Abstract
Purpose
Comparison of long-term outcomes in patients with refractory/relapsed grade 1-2 follicular lymphoma (FL) after allogeneic (allo-HCT) vs. autologous hematopoietic cell transplantation (auto-HCT) in the rituximab-era.
Methods
Adult patients with relapsed/refractory grade 1-2 FL undergoing 1st reduced-intensity allo-HCT or 1st autograft during 2000-2012 were evaluated.
Results
A total of 518 rituximab-treated patients were included. Allo-HCT patients were younger; more heavily pretreated, and more patients had advanced stage and chemoresistant disease. The 5-year adjusted probabilities, comparing auto- vs. allo-HCT groups for non-relapse mortality (NRM) were 5% vs. 26% (p<0.0001); relapse/progression: 54% vs. 20% (p<0.0001); progression-free survival (PFS): 41% vs. 58% (p<0.001) and overall survival (OS): 74% vs. 66% (p=0.05). Auto-HCT was associated with a higher risk of relapse/progression beyond 5 months post-HCT (RR=4.4; p<0.0001), and worse PFS (RR=2.9; p<0.0001) beyond 11 months post HCT. In the first 24 months post HCT, auto-HCT was associated with improved OS (RR=0.41; p<0.0001), but beyond 24 months with inferior OS (RR=2.2; p=0.006). A landmark analysis of patients alive and progression-free at 2-years post-HCT confirmed these observations, showing no difference in further NRM between both groups, but significantly higher risk of relapse/progression (RR=7.3; p<0.0001) and inferior PFS (RR=3.2; p<0.0001) and OS (RR=2.1; p=0.04) following auto-HCT. The 10-year cumulative incidence of second hematological malignancies following allo- and auto-HCT was 0% and 7%, respectively.
Conclusion
Auto- and RIC-allo-HCT as 1st transplantation approach can provide durable disease control in grade 1-2 FL patients. Continued disease relapse-risk following auto-HCT translates into improved PFS and OS following allo-HCT, in long-term survivors.
Keywords: grade 1-2 follicular lymphoma, reduced intensity allo-HCT, auto-HCT, long-time survival
Introduction
Follicular lymphoma (FL), with its long natural history and indolent course, is a heterogeneous malignancy. While many patients survive for decades, a significant portion has a more aggressive course and ∼20% of patients die within 2-3 years of diagnosis. For patients with repeated relapses and short remissions, hematopoietic cell transplantation (HCT) remains a vital tool. However, there is continued debate on the optimal timing and most effective HCT modality. Autologous HCT (auto-HCT) is frequently performed in patients with relapsed/refractory FL, but therapy failure remains a challenge.1-3 To mitigate the relapse-risk, allogeneic HCT (allo-HCT) is often considered in relapsed/refractory FL.4 Whether auto- or allo-HCT represent the preferred first transplantation approach in FL remains to be determined, especially in the rituximab-era. In pre-rituximab era, the EBMT (European Group for Blood and Marrow Transplantation) CUP trial (Chemotherapy, Unpurged or Purged auto-HCT) showed a progression-free survival (PFS) and overall survival (OS) benefit for auto-HCT compared to salvage chemotherapy alone in relapsed FL.5 Some, but not all retrospective studies from the pre rituximab-era have shown durable disease control following auto-HCT, especially among FL patients in first or second complete remission (CR).1,6,7 Unfortunately similar randomized or retrospective data in the rituximab-era are not available. The post-hoc analysis of two successive Groupe d'Etude des Lymphomes Folliculaires (GELF-86/-94) trials suggest that in relapsed FL patients receiving rituximab-containing salvage therapies, auto-HCT does not provide a PFS or OS advantage compared to chemoimmunotherapy alone, with neither strategy resulting in apparent cures.8 Furthermore, the risk of second malignancies post auto-HCT is not insignificant, ranging from 5–20%.1,2
Allo-HCT provides a lymphoma-free graft devoid of prior chemotherapy-induced DNA damage and has the potential to mediate a graft-vs-lymphoma (GvL) effect. Allo-HCT has been shown to confer long-term remissions in patients with FL, with a plateau for PFS after 2–3 years from transplantation, suggesting clinical evidence of durable GvL effects and likely cure.9-12
Historically myeloablative allo-HCTs in FL were associated with increased non-relapse mortality (NRM; ∼30-40%).12,13 To exploit the beneficial GvL effects, without high rates of NRM, reduced-intensity/non-myeloablative conditioning (RIC/NMA) HCTs have been widely adopted.11,14-16 However, as the toxicity of RIC allo-HCT still remains higher than that of auto-HCT, the question arises whether the potential benefit of the GvL effects associated with the allo-HCT justifies its application as the first transplantation approach in FL. The only prospective comparison between auto- and allo-HCT for relapsed FL performed by Bone and Marrow Transplantation Clinical Trials Network closed early because of poor accrual.17 A recent retrospective EBMT study did not show improved OS in FL patients after RIC allo-HCT compared to auto-HCT, when either modality was applied as the first transplantation procedure.18 However, >50% of patients in the EBMT analysis never received rituximab before HCT, a scenario which is no longer clinically relevant.
We utilized the Center for International Blood and Marrow Transplant Research (CIBMTR) registry to assess the relative efficacy of auto-HCT against RIC/NMA allo-HCT, when either modality is used as the first transplantation procedure, in relapsed/refractory FL in the rituximab-era.
Patients and Methods
Data sources
The CIBMTR is a working group of more than 450 transplantation centers worldwide that contribute detailed data on HCTs to a statistical center at the Medical College of Wisconsin. Centers report HCTs consecutively, with compliance monitored by on-site audits. Patients are followed longitudinally with yearly follow-up. Observational studies by the CIBMTR are performed in compliance with federal regulations with ongoing review by the institutional review board of the Medical College of Wisconsin.
Patients
Patients with a histologically proven diagnosis of relapsed/refractory grade 1-2 FL, undergoing a first auto-HCT or a first RIC/NMA allo-HCT, reported to the CIBMTR between 2000-2012 were eligible. RIC/NMA allo-HCT patients with a history of prior auto-HCT were not included. Donor-source for the allo-HCT cohort was restricted to either HLA-identical siblings or at least a 7/8 (antigen or allele-level) matched unrelated donors (URD). Pediatric patients (<18 years), those undergoing alternative donor HCT (e.g. umbilical cord blood, haploidentical, mismatched URD), and patients receiving ex vivo graft manipulation (T-cell depletion or CD34 selection) were not included in the analysis. In addition FL patients undergoing histological transformation to diffuse large B-cell lymphoma and those not receiving rituximab-containing therapies before HCT were excluded.
