Abstract
Originally studied for its role in energy homeostasis, the paraventricular nucleus of the thalamus (PVT) has recently gained attention because of its involvement in the modulation of drug-directed behavior. The posterior part of the PVT (pPVT) is connected with brain structures that modulate motivated behavior, and we tested whether the pPVT plays a pivotal role in cocaine seeking. The aim of the present study was to investigate whether transient inactivation of the pPVT prevents cue-induced reinstatement of cocaine seeking but not natural reward seeking. Male Wistar rats were trained to associate a discriminative stimulus (S+) with the availability of cocaine or a highly palatable conventional reinforcer, sweetened condensed milk (SCM). Following extinction, the cocaine S+ and SCM S+ elicited comparable levels of reinstatement. Intra-pPVT administration of the γ-aminobutyric acid-A (GABAA) and GABAB receptor agonists muscimol and baclofen (0.06 and 0.6 mM, respectively) prior to the presentation of the cocaine or SCM S+ completely prevented the reinstatement of cocaine seeking, with no statistically significant effects on SCM seeking. These data show that the pPVT plays an important role in neuronal mechanisms that drive cocaine-seeking behavior.
Keywords: paraventricular nucleus of the thalamus, cocaine, natural reward, conditioned reinstatement
INTRODUCTION
The high relapse rate and long-lasting vulnerability to relapse in abstinent individuals are among the challenges encountered in the effective treatment of drug addiction [17, 25, 27, 28]. One hypothesis that can explain the long-lasting nature of drug-seeking behavior is that the neuronal circuits that mediate the control of drug-seeking and drug-taking behaviors have common motivational neuronal substrates that are not specific to addiction-related processes but are robustly activated by drugs.
The thalamus was recently proposed to be included in the neurocircuitry of addiction [15, 24]. The paraventricular nucleus of the thalamus (PVT), considered a communication point between the ventral and dorsal striatum and lateral hypothalamus, has drawn attention because it plays a key role in energy homeostasis, arousal, endocrine regulation, and reward [3, 14, 30, 35] and has been reported to be engaged in the effects of drugs (e.g., cocaine and ethanol). The posterior part of the PVT (pPVT) projects to the nucleus accumbens (NAC), extended amygdala, and medial prefrontal cortex (mPFC) [18], placing the pPVT at a pivotal point to modulate motivated behavior.
Earlier findings showed that the PVT is activated by the presentation of cocaine-paired cues [5]. Recent findings demonstrated that transient inactivation of the PVT prevented cocaine prime-induced reinstatement [12] and the expression of conditioned place preference [6]. Moreover, data from this laboratory demonstrated a positive correlation between cocaine-seeking behavior that was induced by cocaine-related stimuli and PVT activation (measured by Fos-expressing neurons), whereas presentation of the stimulus that was predictive of a potent food reward (SCM S+) elicited reinstatement but induced only nonspecific Fos expression that was not correlated with behavior, confirming that the PVT, as a whole, is recruited during the conditioned reinstatement of cocaine seeking [22].
Based on the previous observations that the PVT is differentially recruited by stimuli that are conditioned to the availability of cocaine vs. palatable food reward and that the pPVT is specifically connected to brain structures that are involved in the regulation of motivated behavior, the aim of the present study was to evaluate the importance of pPVT integrity in cocaine-seeking behavior vs. behavior that is motivated by stimuli that are conditioned to a highly palatable conventional reinforcer (sweetened condensed milk [SCM]). This was achieved by transiently inactivating the pPVT using the γ-aminobutyric acid-A (GABAA) and GABAB receptor agonists muscimol and baclofen, respectively, administered together before conditioned reinstatement.
