Abstract
DAS181, (study drug, Fludase®) was developed for treatment of influenza and parainfluenza infections. Delivered by inhalation, DAS181 cleaves sialic acid receptors from respiratory epithelial cells. Treatment of influenza for three days with DAS181 reduced viral shedding. To increase deposition in the upper airways and decrease systemic absorption, the particle size was increased to 10 microns. We conducted two Phase I trials with three cohorts, randomized 2:1, active drug to placebo. The initial cohort got a single 20 mg dose of DAS181, or placebo; the second, 20 mg DAS181 or placebo for 10 days, and the third got 20 mg of DAS181or placebo for 3 days. Formulations differed slightly in their excipients. Subjects in the 1- and 3-day cohorts completed dosing without serious adverse events. Two subjects in the 10-day cohort stopped at Day 9 after developing respiratory and systemic symptoms, and a third experienced a decrease in FEV1 (Forced Expiratory Volume in 1 second) after the 9th dose and a further decline after the 10th dose. Plasma DAS181, in the 10-day cohort, peaked and began falling before the last dose. Antibodies, predominately IgG with neutralizing activity, were detected in 15/18 subjects by Day 30. The highest IgG concentrations were in the 10-day cohort. The respiratory adverse events occurring after seven days and rapid drug clearance during continued dosing are consistent with the induction of DAS181 antibodies. This could preclude use of this medication for longer than seven days or for repeated courses. (These studies have been registered at ClinicalTrials.gov under registration nos. NCT 00527865 and NCT 01651494.)
Keywords: Influenza, Parainfluenza, Sialidase, DAS-181
1.0 INTRODUCTION
Influenza vaccines have limited effectiveness (1); this and the increasing resistance to influenza antivirals emphasizes the need to develop alternative approaches (1,2). DAS181 is such an alternative; it targets the sialic acid adornments on respiratory epithelial cells to which influenza and parainfluenza viruses bind and has the potential for preventing and treating infections caused by both viruses.
DAS181 is a recombinant sialidase, derived from Actinomyces viscosus, fused to an anchoring domain from the binding sequence of human amphiregulin (3). Administered by inhalation, DAS181 removes sialic acid from the respiratory epithelium (4) and thereby prevents the binding of influenza (5,6) and parainfluenza (7) viruses. This potentially could provide prophylaxis and treatment for all strains of influenza, including those that are resistant to neuraminidase inhibitors (8).
Phase I trials of inhaled DAS181, conducted during its development, evaluated various doses, formulations and particle sizes (9). The primary adverse event noted during these trials was elevation of serum alkaline phosphatase (ALP) which was thought to result from the systemic absorption of the drug and de-sialylation of circulating glycoproteins (9). In addition, circulating, and neutralizing, antibodies were induced after a single dose of DAS181. Increasing the particle size from ~3.5 μ (DAS181-F01) to ~6 μ (DAS181-F02) reduced systemic absorption by depositing the drug higher in the respiratory tract, but did not eliminate elevation of ALP or antibody induction. DAS181-F02 was used in a randomized, double-blind, Phase II trial to determine the safety and tolerability of 10 mg of DAS181, inhaled once or over three days, in otherwise healthy adults with laboratory confirmed influenza (10). As compared with placebo, DAS181 reduced the influenza viral load in pharyngeal washes, but the reduction after a single dose was no longer significant after 48 h. After three daily doses, reduction in viral load remained significant for 48 h, or to Day 5. No significant differences were noted in time to resolution of clinical symptoms, and ALP elevations were noted in 19% of the multi-dose recipients.
The results of the Phase II trial lead to the formulation of DAS181-F03 which has a particle size of 10 μ, designed to reduce deep lung deposition and the potential for systemic absorption, and DAS181-F04 which differs from DAS181-F03 only in the addition of MgSO4 as a counter ion excipient (Table 1). In hopes of increasing the effectiveness and duration of its antiviral effect, the dose of DAS181 was increased to 20 mg in the clinical trials reported here, and a duration of ten daily doses was assessed. In the first trial, DAS181-F03 was administered once (1 Day Cohort) or daily for ten days (10 Day Cohort); in the second trial DAS181-F04 was administered daily for three days (3 Day cohort). We assessed the toxicity, systemic absorption and immunogenicity of the two formulations dosed at 20 mg/d. The results of the two trials are combined in this report.