Definitions
The intensity of allo-HCT conditioning regimens was categorized RIC/NMA using consensus criteria.19 Previously established criteria for categorizing the degree of HLA matching were used20 for URD transplants. CR to last therapy line before HCT on CIBMTR forms is defined as complete resolution of all known disease on radiographic (CAT-scan) assessments, while partial remission (PR) is defined as ≥50% reduction in the greatest diameter of all sites of known disease and no new sites of disease. Resistant disease is defined as <50% reduction in the diameter of all disease sites, or development of new disease sites. Rituximab resistance was defined as (a) failure to achieve at least a PR to a rituximab-containing therapy line or (b) relapse/progression during or within six months of finishing a rituximab-based therapy.21
Study Endpoints
Primary outcomes were NRM, progression/relapse, PFS and OS. NRM was defined as death without evidence of lymphoma progression/relapse; relapse was considered a competing risk. Progression/relapse was defined as progressive lymphoma after HCT or lymphoma recurrence after a CR; NRM was considered a competing risk. For PFS, a patient was considered a treatment failure at the time of progression/relapse or death from any cause. Patients alive without evidence of disease relapse or progression were censored at last follow-up. The OS was defined as the interval from the date of transplantation to the date of death or last follow-up. Acute GvHD was defined and graded based on the pattern and severity of organ involvement using established criteria.22 Chronic GvHD was defined as the development of any evidence of chronic GvHD based on clinical criteria.23 Neutrophil recovery was defined as the first of 3 successive days with absolute neutrophil count ≥500/μL after post-transplantation nadir. Platelet recovery was considered to have occurred on the first of three consecutive days with platelet count 20,000/μL or higher, in the absence of platelet transfusion for 7 consecutive days. For neutrophil and platelet recovery, death without the event was considered a competing risk.
Statistical analysis
Adjusted probabilities of PFS and OS were calculated as described previously.24 Adjusted cumulative incidences (CIs) of NRM, lymphoma progression/relapse, hematopoietic recovery and second malignancies were calculated to accommodate for competing risks.25 Patient-, disease- and transplant-related factors were compared between auto-HCT and allo-HCT groups using the Chi-square test for categorical variables and the Wilcoxon sample test for continuous variables. Associations among patient-, disease, and transplantation-related variables and outcomes of interest were evaluated using Cox proportional hazards regression. Backward elimination was used to identify covariates that influenced outcomes. Covariates with a p<0.05 were considered significant. The proportional hazards assumption for Cox regression was tested by adding a time-dependent covariate for each risk factor and each outcome. Covariates violating the proportional hazards assumption were added as time-dependent covariates in the Cox regression model. Interactions between the main effect and significant covariates were examined. Results are expressed as relative risk (RR). All analyses were performed using SAS 9.3. The variables considered in multivariate analysis are shown in Supplemental Table-1S.
Results
Patients' characteristics
Between 2000 and 2012, a total of 250 patients with relapsed/refractory grade 1-2 FL undergoing a first auto-HCT and 268 undergoing a first RIC-allo-HCT met the inclusion criteria. Patient-, disease-and transplant-related characteristics are detailed in Table-1. Recipients of allo-HCT were younger; more heavily pretreated, had more advanced stage disease, longer intervals between diagnosis and HCT, more frequent extranodal involvement and were more likely to be chemo-resistant before HCT. There was no significant difference in duration of remission to first-line therapy between both groups.
Table 1. Characteristics of patients who underwent auto- or RIC-allo-HCT for relapsed/refractory grade 1-2 FL from 2000-2012 reported to the CIBMTR.
| Variable | Allo-HCT | Auto-HCT | P-value |
|---|---|---|---|
| Number of patients | 268 | 250 | |
| Age at HCT | 0.01 | ||
| Mean/Median (range), years | 51.7/52 (27-74) | 53.7/54 (22-79) | |
| Male gender (no; %) | 149 (56) | 147 (59) | 0.46 |
| Karnofsky Performance Score | |||
| <90% | 62 (23) | 56 (22) | 0.06 |
| 90-100% | 193 (72) | 168 (67) | |
| Missing | 13 (5) | 26 (10) | |
| Race | |||
| Caucasian/White (no; %) | 241 (90) | 227 (91) | 0.12 |
| Black | 7 (3) | 5 (2) | |
| Others | 20 (7) | 18 (7) | |
| Disease stage at diagnosis | |||
| I-II | 38 (14) | 59 (24) | <0.001 |
| III-IV | 217 (81) | 185 (74) | |
| Unknown | 13 (5) | 6 (2) | |
| Histological grade | |||
| 1 | 143 (53) | 115 (46) | 0.09 |
| 2 | 125 (47) | 135 (54) | |
| Time from diagnosis to HCT, months | 43 (4-352) | 34 (6-315) | 0.001 |
| B symptoms at diagnosis | 89 (33) | 82 (33) | 0.25 |
| Elevated LDH at HCT | 79 (29) | 72 (29) | 0.10 |
| Unknown | 22 (8) | 35 (14) | |
| Bulky disease at diagnosis | 25 (9) | 20 (8) | 0.17 |
| Bone marrow involvement at HCT | 39 (15) | 10 (4) | <0.001 |
