Abstract
Outcomes of non-myeloablative (NMA), HLA-haploidentical (haplo) related-donor allogeneic blood or marrow transplantation (allo BMT) with high-dose posttransplantation cyclophosphamide (PTCy) appear to be similar to those using HLA-matched donors. Thus, it may be possible to prioritize donor factors other than HLA-matching that could enhance antitumor activity. The Fc receptor polymorphism FCGR3A-158VV may confer greater sensitivity to rituximab than FCGR3A-158FF. In a prospective, phase II study of NMA, related-donor allo BMT with PTCy and posttransplantation rituximab for patients with B-cell lymphomas, we hypothesized that donor selection that prioritized FCGR3A-158 polymorphism over HLA-matching would be feasible, safe and might improve outcomes. The primary endpoint was 1-year progression-free survival (PFS). Of 83 patients transplanted (median age 59 years), 69 (83%) received haplo grafts. Fifty-four (65%) received a graft that maintained or improved their Fc receptor polymorphism status. With 2.6-year median follow-up, the 1-year PFS and overall survival (OS) probabilities were 71% and 86%, respectively, with 1-year relapse and non-relapse mortality (NRM) probabilities of 20% and 8%. At 1-year, the probability of acute grades 2–4 graft-versus-host disease (GVHD) was 41%, with grade 3–4 acute GVHD probability of 5% and chronic GVHD probability of 11%. Among haplo transplants, the 1-year probabilities of PFS, OS, relapse, and NRM were 70%, 83%, 20%, and 10%, respectively. No differences in outcomes were observed based on donor FCGR3A-158 polymorphism. Excess infection risk was not apparent with posttransplantation rituximab. Although donor selection based on FCGR3A-158 polymorphism was not shown to influence PFS, this study suggests that donor selection based on criteria other than best HLA-match is feasible and safe. This study opens the way for the future investigation of donor prioritization based on promising non-HLA factors that may improve anti-tumor activity and decrease relapse after allo BMT. This study was registered at www.clinicaltrials.gov as NCT00946023.
Introduction
Relapse after allogeneic blood or bone marrow transplantation (allo BMT) remains a major challenge and strategies are needed that can enhance anti-tumor alloimmunity without excess toxicity. Non-HLA factors such as donor killer-cell immunoglobulin-like receptor genotype have been shown to influence relapse rates and survival outcomes after unrelated donor BMT for acute leukemia.1 However, such an approach ideally requires the availability of several potential donors to make selection feasible, as well as clinical equipoise in prioritizing non-HLA donor factors over the degree of HLA matching. High-dose, posttransplantation cyclophosphamide (PTCy) as graft-versus-host disease (GVHD) prophylaxis for HLA-haploidentical (haplo), T-cell replete allo BMT is associated with acceptable rates of acute GVHD (aGVHD), non-relapse mortality (NRM), and graft failure as well as low rates of chronic GVHD (cGVHD), yielding results that appear comparable to other alternative donor sources and even HLA-matched related donors.2–11 Allo BMT using PTCy thus provides a platform upon which to investigate outcomes when donors are selected based on factors other than best HLA-match.
FCGR3A is a low-affinity immunoglobulin gamma Fc receptor involved in natural killer (NK) cell-mediated antibody-dependent cellular cytotoxicity and is mainly expressed on NK cells. As compared to the FCGR3A-158FF (FF) polymorphism, FCGR3A-158VV (VV) and to a lesser extent FCGR3A-158VF (VF) have been associated with greater affinity to monoclonal antibodies and more favorable clinical responses to rituximab.12–18 We hypothesized that the efficacy of posttransplantation rituximab in patients with B-cell lymphoma might be enhanced if donors were selected based on the affinity of their FCGR3A-158 polymorphism for rituximab, as a means of enhancing a graft-versus-lymphoma effect.
In this phase II prospective clinical trial of NMA, related donor allo BMT using PTCy for patients with relapsed/refractory B-cell lymphoma, we integrated posttransplantation rituximab and evaluated a donor selection strategy prioritizing FCGR3A-158 polymorphisms over degree of HLA matching. It was hypothesized that non-HLA graft characteristics which might enhance antitumor activity can be feasibly and safely prioritized over HLA matching and that donor selection based on FCGR3A-158 polymorphisms coupled with posttransplantation rituximab could improve progression-free survival (PFS). Herein, we report the results of what is, to our knowledge, the first allo BMT study where donors were first prioritized using a factor other than HLA-matching.
Materials and Methods
Study Design
This was a single-center, prospective phase II clinical trial approved by the Johns Hopkins Institutional Review Board and conducted at the Sidney Kimmel Comprehensive Cancer Center of Johns Hopkins Hospital (clinicaltrials.gov #NCT00946023). Patients were enrolled between August 2009 and April 2013. The primary objective was to determine the 1-year PFS, for the cohort and by donor FCGR3A-159 polymorphism, of patients with B-cell lymphoma following NMA, related-donor allo BMT using PTCy, integrated with posttransplantation rituximab and the prioritization of donors having a favorable FCGR3A-158 polymorphism. Pre-defined secondary endpoints included the feasibility of identifying and selecting donors with the more favorable VV or VF FCGR3A-158 polymorphism, estimates of overall survival (OS) and longer-term PFS, and estimates of the cumulative incidences of relapse, non-relapse mortality (NRM), aGVHD, and cGVHD. All patients gave written informed consent both for donor selection based on FCGR3A-158 polymorphism status and for allo BMT. Outcomes of all 83 patients transplanted on this clinical trial are presented.