Table 1.
Composition of DAS181-F03 and DAS181-F04 Dry Powder
| Component wt/wt (%) | DAS181-F03 | DAS181-F04 | Function |
|---|---|---|---|
| DAS181 | 70.08 | 65.06 | Active Pharmaceutical Ingredient |
| Histidine | 10.11 | 10.09 | Prevent oligomerization |
| Trehalose | 9.23 | 8.50 | Moisture binding |
| MgSO4 | 0.00 | 6.16 | Counter ion |
| Citric Acid | 2.53 | 2.13 | Counter ion |
| Sodium Acetate | 0.04 | 0.03 | Maintaining pH |
| Acetic Acid | 0.03 | 0.01 | Maintaining pH |
| Water | 8.00 | 8.00 | N/A |
| Total | 100.00 | 100.00 |
2.0 MATERIALS AND METHODS
We conducted three randomized, double-blind, placebo-controlled trials, each consisting of nine healthy adult volunteers who received DAS181 or placebo at a ratio of 2:1. The first cohort received a single dose of 20 mg DAS181-F03, or placebo. The second was to receive 20 mg DAS181-F03, or placebo, daily for 10 days. The third was administered DAS181-F04, 20 mg per day, or placebo, for 3 days. The DAS181 formulations were provided by Ansun Biopharma, Inc., San Diego, CA.
The studies were approved by the Johns Hopkins University Institutional Review Board, and written informed consent was obtained from all participants prior to screening.
2.1 Subjects
The studies enrolled 18 healthy adults, who were, non-smokers with no recent respiratory illness and no lactose intolerance, and who had normal complete blood counts, chemistries, coagulation profiles, complement tests, urinalyses, drug abuse screens, HIV, hepatitis B and hepatitis C serologies, chest radiographs (CXR), and electrocardiograms (ECG). Women had negative pregnancy tests. Subject pulmonary function tests (PFTs) had peak expiratory flow rates, and FEV1s >80% predicted. Subjects were trained to use the Cyclohaler® (N.V. Medicopharma, NL) device and achieve an inspiratory flow rate of > 60 L/min, as measured by In-Check DIAL low-range inspiratory flow meter (Alliance Tech Medical, Inc., Grandbury, TX).
2.2 Study design
Subjects inhaled 20 mg of DAS181 powder, or lactose monohydrate as placebo, once daily, for one (DAS181-F03), three (DAS181-F04) or ten (DAS181-F03) days. Dosing was done under direct supervision. An independent Safety Monitoring Committee reviewed clinical and safety data from each cohort.
Safety laboratory tests were obtained at pre-determined time. PFTs were performed at screening, on admission (Day -1), Day 0 before dosing, and 1, 2, 4, and 8 h after the first dose, for all three cohorts. The 1-day cohort also had PFTs at 24 and 48 h after the dose, and again at Day 7. The 3-day cohort had additional PFTs before the second and third doses, one hour after those doses, and on days 3, 4 and 9 after the initial dose. The 10-day cohort had PFTs gotten before the second and fourth doses, on Day 7, pre-dose and 1 hour post-dose on Day 9, and on Days 10 and 17. CXRs were performed one week after the last dose.
Serum samples for the 10-day cohort were collected before each of the 10 doses; 4, 8, and 24 hours after the 1st dose; 4, 8, and 24 hours after the 10th dose, and on Days 11, 13, 15, 17, 20, 22, 24 and 27. Drug concentrations were measured by a biological sialidase assay (10). Sera for antibody measurements were obtained before the first dose and 30 and 90 days after the first dose.
2.3 Immunogenicity
Enzyme-linked immunoassays were used to quantify antibodies (12). DAS181 was adsorbed onto microtiter plates at 20 μg/mL in phosphate-buffered saline (PBS). After 16–18 hours at 4°C, wells were washed with PBS 0.05% Tween 20 and blocked with bovine serum albumin. Serum (100 μL) was added at 1:100, 1:25 and 1:2 dilutions for IgG, IgA and IgE assays, respectively. After 16–18 hours at 4°C, plates were washed, horseradish peroxidase conjugated monoclonal anti-human IgG Fc- (clone HP6043-HRP) or monoclonal anti-IgA Fd (clone HP6123-HRP, Hybridoma Reagent Laboratory, Baltimore, MD) were pipetted into the wells of the IgG or IgA antibody plates (1 μg/mL, 0.1 mL/well). After 1 hour, plates were washed and. substrate (ABTS + H2O2) was added. Color development was stopped with 100 μL of 1M NaN3. For the IgE assay, after the serum incubation, biotin-conjugated monoclonal anti-human IgE Fc (clone HP6029B) was added. After 1 hour and a buffer wash, streptavidin HRP (1 μg/mL) was added and allowed to bind to the bound biotin-anti-IgE. The plates were developed with substrate.