| Missing | 200 (75) | 212 (85) | |
| Extranodal involvement at HCT | 70 (26) | 40 (16) | 0.002 |
| Missing | 10 (4) | 3 (1) | |
| Rituximab-resistant | 118 (44) | 161 (64) | <0.001 |
| Not evaluable | 22 (8) | 9 (4) | |
| Median chemotherapy lines (range) | 4 (1-5) | 3 (1-5) | 0.001 |
| Anthracycline-based therapies before HCT | 188 (70) | 207 (83) | 0.001 |
| Platinum-based therapies before HCT | 91 (34) | 87 (35) | 0.84 |
| Duration of first-line therapy response | |||
| <1 year | 79 (29) | 68 (27) | 0.76 |
| ≥1 year | 173 (65) | 164 (66) | |
| Missing | 16 (6) | 18 (7) | |
| History of radiation therapy before HCT | 54 (20) | 54 (22) | 0.68 |
| History of doxorubicin-based therapies | 188 (70) | 207 (83) | 0.001 |
| Disease response at transplant | |||
| CR | 87 (32) | 108 (43) | <0.001 |
| PR | 115 (43) | 118 (47) | |
| Chemoresistant/untreated relapse | 66 (25) | 24 (10) | |
| Donor type | |||
| HLA-identical sibling | 143 (53) | N/A | N/A |
| Unrelated well-matched (8/8 alleles) | 103 (38) | ||
| Unrelated partially matched (7/8 antigens or 7/8 alleles) | 22 (8) | ||
| TBI-based conditioning | 48 (18) | 36 (14) | 0.28 |
| Conditioning regimens (Allo-HCT) | |||
| TBI low dose (<500cGY single or <800cGY fractionated) | 9 (3) | N/A | N/A |
| Melphalan ≤150 mg/m2 | 44 (16) | ||
| Busulfan ≤9 mg/kg (with TBI n=5) | 66 (25) | ||
| TBI 200cGY + fludarabine | 35 (13) | ||
| Fludarabine/cyclophosphamide | 108 (40) | ||
| Fludarabine/cytarabine | 1 (<1) | ||
| CBV | 5 (2) | ||
| Conditioning regimens (Auto-HCT) | |||
| TBI-based | N/A | 36 (14) | N/A |
| BEAM and similar | 170 (68) | ||
| CBV or similar | 33 (13) | ||
| BuMEL/BuCy | 7 (3) | ||
| Others* | 4 (2) | ||
| Graft type | |||
| Bone marrow | 27 (10) | 0 | <0.001 |
| Peripheral blood | 241 (90) | 250 | |
| GVHD prophylaxis | |||
| Tacrolimus-based | 194 (72) | N/A | N/A |
| Cyclosporine-based | 67 (25) | ||
| Others** | 7 (2) | ||
| ATG or alemtuzumab used | 53 (20) | N/A | N/A |
Abbreviations: CR = complete remission; GVHD = graft versus host disease; N/A=not applicable.
Busulfan only (n=1), Busulfan + fludarabine (n=1), melphalan alone (n=1) and melphalane + mitoxantrone (n=1).
Other GVHD prophylaxis: steroids + MTX (n=1), steroids + MTX + MMF (n=1), steroids + MTX + sirolimus (n=1), monoclonal + MMF (n=1) not specified (n=3).
Engraftment and GvHD
The cumulative incidence of neutrophil and platelet engraftment was similar between both groups (Table-2). The cumulative incidence of acute GvHD (grade II-IV) at day +100 was 28% (95%CI:23-34%). The cumulative incidence of chronic GvHD at 5-years post-transplant was 60% (95%CI:54-66%). GvHD was the most frequent cause of death in the allo-HCT group (Supplemental Table-2S).
Table 2. Results of univariate analysis.
| Outcomes | Allo-HCT (N = 268) | Auto-HCT (N = 250) | |||
|---|---|---|---|---|---|
| N Eval | Prob (95%CI) | N Eval | Prob (95%CI) | P-value | |
| ANC recovery >0.5 × 109/L | 268 | 245 | |||
| 28-day | 97 (93-98)% | 99 (96-100)% | 0.07 | ||
| 100-day | 99 (96-100)% | 99 (96-100)% | 0.99 | ||
| Platelet recovery ³20 × 109 | 205 | 112 | |||
| 28-day | 89 (84-93)% | 84 (75-90)% | 0.19 | ||
| 100-day | 92 (87-95)% | 95 (89-98)% | 0.19 | ||
| Acute GVHD (II-IV) | 268 | N/A | |||
| 100-day | 28 (23-34)% | ||||
| Chronic GVHD | 262 | N/A | |||
| 1-year | 46 (39-52)% | ||||
| 3-year | 60 (53-65)% | ||||
| 5-year | 60 (54-66)% | ||||
| Second malignancy | 260 | 232 | |||
| 1-year | 2 (1-4)% | 0 (0-2)% | 0.21 | ||
| 3-year | 4 (2-7)% | 5 (2-8)% | 0.60 | ||
| 5-year | 8 (5-13)% | 5 (3-9)% | 0.22 | ||
| Second hematologic malignancy | 260 | 232 | |||
| 1-year | 0% | 0% | N/A | ||
| 3-year | 0 (0-2)% | 2 (1-5)% | 0.15 | ||
| 5-year | 0 (0-2)% | 2 (1-5)% | 0.15 | ||
| 10-year | 0 (0-2)% | 7 (2-17)% | 0.12 | ||
| Adjusted probabilities | |||||
| NRM | 265 | 249 | |||
| 1-year | 19 (14-23)% | 3 (1-5)% | <0.001 | ||
| 3-year | 23 (17-28)% | 2 (1-6)% | <0.001 | ||
| 5-year | 26 (20-31)% | 5 (2-7)% | <0.001 | ||
| Relapse/Progression | 265 | 249 | |||
| 1-year | 13 (10-17)% | 22 (17-27)% | 0.005 | ||
| 3-year | 19 (14-24)% | 44 (38-51)% | <0.001 | ||
| 5-year | 20 (15-24)% | 54 (48-60)% | <0.001 | ||
| PFS | 265 | 249 | |||
| 1-year | 70 (65-76)% | 74 (69-80)% | 0.31 | ||
| 3-year | 61 (55-67)% | 51 (45-57)% | 0.02 | ||
| 5-year | 58 (52-64)% | 41 (34-47)% | <0.001 | ||
| Overall survival | 267 | 249 | |||
| 1-year | 77 (72-82)% | 91 (88-95)% | <0.001 | ||
| 3-year | 69 (63-74)% | 82 (77-87)% | <0.001 | ||
| 5-year | 66 (60-71)% | 74 (68-79)% | 0.05 | ||
Abbreviations: ANC = neutrophil recovery; NRM = non-relapse mortality; PFS = progression-free survival; CI = confidence interval; *Log-rank test
Non-relapse mortality
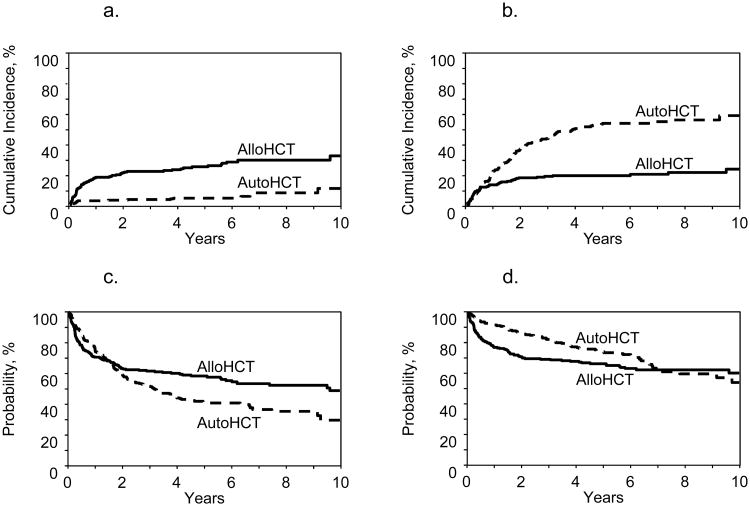
Seventy-four patients in the allo-HCT group and 27 in the auto-HCT group experienced NRM (Supplemental Table-2S). The 5-year adjusted probability of NRM was significantly higher in the allo-HCT group (26% vs. 5%; p<0.001) (Table-2; Figure-1a). On multivariate analysis, auto-HCT (RR=0.21, 95%CI:0.12-0.37; p<0.0001) and normal LDH level (RR=0.55, p=0.02) were associated with lower NRM, while age ≥60years (RR=3.47; p=0.02) and Karnofsky performance score (KPS) <90 (RR=1.85; p=0.01) were associated with higher NRM (Table-3).