Patients
Eligibility for allo BMT included age ≤ 75 years old with a diagnosis of a CD20(+) B-cell lymphoma with at least one related donor who was at a minimum HLA-haploidentical at the HLA-A, -B, -C, -DR, and -DQ alleles. For low-grade B-cell lymphomas or chronic lymphocytic lymphoma/small lymphocytic leukemia (CLL/SLL), patients had to have failed at least two prior therapies excluding single agent rituximab, progressed through multi-agent chemotherapy, or undergone histologic transformation, unless the CLL/SLL harbored an 11q or 17p deletion. For aggressive B-cell non-Hodgkin lymphoma (NHL) or mantle cell lymphoma, patients had to have failed at least one prior multi-agent chemotherapy regimen. Patients were considered for allo BMT after either failing a prior autologous stem cell transplant or exhibiting disease features that made cure with autologous transplant seem unlikely, such as history of chemotherapy refractoriness, short remission after prior chemotherapy, or histologic transformation. For aggressive lymphomas, partial or complete remission (PR or CR) was required prior to allo BMT. Patients with active central nervous system involvement, HIV infection, or uncontrolled infection were excluded. End-organ function requirements included left ventricular ejection fraction of ≥ 35%, bilirubin ≤ 3.0 mg/dL, liver transaminases ≤ five times the upper limit of normal, and FEV1 and FVC ≥ 40% of predicted.
Donor selection algorithm
Donors were first-degree relatives or half-related siblings who were at least HLA-haploidentical, defined as having a shared HLA-haplotype between donor and patient at HLA-A, -B, -C, -DR, and –DQ based on allele or allele-group level typing. Relatives with no known contraindication to donation underwent FCGR3A-158 polymorphism testing. Suitable donors were then prioritized as follows: 1) medically fit to donate, 2) absence of anti-donor HLA antibodies, 3) VV polymorphism, 4) VF polymorphism, 5) lack of major ABO incompatibility, 6) matched cytomegalovirus (CMV) IgG serostatus, 7) lack of minor ABO incompatibility, and 8) male donor preferred for male patients. In cases of more than one potential VV donor (or VF donor, in the absence of VV donors), HLA-matched donors were first prioritized over haplo donors and then donor age ≥ 18 was prioritized over donor age < 18, with donors ≤ 70 preferred.
FCGR3A-158 genotyping
The methods used for genotyping the FCGR3A-158 polymorphism of patients and potential donors are provided as supplementary material (Supplementary Methods S1).
Transplantation regimen
NMA conditioning consisted of fludarabine, cyclophosphamide, and 200 cGy total body irradiation as previously described.5 All grafts were T-cell replete and bone marrow-derived, with a target of 4 × 108 total nucleated cells per kilogram (kg) of recipient ideal body weight. GVHD prophylaxis in all cases consisted of high-dose PTCy (50 milligrams (mg)/kg/day intravenously (IV) on days +3 and +4), mycophenolate mofetil on days +5 through +35, and tacrolimus starting day +5. In the absence of GVHD or graft failure, tacrolimus was continued through day +180 with goal trough of 5 to 15 nanograms/milliliter, then discontinued without taper.
Supportive care measures were administered according to institutional standards. Granulocyte-colony stimulating factors (G-CSF) were given starting day +5 until the absolute neutrophil count (ANC) was ≥ 1000/microliter for three days. Prophylactic IV immunoglobulin G was permitted for hypogammaglobulinemia.
Rituximab 375 mg/m2 IV was scheduled once weekly for eight weeks starting on day +30 +/− 3 days, contingent on neutrophil recovery. The start of rituximab was otherwise delayed. The rituximab schedule could also be delayed or interrupted at physician discretion if there were concerns about its safety, including severe unexplained cytopenias, active significant infection, or significant GVHD. Rituximab was administered early after allo BMT, as this is the period of highest relapse risk.
Definition of endpoints
Disease status was defined per standardized response criteria.19,20 Patients with stable or progressive disease by the referenced criteria were categorized as having “active disease” at the time of allo BMT. Acute GVHD was graded per Keystone criteria.21 Chronic GVHD was evaluated using the National Institutes of Health Working consensus criteria and Seattle criteria.22,23 The hematopoietic cell transplantation-specific comorbidity index (HCT-CI),24 comorbidity-age index,25 and the refined disease risk index (DRI)26 were retrospectively determined.
Neutrophil recovery was the first of three consecutive days with an ANC ≥ 500/mm3. Platelet recovery was the first day with a platelet count of ≥ 20,000/mm3 without platelet transfusion in the preceding week. Death before count recovery was considered a competing risk for count recovery. Full donor chimerism was defined as ≥ 95% donor cells in the blood or bone marrow. Primary graft failure was defined as < 5% donor chimerism by ~ day +60 in unsorted cells and/or CD3 cells, without detectable bone marrow malignancy. Secondary graft failure was defined as < 5% donor chimerism after the initial presence of donor cells. Late-onset neutropenia (LON) was defined as an ANC of ≤ 1000/mm3 (Grade 3) or ≤ 500/mm3 (Grade 4) starting four weeks after last rituximab therapy through 12 months after the last rituximab dose, or until relapse.27,28 Attribution of LON to rituximab is further described in the results section.