IgG in a 1:100 dilution of a high-titred reference serum was arbitrarily assigned a value of 1000 U, and an 11 point calibration curve constructed. Concentrations of Ig in the samples were determined from the calibration curve by heterologous interpolation and reported as arbitrary units (AU) of antibody relative to the 1:100 dilution of the reference serum. The lower limit of detection for IgG was 200 AU; that for IgA was 100 AU. The specificity of antibody responses was verified by pre-incubation of serum with or without soluble DAS181 (1 mg/mL).
2.4 Neutralizing Antibody
A competitive assay was used to determine whether DAS181 antibodies neutralized the enzymatic activity of the drug. Sera were diluted in acid citrate dextrose. Sialidase activity was determined using 2′-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid (MuNaNa), VWR (Biosynth) Cat # 101369-938 (M-5507), a fluorogenic substrate. DAS181 cleaves the sialic acid from the substrate, and the assay detects free sialic acid. The samples were incubated with DAS181 and MuNaNa substrate solution. After 20 minutes, the reaction was stopped with 0.5 M Glycine, pH 10.2, and a read-out, given as the titer that prevented DAS181 from cleaving sialic acid, was obtained with a fluorescence plate reader.
2.5 Statistical Analysis
Statistical analyses were performed using SAS® 9.3 (Cary, North Carolina, USA).
3.0 RESULTS
3.1 Study Population
Table 2 compares the combined nine placebo recipients with the six subjects in each cohort who got active drug.
Table 2.
Demographics
| DAS181-F03 | DAS181-F04 | DAS181-F03 | Placebo | |
|---|---|---|---|---|
| 1-Day Cohort | 3-Day Cohort | 10-Day Cohort | Combined Cohorts | |
| N = 6 | N = 6 | N = 6 | N = 9 | |
| Mean Age (yr) | 42a | 32 | 30 | 29 |
| Gender-Male, # (%) | 6 (100%) | 3 (50%) | 1b (17%) | 7 (78%) |
| Race, # (%) | ||||
| African American | 5 (83%) | 4 (67%) | 2 (33%) | 3 (33%) |
| Caucasian | 0 | 2 (33%) | 4 (67%) | 4 (44%) |
| Asian | 0 | 0 | 0 | 2 (22%) |
| Other | 1 (17%) | 0 | 0 | 0 |
| Ethnicity, # (%) | ||||
| Hispanic or Latino | 0 | 1 (17%) | 1 (17%) | 1 (11%) |
| Non-Hispanic or Latino | 6 (100%) | 5 (83%) | 5 (83%) | 8 (89%) |
The mean age of the 1-day cohort was greater than the mean age of the placebo group (p=0.002)
The % men in the 10-day cohort was less than in the placebo group (p=0.04)
3.2 Safety
Six subjects in each cohort received at least one dose of DAS181 and are included in the safety analysis (Table 3).
Table 3.
Number and Percentage of Subjects with Treatment-Emergent Adverse Events (TEAEs)
| DAS181-F03 | DAS181-F04 | DAS181-F03 | Placebo | |
|---|---|---|---|---|
| 1-Day Cohort | 3-Day Cohort | 10-Day Cohort | Combined Cohorts | |
| N = 6 | N = 6 | N = 6 | N = 9 | |
|
|
||||
| TEAEs | 4 (67%) | 6 (100%) | 6 (100%) | 9 (100%) |
| Mild | 4 (67%) | 3 (50%) | 0 | 9 (100%) |
| Moderate | 0 | 3 (50%) | 5 (83%) | 0 |
| Severe | 0 | 0 | 1 (17%) | 0 |
| Serious | 0 | 0 | 0 | 0 |
| Leading to study treatment withdrawal | 0 | 0 | 3 (50%) | 0 |
| Treatment-related TEAEs | 0 | 6 (100%) | 6 (100%) | 7 (100%) |
| Mild | 0 | 4 (67%) | 2 (33%) | 7 (100%) |
| Moderate | 0 | 2 (33%) | 4 (67%) | 0 |
| Severe | 0 | 0 | 0 | 0 |
| Serious | 0 | 0 | 0 | 0 |
| Leading to study treatment withdrawal | 0 | 0 | 2 (33%) | 0 |
A single dose of DAS181-F03 was well tolerated. Five of the six subjects had mild laboratory abnormalities, not considered related to DAS181. No significant changes in vital signs, FEV1 or ECG were observed, and no SAEs were reported.
