tissue and then to contribute to that tissue (Lim and Thiery, 2012; Reig et al., 2014). EMT and MET can also be coopted by cancer cells to metastasize and form secondary tumors at distant sites (Micalizzi et al., 2010; Nieto, 2013). For a normal or transformed cell to successfully complete the EMT, it must coordinate diverse subcellular processes in space and time, which include altering the types and locations of adhesions, changing apico-basal epithelial polarity to front-back migratory polarity, and modifying cytoskeletal organization (Bear and Haugh, 2014; Saunders and McClay, 2014).
Most of our understanding of the molecules and signaling networks that coordinate the EMT concerns the initiation stages when a cell detaches from its epithelial neighbors (Craene and Berx, 2013; Lamouille et al., 2014; Saunders and McClay, 2014). Just as important is how a cell acquires appropriate migratory abilities during the later stages of the process. Relatively little is known about these stages. Moreover, much of what we know about the mechanisms that drive the EMT is based on work in various cell culture models where cells are observed in artificial environments. In most cases it is very difficult to closely observe the EMT in vivo, particularly during the late stages of the process.
We utilized zebrafish mesodermal progenitor cells to investigate these late stages of the EMT in vivo. Bipotential neuro-mesodermal progenitor cells reside in a pseudo-epithelium at the dorsal posterior end of the embryo (the tailbud) during somitogenesis (Kanki and Ho, 1997; Kimelman and Martin, 2012; Martin and Kimelman, 2012). As cells make the fate choice to become mesoderm, they undergo a developmentally programmed EMT and move ventrally and anteriorly into the maturation zone (MZ) where they become highly migratory (Griffin and Kimelman, 2002; Kanki and Ho, 1997; Lawton et al., 2013). The overall flow of maturing mesodermal cells continues anteriorly as cells progress through the pre-somitic mesoderm, where cell motility gradually declines (Dray et al., 2013; Lawton et al., 2013). Finally, cells re-epithelialize, undergoing an MET to form somites.
We have taken advantage of a unique zebrafish mutant that prevents cells from moving past the MZ in this developmental progression. The transcription factors T-box16/Spadetail (Tbx16) and Mesogenin1 (Msgn1) are together required for both the differentiation and morphogenesis of mesoderm in zebrafish (Fior et al., 2012; Yabe and Takada, 2012). In tbx16 mutants, cells that should contribute to the trunk somites pile up in the tailbud, forming a ball of undifferentiated cells (Amacher et al., 2002; Griffin et al., 1998; Griffin and Kimelman, 2002; Ho and Kane, 1990; Kimmel et al., 1989). While there is a partial recovery of somite formation in the tail of tbx16 single mutants, tbx16;msgn1 double mutants show a complete lack of trunk and tail somite formation and a correspondingly larger mass of undifferentiated cells in the tailbud (Fior et al., 2012; Yabe and Takada, 2012). In contrast, msgn1 single mutants show almost no phenotype (Fior et al., 2012). The orthologues of tbx16 and msgn1 play similar roles in mesoderm development in mouse and other vertebrates, demonstrating the conservation of this process (Chalamalasetty et al., 2014; Chapman et al., 2003; Liu et al., 2004; Nowotschin et al., 2012; Tazumi et al., 2008; Yoon and Wold, 2000). Of particular note, the mouse tbx6;msgn1 mutant strongly resembles the zebrafish tbx16;msgn1 mutant, with a large mass of undifferentiated cells at the posterior end of the embryo (Fior et al., 2012; Nowotschin et al., 2012).
Little is known about the specific roles of tbx16 and msgn1 in mesodermal cell movement. The defect is cell autonomous, such that individual cells lacking tbx16 remain posterior even in a wild-type environment (Ho and Kane, 1990; Row et al., 2011). A previous study examined the protrusive activity of tbx16-deficient mesodermal precursor cells during gastrulation and observed that these cells entered a highly blebbing intermediate state as they involuted to become mesoderm, but that they never downregulated the blebbing as wild-type cells do, and were consequently unable to migrate directionally toward the dorsal midline (Row et al., 2011). Tbx16 was therefore proposed to play a critical role in converting a highly blebbing, transient intermediate state to one where cells could produce the lamellipodia and filopodia necessary for directional migration during mesoderm specification. However, during somitogenesis it is very difficult to know precisely which cells in the tailbud are fated to become mesoderm and the tailbud constantly moves as the body axis extends. Therefore, the phenotype of mesodermal cells during somitogenesis could not be compared to these earlier stages to determine whether the same mechanism is used.
Here we present a novel tailbud explant method that eliminates the substantial tissue movement that occurs during anterior-posterior (A-P) body axis elongation and avoids the visual obstruction from the yolk that previously made it difficult to image these cells with high spatio-temporal precision. We have utilized this approach in combination with a tbx16 promoter driving the expression of the fluorescent actin marker LifeAct (Riedl et al., 2008) and a fluorescent membrane marker. We can now image protrusive activity specifically in newly differentiating tailbud mesodermal cells. Surprisingly, we find that tbx16;msgn1-deficient cells are highly motile despite their inability to exit the posterior end of the embryo. Unlike during gastrulation, they are not stuck in a blebbing intermediate and form protrusions fairly normally. Instead, tbx16;msgn1–deficient lamellipodia do not produce functional cell movement as do wild-type lamellipodia. These results establish a key role for Tbx16, together with Msgn1, in cells’ acquisition of directional migratory ability during completion of the EMT. They also reveal how the same transcription factors play major, but very different, roles in mesoderm morphogenesis during gastrulation and somitogenesis.
Materials and methods
Fish lines
All fish are hybrid WIK/AB. Tg(Ptbx16-3.3:memRFP) was constructed by placing a fragment of the tbx16 promoter (a gift from S. Wells; Wells et al., 2011) from approximately 1200 bp upstream of the transcription initiation site through the second exon in front of TagRFP with a C-terminal prenylation sequence. Morpholinos directed towards tbx16 and msgn1 were combined as follows: 1.1 ng tbx16 MO1 and 0.58 ng tbx16 MO2 from Lewis and Eisen (2004), and 2 ng msgn1 MO from Fior, et al. (2012). For analysis of actin based protrusions, Tg(Ptbx16-3.3:memRFP) embryos were injected with 25 pg Ptbx16-3.3:LifeAct-GFP plasmid at the one-cell stage. This plasmid was made by using Gateway cloning to insert the Ptbx16-3.3 fragment in front of LifeAct-GFP (a gift from C.-P. Heisenberg).
Cell transplantation
Donor embryos were injected with fluorescently labeled dextran with or without the morpholino mix at the one-cell stage. When donors were at sphere stage, approximately 30 cells were transplanted into the ventral margin of shield stage Tg(Ptbx16-3.3:memRFP) hosts with or without the morpholino mix. 25 to 30 embryos were analyzed for each condition at 24 hours post fertilization (hpf). For transplants into wild-type embryos at 24 hpf, if any donor cell took on an elongated muscle phenotype the embryo was counted as having donor cells contributing to Somite. If an embryo did not have donor cells in the somites but any donor cell took on a clear differentiated morphology the embryo was counted as having donor cells contribute to Fin/epithelium. If all donor cells were clearly distinct from the surrounding host tissue without contributing to the tissue the embryo was counted as having donor cells Undifferentiated.
For immunofluorescence, mouse monoclonal MF20 antibody directed towards muscle myosin (DSHB; Bader et al., 1982) was used at 1:50 dilution. Goat anti-mouse conjugated to Alexa568 was used at a 1:500 dilution. Embryos were imaged with an Olympus Fluoview 1200 microscope with 10x dry and 60x oil immersion lenses.
Tailbud explants
Embryos at the 12–13 somite stage were dissected in Modified Barth’s Saline (MBS: 8.8mM NaCl, 0.1mM KCl, 0.1mM MgSO4, 0.5mM HEPES, 0.25mM NaHCO3, 0.07mM CaCl2-2H2O, pH 7.8) plus penicillin/streptomycin (pen/strep) in an agarose-coated Petri dish at 25°C. To isolate tail fragments, embryos were first dechorionated. The epithelium was removed by using two fine forceps to grasp it at an anterior dorsal position and gently peel it off of the embryo. The forceps were then used to make a transverse cut through the body about three-quarters of the way from anterior to posterior, avoiding puncturing the yolk. The posterior portion of the body was gently peeled off of the yolk by holding the anterior, cut end of the posterior body section with one forceps and using the other forceps to hold the anterior body/yolk still. The anterior body and yolk were discarded.
For migration tracking, 5–6 tailbud explants were mounted in a Petri dish as follows. A drop of 2% methylcellulose in embryo medium plus a drop of glass bead risers (75–150 μm dry glass beads; G-3753, Sigma) in distilled H2O were placed in the center of the dish. Explanted tailbuds were added and covered with a coverslip. The dish was filled with MBS plus pen/strep and tricaine. Tailbuds were imaged beginning about 45 minutes after dissection, or around the 14 somite stage on an Olympus Fluoview 1200 microscope with a 40x dipping lens and multi-area time lapse imaging at 28°C. Images were taken at 1 μm intervals over 10 μm in the Z-axis for each tailbud every 5 minutes for 2–4 hours.
For imaging of protrusions, 1–2 tailbuds were mounted on a slide with premixed 1:1 1x MBS:1.5% methylcellulose, plus glass beads, pen/strep, and tricaine, covered with a coverslip, and sealed with nail polish. Tailbuds were imaged beginning about 45 minutes after dissection, or around the 14 somite stage. A spinning disc confocal (3I) was used with a 40x water immersion lens at 28°C with Z-axis intervals of 0.5μm every 30 seconds for 30 minutes.
For all imaging and analysis cells had to start in the maturation zone, which is defined by being posterior to the end of the notochord and expressing a fluorescent marker driven by the Ptbx16-3.3 promoter.