Figure 1. Survival outcomes for all patients (n=518).
1a: Adjusted probabilities for NRM (5 years; p<0.001)
1b: Adjusted probabilities for relapse (5 years; p<0.001)
1c: Adjusted probabilities for PFS (5 years; p<0.001)
1d: Adjusted probabilities for OS (5 years; p=0.05)
Table 3. Results of multivariate analysis.
| Variable | N | Relative risk | 95%CI Lower Limit | 95%CI Upper Limit | P-value |
|---|---|---|---|---|---|
| Non-relapse mortality | |||||
| Allo-HCT | 265 | 1 | |||
| Auto-HCT | 249 | 0.21 | 0.12 | 0.37 | <0.0001 |
| Age at HCT (years) | |||||
| <40 | 48 | 1 | |||
| 40-49 | 145 | 1.85 | 0.63 | 5.38 | 0.26 |
| 50-59 | 200 | 2.02 | 0.71 | 5.77 | 0.20 |
| ≥60 | 121 | 3.47 | 1.19 | 10.09 | 0.02 |
| KPS | |||||
| ≥90% | 360 | 1 | |||
| <90% | 115 | 1.85 | 1.14 | 3.02 | 0.01 |
| Missing | 39 | 2.14 | 0.99 | 4.60 | 0.05 |
| Elevated LDH | |||||
| Yes | 148 | 1 | |||
| No | 309 | 0.55 | 0.34 | 0.90 | 0.02 |
| Missing | 57 | 0.98 | 0.46 | 2.12 | 0.97 |
| Progression/Relapse | |||||
| ≤5 months | |||||
| Allo-HCT | 265 | 1 | |||
| Auto-HCT | 249 | 0.80 | 0.44 | 1.44 | 0.45 |
| >5 months | |||||
| Allo-HCT | 206 | 1 | |||
| Auto-HCT | 217 | 4.38 | 2.87 | 6.68 | <0.0001 |
| Duration of 1st line therapy response | |||||
| <1 year | 144 | 1 | |||
| ≥1 year | 336 | 0.66 | 0.48 | 0.91 | 0.01 |
| Missing | 34 | 0.88 | 0.48 | 1.62 | 0.69 |
| Extranodal Involvement | |||||
| Yes | 107 | 1 | |||
| No | 394 | 0.47 | 0.34 | 0.67 | <0.0001 |
| Missing | 13 | 1.34 | 0.60 | 2.99 | 0.47 |
| Progression free survival | |||||
| ≤11 months | |||||
| Allo-HCT | 265 | 1 | |||
| Auto-HCT | 249 | 0.70 | 0.49 | 0.99 | 0.05 |
| >11 months | |||||
| Allo-HCT | 179 | 1 | |||
| Auto-HCT | 192 | 2.92 | 1.99 | 4.28 | <0.0001 |
| Chemosensitivity at HCT | |||||
| CR | 195 | 1 | |||
| PR | 231 | 1.13 | 0.84 | 1.51 | 0.42 |
| Chemoresistant/untreated | 88 | 1.59 | 1.1 | 2.29 | 0.01 |
| Extranodal Involvement | |||||
| Yes | 107 | 1 | |||
| No | 394 | 0.53 | 0.40 | 0.72 | <0.0001 |
| Missing | 13 | 0.81 | 0.39 | 1.68 | 0.56 |
| Overall survival | |||||
| ≤24 months | |||||
| Allo-HCT | 267 | 1 | |||
| Auto-HCT | 249 | 0.41 | 0.27 | 0.62 | <0.0001 |
| >24 months | |||||
| Allo-HCT | 168 | 1 | |||
| Auto-HCT | 198 | 2.22 | 1.25 | 3.93 | 0.006 |
| Age (years) | |||||
| <40 | 48 | 1 | |||
| 40-49 | 146 | 1.50 | 0.75 | 3.03 | 0.25 |
| 50-59 | 201 | 2.02 | 1.04 | 3.94 | 0.04 |
| >60 | 121 | 2.84 | 1.43 | 5.66 | 0.003 |
| Extranodal involvement | |||||
| Yes | 109 | 1 | |||
| No | 394 | 0.42 | 0.30 | 0.58 | <0.0001 |
| Missing | 13 | 0.59 | 0.23 | 1.48 | 0.26 |
| Year of HCT | |||||
| 2000-2003 | 117 | 1 | |||
| 2004-2007 | 233 | 1.11 | 0.77 | 1.61 | 0.57 |
| ≥2008 | 166 | 0.58 | 0.37 | 0.92 | 0.02 |
Abbreviations: KPS, Karnofsky Performance Score; LDH, lactate dehydrogenase
Disease progression/relapse
The adjusted probability of disease progression/relapse at 5-years was significantly higher in the auto-HCT group (54% vs. 20%; p<0.001) (Table-2, Figure-1b). In multivariate models, the main effect (auto-HCT vs. allo-HCT) displayed a time-varying effect on the risk of lymphoma progression/relapse. During the first 5-months post-transplant no difference between the two groups was seen in terms of progression/relapse risk (RR=0.80, 95%CI:0.44-1.44; p=0.45). Beyond 5-months, auto-HCT was associated with a higher risk of progression/relapse (RR=4.38, 95%CI:2.87-6.68; p<0.0001). Other factors associated with reduced risk of progression/relapse included duration of first-line therapy response ≥1year and absence of extranodal involvement at transplantation (Table-3). Relapse/progression was the most frequent cause of death in the auto-HCT group (Table-2S).