Three consecutive doses of DAS181-F04 were well tolerated. Two subjects reported four moderate treatment–related adverse events: headache after the first dose only, lightheadedness after the first two doses, 2+ proteinuria in a single urine sample, and an isolated creatinine elevation. Two subjects had mild, transient elevations in alkaline phosphatase without abnormalities in transaminases or bilirubin. No clinically relevant changes in vital signs, FEV1sor ECGs were observed and there were no SAEs.
All six subjects in the 10-day cohort who got DAS181 reported adverse events: severe for one subject, moderate for four and mild for one. The severe AE was an exacerbation of an underlying psychiatric condition not related to the study product. This subject stopped after the 4th dose.
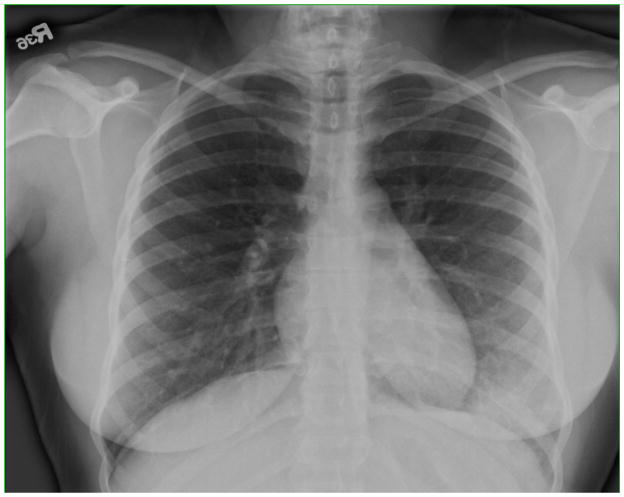
Two subjects stopped early for moderate respiratory events thought to be related to DASA181.. One subject reported a low-grade fever, chills, dizziness and fatigue beginning about 8 hours after the 8th dose. Approximately 7 hours after the 9th dose, this subject developed a fever (102.1°F), but no shortness of breath, cough, or sputum production. Crackles were heard in the left lower chest, and a CXR revealed patchy consolidation in the anterior left lower lobe (Figure 1). The FEV1 dropped to 79% predicted from 104% predicted. This subject was started empirically on levofloxacin, and did not receive the 10th dose of DAS181-F03. Fever resolved within 2 days, and the FEV1 gradually returned to normal over the next 7 days. Levofloxacin was discontinued; the clinical course was thought to represent hypersensitivity pneumonitis and not bacterial pneumonia.
Figure 1.
CXR of subject 2006 obtained after the 9th dose of DAS181 showing a left lower lobe infiltrate.
A second subject developed a mild cough on Day 2 after the 3rd dose which continued through Day 8. On Day 8, after exposure to a dog, the subject reported mild wheezing that progressed to moderate wheezing. The 10th dose was held. The subject remained afebrile, and his CXR was normal. His FEV1 decreased to 82% of predicted from 98%, but returned to baseline within 3 days of his last dose. This subject had had childhood asthma and allergy to dogs, which he had not reported.
Three other subjects in the 10-day group had moderate adverse events. One had a drop in FEV1 from a baseline of 84% of predicted to 76% of predicted 24 hours after the 9th dose, to 68% one hour after the 10th dose, and to 75% 24 hours after the 10th dose. During this time she reported a recurrence of her usual seasonal allergy symptoms with congestion, rhinorrhea, and post-nasal drip, but no cough or wheezing. FEV1 returned to baseline, and CXR one week after completion of dosing was normal. A second subject developed a moderate increase in alkaline phosphatase to 350U/L (normal, 30–120U/L). Fractionation showed an elevation in the intestinal but not the liver or bone isoenzymes.
The final subject in the 10-day cohort experienced a headache of moderate intensity that was considered unrelated to study product.