All time-to-event endpoints were measured from the date of allo BMT. PFS was the time to disease relapse, progression, unplanned treatment of persistent disease, or death from any cause. OS was the time to death from any cause. Competing risks for aGVHD and cGVHD included any PFS failure or < 5% donor chimerism from any cause. Relapse/progression and NRM were considered competing risks. Patients without events were censored at the date of their last evaluation.
Statistical analysis
Differences in group characteristics were compared using the Fisher’s exact test for categorical variables and the Kruskall-Wallis test for continuous variables. PFS, DFS, and OS were estimated using the Kaplan-Meier method with 95% confidence intervals (CIs) and compared between groups using the log-rank statistic.29 Cumulative incidences of relapse, NRM, GVHD, and count recovery were estimated by competing risk analysis using Fine and Gray’s method.30,31 Two-sided p-values < 0.05 were considered significant. Data were analyzed using the R program, version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria, http://R-project.org/). The dataset was locked for analysis on October 27, 2014.
Results
Patient characteristics
Eighty-three consecutive patients with B-cell lymphoma, median age 59 years (range, 34–75), were transplanted on this trial. Patient and transplant characteristics overall and according to donor FCGR3A-158 polymorphism are summarized in Table 1. Sixty-nine patients (83%) received haplo grafts, and 13 (17%) patients received grafts from HLA-matched related donors, with no difference by FCGR3A-158 donor polymorphism in the proportion receiving haplo grafts. Diagnoses included aggressive, de novo or transformed NHL (59%), mantle cell lymphoma (17%) and low-grade B-cell malignancies (23%). Recipients of haplo allo BMT (n=69) had patient and transplantation characteristics that were similar to the cohort as a whole, Supplementary Table S2. The majority of patients (60%) had intermediate-risk disease by the refined DRI, which was similarly distributed across the donor FCGR3A-158 polymorphism groups. More patients with a high age-comorbidity index received FF grafts (p=0.03).
Table 1.
Patient and transplant characteristics overall and by donor FCGR3A-158 polymorphism
| All patients (n=83) | VV donor (n=17) | VF donor (n=43) | FF donor (n=23) | Pa | |
|---|---|---|---|---|---|
| Patient age at BMT | |||||
| Median, years (range) | 59 (34–75) | 55 (38–69) | 59 (34–75) | 59 (40–75) | 0.82 |
| Age ≥ 60, n (%) | 39 (47%) | 8 (47%) | 20 (47%) | 11 (49%) | 0.99 |
| Male sex, n (%) | 56 (67%) | 12 (71%) | 24 (56%) | 20 (87%) | 0.03 |
| Disease, n (%) | |||||
| Aggressive lymphomab | 49 (59%) | 6 (35%) | 31 (72%) | 12 (52%) | |
| MCL | 13 (17%) | 5 (29%) | 4 (9%) | 4 (17%) | |
| CLL/SLLc | 10 (12%) | 2 (12%) | 2 (5%) | 6 (26%) | |
| FL, grade 1–2 or MZLc | 9 (11%) | 3 (18%) | 5 (12%) | 1 (4%) | |
| nLP HL | 2 (2%) | 1 (6%) | 1 (2%) | 0 (0%) | |
| Disease status at allo BMT, n (%) | 0.54 | ||||
| Complete remission | 43 (52%) | 12 (71%) | 20 (47%) | 11 (48%) | |
| Partial remission | 29 (35%) | 4 (24%) | 17 (40%) | 8 (35%) | |
| Active disease | 11 (13%) | 1 (6%) | 6 (14%) | 4 (17%) | |
| Transformed lymphoma, n (%)d | 22 (27%) | 1 (6%) | 14 (33%) | 7 (30%) | 0.10 |
| Prior autografting, n (%) | 12 (14%) | 4 (24%) | 5 (12%) | 3 (13%) | 0.46 |
| Refined DRI, n (%) | 0.10 | ||||
| Low risk | 28 (34%) | 10 (59%) | 10 (23%) | 8 (35%) | |
| Intermediate risk | 50 (60%) | 7 (41%) | 30 (70%) | 13 (57%) | |
| High/very high risk | 5 (6%) | 0 (0%) | 3 (7%) | 2 (9%) | |
| HCT-CI score, n (%) | 0.12 | ||||
| 0 (low risk) | 17 (20%) | 2 (12%) | 12 (28%) | 3 (13%) | |
| 1–2 (intermediate risk) | 41 (49%) | 12 (71%) | 20 (47%) | 9 (39%) | |
| ≥ 3 (high risk) | 25 (30%) | 3 (18%) | 11 (26%) | 11 (48%) | |
| Age-comorbidity index, n (%) | 0.03 | ||||
| 0–2 (low risk) | 41 (49%) | 10 (59%) | 25 (58%) | 6 (26%) | |
| ≥ 3 (high risk) | 42 (51%) | 7 (41%) | 18 (42%) | 17 (74%) | |
| HLA matching | 0.93 | ||||
| HLA-haploidentical | 69 (83%) | 14 (82%) | 35 (81%) | 20 (87%) | |
| HLA-matchede | 14 (17%) | 3 (18%) | 8 (19%) | 3 (13%) | |
| Median donor age, years (range) | 41 (13–72) | 41 (16–64) | 42 (13–72) | 42 (20–65) | 0.78 |
| Female into male, n (%) | 22 (27%) | 3 (18%) | 11 (26%) | 8 (35%) | 0.51 |
| Patient CMV seropositive, n (%) | 30 (36%) | 10 (59%) | 15 (35%) | 5 (22%) | 0.06 |