Migration tracking
Slidebook software (3I) was used to concatenate time lapse images and to create a maximum intensity projection over the Z-axis. Then, Fiji software (NIH) was used to combine channels corresponding to fluorescent dextran labeled donor cells and fluorescent labeled mesodermal cell membranes (as in Figure 2B). Images were aligned using the StackReg plugin using rigid body transformation (Thévenaz et al., 1998) and then rotated so that the anterior was to the left and the notochord horizontal. Cells were manually tracked with the MTrackJ plugin, which provides X–Y coordinates for all points (Meijering et al., 2012). For analysis, the DiPer macros were used in Excel on tracks 2 hours long (Gorelik and Gautreau, 2014). At least four embryos over two independent experiments were used; 25 to 30 cells total were analyzed for each condition. Pairwise χ-squared tests or ANOVA tests were used to determine statistics, with a p-value cutoff of 0.01. For Figures 3B, 4B, and S5B the Bonferroni method of correction for multiple comparisons was used.
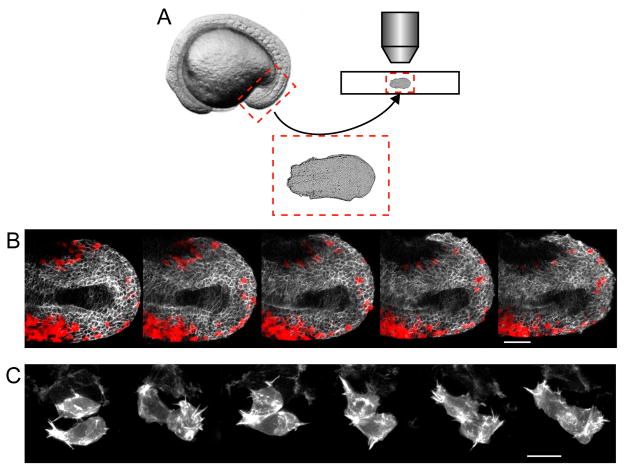
Figure 2.
A novel tailbud explant method allows for high spatio-temporal imaging of migrating cells in vivo. A. The posterior portion of an embryo in mid-somitogenesis is dissected away from the anterior tissue and yolk, mounted, and imaged. B. Time lapse image series of an explant from a wild-type host expressing a fluorescent membrane marker driven by the tbx16 promoter (white) with wild-type donor cells (red) taken at mid-somitogenesis. Anterior is to the left with the notochord running down the middle of the tissue. These images correspond to frames from Movie S1. Scale bar = 50 μm. C. Time lapse image series of two cells in an explant from a wild-type embryo mosaically expressing the fluorescent actin marker LifeAct driven by the tbx16 promoter. Scale bar = 10 μm.
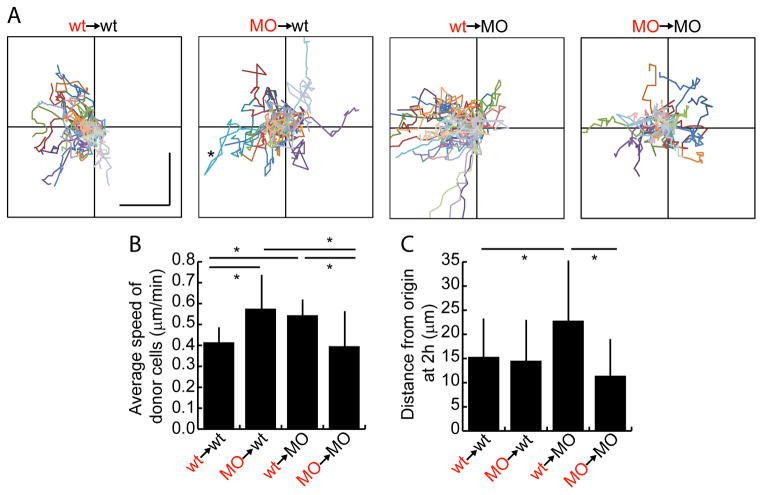
Figure 3.
Spt and Msgn have cell non-autonomous effects on speed. A. Charts show tracks of individual donor cells migrating over two hours. Each cell is in a different color. Anterior is to the left and each axis is 70 μm total. Vertical and horizontal scale bars in wt→wt plot are each 20 μm. Asterisk in MO→wt plot indicates a cell that moved significantly anteriorly and then reversed direction. B. Average speed of donor cells measured over two hours. C. Average net distance of donor cells from their starting points at the end of two hours. Bars in B, C show standard deviation. *: p<0.01 by pairwise Anovas. Data in B, C also displayed in box and whisker plots in Figure S3A, B.
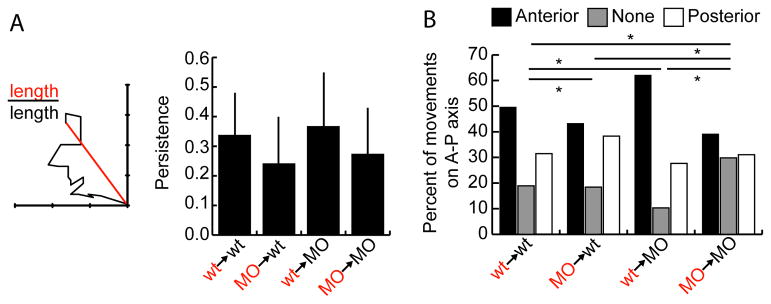
Figure 4.
Tbx16 and Msgn have cell autonomous effects on migratory persistence. A. The persistence of donor cell movement at two hours is equal to the ratio of the straight line distance between starting and ending points to the total distance travelled. Data is taken from the two-dimensional tracks in Figure 3A. Bars show standard deviation. Data analyzed by pairwise Anova and is also shown in box and whisker plot in Figure S3C. B. The percentage of time points that cells moved anteriorly, posteriorly, or did not move along the A-P axis. Data is taken from the one-dimensional A-P tracks in Figure S4. *: p<0.01 by χ2 test.
Analysis of protrusions
Slidebook software was used to create 3-dimensional renderings of fixed images to determine protrusion numbers and orientations. Protrusions were only counted if they extended at least 1 μm from the cell body. Lamellipodia were defined as at least twice as wide in one axis tangential to the cell surface than the other tangential axis. Filopodia were defined as much longer (orthogonal to the cell surface) than they were wide and with a similar width in every direction tangential to the cell surface. Blebs were defined as having similar length in every dimension, particularly tangential to the cell surface. At least 10 embryos across three independent experiments were used for fixed analyses; over 100 total protrusions were used for each condition. For time lapse images, areas around single fluorescent cells were cropped and 3-dimensionally rendered to analyze protrusion dynamics. At least four embryos over two independent experiments were used for live analyses; over 100 protrusions were used for each condition. Pairwise χ-squared, t-tests, or z-tests were used to determine statistics, with a p-value cutoff of 0.01. For Figure 6D the Bonferroni method of correction for multiple comparisons was used.
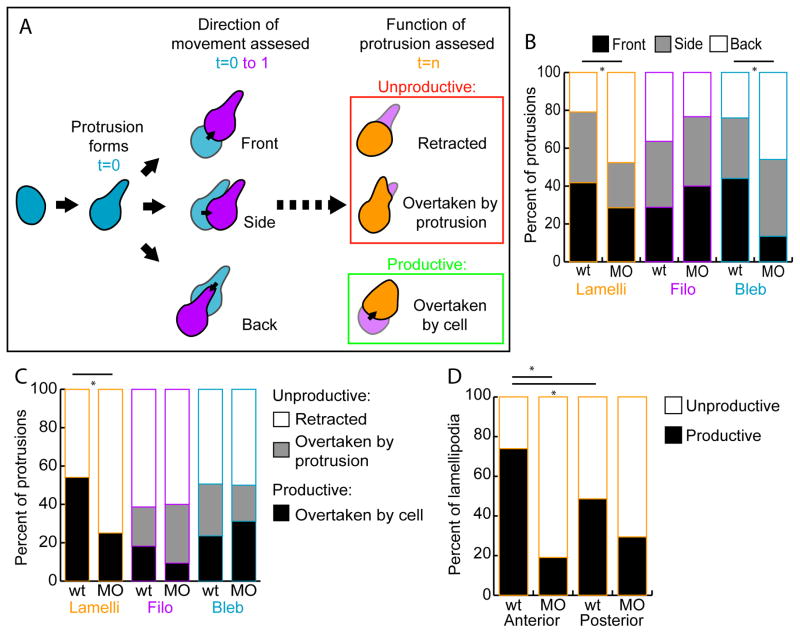
Figure 6.
tbx16;msgn1-deficient lamellipodia are less productive than wild-type lamellipodia. A. Diagram of how measurements of protrusion functionality were assessed. The direction of cell body movement was determined in the frame following the formation of each protrusion and the region of the cell (Front, Side, or Back) on which the protrusion formed relative to this direction was recorded. Then, at the last frame of a protrusion’s lifetime it was noted whether the protrusion was Unproductive (Retracted or Overtaken by the formation of another protrusion) or Productive (Overtaken by the cell body moving in that direction). Whereas the first measurement scores the initial trajectory of the cell, the later measurement reveals the action of the cell when the protrusion is no longer present. B. The percentage of protrusions formed in each direction, front, side, or back, relative to instantaneous cell body movement. *: p<0.01 by χ2 test. C. The percentage of protrusions that were retracted, overtaken by another protrusion, or overtaken by the cell body. *: p<0.01 by χ2 test. D. The percentage of unproductive and productive lamellipodia formed in the anterior and posterior directions. *: p<0.01 by z-test.