Three other subjects in the 10-day group had mild elevations in alkaline phosphatase, which resolved 2 to 19 days after the last dose. There were no elevations in transaminases or bilirubin in these three subjects.
Seven of the nine placebo recipients reported mild adverse events considered to be related to study product. These included altered taste, coughing, throat dryness, and throat irritation soon after inhalation of placebo. One subject reported a one-hour episode of mild wheezing, coughing, and throat irritation beginning about 12 hours after the 10th dose. The next morning his chest was clear, and his FEV1 was 117% predicted and not significantly changed from baseline.
3.3 Chest Radiographs, Electrocardiograms, and Pulmonary Function Tests
The subject who developed hypersensitivity pneumonitis was the only subject with an abnormal CXR. The patchy consolidation in the anterior left lower lobe following the ninth dose of DAS181-F03 (Figure 1) diminished on CXRs taken 2 and 9 days later and was not present 7 weeks later.
There were no significant ECG abnormalities.
In the 3-day group one subject had an FEV1 of 89% predicted just before the first dose, which dropped to grade 1 toxicity (79% and 80%) before and one hour after the third dose. Three of the five subjects in the 10-day group who received 9 or 10 doses of DAS181 had decreases in FEV1. No placebo-recipient had a decrease in FEV1.
3.4 Plasma DAS181
DAS181 was detected in the plasma of 6/12 subjects in the 1-day and 3-day cohorts. In the 10-day cohort, 5 of the 6 subjects in the active arm had measurable plasma levels of DAS181. Plasma levels peaked between the 6th and 9th dose and declined thereafter, even as dosing continued.
3.5 Immunogenicity
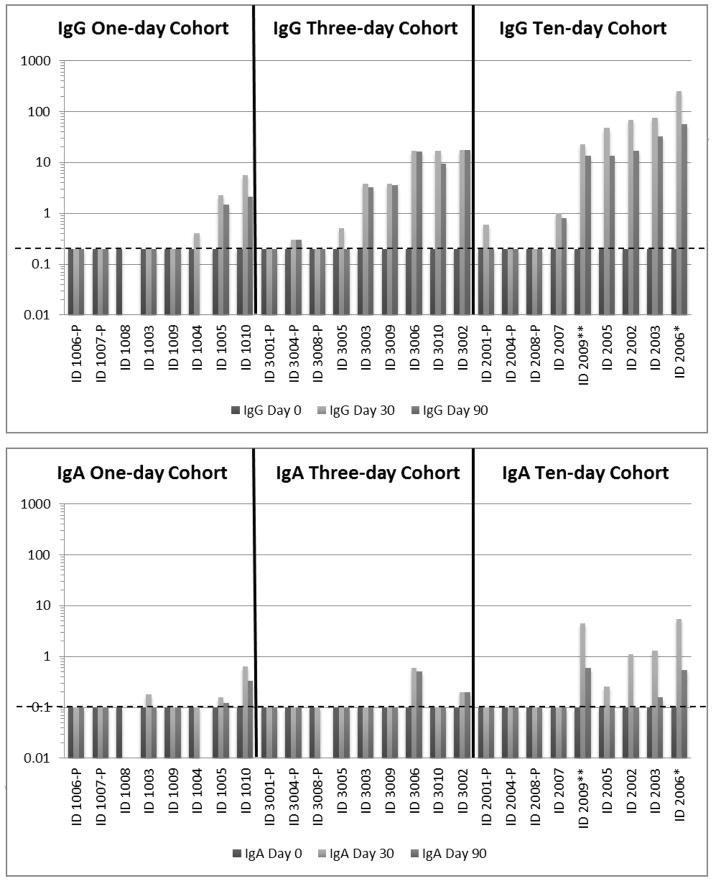
IgG and/or IgA developed in 16 of 18 individuals who received DAS181 (Figure 2). In the single-dose cohort IgG was detected 30 days after dosing in three subjects and IgA in three subjects. Three subjects’ sera had neutralizing activity. By Day 30, six subjects in the active arm of the 3-day group had IgG, and two also had IgA. Four had serum neutralizing activity.
Figure 2.
IgG and IgA levels in serum of subjects before, 30 days after and 90 days after receiving DAS181 by inhalation for 1, 3 or 10 days.