| CMV matching, n (%) | 0.30 | ||||
| Donor (−)/Recipient (−) | 36 (43%) | 4 (24%) | 18 (42%) | 14 (61%) | |
| Donor (+)/Recipient (−) | 17 (20%) | 3 (18%) | 10 (23%) | 4 (17%) | |
| Donor (−)/Recipient (+) | 15 (18%) | 5 (29%) | 7 (16%) | 3 (13%) | |
| Donor (+)/Recipient (+) | 15 (18%) | 5 (29%) | 8 (19%) | 2 (9%) | |
| Infused cell dose, median (range) | |||||
| TNC x 108/kg | 4.5 (2.2–7.7) | 4.5 (3.1–6.1) | 4.3 (2.2–7.7) | 4.6 (3.2–6.1) | |
| CD34+ cells x 106/kg | 4.5 (1.8–25.1) | 4.1 (1.8–25.1) | 4.7 (2.1–9.8) | 4.4 (2.1–8.6) | |
| CD3+ cells × 107/kg | 4.1 (1.6–10.4) | 5.1 (2.3–6.8) | 3.7 (1.6–10.4) | 4.1 (2.8–9.7) | |
| Doses of post-BMT rituximab, n (%) | 0.92 | ||||
| 8 | 67 (81%) | 15 (88%) | 34 (79%) | 18 (78%) | |
| 6–7f | 8 (10%) | 2 (12%) | 4 (9%) | 2 (9%) | |
| 4–5f | 4 (5%) | 0 (0%) | 3 (7%) | 1 (4%) | |
| 1–3f | 3 (4%) | 0 (0%) | 2 (5%) | 1 (4%) | |
| 0g | 1 (1%) | 0 (0%) | 0 (0%) | 1 (4%) | |
Abbreviations: VV, donor homozygous for the V FCGR3A-158 allele; VF, donor with V and F FCGR3A-158 alleles; FF, donor homozygous for the F FCGR3A-158 allele; BMT, bone marrow transplantation; MCL, mantle cell lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; FL, follicular lymphoma; MZL, marginal zone lymphoma; nLP HL, nodular lymphocyte predominant Hodgkin lymphoma; DRI, disease risk index; HCT-CI, hematopoietic cell transplant-comorbidity index; CMV, cytomegalovirus; TNC, total nucleated cells
P values are for differences between the three FCGR3A polymorphism groups
Diffuse large B-cell lymphoma (DLBCL) de novo (n=16) or transformed (n=21), T-cell/histiocyte rich large B-cell lymphoma (n=2), primary mediastinal large B-cell lymphoma (n=2), FL grade 3 (n=8)
Excluding transformed lymphoma
Transformation was defined as evolution of a low-grade lymphoma into an aggressive lymphoma, including transformation of FL grade 1–2 into FL grade 3. Changes from FL grade 3 to DLBCL were not considered a transformation. Transformations included DLBCL out of low-grade lymphoma (n=21) and aggressive B-cell lymphoma not otherwise specified out of CLL (n=1)
Including two patients who received grafts from genetically haplo donors who were phenotypically 10/10 HLA-matched
Reasons that 1–7 doses of rituximab were given: unconfirmed (n=4), neutropenia (n=2), graft-versus-host disease (n=2), musculoskeletal pains (n=2), death prior to completion of rituximab (n=2), inadequate intravenous access (n=1), graft failure (n=1), relapse (n=1)
Rituximab not given due to early death prior to neutrophil recovery (n=1)
Donor selection
The distribution of the FCGR3A-158 polymorphism among patients was 11 (13%) VV, 43 (52%) VF, and 29 (35%) FF. Of 270 donors tested for these polymorphisms (median 3 donors tested per patient, range 1–7), 35 (13%) had the VV polymorphism, 121 (45%) were VF, 111 (41%) were FF, and 3 (1%) had unverified polymorphism results. From this, 54 patients (65%) were able to receive a VF or VV graft, of which 29 (54%) received a graft with a more favorable FCGR3A-158 polymorphism and 25 (46%) received a graft with the same favorable VV (n=5) or VF (n=20) polymorphism. In five cases (6%), a haplo donor was selected over a matched donor in order to use a more favorable polymorphism. In 12 cases (14%), the person with the most favorable polymorphism was unsuitable to donate, resulting in five cases (6%) where a donor with a less favorable polymorphism than the recipient was used.
Engraftment and Chimerism
The estimated cumulative incidence of neutrophil recovery was 98% (95% confidence interval (CI) 94–101%) by day +30 (median, 16 days). The cumulative incidence of platelet recovery was 98% (95% CI 94–101%) by day +60 (median, 24 days). Of 81 evaluable patients, primary graft failure occurred in two patients (2.5%), with autologous recovery of hematopoiesis in both cases. Unsorted chimerism at day +60 was 95% donor or greater for 70 patients; an additional 10 patients had mixed chimerism (5–94% donor cells) and one patient had 0% donor cells. CD3 chimerism at day +60 was 95% donor or greater for 69 patients, mixed for nine patients, 0% for two patients, and not evaluated in one patient who was 100% donor in unsorted blood. One patient with mixed chimerism on day +60 developed secondary graft failure on day +112, with autologous hematopoietic recovery and ongoing disease-free survival.