Results
Environmental cues trigger mesodermal cell anterior migration
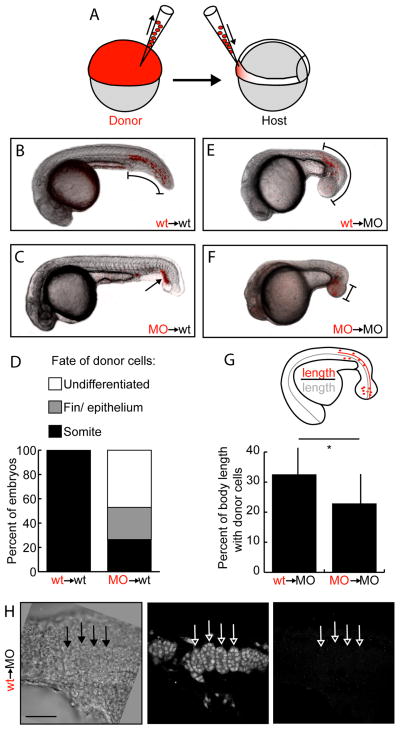
Previous studies have shown that Tbx16 is cell-autonomously required for mesodermal cells to migrate into the body from the progenitor zone (Amacher et al., 2002; Griffin et al., 1998; Ho and Kane, 1990; Row et al., 2011). Additionally, two recent studies established that tbx16;msgn1 double mutant embryos develop no trunk or tail somites because all mesodermal cells stalled in the tailbud in a state where they expressed markers of the MZ and neither differentiated nor moved further (Fior et al., 2012; Yabe and Takada, 2012). These studies also showed that a combination of tbx16 and msgn1 morpholino oligonucleotides (MOs) completely recapitulates the double mutant phenotype, the current standard for the use of MOs (Schulte-Merker and Stainier, 2014). We therefore asked if prospective tail somite cells lacking tbx16 and msgn1 would fail to migrate from the progenitor zone as do prospective trunk cells lacking tbx16. We first performed cell transplant experiments using either wild-type donor embryos or donors injected with MOs targeting both tbx16 and msgn1 (hereafter referred to as MO embryos; Fior et al., 2012; Yabe and Takada, 2012). Donor cells were transplanted into the ventral region of gastrulating host embryos (Figure 1A). These ventral cells are fated to become posterior trunk and tail somite tissue whereas the lateral regions primarily contribute to the more anterior trunk somites (Kimmel et al., 1990; Warga and Nusslein-Volhard, 1999). Wild-type donor cells formed tail muscle fibers, but tbx16;msgn1 MO cells mostly remained in clusters discrete from differentiated host tissues (Undifferentiated) or contributed to non-somite tissue at the posterior end of the tail in wild-type hosts (Figure 1B–D). This result is consistent with the published tbx16;msgn1 phenotype (Fior et al., 2012; Yabe and Takada, 2012). Donor cell fate for each host was scored as Somite if at least one cell contributed to a host somite. MO donor cells in wild-type embryos scored as having a somite fate always had many more cells contributing to other tissues than to somites. Thus, knocking down Tbx16 and Msgn1 together provides an effective system for analyzing the failure of cell migration during tail somite formation.
Figure 1.
Tbx16 and Msgn1 act cell-autonomously in migration out of the tailbud. A. Diagram of transplant scheme. Labelled undifferentiated cells are removed from donor embryos and placed in the ventral margin (fated to become tail somites) of unlabeled gastrulating embryos. B–C,E–F. Fluorescently labelled donor cells (red) overlaid on bright field images of host embryos at 24 hours post fertilization. B. Wild-type donor cells in wild-type host. C. MO donor cells in wild-type host. E. Wild-type donor cells in MO host. F. MO donor cells in MO host. Brackets and arrow indicate locations of donor cells. D. Percentage of wild-type host embryos containing donor cells in somite, fin or epithelium, and undifferentiated groups. *: p<0.01 by χ2 test. G. Percentage of MO host A-P body length containing donor cells. (Distance from posterior of embryo to anterior-most donor cell divided by total A-P body length.) *: p<0.01 by Anova. Bars show standard deviation. H. 24 hours post fertilization MO host with somite-like organization of wild-type donor cells. Left panel is a single frame bright field image; middle is a Z projection through 4 μm of fluorescent dextran-labeled donor cells; right is a Z projection through 4 μm of embryos stained with a muscle myosin antibody. Arrows show somite-like structures formed from donor cells. This is the same embryo as in Figure S1A. Scale bar is 50 μm.
We also asked whether transplanted wild-type cells would be able to migrate out of the posterior end when transplanted into tbx16;msgn1-deficient embryos to determine if these factors are required to establish the environment necessary for migration. As expected, MO cells transplanted into MO hosts remain in the expanded MZ, or “spade” of undifferentiated cells, at the posterior end of the embryo (Figure 1F). Surprisingly, wild-type cells transplanted into a MO host migrated out of the tailbud and into the tail (Figure 1E). To quantify the ability of cells to leave the tailbud in MO hosts, the distance that transplanted cells migrated was measured from the posterior end of the embryo and expressed as a percentage of the total anterior-posterior (A-P) body length (Figure 1G). Wild-type cells move significantly farther anteriorly than do MO cells. These data indicate that there is a signal still present in tbx16;msgn1–deficient embryos that directs wild-type cells to migrate anteriorly. In contrast, tbx16;msgn1-deficient cells either cannot receive or cannot respond to this signal.
The surprising result that wild-type cells can leave the tailbud in tbx16;msgn1-deficient embryos lead us to ask how far these anteriorly migrating cells can progress along the normal mesodermal differentiation pathway. Upon closer examination we saw that most MO hosts only had scattered, mesenchymal donors anterior to the MZ (Figure S1A). However, when there were higher numbers of donors present they could organize into rudimentary segmented somite-like structures (Figure 1H). We then used an antibody against muscle myosin heavy chain that stains both pre-somitic mesoderm and somites, but not the undifferentiated MZ cells, in zebrafish embryos beginning at the 14 somite stage (Figure S1B; Windner et al., 2012) to determine whether any cells from wild-type donors or their MO hosts differentiate into muscle. We never saw any wild-type donor cells that stained for myosin, whether mesenchymal or somite-like (Figures 1H and S1A), although very rarely some MO host cells showed some myosin staining (data not shown). These results reveal that wild-type cells in a tbx16;msgn1-deficient background have all of the machinery necessary to cell-autonomously undergo an EMT, migrate anteriorly, organize into a mesodermal column, and segment periodically. However, they do not differentiate into muscle.
A novel explant method for imaging the EMT in vivo
Our results, combined with previous studies, make it clear that there are differences in the migratory abilities of wild-type and MO cells, even in the same environment (Amacher et al., 2002; Ho and Kane, 1990; Row et al., 2011; Yabe and Takada, 2012). To date, it has been difficult to observe subcellular details of morphogenesis in vivo during EMT, particularly during vertebrate development. While studies of the bulk flow of mesodermal progenitor cells have provided insights into the general properties of cell movement during this stage, these observations remained at the tissue or whole-cell level (Kanki and Ho, 1997; Lawton et al., 2013). Therefore, a new method enabling close examination of the migratory and protrusive behaviors of individual mesodermal cells during axis elongation was needed.
In order to observe cells with high spatio-temporal resolution, we developed a method to image isolated tailbuds which increased visual accessibility and reduced the large-scale tissue movement due to A-P axis elongation that has precluded this type of analysis in the past (Figure 2A). Briefly, embryos were dechorionated and dissected to discard the yolk and the anterior three-quarters of the body. Embryos at the 14 somite stage were used for all explant experiments since at this stage the tailbud has everted from the yolk and can be easily dissected. Tailbud-containing explants were mounted in a dish to track cell migration, or on a slide to image protrusions with high resolution (Figure 2B, C). Precisely-sized glass beads were used to support a coverslip that was sealed to the underlying support to prevent desiccation of tailbuds during imaging. When mounted this way, tailbuds lay flat, affording a clear view of cells from the ventral side of the embryo. Tailbuds cultured in this way continue to form somites for several hours, showing that they retain normal function on the time scales necessary for the analyses presented here (Figure S2).
In order to focus specifically on newly differentiating mesodermal cells, a transgenic line was used which expresses a fluorescent membrane marker under the control of a portion of the tbx16 promoter. This promoter is first activated in the tailbud at the end of gastrulation (Ptbx16-3.3:memRFP; Kimelman, et al., in preparation). Embryos stably carrying Ptbx16-3.3:memRFP served as hosts for transplanted cells during time-lapse imaging of cell migration, as shown in Figure 2B. Additionally, to image cellular protrusions, embryos stably carrying Ptbx16-3.3:memRFP were injected at the single cell stage with a plasmid encoding the fluorescent actin cytoskeleton marker LifeAct-GFP (Riedl et al., 2008) under the control of the same promoter (Ptbx16-3.3:LifeAct-GFP). Injected embryos show mosaic labeling of the actin cytoskeleton so that both the protrusions and shapes of individual cells can be delineated (Figure 2C). This novel explant technique allows the morphologies and dynamics of individual cells to be imaged in fine detail in vivo as they move through the EMT. tbx16 and msgn1 are required for mesodermal cell anterior persistence but not for motility
The observation that tbx16;msgn1-deficient cells do not leave the tailbud in both wild-type and MO environments, lead to the hypothesis that during somitogenesis mesodermal cells are unable to migrate, as was seen during gastrulation in tbx16-deficient embryos. We note that the previous study used tbx16 morphant gastrula stage embryos (Row et al., 2011), but Tbx16 is required for msgn1 expression during this early stage (Goering et al., 2003; Griffin and Kimelman, 2002). Therefore both the gastrula stage tbx16 morphants used previously and the double morphant somitogenesis stage embryos used here lacked both Tbx16 and Msgn1 function.
The movement of wild-type or MO donor cells in the MZ of wild-type or MO hosts was tracked for two hours beginning at the 14 somite stage in time lapse image series oriented with the anterior to the right (as in Figure 2B and Movies S1–4). The two-dimensional track of each cell’s movement in the A-P and medio-lateral directions was plotted starting at the origin of the graphs in Figure 3A. Wild-type cells transplanted into wild-type hosts show varying paths but generally end up anterior to where they started (to the left of the Y-axis; Movie S1). Notably, MO cells in wild-type hosts are highly motile (Movie S2). This result is strikingly different from what was observed during gastrulation (Row et al., 2011). Indeed, the speed and the net distance travelled by MO cells from their starting points in two hours are the same as for wild-type cells (Figures 3B, C, and S3A, B). However, MO cells do not consistently end up anterior to where they started. For example, the cell shown in the turquoise trace moves anteriorly but then doubles back and moves posteriorly (asterisk in Figure 3A MO→wt). Thus, the cell autonomous defect in cells lacking Tbx16 and Msgn1 during somitogenesis is not a failure to move but instead is a defect in migrating in the correct direction.