IgG and IgA levels are arbitrary units based on a standard curve of dilutions of a serum with high titers. The lower limit for each assay is shown by the horizontal hatched line: 0.2 for IgG titers and 0.1 for IgA titers.
Subject number followed by “P” = placebo recipient
Three subjects in the 10-day cohort stopped dosing early: Subject 2007 after the 4th dose for psychiatric symptoms considered to be treatment-unrelated, Subject 2009 (identified by **) after the 9th dose for prolonged wheezing and coughing, and Subject 2006 (identified by *) after the 9th dose for a hypersensitivity pneumonitis syndrome.
All six subjects who received DAS181 in the10-day cohort developed serum IgG and five had detectable IgA. Four had serum neutralizing activity. The subject who developed pneumonitis had the highest serum IgG concentration1:1024; 1:2048 neutralizing antibody). Two of 8 placebo recipients had detectable IgG, and none had detectable IgA. Among subjects with DAS181 antibody at Day 30, most had lower levels at Day 90.
Only one subject, in the single-dose group, had detectable DAS-181 IgE.
4.0 DISCUSSION
DAS181 is a novel compound that directs its action at the host sialic acid receptor to which all influenza and parainfluenza viruses must bind in order to cause a productive infection in humans (4). It differs from neuraminidase inhibitors that prevent the virus from binding to and cleaving this receptor and is effective against influenza viruses that are resistant to oseltamivir (6). Earlier formulations of DAS181 were used to treat six patients who developed parainfluenza virus (PIV) infections, primarily PIV-3, following hematopoietic stem cell or lung transplants, all but one on of whom were on prolonged immunosuppression (13–16). These patients were dosed for three (1 patient) or five days (5 patients), either with 10 mg/d of powder given by inhalation, as in the present study (4 patients), or, if intubated, with 3.2 mg/d of DAS181, formulated for nebulization, for three days, followed by 4.5 mg/d by nebulization for two days (2 patients). In every case, PIV viral shedding diminished and the patients improved, clinically, although two subsequently succumbed to GVHD and recurrence of AML. In a Phase II trial three daily 10 mg doses of DAS181-F02 significantly reduced influenza virus shedding, as compared with placebo, and as measured by quantitative polymerase chain reaction, but did not affect resolution of clinical symptoms (10). These promising virologic results raise the possibility that DAS181 could be useful in the prevention and treatment of influenza, if the effective dose and duration of therapy could be established. The present trials examined the safety and immunogenicity of 20 mg of 10 μ particles of DAS181 inhaled daily for up to 10 days. The rationale was that the larger particles would lodge in the upper airway and thereby reduce the amount of drug that would be absorbed, and that the higher dose would provide greater and more prolonged reductions in viral loads when used clinically.
The one-day and three-day courses of 20 mg of DAS181 were well tolerated, consistent with the Phase II trial. However, administration of DAS181 for more than 7 days was associated with adverse respiratory events that required termination of dosing in 2 of 5 subjects, and that caused a drop in FEV1 in a third. One subject developed fever, pulmonary crackles and an infiltrate on CXR. The onset of these symptoms, seven days after inhaling the first dose, with increased severity and worsening of the FEV1 after repeat dosing, suggest a hypersensitivity-like syndrome (17). Precipitins were not measured.
The humoral antibody response to a newly experienced antigen appears between five and ten days after exposure (18). By Day 30 all three subjects who developed respiratory symptoms and decreased FEV1 had IgG and IgA antibodies to DAS181; the subject with presumed hypersensitivity pneumonitis had the highest titers of DAS181 IgG. Most samples with detectable antibody also had sialidase neutralizing activity, which suggests that should these antibodies be on the luminal surface of the respiratory tract, they would inactivate the drug, but antibodies in bronchial secretions were not sought.
The excipients in the DAS181-F03 and FO4 formulations were unlikely to have affected the immunogenicity of DAS181, as serum antibodies were also induced by the FO2 formulation, as well (9) which had different excipients.
Surprisingly, the three subjects who experienced respiratory symptoms did not have measurable levels of DAS181 IgE. This supports the clinical diagnosis of an IgG-mediated hypersensitivity pneumonitis and broncho-constriction. The only subject who developed DAS181 IgE received only a single dose of the drug and had no associated respiratory symptoms.