Progression-free survival
The adjusted probability of 5-year PFS was significantly better following allo-HCT (58% vs. 41%; p<0.001) (Table-2, Figure-1c). On multivariate analysis, the main effect (auto-HCT vs. allo-HCT) displayed a time-varying effect on the risk of treatment failure. During the first 11-months post HCT, auto-HCT was associated a marginally reduced risk of treatment failure (RR=0.70, 95%CI: 0.49-0.99; p=0.05). But beyond 11-months, auto-HCT was associated with significantly higher risk of treatment failure (i.e. inferior PFS) (RR=2.92, 95%CI:1.99-4.28; p<0.0001). Other factors predictive of improved PFS in the whole cohort were chemosensitive disease and absence of extranodal involvement at transplantation (Table-3).
Overall survival
The median follow-up was similar in both groups (61 months; range=3-169). In the univariate analysis, the 5-year adjusted probability of OS for the auto-HCT and allo-HCT groups was 74% and 66% (p=0.05), respectively (Table-2; Figure-1d). In the multivariate analysis, within the first 24-months after transplantation, auto-HCT was associated with reduced risk of mortality (i.e. improved OS) (RR=0.41; 95%CI:0.27-0.62; p<0.0001). In contrast, beyond 24-months, auto-HCT was associated with a higher risk for mortality (i.e. inferior OS) (RR=2.21; 95%CI:1.25-3.93; p=0.006). Other factors positively impacting on OS in the whole cohort were younger age (<60 years), absence of extranodal involvement, and HCT performed from 2008 onwards (Table-3).
Allo-HCT outcomes according to donor type
NRM, disease progression/relapse, PFS and OS following allo-HCT, stratified according to donor source is shown in Table 5.
Table 5. Univariate outcomes following allogeneic HCT stratified according to donor type.
| HLA-identical Siblings (N = 143) | 8/8 allele matched URD (N = 103) | <8/8 URD (N = 22) | |||||
|---|---|---|---|---|---|---|---|
| Outcomes | N Eval | Prob (95% CI) | N Eval | Prob (95% CI) | N Eval | Prob (95% CI) | P-value |
| Non-relapse mortality | 143 | 101 | 22 | ||||
| 1-year | 14 (9-21)% | 21 (13-29)% | 23 (8-42)% | 0.34 | |||
| 3-year | 18 (12-25)% | 25 (17-34)% | 23 (8-42)% | 0.43 | |||
| 5-year | 20 (14-28)% | 30 (21-40)% | 23 (8-42)% | 0.30 | |||
| Progression/relapse | 143 | 101 | 22 | ||||
| 1-year | 15 (10-21)% | 15 (9-23)% | 5 (0-19)% | 0.13 | |||
| 3-year | 20 (13-27)% | 23 (15-32)% | 9 (1-26)% | 0.23 | |||
| 5-year | 20 (13-27)% | 24 (16-33)% | 9 (1-26)% | 0.18 | |||
| Progression-free Survival | 143 | 101 | 22 | ||||
| 1-year | 71 (63-78)% | 64 (55-73)% | 72 (52-89)% | 0.52 | |||
| 3-year | 62 (54-70)% | 52 (42-62)% | Not evaluable | 0.20 | |||
| 5-year | 60 (51-68)% | 46 (36-57)% | Not evaluable | 0.06 | |||
| Overall Survival | 143 | 103 | 22 | ||||
| 1-year | 77 (70-84)% | 76 (67-83)% | 73 (53-89)% | 0.88 | |||
| 3-year | 70 (62-77)% | 65 (55-74)% | 73 (53-89)% | 0.68 | |||
| 5-year | 67 (59-75)% | 60 (50-69)% | 73 (53-89)% | 0.36 | |||
Outcomes of <8/8 allele matched group showed be interpreted with caution, considering the small sample size.
Landmark analysis in long-term survivors
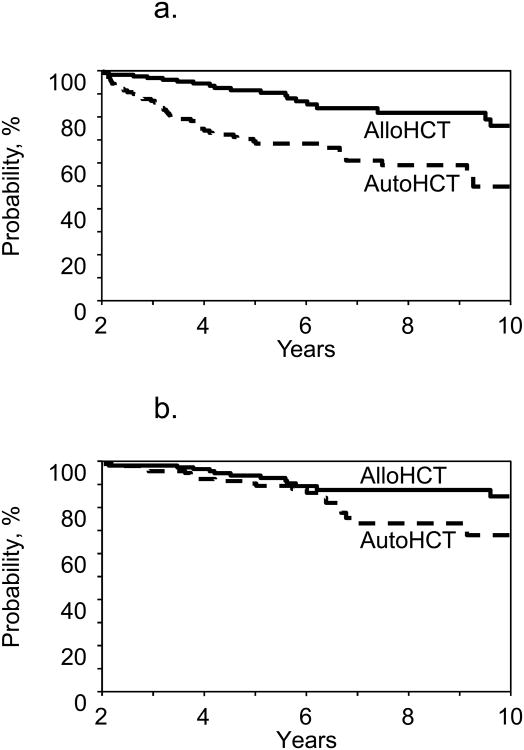
To further evaluate the time-varying effect seen on multivariate models, we performed a landmark analysis, including only patients surviving at least 24-months post HCT without disease progression/relapse (Table 4; Figure-2). When starting the analysis from the 24-months post HCT time point, we observed no significant difference in the risk of NRM between auto- and allo-HCT (RR=0.90; p=0.82). Auto-HCT was found to be associated with a significantly increased risk progression/relapse (RR=7.35; 95%CI:3.10-17.42; p<0.0001), treatment failure (RR=3.23; 95%CI:1.87-5.58; p<0.0001; Fig2a) and mortality (RR=2.09; 95%CI:1.04-4.22; p=0.04; Figure-2b). Among patients surviving 24-months post HCT without disease progression/relapse, 35 subjects died (allo-HCT=13 and auto-HCT=22). Most common cause of death in allo-HCT cohort was GVHD (n=8), while relapsed FL was the most common cause of death for the auto-HCT group (n=11). Detailed causes of death of patients included in the landmark analysis are shown in Supplemental Table-3S.