Interestingly, wild-type cells transplanted into MO hosts move significantly farther anteriorly than those in a wild-type environment (Figure 3A, C wt→MO and Movie S3). This result strengthens our conclusion that the directional cues for anteriorward migration are still present in tbx16;msgn1-deficient embryos. Another striking observation is that wild-type cells in MO hosts and MO cells in wild-type hosts move faster than cells transplanted into a homotypic (same genotype) environment (Figure 3B and Movie S4). This demonstrates that the identity of the host environment has a significant effect on a cell’s migration speed, with a homotypic environment actually restricting the rate of migration.
An informative way to measure cell movement is by examining directional persistence, or the ability of a cell to continuously move in a given direction. The persistence of a cell is determined by dividing the straight line distance between its starting and ending points by the total distance it travelled. A cell that travels in a straight line has a persistence of 1, while a cell that moves randomly has persistence close to zero. Analysis of the migration tracks above showed that wild-type cells have higher persistence than MO cells in both wild-type and MO backgrounds (Figures 4A and S3C). Therefore, MO cells have trouble consistently moving in the same direction over time, which likely contributes to their inability to move out of the tailbud during axis elongation. Wild-type cells in a MO background have only slightly higher persistence than the same cells in a wild-type background, though they move significantly farther anteriorly (Figures 3B, 4A). Therefore, the faster migration speed of wild-type cells in a MO environment contributes substantially to the ultimate ability of these cells to move farther (Figure 3C).
In addition to general persistence defects, MO cells have specific problems coordinating anterior-directed movement in order to exit the tailbud. To measure A-P movement alone, the lateral movements from the tracks in Figure 3A were removed (Figure S4). Counting the number of time points in which cells moved anteriorly, posteriorly, or were stationary along the A-P axis shows clear differences among all transplant conditions (Figure 4B). Wild-type cells in their normal environment move anteriorly about half the time, with less time either moving posteriorly or not moving. MO cells in wild-type hosts move anteriorly and posteriorly for about equal amounts of time, confirming their lack of anterior bias. Similarly, MO cells in a MO background have no A-P bias, but spend more time than any other group not moving either anteriorly or posteriorly. Finally, wild-type cells in MO hosts have a greater bias for anterior movement than they do in wild-type hosts. Strikingly, they also spend less time remaining stationary on the A-P axis. This result suggests that wild-type cells are normally in competition with each other to move anteriorly out of the tailbud, but that a wild-type cell surrounded by MO cells has a competitive advantage.
We also investigated whether Spt and Msgn have any effects on medio-lateral migration by removing the cells’ A-P movement from the tracks (Figure S5). Wild-type donor cells in wild-type hosts move laterally (towards the sides of the tissue) slightly more often than medially (towards the notochord; Figure S4), which is consistent both with their needing to migrate around the notochord and with previous bulk flow studies (Kanki and Ho, 1997; Lawton et al., 2013). Donor cells in the two heterotypic (different genotype) transplant situations had no medio-lateral bias whereas MO cells in MO hosts moved medially more frequently than laterally. Taken together, the tracking data show that wild-type cells move persistently anteriorly during body axis elongation and therefore must be responding to an environmental cue. Cells lacking tbx16 and msgn1 do not respond to that directional cue but it is present even when Tbx16 and Msgn1 expression is disrupted since wild-type cells can move anteriorly in a host lacking Tbx16 and Msgn1. In addition, this detailed quantitative analysis shows that the speed of mesodermal cell movement depends on whether cells are in a homotypic or heterotypic environment.
tbx16 and msgn1 contribute to lamellipodial productivity
Tracking migrating cells provided a view at the cellular level of the defects in motility that occur in tbx16;msgn1-deficient cells. To investigate the mechanisms underlying these defects, we next asked how maturing mesodermal cells form and utilize actin-based protrusions during this stage of development. Protrusions were categorized into three main types, each known to have different modes of formation and function during cell motility. Lamellipodia are broad and flat protrusions that extend by growing branched actin networks (Figure S6A; Petrie et al., 2009). They are generally considered the main drivers of persistent, directional cell migration. Filopodia are finger-like protrusions formed by linear actin filament bundles (Figure S6B; Arjonen et al., 2011). They often probe the environment before other protrusions enact cell movement, but can also drive it independently. Blebs are dome shaped bubbles of membrane pushed away from the underlying actin cortex by intracellular pressure (Figure S6C; Paluch and Raz, 2013). They often produce non-directional, short-term, or random migration.
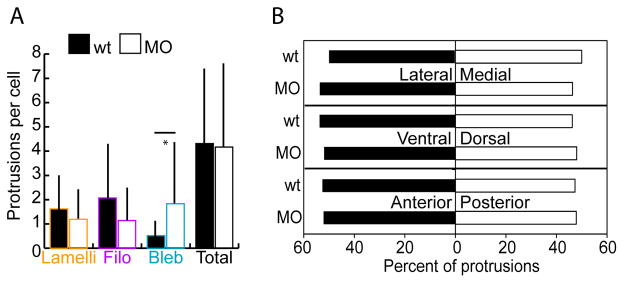
Given the previously reported differences in the types of protrusions produced by wild-type and tbx16-deficient cells during gastrulation (Row et al., 2011), we first asked whether protrusions are also altered during body axis elongation. Embryos were injected with Ptbx16-3.3:LifeAct-GFP plasmid to mosaically label the actin cytoskeleton in tailbud cells and fixed at the 14 somite stage. Then, the number of each of the three protrusion types on wild-type and MO cells was quantified. This analysis revealed that cells of both genotypes produce approximately the same numbers of each type of protrusion (Figure 5A). MO cells do make more blebs than wild-type cells, but there is no significant reduction in the number of lamellipodia and filopodia in the MO cells. Therefore, the protrusion phenotype during somitogenesis is distinct from the extreme blebbing that occurs at the cost of all other protrusions during gastrulation (Row et al., 2011). This distinction suggests that Tbx16 controls cell migration via different mechanisms at these two developmental stages.
Figure 5.
tbx16;msgn1-deficient cells form protrusions fairly normally during axis elongation. A. Number of lamellipodia, filopodia, and blebs per cell measured in the MZ of embryos mosaically labelled with LifeAct and fixed at the 14 somite stage. Bars show standard deviation. *: p<0.01 by two-tailed heteroscedastic t-tests. B. Percent of protrusions in each body axis direction: lateral versus medial; ventral versus dorsal; and anterior versus posterior using the same labelling conditions as for A. Data were analyzed with z-tests.
Since the bulk flow of cells in the MZ is towards the anterior, ventral, and slightly lateral (Kanki and Ho, 1997; Lawton et al., 2013), and since there are major differences in the directions wild-type and MO cells migrate along the A-P body axis (Figure 4B), we asked whether cells bias their protrusion formation with respect to the body axes. Surprisingly, all wild-type protrusions were equally distributed on each of the three body axes and no differences were observed between wild-type and MO embryos (Figure 5B). Even when this data was examined for each protrusion type separately the only difference seen was between wild-type and MO cells’ blebs along the A-P axis. Wild-type cells made blebs more frequently toward the anterior whereas MO cells did not show a bias (data not shown). Thus, since tbx16;msgn1-deficient cells can make protrusions relatively normally during somitogenesis another explanation is needed to account for their altered migration.
Next, the protrusions that mesodermal cells form were examined to determine whether they generate cell movement. Since wild-type cells migrate more persistently through the MZ than do MO cells, we hypothesized that their protrusions would be more effective at causing migration. To test this hypothesis, live cells expressing LifeAct from injected Ptbx16-3.3:LifeAct-GFP plasmid were imaged using our explant system (Figure 2C, Movies S5–9). The direction a cell body moved in the frame after the formation of a protrusion was determined (t=1; Figure 6A). The direction a cell body moved in three-dimensional space was defined as the “front” of the cell. Then, the protrusion was scored (at t=0) on whether it was formed in the direction the cell was moving (Front; Movie S5), to the side relative to the direction the cell moved (Side), or in the opposite direction compared to the movement of the cell (Back; Movie S6). These measurements indicate the initial trajectory of the cell relative to the direction of the protrusion. This analysis revealed that lamellipodia and blebs formed most often on the front and side of wild-type cells. In contrast, these same protrusion types formed primarily on the back of MO cells (Figure 6B). The orientations of filopodia were the same for wild-type and MO cells. Thus, it is probable that lamellipodia and blebs, which play a major role in directing where a cell will move, contribute to the ability of wild-type cells to migrate consistently in the same direction. MO cells, however, form protrusions that are out of line with the movement of the cell body, which likely causes them to change directions more frequently and be unable to maintain persistent directional movement.
In order to assess the functionality of these protrusions, we next examined what happens at the end of a protrusion’s lifetime (Figure 6A). The observed behaviors fell into three categories, the first two of which did not produce cell body movement and were collectively labeled as Unproductive. First, a protrusion could retract without the cell moving in the direction of the protrusion (scored as Retracted; Movie S7). Alternatively, a second protrusion could form very close to or overlapping the original protrusion without the cell exhibiting movement in that direction (scored as Overtaken by protrusion). This second category was most frequently observed when multiple blebs formed in quick succession on one area of a cell (Movie S8) or when a lamellipodium formed to encompass a filopodium (Movie S9). Lastly, a protrusion could generate functional movement of the cell and be overtaken as the cell body moved in the direction of the protrusion (scored as Overtaken by cell; Movies S5,6). This fate was labeled as Productive in producing cell movement. The quantification of protrusion fates shows that wild-type lamellipodia are productive about half the time, and are retracted about half the time (Figure 6C). In contrast, MO lamellipodia are productive much less frequently, demonstrating that they are defective in driving functional cell movement. Filopodia and blebs have similar fates in both genotypes. Both the high proportion of productive lamellipodia in wild-type embryos and the significant difference between lamellipodial function in wild-type and MO embryos shows that this protrusion type is the primary driver of directional migration in mesodermal cells.