MATERIALS AND METHODS
Animals
Forty-two male Wistar rats (Charles River, Wilmington, MA, USA), weighing 200–225 g upon arrival, were housed two per cage in a temperature- and humidity-controlled vivarium on a reverse 12 h/12 h light/dark cycle with ad libitum access to food and water. All of the procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Drugs
Cocaine hydrochloride (COC; National Institute on Drug Abuse, Bethesda, MD, USA) was dissolved in 0.9% sodium chloride (Hospira, Lake Forest, IL, USA; 2.5 mg/ml). Sweetened condensed milk (Nestlé, Solon, OH, USA) was diluted 2:1 (v/v) in water. Muscimol and baclofen (M/B; Tocris Bioscience, Bristol, United Kingdom) were dissolved in 0.9% sodium chloride at concentrations of 0.06 and 0.6 mM, respectively.
Self-administration, conditioning, and extinction
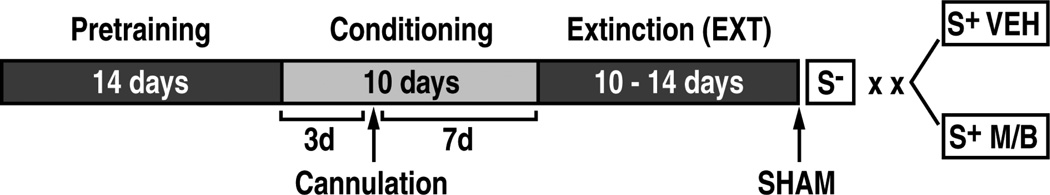
Behavioral training and testing were conducted as previously described [20, 21] (Fig. 1). Rats that were designated for COC self-administration training were surgically prepared with indwelling silastic catheters that were inserted in the right jugular vein. Rats that were designated for testing with the highly palatable food reward, SCM, were not subjected to surgical procedures. Following 7 days of postsurgical recovery, the rats began self-administration training. Each session was initiated by extending two retractable levers into the operant conditioning chamber. The self-administration of COC (0.25 mg per 0.1 ml infusion, delivered over 4 s) or SCM (0.1 ml delivered into a 0.2 ml receptacle) began on a fixed-ratio 1 (FR1) schedule of reinforcement in daily 120-min (COC) or 40-min (SCM) sessions, 5 days per week. Responses at the right, active lever were reinforced, followed by a 20-s timeout (20 s TO) period that was signaled by illumination of a cue light above the active lever. During this time, the lever remained inactive to prevent accidental overdosing with COC. To maintain identical training and experimental conditions, the 20 s signaled TO period was implemented during SCM self-administration. Responses at the left, inactive lever were without programmed consequences. Following 10 days of COC or SCM self-administration training, a contingency was introduced whereby responses at the active lever were differentially reinforced in the presence of discriminative stimuli (SD) that signaled reward availability vs. non-availability. Constant 70 dB white noise served as a discriminative stimulus (S+) that signaled availability of the reinforcer (COC or SCM), whereas illumination of a 2.8 W house light located at the top of the chamber’s front panel served as a discriminative stimulus (S−) that signaled non-availability of the reinforcer (i.e., saline solution instead of COC or no consequence instead of SCM). Each session was initiated by presenting the respective SD and extending the levers into the chambers. The SD remained present until termination of the session by retraction of the levers. In the presence of the S+, responses at the right, active lever were reinforced by COC or SCM on an FR1 schedule, followed by a 20 s TO period that was signaled by illumination of a cue light above the lever. In the presence of the S−, responses at the right, active lever were followed by an intermittent tone, during which the lever remained inactive for 20 s. Three daily sessions (each lasting 1 h for the COC group and 20 min for the SCM group), separated by 30 min intervals, were conducted, with two S+ (reward) sessions and one S− (non-reward) session sequenced in random order. The SCM sessions were restricted to 20 min to avoid satiety by the excessive ingestion of SCM and to ensure that the number of responses were comparable during the first and second S+ sessions [20, 21]. Three days after the beginning of the conditioning training, the rats were implanted with a guide cannula (23-gauge, 15 mm, Plastics One, Roanoke, VA, USA) aimed at the pPVT (anterior/posterior, −3.3 mm; medial/lateral, ±2.72 mm from bregma; dorsal/ventral, −2.96 mm from dura, at an angle of 25° [31], positioned 3.5 mm above the target injection point; Fig. 2). After 7–10 days of recovery, the animals resumed conditioning training for an additional 7 days. Following a total of 10 days of conditioning training (i.e., a total of 20 S+ and 10 S− sessions), both the COC and SCM groups were placed on extinction (EXT) conditions in daily 1 h sessions. Each EXT session was initiated by extension of the active and inactive levers in the absence of either SD. During an EXT session, the rats were allowed to lever press, but they did not receive reinforcement. The EXT criterion was less than five responses per session for 3 consecutive days.