Table 4. Landmark analysis of patients surviving 2 years progression-free post transplantation.
| N | Relative Risk | 95%CI Lower Limit | 95%CI Upper Limit | P-value | |
|---|---|---|---|---|---|
| Non-relapse mortality | |||||
| Allo-HCT | 138 | 1 | |||
| Auto-HCT | 138 | 0.90 | 0.36 | 2.22 | 0.82 |
| Extranodal involvement | |||||
| Yes | 44 | 1 | |||
| No | 232 | 0.27 | 0.11 | 0.66 | 0.004 |
| Progression/Relapse | |||||
| Allo-HCT | 138 | 1 | |||
| Auto-HCT | 138 | 7.35 | 3.10 | 17.42 | <0.0001 |
| Progression-free survival | |||||
| Allo-HCT | 138 | 1 | |||
| Auto-HCT | 138 | 3.23 | 1.87 | 5.58 | <0.0001 |
| Extranodal involvement | |||||
| Yes | 44 | 1 | |||
| No | 232 | 0.40 | 0.22 | 0.71 | 0.002 |
| Overall survival | |||||
| Allo-HCT | 138 | 1 | |||
| Auto-HCT | 138 | 2.09 | 1.04 | 4.22 | 0.04 |
| Extranodal involvement | |||||
| Yes | 44 | 1 | |||
| No | 232 | 0.31 | 0.15 | 0.65 | 0.002 |
Figure 2. Landmark analysis in long-term survivors (≥24 months).
2a: Progression-free survival (p<0.0001)
2b: Overall survival (p=0.0063)
Second malignancies
The 5-year cumulative incidence of second malignancies did not differ significantly (allo-HCT=8%, auto-HCT=5%; p=0.22) (Table-2; in detail Supplemental Table-4S). Non-melanoma skin cancers were the most frequent second malignancy type in both groups when only non-hematologic malignancies were considered. The 10-year cumulative incidence of second hematological malignancies for allo-HCT and auto-HCT cohorts was 0% and 7% respectively. Four (4%) patients in the allo- and eight (10%) patients in the auto-group died because of a second malignancy (Table-2S).
Discussion
In the current study we assessed the role of auto- vs. allo-HCT as the first transplantation strategy in rituximab-treated grade 1-2 FL, and make several important observations. First, despite a higher initial NRM, allo-HCT provides a survival benefit in long-term survivors. Second, in 2-year survivors, auto-HCT was associated with higher relapse-risk and inferior PFS and OS. Third, disease relapse 2-3 years post allo-HCT was rare, but no such plateau for auto-HCT was identified. Fourth, HCT survival in the most recent era (2008 onwards) has improved. Finally, risk of hematological second malignancies was largely confined to auto-HCT.
The EBMT registry study comparing 1st auto-HCT with 1st RIC allo-HCT in relapsed FL showed significantly increased toxicity following allo-HCT due to infectious complications and GvHD18. Allo-HCT was associated with improved PFS, but OS did not differ significantly between the two groups. However, the EBMT analysis covered an earlier period (1998-2005) and >50% of included patients were rituximab-naïve. In contrast, the present CIBMTR analysis spanned a more recent era (2000-2012), and was restricted to rituximab-treated patients with grade 1-2 histologies to minimize biologic heterogeneity. Unlike prior studies we also analyzed the duration of disease control after first-line therapies, number of prior therapy lines and the presence of rituximab-resistance before HCT to assess therapy differences across the two cohorts in order to adjust for them in our multivariate models.
The observed lower NRM, increased relapse-risk and inferior PFS following auto-HCT in our analysis, is generally in line with published data.12,18,26 Unlike previous retrospective studies with long-term follow-up, where majority of included patients were rituximab-naïve,1,2,6,7 we found no plateau in PFS of FL patients after auto-HCT. Crossing adjusted survival curves (Figure-1c-d) 5-6 years post-transplant in our study underscore the importance of mature follow-up in assessing long-term outcomes in FL. Further, in agreement with previous studies,11,12,18 the allograft recipients in our study had improved PFS achieving a plateau 2-3 years post-transplant. Advanced patient age, lower KPS, high LDH and allo-HCT were associated with increased NRM, in line with earlier reports.12,26
In a small series of indolent lymphoma patients (n=112), Hosing et al. observed crossing OS curves after auto- and allo-HCT at 5-years post-transplant, suggesting improved survival for allo-HCT.10 In the current study, we focused on the outcomes of long-time survivors (≥24 months). Our landmark analysis showed that beyond 2-years post-HCT the early benefit of NRM with auto-HCT was no longer present, but the sustained lower relapse/progression rates following allo-HCT translated into long-term PFS and OS benefit in favor of allografting. The lower risk of relapse/progression following allo-HCT on multivariate models becomes apparent 5-months post-transplant. This somewhat delayed effect could be explained by intensive immunosuppression during the first few months post-HCT that may attenuate the development of GvL early after allo-HCT, or alternately by inherently higher relapse risk following auto-HCT, that does not diminish over time.
Interestingly, the survival curves in our study are reminiscent of ones seen in the pre rituximab-era CIBMTR study comparing auto-HCT against allo-HCT in a much younger patient cohort.12 This not only confirms the impressively low relapse rates in FL following allo-HCT, but also suggests that over the past decade, NRM associated with allografting has not changed substantially and remains the main barrier for the wider application of allo-HCT in FL. Having said that, compared to previous CIBMTR study,12 owing to wider adoption of RIC/NMA strategies the allografted FL patients in current study represent a much older cohort. The improved OS among long-term survivors following allo-HCT in our analysis are in contrast to the findings of National Comprehensive Cancer Network (NCCN) retrospective study where allo-HCT (n=49) was associated with higher mortality.26 The reasons for this difference are not readily apparent. Conditioning intensity and GvHD prophylactic approaches were not available in the NCCN study and could account for observed differences.