Toxicity
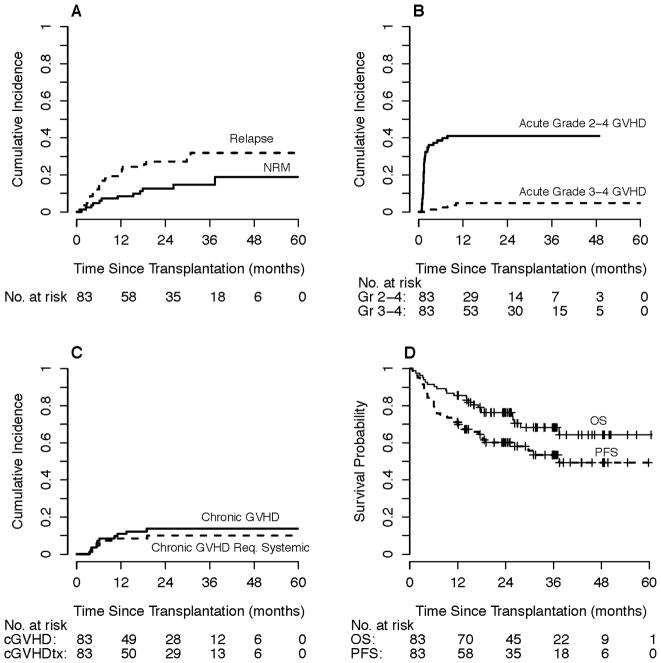
By competing risk analysis, the estimated probability of NRM for the cohort was 5% (95% CI 0–9%) at day +180 and 8% (95% CI 2–14%) at one year, Figure 1A. Causes of NRM (n=12) were non-neutropenic infection (n=5, with three in the setting of GVHD treatment), neutropenic infection (n=3), treatment-related myeloid neoplasm (n=2), pulmonary embolism (n=1), and decompensated alcoholic cirrhosis (n=1). At one year, the estimated probability of grade 2–4 aGVHD was 41% (95% CI 30–52%), although most cases were grade 2 with an estimated probability of grade 3–4 aGVHD at 1-year of 5% (95% CI 0–9%), Figure 1B. The estimated probability of any cGVHD was 11% (95% CI 4–18%) at one year and 14% (95% CI 6–21%) at two years. The probability of systemic therapy for cGHVD was 9% (95% CI 2–15%) at one year and 10% (95% CI 3–17%) at two years, Figure 1C.
Figure 1. Overall outcomes of non-myeloablative, related-donor bone marrow transplantation with posttransplantation high-dose cyclophosphamide and rituximab (n=83).
A) Relapse and non-relapse mortality by competing risk analysis. B) Acute graft-versus-host disease (grades 2–4 and grades 3–4) by competing risk analysis. C) Chronic graft-versus-host disease (overall and requiring systemic therapy) by competing risk analysis. D) Progression-free and overall survival. Of these 83 patients, 69 received HLA-haploidentical grafts.
Sixty-seven patients (81%) received all eight planned doses of posttransplantation rituximab. Reasons for fewer doses of rituximab are outlined in the footnote of Table 1. Grade 3–4 LON was documented in 38 of the 82 (46%) patients who received posttransplantation rituximab, 25 (68%) of whom had minimal or no associated clinical findings; 23 were completely asymptomatic, one had oral ulcers requiring no therapy, and one required oral antibiotics for sinusitis. At the time of LON diagnosis, 22 of 38 patients were assessed for donor chimerism, of which 19 had 100% donor cells, two had mixed chimerism (81% and 95% donor cells in unsorted peripheral blood), and one had secondary graft failure. An additional 11 patients were not assessed for chimerism at the time of LON, but were assessed in the two months preceding or following LON; of these, ten had full donor chimerism on all assessments while one had 93%/81% donor cells in the unsorted peripheral blood in the two months preceding/following LON. By FCGR3A-158 polymorphism, there was no difference detected in the percentage of patients with known grade 4 LON (p=0.79); the rates of hospitalization for serious grade 4 LON-associated infections and fatal infections were also similar between groups. Thirteen patients (16%) were hospitalized for grade 4 LON. Of those hospitalized, six patients had LON that was likely not attributable to rituximab; five patients had neutropenia in the setting of multilineage cytopenias during treatment for CMV (n=4) and/or GVHD (n=3), one of which was further complicated by fatal adenovirus encephalitis, and one patient had pancytopenia in the setting of toxoplasmosis infection. There were seven patients hospitalized with LON-associated infections potentially attributable to rituximab including three fatal infections (one Pseudomonas pneumonia, one presumed nocardiosis, and one presumed histoplasmosis).
Survival outcomes and relapse
The median follow-up was 2.6 years for all patients by the reverse Kaplan-Meier method and 2.4 years (range 1.0 to 5.0 years) among surviving patients. PFS, OS, and relapse point estimates with 95% CIs are shown in Table 2. In the group overall, the estimated 1-year PFS and OS were 71% and 86%, with corresponding 2-year probabilities of 60% and 76%, Figure 1D. By competing risk analysis, the estimated 1-year cumulative incidence of relapse for the cohort was 20% at one year and 27% at two years, Figure 1A.