Lastly, we wanted to assess whether there was a directional bias of lamellipodia that produced functional cell movement versus those that retracted along the A-P axis. All lamellipodia that formed on the anterior or posterior of a cell were examined and their fates were recorded (Figure 6D). The lamellipodia of wild-type cells were very often productive when they formed towards the anterior. However, when lamellipodia formed on the posterior of a cell, they were unproductive more frequently. Thus, wild-type cells are biased; they form productive lamellipodia toward the anterior (the direction cells need to move) more often than toward the posterior. Strikingly, MO cells not only produced unproductive lamellipodia a majority of the time, but the ineffective lamellipodia showed no preference for the anterior or posterior directions. Therefore, a major defect of MO cells is that they are unable to produce productive lamellipodia to drive cell movement anteriorly. In summary, our analysis of protrusive activity shows that wild-type cells produce protrusions in the direction of migration more frequently than cells lacking Tbx16 and Msgn1. Additionally, wild-type lamellipodia are more productive, especially when pointing anteriorly, than those made by tbx16;msgn1-deficient cells.
Discussion
This work addresses many of the challenges faced previously when studying the EMT. The novel tissue explant method described here makes it possible to observe a developmentally programmed EMT in the early zebrafish embryo at high spatial and temporal resolution. This system was used to analyze how actin-based protrusions act to create functional cell movement in a three dimensional in vivo context, and how these protrusions affect migratory directionality relative to the development of an entire tissue. Our results show that the Tbx16 and Msgn1 transcription factors act cell-autonomously in morphogenesis during embryonic development to control directed migration, but they also have a cell-non-autonomous effect on migration speed and differentiation. Together, this work outlines requirements for cells to become migratory during an EMT, and what defects lead to a failure in this transition.
A novel tailbud explant system allows high resolution imaging of the EMT
While a significant amount of work has investigated mechanisms underlying the various cellular processes that must be coordinated to initiate the EMT (Craene and Berx, 2013; Lamouille et al., 2014; Saunders and McClay, 2014), very little is known about late stages of this crucial developmental process when cells become mesenchymal. Additionally, it has been technically challenging to study details of a physiological EMT in an in vivo setting. We overcame many of these difficulties by developing a method to culture zebrafish tailbuds that makes them amenable to live imaging studies. This method prevents the large scale movements of the tailbud away from the rest of the body and uses a robust mesoderm-specific promoter to drive the expression of fluorescent reporters in our cells of interest. The tissue explants continue to develop in culture for several hours, demonstrating that they carry out normal processes under these conditions. This method allowed tracking of the migration of individual maturing mesodermal cells and the dynamics of the protrusions they form when transplanted into different host environments. This explant system should also be very useful for studying other aspects of morphogenesis during this developmental period.
Differential control of mesoderm during gastrulation and somitogenesis
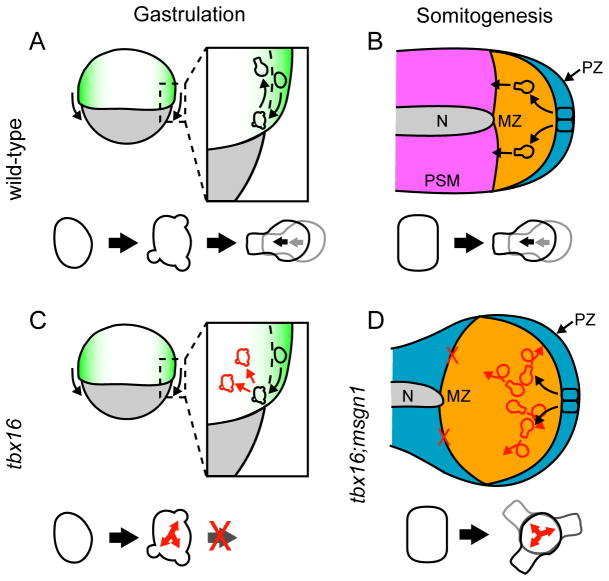
Depleting cells and embryos of Tbx16 provides a great opportunity to understand the defects that occur when cells can’t complete the EMT. Previously it was difficult to examine this process during somitogenesis because of the imaging problems discussed above, and because of the partial recovery of the tbx16 phenotype after the 4–6 somite stage (Griffin et al., 1998; Kimmel et al., 1989). However, the recent finding that the recovery of tail somites in tbx16 mutants is due to Msgn1 (Fior et al., 2012; Yabe and Takada, 2012), combined with the data presented here showing that tbx16;msgn1-deficient cells are completely unable to leave the MZ during somitogenesis, now gives us the ability to examine the completion of the EMT during somitogenesis. Studying the migration of wild-type and tbx16;msgn1-deficient cells revealed several intriguing behaviors, which, when combined, lead to the striking differences in the morphogenesis of the mesoderm. While tbx16;msgn1-deficient cells fail to leave the MZ, it is not due to a failure to migrate as is seen in the gastrula stages (Figure 7A, C). Indeed, tbx16;msgn1-deficient cells are highly motile during somitogenesis. However, they do not persistently move anteriorly (Figure 7B, D). The different effects of Tbx16 and Msgn1 on cell movement patterns during gastrulation versus somitogenesis, which were also reflected in the observed protrusive activities, were unexpected and likely reflect important transcriptional differences during these two stages of development. Since Tbx16 and Msgn1 are expressed together throughout mesodermal development (Fior et al., 2012; Yabe and Takada, 2012), our results suggest that other factors must influence their transcriptional targets as the embryo moves from gastrulation to somitogenesis.
Figure 7.
Model of mesodermal cell morphogenesis during gastrulation and somitogenesis. A. In gastrulating wild-type embryos, presumptive mesodermal cells transition through a highly blebbing intermediate state before becoming directionally migratory as they move from the epiblast (green) to the hypoblast (white). Yolk is gray. B. Later, during somitogenesis, neuromesodermal progenitors reside in a posterior pseudo-epithelium (PZ, blue) and transition to an anteriorward migratory state primarily driven by lamellipodia as they make the mesodermal fate choice and move into the MZ (orange). Pre-somitic mesoderm (PSM) is pink; notochord (N) is gray. C. During gastrulation, tbx16-deficient cells (which also don’t express Msgn1) begin to transition by becoming highly blebby but are never able to leave that state to become migratory. Colors are the same as in A. D. During somitogenesis, tbx16;msgn1-deficient cells leave the neuromesodermal progenitor epithelium and become highly motile, but never migrate anteriorly or leave the MZ despite the presence of directional cues. Colors are the same as in B. White is the region that lacks pre-somitic mesoderm. Red arrows and cell outlines denote aberrant behavior. A and C are lateral views; B and D are ventral views.
Cell-non-autonomous control of mesodermal cell migration
Using careful measurements of cell migration speed we discovered that cells move faster in a heterotypic environment than in a homotypic environment. This cell-non-autonomous defect in cell migration speed was surprising as all of the defects previously noted in tbx16- and tbx16;msgn1-deficient mesodermal development were cell-autonomous (Fior et al., 2012; Ho and Kane, 1990; O’Neill and Thorpe, 2013; Row et al., 2011; Yabe and Takada, 2012). However, this is the first time that the contributions of an individual mesodermal cell and its environment on cell migration have been examined in such detail. Cell-non-autonomous effects on migration speed could result from differential adhesion strength or adhesion types between wild-type and tbx16;msgn1-deficient cells, as has been previously hypothesized (Ho and Kane, 1990; Yamamoto et al., 1998). Interestingly, integrins are required for mesoderm morphogenesis, and loss of integrins leads to increased cell migration speed but lower coherence of cell movement in the MZ (Dray et al., 2013; Jülich et al., 2009, 2005). These migratory defects correlate with those we saw in tbx16;msgn1-deficient cells transplanted homotypically and suggest that integrins may play a key role in this process.
Lamellipodia as drivers of directional migration in vivo
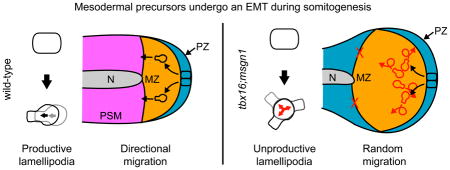
Surprisingly, when examining wild-type and tbx16;msgn1-deficient cells, we found that they make similar numbers and types of protrusions. Both genotypes also displayed a lack of bias in the directions of their protrusion with respect to the body axes. This has been seen previously in wild-type chick pre-somitic mesoderm, as well (Bénazéraf et al., 2010). Though we and others have shown that wild-type MZ cells move directionally through the tailbud (Kanki and Ho, 1997; Lawton et al., 2013; O’Neill and Thorpe, 2013), they do not form protrusions preferentially in the direction of movement. In order to explain this, we looked further into the dynamics of the protrusions. Wild-type cells utilized lamellipodia, generally considered drivers of long-distance directed migration, to frequently produce cell movement (rather than retracting them unproductively) and did so with a directional bias. Productive lamellipodia formed more often in the direction that a cell was moving when the protrusion formed (Figure 7B) and towards the anterior (the direction the cells need to move) compared to the posterior.
Conversely, tbx16;msgn1-deficient lamellipodia were frequently unproductive and did not form on a consistent side of the cell or with a bias along the A-P axis (Figure 7D). The unbiased and low productivity lamellipodial formation resulted in randomized motility without directed migration over long distances and no consistent anteriorward movement. This defect significantly contributes to the inability of tbx16;msgn1-deficient cells to exit the MZ during somitogenesis. We suggest that the lack of directional migration due to random lamellipodial formation in tbx16;msgn1-deficient cells represents the observable effects of an inability to sense or respond to a directional cue in the environment or to maintain consistent cellular polarity.
The polarity and consistent formation of lamellipodia on migrating wild-type MZ cells is likely due to a response to an extracellular signal. However, to transduce tissue-level signaling into functional protrusion formation there is intermediary machinery that regulates front-back polarity as a cell moves. The Par complexes, which define opposing membrane domains, are involved in apical-basal epithelial polarity, but they also help confer front-back migratory polarity (Nelson, 2009). Additionally, regulators of Rho family GTPases often localize asymmetrically in migrating cells and keep the GTPases active only in zones where they must function (Petrie et al., 2009). Mutual antagonism further reinforces the front and back domains. Wild-type mesodermal cells likely use some or all of these mechanisms to maintain an anterior trajectory while migrating through the MZ, and we propose that there are defects in at least one of them in tbx16;msgn1-deficient cells. These cells either cannot receive the directional cue from their environment, or cannot respond to this cue because of a defect in maintaining front-back polarity, organizing Rho GTPase signaling, or related defects.