Fig. 1.
General behavioral procedure.
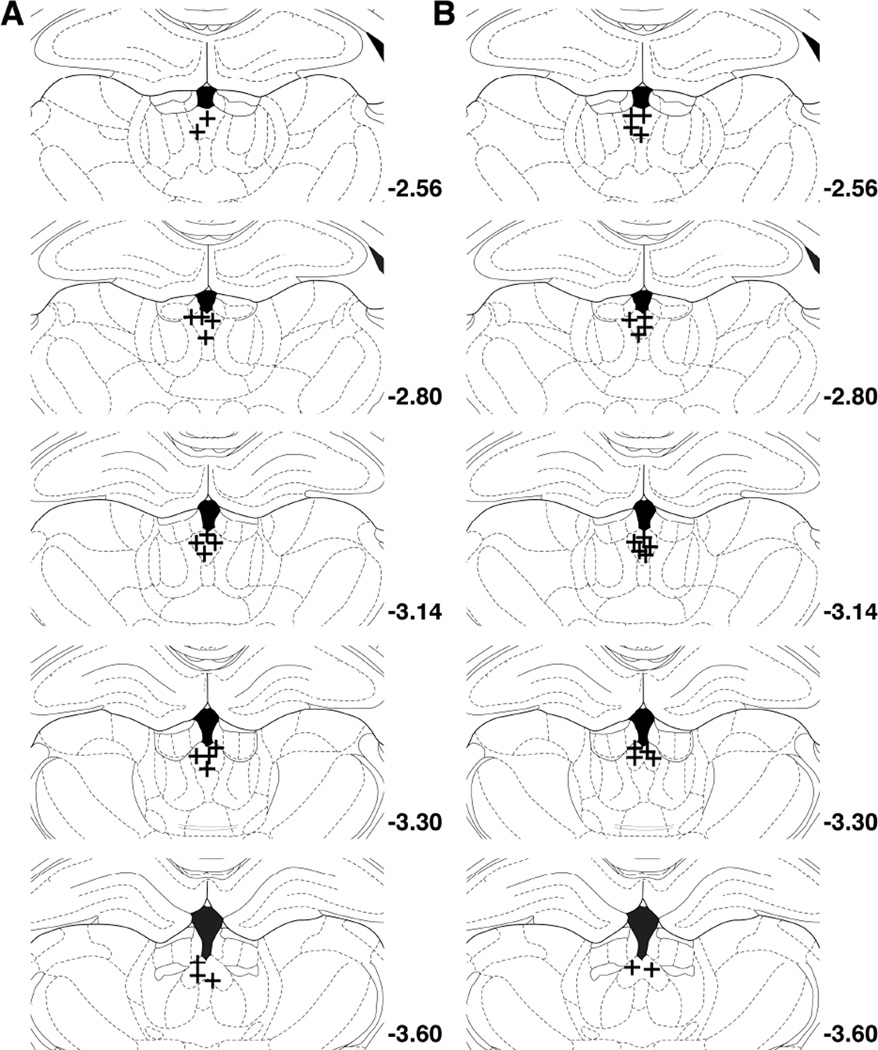
Fig. 2.
pPVT injection sites: (A) cocaine, (B) SCM (sweetened condensed milk).