Table 2.
Point estimates for relapse, progression-free survival, and overall survival for the cohort and subgroups.
| Probability (%) with 95% confidence interval | |||||||
|---|---|---|---|---|---|---|---|
| Relapse | Progression-free survival | Overall survival | |||||
| Group | N | 1 year | 2 years | 1 year | 2 years | 1 year | 2 years |
| All patients | 83 | 20 (13–28) | 27 (17–37) | 71 (62–82) | 60 (52–70) | 86 (78–93) | 76 (67–86) |
| Aggressive NHL | 49 | 22 (11–34) | 25 (12–37) | 69 (58–84) | 62 (49–78) | 86 (76–96) | 74 (62–88) |
| Haplo | 69 | 20 (11–30) | 23 (13–34) | 70 (60–81) | 63 (52–76) | 83 (74–92) | 73 (63–84) |
| VV or VF donor | 60 | 22 (11–32) | 29 (17–41) | 73 (63–85) | 62 (50–76) | 88 (81–97) | 79 (69–90) |
| VV donor | 17 | 18 (0–36) | 25 (3–46) | 82 (66–100) | 68 (48–96) | 94 (84–100) | 87 (71–100) |
| VF donor | 43 | 23 (10–36) | 31 (17–45) | 70 (56–85) | 59 (46–76) | 86 (76–97) | 76 (64–90) |
| FF donor | 23 | 17 (1–33) | 22 (4–39) | 65 (44–88) | 56 (39–81) | 78 (63–97) | 69 (52–91) |
There were no statistically significant differences in relapse, progression-free survival, or overall survival according to donor FCGR3A-158 polymorphism. Disease-free survival was virtually identical to progression-free survival and is therefore not shown separately.
Abbreviations: NHL, non-Hodgkin lymphoma; VV, donor homozygous for the V FCGR3A-158 allele; VF, donor with V and F FCGR3A-158 alleles; FF, donor homozygous for the F FCGR3A-158 allele
Outcomes by subgroups
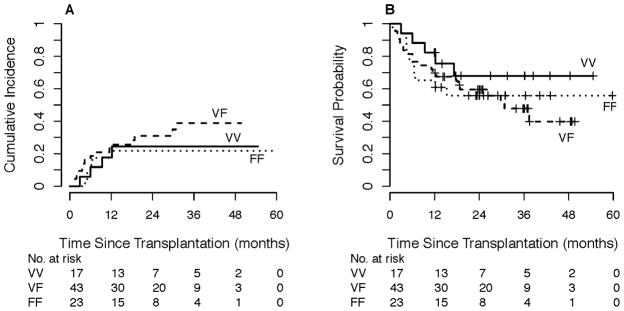
Point estimates for relapse, PFS, and OS for subgroups are shown in Table 2. By donor FCGR3A-158 polymorphism, the 1-year estimated cumulative incidence of relapse was 18% for VV, 23% for VF, and 17% for FF grafts, Figure 2A. The estimated 1-year PFS was 82%, 70%, and 65% for VV, VF, and FF grafts, respectively (Figure 2B, log-rank p=0.44), with corresponding estimated 1-year OS of 94%, 86%, and 78%, log-rank p=0.43. The estimated 1-year probability of NRM was 0% (95% CI 0–0%) for VV, 7% (95% CI 0–15%) for VF, and 17% (95% CI 1–33%) for FF.
Figure 2. Outcomes of non-myeloablative, related-donor bone marrow transplantation according to donor FCGR3A-158 polymorphism.
A) Relapse by competing-risk analysis. B) Progression-free survival.
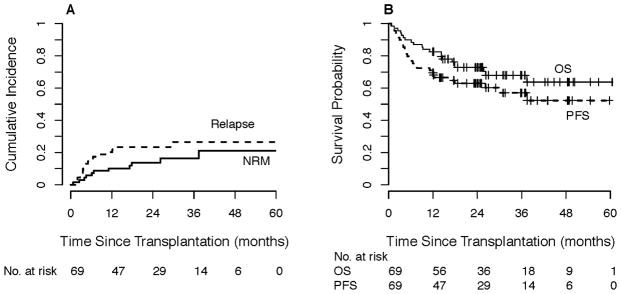
Among those receiving haplo grafts (n=69), the 1-year estimated cumulative incidence of relapse was 20% (Figure 3A, with NRM; Table 2). The estimated 1-year PFS and OS were 70% and 83%, respectively, with corresponding 2-year probabilities of 63% and 73% (Figure 3B, Table 2). At one year, the probability of grade 2–4 aGVHD was 45% (95% CI 33–57%), grade 3–4 aGVHD was 6% (95% CI 0–11%), cGVHD was 13% (95% CI 5–21%), and NRM was 10% (95% CI 3–17%).
Figure 3. Outcomes of non-myeloablative, related HLA-haploidentical donor bone marrow transplantation (n=69).
A) Relapse and non-relapse mortality by competing risk analysis. B) Progression-free and overall survival.
Discussion
Herein we report the outcomes of a phase II study of NMA, related-donor allo BMT for B-cell lymphomas incorporating PTCy, posttransplantation rituximab, and donor selection based on FCGR3A-158 polymorphism. The PFS and OS were favorable for the cohort as a whole and appear improved as compared to the historical outcomes of patients with B-cell lymphomas who received NMA haplo allo BMT using PTCy without rituximab, where the estimated 1-year PFS is lower at around 50%, based on our published and unpublished data.5 The survival outcomes presented here are also consistent with other recent reports using posttransplantation rituximab, suggesting that this approach may improve disease control after allo BMT.32,33 Our study is unique given the use of haplo grafts in the majority of patients and the implementation of a donor selection process based on non-HLA factors.