Very few transcriptional targets of Tbx16, the main driver of mesodermal morphogenesis, have been investigated at this point. Microarray and chromatin immunoprecipitation experiments have defined many loci regulated by Tbx16 (Garnett et al., 2009), but recent work has focused on the roles of these targets in mesodermal fate and the segmentation clock (Bouldin et al., 2015; Jahangiri et al., 2012; Warga et al., 2013). Two protocadherins involved in morphogenesis, pcdh8 and pcdh10b, are targets of Tbx16, but they are involved in convergence-extension and do not appear to affect exit from the tailbud (Garnett et al., 2009; Murakami et al., 2006; Yamamoto et al., 1998). Identifying targets of Tbx16 that contribute to completion of the EMT will significantly advance our understanding of the cellular basis of mesenchymal migration in vivo.
A Tbx16;Msgn1-independent guidance cue
As is true in many cases of EMT in vivo, cues in the environment regulate the directed migration of cells after they leave the epithelium and enable them to reach their appropriate destinations (Roussos et al., 2011; Thiery et al., 2009). In the case of the maturing mesodermal cell model used in these experiments, the nature of these signals is not known. However, our transplants of wild-type cells into tbx16;msgn1-deficient embryos make clear that whatever this signal is, it is present in the tbx16;msgn1-deficient embryos. That wild-type cells can migrate anteriorly in a tbx16;msgn1-deficient environment to the region where somites would form suggests for the first time that there is an attractive or repulsive cue present in both genotypes and that the primary migratory defect stems from tbx16;msgn1-deficient cells not being able to properly respond this cue.
Very little is known about what molecules may act as guidance factors for migrating mesodermal cells during somitogenesis in any vertebrate system. One possibility is FGF signaling, which is important for cell guidance among other functions across species and developmental stages and is active in the tailbud during somitogenesis (Bénazéraf et al., 2010; Ciruna and Rossant, 2001; Griffin et al., 1998; Griffin and Kimelman, 2003; Stulberg et al., 2012; Xu et al., 1999). While FGFs may provide directional cues, they also have complex roles in tissue survival and fate choices. This pleiotropy makes it hard to parse out specific roles for FGF signaling components. During somitogenesis in chick, a gradient of FGF signaling induces a gradient of diffusive cell motility decreasing from posterior to anterior in the pre-somitic mesoderm (Bénazéraf et al., 2010; Delfini et al., 2005). In this case FGF acts primarily as a motility cue, and only indirectly a directional cue, since cells moved randomly without the local directed movements that are characteristic of chemotaxis. In zebrafish, we and others have observed that ubiquitous activation of FGF signaling early in somitogenesis causes some disruption of mesoderm morphogenesis, but does not cause cells to build up in the tailbud (Marques et al., 2008; our unpublished results). These results indicate that FGF signaling is not a key migratory cue. The nature and identity of this cue is an important puzzle to be solved.
Genetic separation of mesoderm morphogenesis and differentiation
Before the work presented here it was not known whether the next phases of mesoderm development, organization into pre-somitic mesoderm and segmentation into somites, and muscle differentiation, were controlled by Tbx16 and Msgn1. Strikingly, by transplanting wild-type cells into tbx16;msgn1-deficient embryos we were able to genetically separate mesoderm morphogenesis and differentiation. Wild-type cells can go through all of the processes required to form somite-like structures, including progression of the segmentation clock and undergoing an MET. However, they do not show any signs of terminal differentiation, such as muscle myosin expression or elongation into muscle fibers. What could account for the disconnect between these cells’ ability to form somites and to continue differentiation? One possibility is that tbx16;msgn1-deficent embryos may lack a differentiation-inducing signal. Secreted signaling molecules required for induction of muscle differentiation may be lacking from neighboring in tbx16;msgn1-deficent tissues. For example, it has been well established in fish that Hedgehog signaling from axial tissues such as notochord is required for slow muscle differentiation (Blagden et al., 1997; Nguyen-Chi et al., 2012). Axial tissues and other non-mesodermal tissues are still present in in tbx16;msgn1-deficent embryos (Fior et al., 2012; Yabe and Takada, 2012), but they may be unable to produce or relay proper signals. Alternatively, tbx16;msgn1-deficent embryos may express too much of an inhibitory molecule. It has been previously shown that Tbx16 can act as a transcriptional repressor (Bouldin et al., 2015) so an inhibitor of muscle differentiation may be overexpressed by tbx16;msgn1-deficent cells and act to block wild-type somite differentiation in the transplant situation. One candidate for this role is the FGF family, established inhibitors of muscle differentiation (Clegg et al., 1987; Florini and Magri, 1989; Nguyen-Chi et al., 2012), several of which are expressed in the MZ. This interesting area of inquiry should be further explored in the future.
Conclusions
Our work here establishes a novel in vivo experimental system with which to explore the cellular dynamics of EMTs: the developing mesodermal cells in cultured zebrafish tailbuds. These cells require Tbx16 and Msgn1 to form productive lamellipodia and migrate persistently anteriorly. Using cell transplant experiments, we show that there is a cue in the environment that is independent of Tbx16 and Msgn1 expression and that directs this anteriorward migration. Additionally, we demonstrate that these transcription factors act differently in the regulation of mesodermal morphogenesis at different developmental stages and that mesodermal morphogenesis and differentiation are genetically separable. Thus, we defined changes that are necessary for successful completion of the later stages of the EMT and showed that simply leaving an epithelial sheet does not automatically result in a functionally migrating cell.
Supplementary Material
Time lapse of wild-type donor cells transplanted into a wild-type host. Movie shows a Z-projection from a 10 μm Z-stack of fluorescent dextran-labeled donor cells (red) overlaid on a single bright field plane. Frames were acquired every five minutes for two hours, and playback is at 5 frames per second. Figure 2B shows stills from this movie with membrane fluorescence images replacing bright field images.
Figure S1. Wild-type cells in MO embryos can segment into somites, but not differentiate into muscle. A. Lateral view of a 24 hour post fertilization MO host with wild-type donor cells. Left panel is a single frame bright field image; middle is a maximum Z projection of fluorescent dextran-labeled donor cells; right is a maximum Z projection of muscle myosin staining. B. Ventro-lateral view of a 14 somite wild-type control embryo stained for muscle myosin, which highlights somite and pre-somitic mesoderm cells. Left panel is a single frame bright field image; right is a maximum Z projection of muscle myosin staining. Arrows show somite boundaries and arrowheads show newly forming somite boundary.
Figure S2. Tailbud explants continue to form somites in culture. Tailbud explanted from a Tg(Ptbx16-3.3:memRFP) embryo imaged every hour after dissection, beginning immediately after mounting. Arrows denote somite boundaries; arrowheads denote newly forming somite boundaries.
Figure S3. Box and whisker plots of migration data shown in bar graphs. A. Average speed of donor cells measured over two hours as in Figure 3B. B. Average net distance of donor cells from their starting points at the end of two hours as in Figure 3C. C. The persistence of donor cell movement at two hours is equal to the ratio of the straight line distance between starting and ending points to the total distance travelled as in Figure 4A. Horizontal lines show means, boxes show 1st and 3rd quartiles, and whiskers show minimums and maximum. “x”s show outliers.
Figure S4. Tbx16 and Msgn1 have cell autonomous effects on migration direction. Tracks of transplanted cells from Figure 3A only showing A-P (X-axis) movement with lateral (Y-axis) component of movement removed. Tracks are plotted so that they change Y-axis value every time the direction of A-P movement reverses. The X-axis is 70 μm total, with scale bar equaling 20 μm. Asterisk in MO→wt condition indicates a cell that moved significantly anteriorly and then reversed direction (same cell indicated with asterisk in Figure 3A).
Figure S5. Tbx16 and Msgn1 have cell autonomous and non-autonomous effects on medio-lateral migration. A. Tracks of transplanted cells from Figure 3A only showing medio-lateral (Y-axis) movement with A-P (X-axis) component of movement removed. Tracks are plotted so that they change X-axis value every time the direction of medio-lateral movement reverses. The Y-axis is 70 μm total, with scale bar equaling 20 μm. B. The percentage of time points that cells moved medially, laterally, or did not move along the medio-lateral axis. *: p<0.01 by χ2 test.
Figure S6. Protrusion examples. 3D renderings of LifeAct-GFP in individual cells show a lamellipodium (A), filopodia (B), and blebs (C), indicated by arrows.
Time lapse of tbx16;msgn MO donor cells transplanted into a wild-type host. Movie shows a Z-projection from a 10 μm Z-stack of fluorescent dextran-labeled donor cells (red) overlaid on a single bright field plane. Frames were acquired every five minutes for two hours, and playback is at 5 frames per second.
Time lapse of wild-type donor cells transplanted into a tbx16;msgn MO host. Movie shows a Z-projection from a 10 μm Z-stack of fluorescent dextran-labeled donor cells (red) overlaid on a single bright field plane. Frames were acquired every five minutes for two hours, and playback is at 5 frames per second.
Time lapse of tbx16;msgn MO donor cells transplanted into a tbx16;msgn MO host. Movie shows a Z-projection from a 10 μm Z-stack of fluorescent dextran-labeled donor cells (red) overlaid on a single bright field plane. Frames were acquired every five minutes for two hours, and playback is at 5 frames per second.
Time lapse of lamellipodia forming on the front of a cell, relative to instantaneous cell body movement, and then being overtaken by the cell body. Maximum Z-projection through a cell expressing LifeAct-GFP is shown. Frames were acquired every 30 seconds, and playback is at 5 frames per second.
Time lapse of lamellipodia forming on the back of a cell, relative to instantaneous cell body movement, and then being overtaken by the cell body. Maximum Z-projection through a cell expressing LifeAct-GFP is shown. Frames were acquired every 30 seconds, and playback is at 5 frames per second.
Time lapse of lamellipodia being retracted without producing cell body movement. Maximum Z-projection through a cell expressing LifeAct-GFP is shown. Frames were acquired every 30 seconds, and playback is at 5 frames per second.