DAS181 was detected in the plasma of 12 of the 18 subjects who received the drug, so the increase in particle size to 10 μ did not prevent absorption. Among five subjects who received 9 or 10 doses, drug levels peaked 5–8 days after dosing began and then declined as dosing continued. Although antibodies were not measured until Day 30, it is likely that they caused the removal of DAS181 from plasma, as they would be expected to appear in that time-frame (18), and DAS-181 antibodies peaked at 14 days in Phase I trials of other formulations (9).
Several subjects had an elevation in serum alkaline phosphatase. Isolated elevations in alkaline phosphatase seen in previous trials were attributed to desialylation of serum proteins leading to saturation of the asiaglycoprotein receptors in the liver and spleen with reduced clearance of desialylated alkaline phosphatase (19). The alkaline phosphatase in the subject with the highest increase was of intestinal origin, which suggests a different mechanism. In any event, formulation of DAS181 as 10 micron particles did not prevent absorption and antibody induction or prevent increases in alkaline phosphatase.
4.1 Conclusions
DAS181 was well tolerated for up to seven days when 20 mg was administered daily, and although our findings need to be tempered by the small sample size, we believe the drug could safely be used to prevent or treat influenza virus infections so long as the duration of dosing was limited to 5–7 days. Longer dosing periods, however, would be inadvisable, as increasing the particle size to 10 μ did not prevent the drug from being absorbed and inducing antibodies that can precipitate respiratory symptoms and decreases in FEV1. Repeated courses of DAS181 also would seem inadvisable. However, immunocompromised patients, such as transplant recipients, might tolerate longer exposures (13–16).
DAS181 is a sialidase with activity against influenza and parainfluenza
Daily inhalation of 20 mg/day was well tolerated for up to seven days.
Daily Inhalation of 20mg/day for >7 days was associated with respiratory toxicity
Longer dose schedules were associated with immunogenicity
Acknowledgments
This work was supported by Public Health Service Contract No. HHSN272200800026 from the National Institute of Allergy and Infectious Diseases, J.McL.Griffiss, PI. Dr. Jeffrey Blumer reviewed the manuscript and provided excellent advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 2.Garg S, Moore Z, Lee N, McKenna J, Bishop A, Fleischauer A, Springs CB, Nguyen HT, Sheu TG, Sleeman K, Finelli L, Gubareva L, Fry AM. A cluster of patients infected with I221V influenza b virus variants with reduced oseltamivir susceptibility--North Carolina and South Carolina, 2010–2011. J Infect Dis. 2013;207:966–973. doi: 10.1093/infdis/jis776. [DOI] [PubMed] [Google Scholar]
- 3.Belser JA, Lu X, Szretter KJ, Jin X, Aschenbrenner LM, Lee A, Hawley S, Kim do H, Malakhov MP, Yu M, Fang F, Katz JM. DAS181, a novel sialidase fusion protein, protects mice from lethal avian influenza H5N1 virus infection. J Infect Dis. 2007;196:1493–1499. doi: 10.1086/522609. [DOI] [PubMed] [Google Scholar]
- 4.Triana-Baltzer GB, Babizki M, Chan MC, Wong AC, Aschenbrenner LM, Campbell ER, Li QX, Chan RW, Peiris JS, Nicholls JM, Fang F. DAS181, a sialidase fusion protein, protects human airway epithelium against influenza virus infection: an in vitro pharmacodynamic analysis. J Antimicrob Chemother. 2010;65:275–284. doi: 10.1093/jac/dkp421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan RW, Chan MC, Wong AC, Karamanska R, Dell A, Haslam SM, Sihoe AD, Chui WH, Triana-Baltzer G, Li Q, Peiris JS, Fang F, Nicholls JM. DAS181 inhibits H5N1 influenza virus infection of human lung tissues. Antimicrob Agents Chemother. 