In a recently published prospective phase II trial of NMA HLA-matched allo BMT for B-cell lymphoma with pre- and posttransplantation rituximab, the 2-year event-free survival and OS probabilities were similar to ours at 72% and 78%, respectively.32 As these data were not available in planning the trial, we had not anticipated such good survival outcomes. The overall favorable outcomes virtually precluded finding significant differences based on FCGR3A-158 polymorphism. The potential benefits of FCGR3A-158-guided donor selection might be further obscured if such benefits are associated only with particular subtypes of B-cell lymphoma or are associated with only particular regimens as has been suggested.15,17,34
LON was documented in nearly half of patients, as has been shown by others.32 However, it can be difficult to attribute neutropenia to rituximab versus other etiologies (e.g. infection) in the allo BMT setting. Importantly, LON was asymptomatic in the majority of the patients, and the use of posttransplantation rituximab did not appear to translate into an increased risk of severe infection.
The favorable PFS and OS, as well as low relapse rates, suggest that posttransplantation rituximab may have a direct anti-tumor effect or modulate graft related anti-tumor immune effects. With regard to the latter, we note that the incidence of grades 2–4 aGVHD in the present study appears higher than has previously been reported for NMA haplo BMT with PTCy but no rituximab. However, this was attributable to higher rates of grade 2 aGVHD, with rates of grade 3–4 aGVHD that were not higher than previous reports.5,6,8,10 As the principal difference between the platform in prior studies and the current study is the inclusion of posttransplantation rituximab here, this suggests that rituximab may impact aGVHD risk. While there is evidence to support the role of posttransplantation rituximab in preventing or treating cGVHD,35–42 less is known about how rituximab might influence aGVHD risk.43 The cellular interactions mediating acute and chronic GVHD are complicated and the relative roles of donor and host B cells, including lymphoma cells, in GVHD pathogenesis are incompletely understood. We hypothesize that rituximab-induced killing of lymphoma cells early after allo BMT promotes the immunogenic cross-presentation of lymphoma-derived minor histocompatibility and tumor antigens to non-tolerant donor T-cells, thereby augmenting both aGVHD and the graft-versus-lymphoma effect, respectively.44 However, other interpretations of our data are certainly possible.
Of broader significance, this study demonstrates the feasibility and safety of selecting related donors based on factors other than degree of HLA matching. The haplo outcomes presented here are similar to those previously published for matched related or unrelated donor allo BMT.2,7 The relatively low rates of grade 3–4 aGVHD, cGVHD requiring systemic therapy, graft failure, and NRM with haplo grafts suggests that HLA matching need no longer be a barrier to optimal donor selection. This study provides support for future trials that similarly aim to identify and select donors based on factors apart from HLA that are hypothesized to improve the antitumor activity of the graft and decrease relapse after allo BMT.
Supplementary Material
Table S2. Patient and transplant characteristics for those receiving HLA-haploidentical grafts.
Highlights.
Donor selection prioritizing non-HLA factors can be feasible and safe
Rituximab after allo BMT is associated with favorable survival outcomes
Donor FCGR3A-158 polymorphism does not appear to influence outcomes after allo BMT
Acknowledgments
This study was supported by National Institutes of Health grants P50 CA096888 to RFA, P01 CA015396 to RJJ, K23 CA124465 to YLK, and P30 CA006973. We thank the Cell Therapy Laboratory at Johns Hopkins for graft data.
Footnotes
Financial Disclosures Statement: The authors have no financial disclosures or conflicts of interest.