Time lapse of blebs repeatedly forming to overtake other blebs. Maximum Z-projection through a cell expressing LifeAct-GFP is shown. Frames were acquired every 30 seconds, and playback is at 5 frames per second.
Time lapse of a filopodium being overtaken by a lamellipodium. Maximum Z-projection through a cell expressing LifeAct-GFP is shown. Frames were acquired every 30 seconds, and playback is at 5 frames per second.
Highlights.
A method to image the EMT in zebrafish tailbuds at high resolution is established.
Wild-type cells use productive lamellipodia to migrate persistently anteriorly.
Cells lacking Tbx16 and Msgn1 are motile but cannot leave the tailbud.
These mutant cells do not migrate directionally or form productive lamellipodia.
Embryos have a directional migratory cue that is independent of Tbx16 and Msgn1.
Acknowledgments
We thank Dale Hailey and Gregory Morgan for comments on the manuscript and Carl-Philippe Heisenberg, Cecilia Moens, Simon Wells, and David Raible for reagents. This work was supported by a National Institutes of Health grant (RO1GM079203) to DK.
Abbreviations
- EMT
epithelial to mesenchymal transition
- MET
mesenchymal to epithelial transition
- Tbx16
T-box 16/Spadetail
- Msgn1
tbx16;msgn1, Mesogenin1
- MO
morpholino-treated
- A-P
anterior-posterior
- MZ
maturation zone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amacher SL, Draper BW, Summers BR, Kimmel CB. The zebrafish T-box genes no tail and spadetail are required for development of trunk and tail mesoderm and medial floor plate. Development. 2002;129:3311–3323. doi: 10.1242/dev.129.14.3311. [DOI] [PubMed] [Google Scholar]
- Arjonen A, Kaukonen R, Ivaska J. Filopodia and adhesion in cancer cell motility. Cell Adhes Migr. 2011;5:421–430. doi: 10.4161/cam.5.5.17723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader D, Masaki T, Fishman Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear JE, Haugh JM. Directed migration of mesenchymal cells: where signaling and the cytoskeleton meet. Curr Opin Cell Biol. 2014;30:74–82. doi: 10.1016/j.ceb.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénazéraf B, Francois P, Baker RE, Denans N, Little CD, Pourquié O. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature. 2010;466:248–252. doi: 10.1038/nature09151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagden CS, Currie PD, Ingham PW, Hughes SM. Notochord induction of zebrafish slow muscle mediated by Sonic hedgehog. Genes Dev. 1997;11:2163–2175. doi: 10.1101/gad.11.17.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouldin CM, Manning AJ, Peng YH, Farr GH, Hung KL, Dong A, Kimelman D. Wnt signaling and tbx16 form a bistable switch to commit bipotential progenitors to mesoderm. Development. 2015;142:2499–2507. doi: 10.1242/dev.124024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalamalasetty RB, Garriock RJ, Dunty WC, Kennedy MW, Jailwala P, Si H, Yamaguchi TP. Mesogenin 1 is a master regulator of paraxial presomitic mesoderm differentiation. Development. 2014;141:4285–4297. doi: 10.1242/dev.110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DL, Cooper-Morgan A, Harrelson Z, Papaioannou VE. Critical role for Tbx6 in mesoderm specification in the mouse embryo. Mech Dev. 2003;120:837–847. doi: 10.1016/S0925-4773(03)00066-2. [DOI] [PubMed] [Google Scholar]
- Ciruna B, Rossant J. FGF Signaling Regulates Mesoderm Cell Fate Specification and Morphogenetic Movement at the Primitive Streak. Dev Cell. 2001;1:37–49. doi: 10.1016/S1534-5807(01)00017-X. [DOI] [PubMed] [Google Scholar]
- Clegg CH, Linkhart TA, Olwin BB, Hauschka SD. Growth factor control of skeletal muscle differentiation: commitment to terminal differentiation occurs in G1 phase and is repressed by fibroblast growth factor. J Cell Biol. 1987;105:949–956. doi: 10.1083/jcb.105.2.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craene BD, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- Delfini MC, Dubrulle J, Malapert P, Chal J, Pourquié O. Control of the segmentation process by graded MAPK/ERK activation in the chick embryo. Proc Natl Acad Sci U S A. 2005;102:11343–11348. doi: 10.1073/pnas.0502933102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray N, Lawton A, Nandi A, Jülich D, Emonet T, Holley SA. Cell-Fibronectin Interactions Propel Vertebrate Trunk Elongation via Tissue Mechanics. Curr Biol. 2013;23:1335–1341. doi: 10.1016/j.cub.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fior R, Maxwell AA, Ma TP, Vezzaro A, Moens CB, Amacher SL, Lewis J, Saúde L. The differentiation and movement of presomitic mesoderm progenitor cells are controlled by Mesogenin 1. Development. 2012;139:4656–4665. doi: 10.1242/dev.078923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florini JR, Magri KA. Effects of growth factors on myogenic differentiation. Am J Physiol. 1989;256:C701–711. doi: 10.1152/ajpcell.1989.256.4.C701. [DOI] [PubMed] [Google Scholar]
- Garnett AT, Han TM, Gilchrist MJ, Smith JC, Eisen MB, Wardle FC, Amacher SL. Identification of direct T-box target genes in the developing zebrafish mesoderm. Development. 2009;136:749–760. doi: 10.1242/dev.024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering LM, Hoshijima K, Hug B, Bisgrove B, Kispert A, Grunwald DJ. An interacting network of T-box genes directs gene expression and fate in the zebrafish mesoderm. Proc Natl Acad Sci U S A. 2003;100:9410–9415. doi: 10.1073/pnas.1633548100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik R, Gautreau A. Quantitative and unbiased analysis of directional persistence in cell migration. Nat Protoc. 2014;9:1931–1943. doi: 10.1038/nprot.2014.131. [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Amacher SL, Kimmel CB, Kimelman D. Molecular identification of spadetail: regulation of zebrafish trunk and tail mesoderm formation by T-box genes. Development. 1998;125:3379–3388. doi: 10.1242/dev.125.17.3379. [DOI] [PubMed] [Google Scholar]
- Griffin KJP, Kimelman D. Interplay between FGF, one-eyed pinhead, and T-box transcription factors during zebrafish posterior development. Dev Biol. 2003;264:456–466. doi: 10.1016/j.ydbio.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Griffin KJP, Kimelman D. One-Eyed Pinhead and Spadetail are essential for heart and somite formation. Nat Cell Biol. 2002;4:821–825. doi: 10.1038/ncb862. [DOI] [PubMed] [Google Scholar]
- Ho RK, Kane DA. Cell-autonomous action of zebrafish spt-1 mutation in specific mesodermal precursors. Nature. 1990;348:728–730. doi: 10.1038/348728a0. [DOI] [PubMed] [Google Scholar]
- Jahangiri L, Nelson AC, Wardle FC. A cis-regulatory module upstream of deltaC regulated by Ntla and Tbx16 drives expression in the tailbud, presomitic mesoderm and somites. Dev Biol. 2012;371:110–120. doi: 10.1016/j.ydbio.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jülich D, Geisler R, Holley SA. Integrinα5 and Delta/Notch Signaling Have Complementary Spatiotemporal Requirements during Zebrafish Somitogenesis. Dev Cell. 2005;8:575–586. doi: 10.1016/j.devcel.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Jülich D, Mould AP, Koper E, Holley SA. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development. 2009;136:2913–2921. doi: 10.1242/dev.038935. [DOI] [PubMed] [Google Scholar]
- Kanki JP, Ho RK. The development of the posterior body in zebrafish. Development. 1997;124:881–893. doi: 10.1242/dev.124.4.881. [DOI] [PubMed] [Google Scholar]
- Kimelman D, Martin BL. Anterior posterior patterning in early development: three strategies. Wiley Interdiscip Rev Dev Biol. 2012;1:253–266. doi: 10.1002/wdev.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Kane DA, Walker C, Warga RM, Rothman MB. A mutation that changes cell movement and cell fate in the zebrafish embryo. Nature. 1989;337:358–362. doi: 10.1038/337358a0. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Warga RM, Schilling TF. Origin and organization of the zebrafish fate map. Development. 1990;108:581–594. doi: 10.1242/dev.108.4.581. [DOI] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton AK, Nandi A, Stulberg MJ, Dray N, Sneddon MW, Pontius W, Emonet T, Holley SA. Regulated tissue fluidity steers zebrafish body elongation. Development. 2013;140:573–582. doi: 10.1242/dev.090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KE, Eisen JS. Paraxial mesoderm specifies zebrafish primary motoneuron subtype identity. Development. 2004;131:891–902. doi: 10.1242/dev.00981. [DOI] [PubMed] [Google Scholar]
- Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012;139:3471–3486. doi: 10.1242/dev.071209. [DOI] [PubMed] [Google Scholar]
- Liu C, Knezevic V, Mackem S. Ventral tail bud mesenchyme is a signaling center for tail paraxial mesoderm induction. Dev Dyn. 2004;229:600–606. doi: 10.1002/dvdy.20017. [DOI] [PubMed] [Google Scholar]
- Marques SR, Lee Y, Poss KD, Yelon D. Reiterative roles for FGF signaling in the establishment of size and proportion of the zebrafish heart. Dev Biol. 2008;321:397–406. doi: 10.1016/j.ydbio.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BL, Kimelman D. Canonical Wnt signaling dynamically controls multiple stem cell fate decisions during vertebrate body formation. Dev Cell. 2012;22:223–232. doi: 10.1016/j.devcel.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijering E, Dzyubachyk O, Smal I. Imaging and Spectroscopic Analysis of Living Cells. In: Conn PM, editor. Methods in Enzymology. Elsevier; 2012. pp. 183–200. [DOI] [PubMed] [Google Scholar]
- Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Hijikata T, Matsukawa M, Ishikawa H, Yorifuji H. Zebrafish protocadherin 10 is involved in paraxial mesoderm development and somitogenesis. Dev Dyn. 2006;235:506–514. doi: 10.1002/dvdy.20622. [DOI] [PubMed] [Google Scholar]