Conditioned reinstatement
On the last day of EXT training, every rat received a sham injection (SHAM) for habituation to the microinjection (Fig. 1). Twenty-four hours later, both groups of animals (SCM and COC) underwent the reinstatement test with reintroduction of the S−. Three days later, the animals received an intra-PVT microinjection of the GABAA/GABAB agonists (M/B) or vehicle (VEH; Fig. 1) using a microinfusion pump (Harvard 22 Syringe Pump, Holliston, MA, USA) and injectors that extended 3.5 mm beyond the guide cannula. Injections were made at a flow rate of 0.5 µl/min over 1 min, followed by an additional 1 min with the injector in place to allow for drug diffusion. Reinstatement tests were conducted under extinction conditions but with reintroduction of the S+ only.
Histology
Upon completion of the reinstatement test, the rats were euthanized by CO2 inhalation, and their brains were collected and snap frozen. The brains were then sliced in 40 µm coronal sections, and injector placements within the pPVT were verified (Fig. 2).
Statistical analysis
The data were analyzed using one- or two-way analysis of variance (ANOVA). Significant main effects or interactions were followed by the Protected Least Significant Difference (PLSD) post hoc test or pairwise comparisons when appropriate. Differences in responding at the active lever between the respective reward and non-reward conditions during the last day of the training/conditioning phase were analyzed using paired t-tests.
RESULTS
Two rats in the SCM group and four rats in the COC group were lost because of cannula misplacement (n = 2 for SCM and 1 for COC), health complications (n = 2 for COC), or the lack of acquisition of COC self-administration (n = 1), thus reducing the number of animals to n = 17 for COC and n = 19 for SCM.
Self-administration, conditioning, and extinction
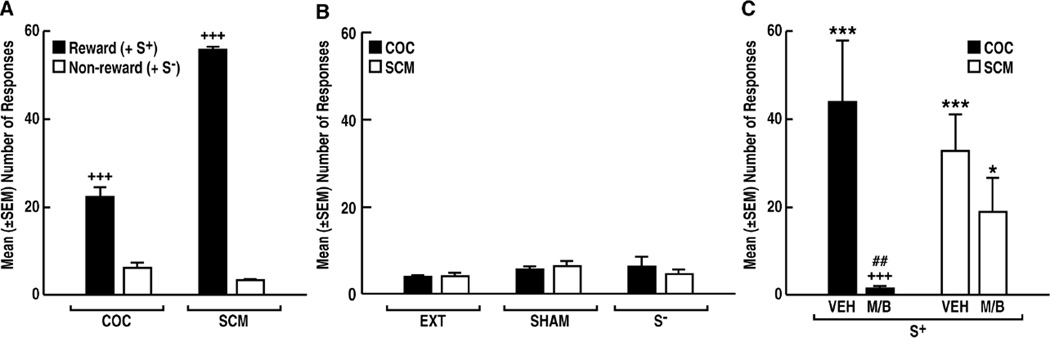
Rats in both the COC (n = 17) and SCM (n = 19) groups acquired robust and stable responding for the reward and showed only negligible responding for the non-reward (paired t-test: COC, t16 = 7.974, p < 0.0001; SCM, t18 = 109.4, p < 0.0001; Fig. 3A). The number of days to reach the extinction criteria (≤ 5 responses) was similar for both the COC and SCM animals (10 ± 5 days and 12 ± 4 days, respectively).
Fig. 3.
Inactivation of the pPVT selectively prevents cocaine-seeking behavior. Following conditioning (+++p < 0.001, vs. respective non-reward) (A) and extinction (B), presentation of the COC S+ or SCM S+ (C) under vehicle (VEH) conditions produced robust reinstatement compared with the non-reward stimulus (S−; ***p < 0.001, *p < 0.05, vs. respective S−). Inactivation of the pPVT with muscimol/baclofen (M/B) prevented cocaine seeking only (+++p < 0.001, vs. VEH; ##p < 0.01, vs. S+ SCM with M/B pretreatment). COC, cocaine; SCM, sweetened condensed milk; EXT, extinction; VEH, vehicle; M/B, muscimol/baclofen.