Presented in part at the 54th American Society of Hematology Annual Meeting.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116(14):2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bashey A, Zhang X, Sizemore CA, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31(10):1310–1316. doi: 10.1200/JCO.2012.44.3523. [DOI] [PubMed] [Google Scholar]
- 3.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burroughs LM, O’Donnell PV, Sandmaier BM, et al. Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14(11):1279–1287. doi: 10.1016/j.bbmt.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munchel A, Kesserwan C, Symons HJ, et al. Nonmyeloablative, HLA-haploidentical bone marrow transplantation with high dose, post-transplantation cyclophosphamide. Pediatr Rep. 2011;3(Suppl 2):e15. doi: 10.4081/pr.2011.s2.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raiola AM, Dominietto A, di Grazia C, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014;20(10):1573–1579. doi: 10.1016/j.bbmt.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Castagna L, Crocchiolo R, Furst S, et al. Bone marrow compared with peripheral blood stem cells for haploidentical transplantation with a nonmyeloablative conditioning regimen and posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2014;20(5):724–729. doi: 10.1016/j.bbmt.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Ciurea SO, Mulanovich V, Saliba RM, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(12):1835–1844. doi: 10.1016/j.bbmt.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasamon YL, Luznik L, Leffell MS, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16(4):482–489. doi: 10.1016/j.bbmt.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCurdy S, Kanakry JA, Showel M, et al. Risk-stratified outcomes of nonmyeloablative, HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015 doi: 10.1182/blood-2015-01-623991. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Congy-Jolivet N, Bolzec A, Ternant D, Ohresser M, Watier H, Thibault G. Fc gamma RIIIa expression is not increased on natural killer cells expressing the Fc gamma RIIIa-158V allotype. Cancer Res. 2008;68(4):976–980. doi: 10.1158/0008-5472.CAN-07-6523. [DOI] [PubMed] [Google Scholar]
- 13.Dall’Ozzo S, Tartas S, Paintaud G, et al. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res. 2004;64(13):4664–4669. doi: 10.1158/0008-5472.CAN-03-2862. [DOI] [PubMed] [Google Scholar]
- 14.Veeramani S, Wang SY, Dahle C, et al. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood. 2011;118(12):3347–3349. doi: 10.1182/blood-2011-05-351411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21(21):3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99(3):754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 17.Kim DH, Jung HD, Kim JG, et al. FCGR3A gene polymorphisms may correlate with response to frontline R-CHOP therapy for diffuse large B-cell lymphoma. Blood. 2006;108(8):2720–2725. doi: 10.1182/blood-2006-01-009480. [DOI] [PubMed] [Google Scholar]
- 18.Treon SP, Hansen M, Branagan AR, et al. Polymorphisms in FcgammaRIIIA (CD16) receptor expression are associated with clinical response to rituximab in Waldenstrom’s macroglobulinemia. J Clin Oncol. 2005;23(3):474–481. doi: 10.1200/JCO.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 20.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 22.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 23.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;32(29):3249–3256. doi: 10.1200/JCO.2013.53.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolach O, Bairey O, Lahav M. Late-onset neutropenia after rituximab treatment: case series and comprehensive review of the literature. Medicine (Baltimore) 2010;89(5):308–318. doi: 10.1097/MD.0b013e3181f2caef. [DOI] [PubMed] [Google Scholar]
- 28.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.03. 2010 Available from: http://ctep.cancer.gov/default.htm.
- 29.Kaplan E, Meier P. Nonparametric-Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 30.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 31.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 32.Sauter CS, Barker JN, Lechner L, et al. A phase II study of a nonmyeloablative allogeneic stem cell transplant with peritransplant rituximab in patients with B cell lymphoid malignancies: favorably durable event-free survival in chemosensitive patients. Biol Blood Marrow Transplant. 2014;20(3):354–360. doi: 10.1016/j.bbmt.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laport GWJ, Logan B, Bachanova V, Hosing C, Fenske T, Longo W, Devine S, Nademanee A, Gersten I, Horowitz M, Lazarus H, Riches M. Reduced Intensity Conditioning (RIC) with rituximab yields excellent outcomes after allogeneic hematopoietic cell transplantation (alloHCT) for relapsed follicular lymphoma: A Phase II multicenter trial from the Blood and Marrow Transplant Network (BMT CTN 0701) Blood. 2014;124(21):Abstract #682. [Google Scholar]
- 34.Weng WK, Negrin RS, Lavori P, Horning SJ. Immunoglobulin G Fc receptor FcgammaRIIIa 158 V/F polymorphism correlates with rituximab-induced neutropenia after autologous transplantation in patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2010;28(2):279–284. doi: 10.1200/JCO.2009.25.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arai S, Sahaf B, Narasimhan B, et al. Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119(25):6145–6154. doi: 10.1182/blood-2011-12-395970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cutler C, Kim HT, Bindra B, et al. Rituximab prophylaxis prevents corticosteroid-requiring chronic GVHD after allogeneic peripheral blood stem cell transplantation: results of a phase 2 trial. Blood. 2013;122(8):1510–1517. doi: 10.1182/blood-2013-04-495895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108(2):756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khoder A, Sarvaria A, Alsuliman A, et al. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood. 2014;124(13):2034–2045. doi: 10.1182/blood-2014-04-571125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kharfan-Dabaja MA, Mhaskar AR, Djulbegovic B, Cutler C, Mohty M, Kumar A. Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2009;15(9):1005–1013. doi: 10.1016/j.bbmt.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Mohty M, Marchetti N, El-Cheikh J, Faucher C, Furst S, Blaise D. Rituximab as salvage therapy for refractory chronic GVHD. Bone Marrow Transplant. 2008;41(10):909–911. doi: 10.1038/bmt.2008.12. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto M, Okano A, Akamatsu S, et al. Rituximab is effective for steroid-refractory sclerodermatous chronic graft-versus-host disease. Leukemia. 2006;20(1):172–173. doi: 10.1038/sj.leu.2403996. [DOI] [PubMed] [Google Scholar]
- 42.Zaja F, Bacigalupo A, Patriarca F, et al. Treatment of refractory chronic GVHD with rituximab: a GITMO study. Bone Marrow Transplant. 2007;40(3):273–277. doi: 10.1038/sj.bmt.1705725. [DOI] [PubMed] [Google Scholar]
- 43.Ratanatharathorn V, Logan B, Wang D, et al. Prior rituximab correlates with less acute graft-versus-host disease and better survival in B-cell lymphoma patients who received allogeneic peripheral blood stem cell transplantation. Br J Haematol. 2009;145(6):816–824. doi: 10.1111/j.1365-2141.2009.07674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abes R, Gelize E, Fridman WH, Teillaud JL. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood. 2010;116(6):926–934. doi: 10.1182/blood-2009-10-248609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S2. Patient and transplant characteristics for those receiving HLA-haploidentical grafts.