We also evaluated the rates of second malignancies after both transplant approaches. Second malignancies are reported in 5-20% of lymphoma patients after auto-1-3,27-29 and in 2-6% of patients after allo-HCT.30,31 Here, we found no significant difference in the incidence and mortality due to second malignancies between auto- and allo-HCT; however second hematological malignancies were seen predominantly after auto-HCT.
The therapeutic landscape of relapsed/refractory FL is undergoing rapid evolution with development of several novel agents including PI3K inhibitors32, Bruton's tyrosine kinase inhibitors33, ABT-199 (NCT02187861) to name a few. The role and timing of HCT, with greater incorporation of these agents in clinical practice in the coming years will warrant reappraisal. However, at the same time it is important to point out that patients included in our study appeared enriched for several high-risk features predicting poor prognosis, where durable disease control without incorporating HCT would have been unlikely. For example, median lines of therapy preHCT were 3-4, approximately 20% were chemorefractory at HCT, ∼50% were rituximab resistant and nearly a third had a first remission lasting <1-year; with the later two predicting especially poor outcomes in the chemoimmunotherapy-era34,35.
Keeping limitations inherent to registry studies, including retrospective nature and selection bias in mind, our data indicate that in relapsed/refractory FL, either auto- or allo-HCT when applied as 1st transplantation modality can provide durable disease control. The choice of the 1st transplantation modality in the current era should take into account several practical considerations. The benefits of auto-HCT include disease control with relatively low morbidity and NRM. These potential advantages need to be weighed against the continued risk of relapse and higher risk of second hematological malignancies. At the other end of the decision spectrum, early NRM and quality-of-life considerations (secondary to chronic GvHD) remain a limitation with allo-HCT. However our data indicate that relapses 2-years after allo-HCT are rare (Figure1b), and patients who are able to survive initial procedure-related toxicities enjoy a long-term survival benefit (Figure-2b). Using our data derived from chemoimmunotherapy treated FL, it is not unreasonable to support continued use of auto-HCT as first transplant modality in less fit or elderly patients, especially at an earlier time point in the disease course (since the auto-cohort in our analysis was less heavily pretreated). However, at the same time our data suggest that in carefully selected individuals, allo-HCT potentially later in the disease course can provide (at least) comparable (if not improved) survival outcomes. Our observations challenge the practice of considering allografting only in FL patients failing a prior autograft. At least in younger/more fit patients, using allo-HCT as the first transplantation approach can not only avoid the costs associated with a prior auto-HCT, but can also mitigate the not-so negligible risk of second hematological malignancies and higher NRM seen in post autograft allo-HCTs. It is also worth mentioning that our data pertain to allografting from matched sibling or adult unrelated donors. Whether these observations can be extrapolated to alternative donor (umbilical cord blood or haploidentical) allo-HCT, warrant investigation.
In conclusion, our study shows that in grade 1-2 relapsed/refractory FL treated with rituximab-based chemoimmunotherapies, RIC-allo-HCT when applied as the first transplantation modality is associated with a survival benefit in long-term survivors.
Supplementary Material
Table 1S. Variables tested in Cox proportional hazards regression models
Table 2S. Causes of death
Table 3S. Causes of death of patients included in the Landmark Analysis
Table 4S. Second malignancies post HCT.
Table 5S: Univariate analysis (chemosensitive patients only)
Largest study comparing autoHCT vs. alloHCT in grade 1 & 2 FL in rituximab era.
In FL patients surviving 2 year post HCT, allografting provided a OS benefit.
Rituximab resistance does not predict HCT outcomes.
OS better in HCT performed from 2008 onward.
Second hematological malignancies develop only post autoHCT.
Acknowledgments
We would like to acknowledge the following for their contributions to the manuscript: Mahmoud Aljurf, Ernesto Ayala, Amanda Cashen, Andy Chen, Yi-Bin Chen, Marcos de Lima, James Gajewski, Nilanjan Ghosh, Rummurti Kamble, Rodrigo Martino, Reinhold Munker, Taiga Nishihori, Nishitha Reddy, David Rizzieri, Bipin Savani, Harry C. Schouten, Peter Wiernik and Baldeep Wirk.
Maggie Simaytis for administrative assistance.
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children's Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick's Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Previous Presentations: Results presented in part as an oral presentation at the 20th Congress of European Hematology Association, Vienna Austria.
Financial Disclosure Statement: There are no relevant conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Montoto S, Canals C, Rohatiner AZ, et al. Long-term follow-up of high-dose treatment with autologous haematopoietic progenitor cell support in 693 patients with follicular lymphoma: an EBMT registry study. Leukaemia. 2007;21:2324–2331. doi: 10.1038/sj.leu.2404850. [DOI] [PubMed] [Google Scholar]
- 2.Rohatiner AZ, Nadler L, Davies AJ, et al. Myeloablative therapy with autologous bone marrow transplantation for follicular lymphoma at the time of second or subsequent remission: longterm follow-up. J Clin Oncol. 2007;25:2554–2559. doi: 10.1200/JCO.2006.09.8327. [DOI] [PubMed] [Google Scholar]
- 3.le Gouill S, de Guibert S, Planche L, et al. Impact of the use of autologous stem cell transplantation at first relapse both in naive and previously rituximab exposed follicular lymphoma patients treated in the GELA/GOELAMS FL2000 study. Haematologica. 2011;96:1128–1135. doi: 10.3324/haematol.2010.030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passweg JR, Baldomero H, Gratwohl A, et al. The EBMT activity survey: 1990-2010. Bone Marrow Transplant. 2012;47:906–923. doi: 10.1038/bmt.2012.66. [DOI] [PubMed] [Google Scholar]
- 5.Schouten HC, Qian W, Kvaloy S, et al. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin's lymphoma: results from the randomized European CUP trial. J Clin Oncol. 2003;21:3918–3927. doi: 10.1200/JCO.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Sabloff M, Atkins HL, Bence-Bruckler I, et al. A 15-Year Analysis of Early and Late Autologous Hematopoietic Stem Cell Transplant in Relapsed, Aggressive, Transformed, and Nontransformed Follicular Lymphoma. Biol Blood Marrow Transplant. 2007;13:956–964. doi: 10.1016/j.bbmt.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Ubieto AJ, García CG, Yáñez L, et al. High Dose Therapy with Autologous Stem Cell Transplantation (HDT/ASCT) Support in Follicular Lymphoma (FL) a Very Long Follow-up Analysis of 640 Patients of GELTAMO Spanish Group Suggests That FL Might be Cured, Even in High-Risk Patients. Blood. 2014 ASH Annual Meetings Abstract. [Google Scholar]