Conditioned reinstatement
Neither SHAM nor S− presentation induced reinstatement (two-way ANOVA; Group [COC, SCM]: F1,102 = 0.009, p > 0.05; Reinstatement [EXT, SHAM, S−]: F2,102 = 1.441, p > 0.05; Group × Reinstatement interaction: F2,102 = 0.51, p > 0.05; Fig. 3B). Following the intra-pPVT VEH injection, S+ presentation produced comparable reinstatement in both COC and SCM animals (two-way ANOVA; Group [COC, SCM]: F1,15 = 0.369, p > 0.05, Reinstatement [S−, S+ VEH]: F1,15 = 34.58, p < 0.0001; Group × Reinstatement interaction: F1,15 = 0.2517, p > 0.05; Fig. 3C).
Following the M/B injection, COC-seeking behavior was completely abolished (two-way ANOVA; Group [COC, SCM]: F1,17 = 4.567, p < 0.05; Reinstatement [S−, S+ M/B]: F1,17 = 2.070, p > 0.05; Group × Reinstatement interaction: F1,17 = 5.369, p < 0.05; Fig. 3C). Post hoc tests confirmed that M/B prevented COC seeking (PLSD p < 0.01, vs. SCM S+ M/B; Fig. 3C). Separate two-way ANOVAs for the COC and SCM groups showed that M/B had no significant effect on the conditioned reinstatement of SCM seeking, although a noticeable reduction was observed (PLSD post hoc test; p < 0.05, vs. S− following two-way ANOVA; Group [VEH, M/B]: F1,17 = 1.04, p > 0.05; Reinstatement [S−, S+]: F1,17 = 18.30, p < 0.001; Group × Reinstatement interaction: F1,17 = 1.69, p > 0.05; Fig. 3B, C). In contrast, M/B completely prevented the effect of the COC S+ (PLSD post hoc test; p < 0.05, vs. S−; p < 0.001, vs. S+ VEH, following two-way ANOVA; Group [VEH, M/B]: F1,15 = 7.12, p < 0.05; Reinstatement [S−, S+]: F1,15 = 14.47, p < 0.001; Group × Reinstatement interaction: F1,15 = 22.09, p < 0.001; Fig. 3B, C).
Interestingly, the three animals with cannula misplacements (n = 1 for SCM S+ VEH, n = 1 for SCM S+ M/B, and n = 1 for COC S+ M/B) exhibited comparable conditioned reinstatement to the S+ VEH groups of rats (27 responses for SCM S+ VEH, 32 responses for SCM S+ M/B, and 34 responses for COC S+ M/B).
DISCUSSION
Although reward-predictive environmental stimuli (S+) produced strong and identical recovery of responding, regardless of whether they were conditioned to a conventional reward (SCM) or drug reward (cocaine), transient inactivation of the pPVT completely inhibited cocaine-seeking behavior but had no significant effect on the motivational actions of stimuli that were conditioned to SCM. These findings implicate the pPVT as an important site that regulates the control of cocaine seeking by environmental stimuli, with a negligible effect on behavior that is controlled by stimuli that are conditioned to a highly palatable food reward.
Before discussing the implications of these findings, examining differences in baseline levels of responding during conditioning is necessary, which were considerably higher with SCM than with cocaine. Despite these differences, the level of reinstatement that was induced by the cocaine- and SCM-associated stimuli was similar. Reinforcing efficacy and the rate of responding on FR schedules are not necessarily correlated [32, 33]. The concentration of SCM that was used in the present study has been shown to maintain breakpoints under a progressive-ratio schedule of reinforcement that are comparable to those measured with the dose of cocaine that was used in the present study [32, 33]. Moreover, the magnitude of the response that was induced by the cocaine and SCM S+ during the reinstatement tests was statistically identical, which was the goal of the present paradigm. This suggests that under the present conditions, reliable and comparable conditioning effects occurred for the cocaine and SCM reinforcers, which is consistent with previous reports [1, 10, 20, 21].