- Nelson WJ. Remodeling Epithelial Cell Organization: Transitions Between Front Rear and Apical Basal Polarity. Cold Spring Harb Perspect Biol. 2009;1:a000513. doi: 10.1101/cshperspect.a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Chi ME, Bryson-Richardson R, Sonntag C, Hall TE, Gibson A, Sztal T, Chua W, Schilling TF, Currie PD. Morphogenesis and Cell Fate Determination within the Adaxial Cell Equivalence Group of the Zebrafish Myotome. PLoS Genet. 2012;8:e1003014. doi: 10.1371/journal.pgen.1003014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA. Epithelial Plasticity: A Common Theme in Embryonic and Cancer Cells. Science. 2013;342:1234850. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- Nowotschin S, Ferrer-Vaquer A, Concepcion D, Papaioannou VE, Hadjantonakis AK. Interaction of Wnt3a, Msgn1 and Tbx6 in neural versus paraxial mesoderm lineage commitment and paraxial mesoderm differentiation in the mouse embryo. Dev Biol. 2012;367:1–14. doi: 10.1016/j.ydbio.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill K, Thorpe C. BMP signaling and spadetail regulate exit of muscle precursors from the zebrafish tailbud. Dev Biol. 2013;375:117–127. doi: 10.1016/j.ydbio.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluch EK, Raz E. The role and regulation of blebs in cell migration. Curr Opin Cell Biol. 2013;25:582–590. doi: 10.1016/j.ceb.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig G, Pulgar E, Concha ML. Cell migration: from tissue culture to embryos. Development. 2014;141:1999–2013. doi: 10.1242/dev.101451. [DOI] [PubMed] [Google Scholar]
- Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, Sixt M, Wedlich-Soldner R. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos ET, Condeelis JS, Patsialou A. Chemotaxis in cancer. Nat Rev Cancer. 2011;11:573–587. doi: 10.1038/nrc3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row RH, Maître JL, Martin BL, Stockinger P, Heisenberg CP, Kimelman D. Completion of the epithelial to mesenchymal transition in zebrafish mesoderm requires Spadetail. Dev Biol. 2011;354:102–110. doi: 10.1016/j.ydbio.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders LR, McClay DR. Sub-circuits of a gene regulatory network control a developmental epithelial-mesenchymal transition. Development. 2014;141:1503–1513. doi: 10.1242/dev.101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Merker S, Stainier DYR. Out with the old, in with the new: reassessing morpholino knockdowns in light of genome editing technology. Development. 2014;141:3103–3104. doi: 10.1242/dev.112003. [DOI] [PubMed] [Google Scholar]
- Stulberg MJ, Lin A, Zhao H, Holley SA. Crosstalk between Fgf and Wnt signaling in the zebrafish tailbud. Dev Biol. 2012;369:298–307. doi: 10.1016/j.ydbio.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazumi S, Yabe S, Yokoyama J, Aihara Y, Uchiyama H. pMesogenin1 and 2 function directly downstream of Xtbx6 in Xenopus somitogenesis and myogenesis. Dev Dyn. 2008;237:3749–3761. doi: 10.1002/dvdy.21791. [DOI] [PubMed] [Google Scholar]
- Thévenaz P, Ruttimann UE, Unser M. A Pyramid Approach to Subpixel Registration Based on Intensity. IEEE Trans Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-Mesenchymal Transitions in Development and Disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Warga RM, Mueller RL, Ho RK, Kane DA. Zebrafish Tbx16 regulates intermediate mesoderm cell fate by attenuating Fgf activity. Dev Biol. 2013;383:75–89. doi: 10.1016/j.ydbio.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warga RM, Nusslein-Volhard C. Origin and development of the zebrafish endoderm. Development. 1999;126:827–838. doi: 10.1242/dev.126.4.827. [DOI] [PubMed] [Google Scholar]
- Wells S, Nornes S, Lardelli M. Transgenic Zebrafish Recapitulating tbx16 Gene Early Developmental Expression. PLoS ONE. 2011;6:e21559. doi: 10.1371/journal.pone.0021559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windner SE, Bird NC, Patterson SE, Doris RA, Devoto SH. Fss/Tbx6 is required for central dermomyotome cell fate in zebrafish. Biol Open. 2012;1:806–814. doi: 10.1242/bio.20121958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Li C, Takahashi K, Slavkin HC, Shum L, Deng CX. Murine Fibroblast Growth Factor Receptor 1α Isoforms Mediate Node Regression and Are Essential for Posterior Mesoderm Development. Dev Biol. 1999;208:293–306. doi: 10.1006/dbio.1999.9227. [DOI] [PubMed] [Google Scholar]
- Yabe T, Takada S. Mesogenin causes embryonic mesoderm progenitors to differentiate during development of zebrafish tail somites. Dev Biol. 2012;370:213–222. doi: 10.1016/j.ydbio.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Amacher SL, Kim SH, Geissert D, Kimmel CB, De Robertis EM. Zebrafish paraxial protocadherin is a downstream target of spadetail involved in morphogenesis of gastrula mesoderm. Development. 1998;125:3389–3397. doi: 10.1242/dev.125.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JK, Wold B. The bHLH regulator pMesogenin1 is required for maturation and segmentation of paraxial mesoderm. Genes Dev. 2000;14:3204–3214. doi: 10.1101/gad.850000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time lapse of wild-type donor cells transplanted into a wild-type host. Movie shows a Z-projection from a 10 μm Z-stack of fluorescent dextran-labeled donor cells (red) overlaid on a single bright field plane. Frames were acquired every five minutes for two hours, and playback is at 5 frames per second. Figure 2B shows stills from this movie with membrane fluorescence images replacing bright field images.
Figure S1. Wild-type cells in MO embryos can segment into somites, but not differentiate into muscle. A. Lateral view of a 24 hour post fertilization MO host with wild-type donor cells. Left panel is a single frame bright field image; middle is a maximum Z projection of fluorescent dextran-labeled donor cells; right is a maximum Z projection of muscle myosin staining. B. Ventro-lateral view of a 14 somite wild-type control embryo stained for muscle myosin, which highlights somite and pre-somitic mesoderm cells. Left panel is a single frame bright field image; right is a maximum Z projection of muscle myosin staining. Arrows show somite boundaries and arrowheads show newly forming somite boundary.
Figure S2. Tailbud explants continue to form somites in culture. Tailbud explanted from a Tg(Ptbx16-3.3:memRFP) embryo imaged every hour after dissection, beginning immediately after mounting. Arrows denote somite boundaries; arrowheads denote newly forming somite boundaries.
Figure S3. Box and whisker plots of migration data shown in bar graphs. A. Average speed of donor cells measured over two hours as in Figure 3B. B. Average net distance of donor cells from their starting points at the end of two hours as in Figure 3C. C. The persistence of donor cell movement at two hours is equal to the ratio of the straight line distance between starting and ending points to the total distance travelled as in Figure 4A. Horizontal lines show means, boxes show 1st and 3rd quartiles, and whiskers show minimums and maximum. “x”s show outliers.
Figure S4. Tbx16 and Msgn1 have cell autonomous effects on migration direction. Tracks of transplanted cells from Figure 3A only showing A-P (X-axis) movement with lateral (Y-axis) component of movement removed. Tracks are plotted so that they change Y-axis value every time the direction of A-P movement reverses. The X-axis is 70 μm total, with scale bar equaling 20 μm. Asterisk in MO→wt condition indicates a cell that moved significantly anteriorly and then reversed direction (same cell indicated with asterisk in Figure 3A).
Figure S5. Tbx16 and Msgn1 have cell autonomous and non-autonomous effects on medio-lateral migration. A. Tracks of transplanted cells from Figure 3A only showing medio-lateral (Y-axis) movement with A-P (X-axis) component of movement removed. Tracks are plotted so that they change X-axis value every time the direction of medio-lateral movement reverses. The Y-axis is 70 μm total, with scale bar equaling 20 μm. B. The percentage of time points that cells moved medially, laterally, or did not move along the medio-lateral axis. *: p<0.01 by χ2 test.
Figure S6. Protrusion examples. 3D renderings of LifeAct-GFP in individual cells show a lamellipodium (A), filopodia (B), and blebs (C), indicated by arrows.
Time lapse of tbx16;msgn MO donor cells transplanted into a wild-type host. Movie shows a Z-projection from a 10 μm Z-stack of fluorescent dextran-labeled donor cells (red) overlaid on a single bright field plane. Frames were acquired every five minutes for two hours, and playback is at 5 frames per second.
Time lapse of wild-type donor cells transplanted into a tbx16;msgn MO host. Movie shows a Z-projection from a 10 μm Z-stack of fluorescent dextran-labeled donor cells (red) overlaid on a single bright field plane. Frames were acquired every five minutes for two hours, and playback is at 5 frames per second.
Time lapse of tbx16;msgn MO donor cells transplanted into a tbx16;msgn MO host. Movie shows a Z-projection from a 10 μm Z-stack of fluorescent dextran-labeled donor cells (red) overlaid on a single bright field plane. Frames were acquired every five minutes for two hours, and playback is at 5 frames per second.
Time lapse of lamellipodia forming on the front of a cell, relative to instantaneous cell body movement, and then being overtaken by the cell body. Maximum Z-projection through a cell expressing LifeAct-GFP is shown. Frames were acquired every 30 seconds, and playback is at 5 frames per second.
Time lapse of lamellipodia forming on the back of a cell, relative to instantaneous cell body movement, and then being overtaken by the cell body. Maximum Z-projection through a cell expressing LifeAct-GFP is shown. Frames were acquired every 30 seconds, and playback is at 5 frames per second.
Time lapse of lamellipodia being retracted without producing cell body movement. Maximum Z-projection through a cell expressing LifeAct-GFP is shown. Frames were acquired every 30 seconds, and playback is at 5 frames per second.
Time lapse of blebs repeatedly forming to overtake other blebs. Maximum Z-projection through a cell expressing LifeAct-GFP is shown. Frames were acquired every 30 seconds, and playback is at 5 frames per second.
Time lapse of a filopodium being overtaken by a lamellipodium. Maximum Z-projection through a cell expressing LifeAct-GFP is shown. Frames were acquired every 30 seconds, and playback is at 5 frames per second.