2009;53:3935–3941. doi: 10.1128/AAC.00389-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Triana-Baltzer GB, Gubareva LV, Nicholls JM, Pearce MB, Mishin VP, Belser JA, Chen LM, Chan RW, Chan MC, Hedlund M, Larson JL, Moss RB, Katz JM, Tumpey TM, Fang F. Novel pandemic influenza A(H1N1) viruses are potently inhibited by DAS181, a sialidase fusion protein. PloS One. 2009;4:e7788. doi: 10.1371/journal.pone.0007788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moscona A, Porotto M, Palmer S, Tai C, Aschenbrenner LM, Triana-Baltzer G, Li QX, Wurtman D, Niewiesk S, Fang F. A recombinant sialidase fusion protein effectively inhibits human parainfluenza viral infection in vitro and in vivo. J Infect Dis. 2010;202:234–241. doi: 10.1086/653621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triana-Baltzer GB, Gubareva LV, Klimov AI, Wurtman DF, Moss RB, Hedlund M, Larson JL, Belshe RB, Fang F. Inhibition of neuraminidase inhibitor-resistant influenza virus by DAS181, a novel sialidase fusion protein. PloS One. 2009;4:e7838. doi: 10.1371/journal.pone.0007838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moss RB. DAS181 Investigators Brochure, Version 5.0. NexBio, Inc; 2010. [Google Scholar]
- 10.Moss RB, Hansen C, Sanders RL, Hawley S, Li T, Steigbigel RT. A Phase II study of DAS181, a novel host directed antiviral for the treatment of influenza infection. J Infect Dis. 2012;206:1844–1851. doi: 10.1093/infdis/jis622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malakhov MP, Aschenbrenner LM, Smee DF, Wandersee MK, Sidwell RW, Gubareva LV, Mishin VP, Hayden FG, Kim DH, Ing A, Campbell ER, Yu M, Fang F. Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob Agents Chemother. 2006;50:1470–1479. doi: 10.1128/AAC.50.4.1470-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton RG. Serological (in vitro) testing methods in the diagnosis of human allergic disease. In: Lockey RF, Ledford DK, editors. Allergens and Allergen Immunotherapy. Chapter 8. CRC Press; Boca Raton, FL: 2013. [Google Scholar]
- 13.Chalkias S, Mackenzie MR, Gay C, Dooley C, Marty FM, Moss RB, Li T, Routh RL, Walsh SR, Tan CS. DAS181 treatment of hematopoietic stem cell transplant patients with parainfluenza virus lung disease requiring mechanical ventilation. Transpl Infect Dis. 2014;16:141–144. doi: 10.1111/tid.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YB, Driscoll JP, McAfee SL, Spitzer TR, Rosenberg ES, Sanders R, Moss RB, Fang F, Marty FM. Treatment of Parainfluenza 3 Infection With DAS181 in a Patient After Allogeneic Stem Cell Transplantation. Clin Infect Dis. 2011 doi: 10.1093/cid/cir501. first published online August 31, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Guzman-Suarez BB, Buckley MW, Gilmore ET, Vocca E, Moss R, Marty FM, Sanders R, Baden LR, Wurtman D, Issa NC, Fang F, Koo S. Clinical potential of DAS181 for treatment of parainfluenza-3 infections in transplant recipients. Transpl Infect Dis. 2012 Aug;14(4):427–33. doi: 10.1111/j1399-3062.2012.00718x. Epub 2012 Feb 16. [DOI] [PubMed] [Google Scholar]
- 16.Drozd DR, Limaye AP, Moss RB, Sanders RZL, Hansen C, Edelman JD, Raghu G, Boeckh M, Rakita RM. DAS181 treatment of severe parainfluenza type 3 pneumonia in a lung transplant recipient. Transplant infectious disease [1398–2273] 2013;15 (1):E28–32. doi: 10.1111/tid.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacasse Y, Selman M, Costabel U, Dalphin JC, Ando M, Morell F, Erkinjuntti-Pekkanen R, Muller N, Colby TV, Schuyler MR, Cormier Y HP Study Group. Clinical diagnosis of hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2003;168:952–958. doi: 10.1164/rccm.200301-137OC. [DOI] [PubMed] [Google Scholar]
- 18.Schütz K, Hughes RG, Parker A, Quinti I, Thon V, Cavaliere M, Würfel M, Herzog W, Gessner JE, Baumann U. Kinetics of IgM and IgA antibody response to 23- valent pneumococcal polysaccharride vaccination in healthy subjects. J Clin Immunol. 2013;33 :288–296. doi: 10.1007/s10875-012-9792-y. [DOI] [PubMed] [Google Scholar]
- 19.Blom E, Ali MM, Mortensen B, Huseby NE. Elimination of alkaline phosphatases from circulation by the galactose receptor. Different isoforms are cleared at various rates. Clin Chim Acta. 1998;270:125–137. doi: 10.1016/s0009-8981(97)00217-9. [DOI] [PubMed] [Google Scholar]