One hypothesis that may explain the importance of pPVT inactivation in preventing cocaine seeking vs. SCM seeking is that the pPVT is more recruited/activated during drug-related behavior and essential for mediating cocaine-seeking behavior, but not to the same extent as SCM-seeking behavior. This hypothesis is supported by previous findings [11] and data from this laboratory that showed a correlation between cocaine seeking and PVT activation (i.e., increased Fos expression) but no correlation between SCM seeking and PVT activation [22]. An alternative explanation for the specific blockade of cocaine seeking vs. SCM seeking by M/B is that during self-administration, cocaine alters PVT neurotransmission, which in turn makes the PVT more sensitive to pharmacological manipulations. Supporting this hypothesis, a study from our laboratory investigated the involvement of orexin/hypocretin (Orx/Hcrt) transmission in the pPVT during cocaine-seeking behavior and found that although intra-pPVT orexin-A/hypocretin-1 injections exerted a priming-like effect (i.e., reinstated both cocaine and SCM seeking), it produced different dose-response profiles, with stronger reinstatement of cocaine vs. SCM seeking at moderate doses [23].
The present study shows that the integrity of the pPVT is necessary for the expression of cocaine-seeking behavior but not SCM-seeking behavior. These data extend earlier findings that showed that lesions of the entire PVT disrupted several behavioral effects of drugs of abuse. For example, electrolytic lesions of the PVT prevented cocaine-induced locomotor sensitization [37]. Excitotoxic lesions of the PVT blocked the contextual reinstatement of alcohol seeking [9]. Transient inactivation of the PVT with tetrodotoxin attenuated cocaine-induced reinstatement [12]. Microinjection of a κ opioid receptor agonist into the PVT blocked the contextual reinstatement of alcoholic beer seeking [19]. Moreover, pPVT neurons project to brain structures that mediate craving and cocaine seeking (e.g., medial prefrontal cortex [mPFC], nucleus accumbens shell [NACsh], and amygdala) [7, 15, 26, 36]. The PVT also sends glutamatergic efferents to the NACsh. When the PVT is stimulated, an increase in glutamate levels is observed in the NACsh [13, 29], a phenomenon that has been hypothesized to be linked to cocaine-seeking behavior in animal models [8]. Additional evidence from the ethanol research field also supports a role for PVT projections to the NACsh in the regulation of drug-seeking behavior, such that the context-induced reinstatement of alcoholic beer seeking is associated with PVT-NACsh pathway recruitment [9]. Therefore, the behavioral effect that stems from pPVT inactivation (i.e., full prevention of cocaine-seeking behavior) may result from the blockade of communication between this thalamic nucleus and its projection targets that participate in the mediation of drug-seeking behavior.
The PVT is known to be involved in ingestive behavior [2, 4, 14, 30, 34]. The present study extends these findings, showing that the pPVT participates in the regulation of behavior that is motivated by stimuli that are conditioned to COC and to a lesser extent behavior that is motivated by stimuli that are conditioned to a highly palatable food. Importantly, a functional difference has been reported between the roles of the anterior part of the PVT (aPVT) and pPVT in alcohol and sucrose (another palatable sweet solution) ingestion. For example, Barson et al. found that Orx/Hcrt administration in the aPVT stimulated alcohol intake but had no effect on sucrose, food, or water intake, whereas intra-pPVT Orx/Hcrt injection only increased sucrose intake, with no effect on alcohol, water, or food intake [2]. Earlier studies reported that lesions of the PVT increased food intake and weight gain in rats [4], and intra-pPVT muscimol injections increased food consumption in rats [34]. These studies are difficult to reconcile with the present findings. Nevertheless, the intra-PVT injections in the current study were aimed at the posterior portion of the nucleus. Because different subregions of the PVT may present different connectivity (e.g., [15]), further work will be required to determine whether injections into the rostral or medial poles of the PVT exert similar effects on COC conditioned reinstatement vs. SCM conditioned reinstatement as those observed in the present study.