- 8.Sebban C, Brice P, Delarue R, et al. Impact of rituximab and/or high-dose therapy with autotransplant at time of relapse in patients with follicular lymphoma: a GELA Study. J Clin Oncol. 2008;26:3614–3620. doi: 10.1200/JCO.2007.15.5358. [DOI] [PubMed] [Google Scholar]
- 9.Branson K, Chopra R, Kottaridis PD, et al. Role of nonmyeloablative allogeneic stem-cell transplantation after failure of autologous transplantation in patients with lymphoproliferative malignancies. J Clin Oncol. 2002;20:4022–4031. doi: 10.1200/JCO.2002.11.088. [DOI] [PubMed] [Google Scholar]
- 10.Hosing C, Saliba RM, McLaughlin P, et al. Long-term results favor allogeneic over autologous hematopoietic stem cell transplantation in patients with refractory or recurrent indolent non-Hodgkin's lymphoma. Ann Oncol. 2003;14:737–744. doi: 10.1093/annonc/mdg200. [DOI] [PubMed] [Google Scholar]
- 11.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Besien K, Loberiza FR, Jr, Bajorunaite R, et al. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood. 2003;102:3521–3529. doi: 10.1182/blood-2003-04-1205. [DOI] [PubMed] [Google Scholar]
- 13.Peniket AJ, Ruiz de Elvira MC, Taghipour G, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31:667–678. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 14.Pinana JL, Martino R, Gayoso J, et al. Reduced intensity conditioning HLA identical sibling donor allogeneic stem cell transplantation for patients with follicular lymphoma: long-term follow-up from two prospective multicenter trials. Haematologica. 2010;95:1176–1182. doi: 10.3324/haematol.2009.017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomson KJ, Morris EC, Milligan D, et al. T-cell-depleted reduced-intensity transplantation followed by donor leukocyte infusions to promote graft-versus-lymphoma activity results in excellent long-term survival in patients with multiply relapsed follicular lymphoma. J Clin Oncol. 2010;28:3695–3700. doi: 10.1200/JCO.2009.26.9100. [DOI] [PubMed] [Google Scholar]
- 16.Shea T, Johnson J, Westervelt P, et al. Reduced-intensity allogeneic transplantation provides high event-free and overall survival in patients with advanced indolent B cell malignancies: CALGB 109901. Biol Blood Marrow Transplant. 2011;17:1395–1403. doi: 10.1016/j.bbmt.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomblyn MR, Ewell M, Bredeson C, et al. Autologous versus reduced-intensity allogeneic hematopoietic cell transplantation for patients with chemosensitive follicular non-Hodgkin lymphoma beyond first complete response or first partial response. Biol Blood Marrow Transplant. 2011;17:1051–1057. doi: 10.1016/j.bbmt.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson SP, Canals C, Luang JJ, et al. The outcome of reduced intensity allogeneic stem cell transplantation and autologous stem cell transplantation when performed as a first transplant strategy in relapsed follicular lymphoma: an analysis from the Lymphoma Working Party of the EBMT. Bone Marrow Transplant. 2013;48:1409–1414. doi: 10.1038/bmt.2013.83. [DOI] [PubMed] [Google Scholar]
- 19.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezvani AR, Maloney DG. Rituximab resistance. Best Pract Res Clin Haematol. 2011;24:203–216. doi: 10.1016/j.beha.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 23.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Loberiza FR, Klein JP, et al. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88:95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Zhang MJ. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101:87–93. doi: 10.1016/j.cmpb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evens AM, Vanderplas A, la Casce AS, et al. Stem cell transplantation for follicular lymphoma relapsed/refractory after prior rituximab: a comprehensive analysis from the NCCN lymphoma outcomes project. Cancer. 2013;119:3662–3671. doi: 10.1002/cncr.28243. [DOI] [PubMed] [Google Scholar]
- 27.Lenz G, Dreyling M, Schiegnitz E, et al. Myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission prolongs progression-free survival in follicular lymphoma: results of a prospective, randomized trial of the German Low-Grade Lymphoma Study Group. Blood. 2004;104:2667–2674. doi: 10.1182/blood-2004-03-0982. [DOI] [PubMed] [Google Scholar]
- 28.Sebban C, Mounier N, Brousse N, et al. Standard chemotherapy with interferon compared with CHOP followed by high-dose therapy with autologous stem cell transplantation in untreated patients with advanced follicular lymphoma: the GELF-94 randomized study from the Groupe d'Etude des Lymphomes de l'Adulte (GELA) Blood. 2006;108:2540–2544. doi: 10.1182/blood-2006-03-013193. [DOI] [PubMed] [Google Scholar]
- 29.Gyan E, Foussard C, Bertrand P, et al. High-dose therapy followed by autologous purged stem cell transplantation and doxorubicin-based chemotherapy in patients with advanced follicular lymphoma: a randomized multicenter study by the GOELAMS with final results after a median follow-up of 9 years. Blood. 2009;113:995–1001. doi: 10.1182/blood-2008-05-160200. [DOI] [PubMed] [Google Scholar]
- 30.Leisenring W, Friedman DL, Flowers ME, et al. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol. 2006;24:1119–1126. doi: 10.1200/JCO.2005.02.7052. [DOI] [PubMed] [Google Scholar]
- 31.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Younes A, Thieblemont C, Morschhauser F, et al. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. Lancet Oncol. 2014;15:1019–1026. doi: 10.1016/S1470-2045(14)70311-0. [DOI] [PubMed] [Google Scholar]
- 34.Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after R-CHOP uniquely defines patients at high risk for death: an analysis from the national Lymphocare Study. Blood. 2013 doi: 10.1200/JCO.2014.59.7534. ASH Annual Meetings Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mozessohn L, Cheung MC, Crump M, et al. Chemoimmunotherapy resistant follicular lymphoma: predictors of resistance, association with transformation and prognosis. Leuk Lymphoma. 2014;55:2502–2507. doi: 10.3109/10428194.2014.885513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1S. Variables tested in Cox proportional hazards regression models
Table 2S. Causes of death
Table 3S. Causes of death of patients included in the Landmark Analysis
Table 4S. Second malignancies post HCT.
Table 5S: Univariate analysis (chemosensitive patients only)