Overall, the present data show that pPVT functionality is necessary for the expression of cocaine-seeking behavior. Remaining to be determined, however, is the importance of subpopulations of pPVT neurons that are recruited during cocaine-seeking behavior. The selective inactivation of neuronal ensembles using Daun02 inactivation in c-fos-lacZ transgenic rats [16] will confirm whether the pPVT neurons that are activated during cocaine seeking are necessary for the expression of cocaine-seeking behavior. Such data may reveal valuable targets for the treatment of drug abuse, craving, and relapse, without producing nonspecific side effects that may interfere with natural motivated behavior. Other unresolved issues are whether inactivation of the pPVT will affect conditioned reinstatement for other drugs of abuse (e.g., heroin and alcohol) and whether the aPVT and pPVT are distinctly recruited during the different stages of the addiction process (i.e., consummatory behavior maintained by the drugs vs. conditioned reinstatement induced by drug-paired contextual stimuli).
Highlights.
The paraventricular nucleus of the thalamus (PVT) has recently gained attention because of its involvement in the modulation of drug-directed behavior.
Cue-induced reinstatement of cocaine seeking is more sensitive to temporary inactivation of the pPVT than cue-induced reinstatement of natural reward seeking.
The pPVT plays an important role in neuronal mechanisms that drive cocaine-seeking behavior.
Acknowledgements
This is publication number 28067-MCN from The Scripps Research Institute. Research support: NIH/NIDA DA033344 (R.M.F), DA08467, and DA07348 (F.W.). We thank M. Bainier for technical assistance and M. Arends for assistance with preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barson JR, Ho HT, Leibowitz SF. Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addiction biology. 2015;20:469–481. doi: 10.1111/adb.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 4.Bhatnagar S, Dallman MF. The paraventricular nucleus of the thalamus alters rhythms in core temperature and energy balance in a state-dependent manner. Brain research. 1999;851:66–75. doi: 10.1016/s0006-8993(99)02108-3. [DOI] [PubMed] [Google Scholar]
- 5.Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browning JR, Jansen HT, Sorg BA. Inactivation of the paraventricular thalamus abolishes the expression of cocaine conditioned place preference in rats. Drug and alcohol dependence. 2014;134:387–390. doi: 10.1016/j.drugalcdep.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S, Su HS. Afferent connections of the thalamic paraventricular and parataenial nuclei in the rat--a retrograde tracing study with iontophoretic application of Fluoro-Gold. Brain research. 1990;522:1–6. doi: 10.1016/0006-8993(90)91570-7. [DOI] [PubMed] [Google Scholar]
- 8.Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci. 2009;29:802–812. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- 10.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 11.James MH, Charnley JL, Flynn JR, Smith DW, Dayas CV. Propensity to 'relapse' following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience. 2011;199:235–242. doi: 10.1016/j.neuroscience.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 12.James MH, Charnley JL, Jones E, Levi EM, Yeoh JW, Flynn JR, Smith DW, Dayas CV. Cocaine- and amphetamine-regulated transcript (CART) signaling within the paraventricular thalamus modulates cocaine-seeking behaviour. PLoS One. 2010;5:e12980. doi: 10.1371/journal.pone.0012980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones MW, Kilpatrick IC, Phillipson OT. Regulation of dopamine function in the nucleus accumbens of the rat by the thalamic paraventricular nucleus and adjacent midline nuclei. Experimental brain research. 1989;76:572–580. doi: 10.1007/BF00248914. [DOI] [PubMed] [Google Scholar]
- 14.Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- 15.Kirouac GJ. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neuroscience and biobehavioral reviews. 2015;56:315–329. doi: 10.1016/j.neubiorev.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, Mitchell TB, Farquhar D, Ghosh SC, Mattson BJ, Hope BT. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nature neuroscience. 2009;12:1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. The Journal of comparative neurology. 2008;506:263–287. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- 19.Marchant NJ, Furlong TM, McNally GP. Medial dorsal hypothalamus mediates the inhibition of reward seeking after extinction. J Neurosci. 2010;30:14102–14115. doi: 10.1523/JNEUROSCI.4079-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin-Fardon R, Baptista MA, Dayas CV, Weiss F. Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. J Pharmacol Exp Ther. 2009;329:1084–1090. doi: 10.1124/jpet.109.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology. 2007;32:1967–1973. doi: 10.1038/sj.npp.1301323. [DOI] [PubMed] [Google Scholar]
- 22.Matzeu A, Cauvi G, Kerr TM, Weiss F, Martin-Fardon R. The paraventricular nucleus of the thalamus is differentially recruited by stimuli conditioned to the availability of cocaine versus palatable food. Addiction biology. 2015 doi: 10.1111/adb.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matzeu A, Kerr T, Weiss F, Martin-Fardon R. Society for Neuroscience. San Diego, CA: Neuroscience Meeting Planner; 2013. Orexin/hypocretin in the paraventricular nucleus of the thalamus mediates cocaine-seeking behavior in rats. Program No. 350.19. 2013, Online. [Google Scholar]
- 24.Matzeu A, Zamora-Martinez ER, Martin-Fardon R. The paraventricular nucleus of the thalamus is recruited by both natural rewards and drugs of abuse: recent evidence of a pivotal role for orexin/hypocretin signaling in this thalamic nucleus in drug-seeking behavior. Frontiers in behavioral neuroscience. 2014;8:117. doi: 10.3389/fnbeh.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA : the journal of the American Medical Association. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 26.Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol. 1995;359:221–238. doi: 10.1002/cne.903590204. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? Journal of psychopharmacology. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien CP, McLellan AT. Myths about the treatment of addiction. Lancet. 1996;347:237–240. doi: 10.1016/s0140-6736(96)90409-2. [DOI] [PubMed] [Google Scholar]
- 29.Parsons MP, Li S, Kirouac GJ. Functional and anatomical connection between the paraventricular nucleus of the thalamus and dopamine fibers of the nucleus accumbens. J Comp Neurol. 2007;500:1050–1063. doi: 10.1002/cne.21224. [DOI] [PubMed] [Google Scholar]
- 30.Parsons MP, Li S, Kirouac GJ. The paraventricular nucleus of the thalamus as an interface between the orexin and CART peptides and the shell of the nucleus accumbens. Synapse. 2006;59:480–490. doi: 10.1002/syn.20264. [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd ed. San Diego: Academic Press; 1997. Vol. [DOI] [PubMed] [Google Scholar]
- 32.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of neuroscience methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 33.Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- 34.Stratford TR, Wirtshafter D. Injections of muscimol into the paraventricular thalamic nucleus, but not mediodorsal thalamic nuclei, induce feeding in rats. Brain research. 2013;1490:128–133. doi: 10.1016/j.brainres.2012.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness, Brain research. Brain research reviews. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 36.Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol. 2008;508:212–237. doi: 10.1002/cne.21679. [DOI] [PubMed] [Google Scholar]
- 37.Young CD, Deutch AY. The effects of thalamic paraventricular nucleus lesions on cocaine-induced locomotor activity and sensitization. Pharmacology, biochemistry, and behavior. 1998;60:753–758. doi: 10.1016/s0091-3057(98)00051-3. [DOI] [PubMed] [Google Scholar]