Abstract
There is an ongoing need for new adjuvants to facilitate development of vaccines against HIV, tuberculosis, malaria and cancer, amongst many others. Unfortunately, the most potent adjuvants are often associated with toxicity and safety issues. Inulin, a plant-derived polysaccharide, has no immunological activity in its native soluble form but when crystallized into a stable microcrystalline particulate from (delta inulin) acquires potent adjuvant activity. Delta inulin has been shown to enhance humoral and cellular immune responses against a broad range of co-administered viral, bacterial, parasitic and toxin antigens. Inulin normally crystallizes as large heterogeneous particles with a broad size distribution and variable solubility temperatures. To ensure reproducible delta inulin particles with a consistent size distribution and temperature of solubility, a current Good Manufacturing Practice (cGMP) process was designed to produce Advax™ adjuvant. In its cCMP form, Advax™ adjuvant has proved successful in human trials of vaccines against seasonal and pandemic influenza, hepatitis B and insect sting anaphylaxis, enhancing antibody and T-cell responses while being safe and well tolerated. Advax™ adjuvant represents a novel human adjuvant that enhances both humoral and cellular immunity. This review describes the discovery and development of Advax™ adjuvant and research into its unique mechanism of action.
Keywords: Adjuvant, Vaccine, Delta inulin, Advax™, Immunity
1. Inulin: historical background
Inulin is a simple plant-based fructan polysaccharide comprising a family of linear β(2 → 1) polyfructofuranosyl α-d-glucose polymer chains in which an unbranched chain of fructose rings is terminated with a single glucose (Fig. 1 ). Inulin is utilized as a storage carbohydrate by plants of the Compositae family that include dahlias, chicory, artichoke, onions, and garlic (reviewed in [1]). Inulin's medicinal uses date back to ancient times. Pedanios Dioscoride, a physician with the Roman army, in 100 AD reported the beneficial effects of chicory root extract (∼40% inulin by weight) for treatment of stomach, liver and kidney complaints [2]. In 1804, inulin was extracted by a German scientist from a boiling water extract of Inula helenium [3]. Based on its Inula source, Thomson in 1818 gave this extract the name, inulin [4]. Oral inulin's beneficial effects on metabolic disorders was re-discovered in 1874 with a report that diabetic patients lost their glycosuria when put on a diet of 100 g of inulin per day [3]. Dietary inulin not only has a favourable effect on blood sugar levels in patients with type 2 diabetes but also reduces fasting insulin concentrations, triacylglycerol levels [5] and hepatic lipogenesis [6]. Inulin has also been shown to reduce atherosclerotic lesions in Apo E-deficient mice [7]. Furthermore, short chain fatty acids including butyrate and propionate formed by inulin fermentation in the gut have anti-proliferative effects [8] and stimulate apoptosis of colon cancer cells, enhancing expression of enzymes including histone deacetylases involved in detoxification of carcinogens [9]. Inulin fermentation products in the gut also have anti-inflammatory effects and may help suppress autoimmune disease [10].
Fig. 1.
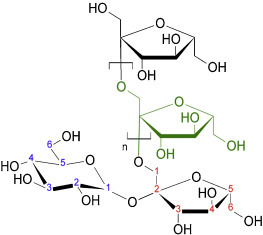
Structure of a single inulin polymer. This shows inulin comprises a variable-length chain of fructose rings terminated with a single glucose ring. In general, inulin polymers isolated from plant sources range in length from 3 to 100 fructose units.
2. Use of inulin for measurement of human glomerular filtration
Although inulin can be metabolized by gut flora, the inability of mammals to metabolize injected inulin led Shannon and Smith in the 1920s to explore inulin's use for measurement of glomerular filtration (GFR) [11]. They confirmed the nontoxicity of inulin by intravenously injecting themselves with 160 g of soluble inulin thereby confirming inulin's exceptional safety [11]. Injection of soluble inulin subsequently became the gold standard for GFR measurement, with intravenous injection of a loading dose of 50 mg/kg body weight followed by an infusion to maintain steady plasma levels. Measurement of urinary inulin then provides an estimate of GFR. Many thousands of patients have safely had this GFR test including pregnant women and newborn babies, with no known adverse effects attributable to inulin other than a mild osmotic diuresis [12]. In the early days hospitals made their own inulin formulations and patients receiving inulin infusions occasionally developed transient hypotensive symptoms. This was found to reflect complement activation that was independent of the only then known (now called classical) complement pathway. A search for the mechanism of complement activation by inulin led to discovery of the alternative complement pathway (ACP) [13]. ACP activation was found to occur when injected inulin was not entirely dissolved with microscopic crystalline inulin contaminants responsible for ACP activation. Hence ACP activation is a unique feature of crystalline but not soluble inulin, a feature critical to inulin's use as a vaccine adjuvant as described below.
3. Development of gamma inulin as an anti-cancer agent
Complement activation has anti-cancer effects in animals and humans. An early demonstration of this anti-cancer effect utilized the complement activator, Staphylococcus aureus protein A Unfortunately, this also had major side effects including pyrexia, nausea, vomiting, and cardiopulmonary toxicity [14]. To avoid the problems of using S. aureus for complement activation, an alternative approach to cancer therapy was developed by Cooper et al. with the idea of instead using inulin crystals for complement activation [15]. This led to the development of a crystalline isoform of inulin given the name gamma inulin to distinguish it from highly soluble alpha and beta inulin isoforms [16]. Gamma inulin was potent at ACP activation in human plasma reaching a maximum at ∼20 μg/ml [17]. In mice, intraperitoneal administration of gamma inulin at doses of 0.2–0.5 mg induced greater than 50% serum complement depletion within 30 min with complement levels returning to normal within 16–24h [18]. Despite potent ACP activation, intraperitoneal or subcutaneous injections of gamma inulin at doses up to 10 mg were well tolerated with mice not exhibiting any adverse effects. Intraperitoneal administration of gamma inulin to mice implanted with a B16 melanoma cell line prolonged survival times and depletion of complement factor 3 (C3) abrogated this protective effect, consistent with gamma inulin's anti-tumour effects being mediated by ACP activation [19]. Gamma inulin induced tumour regression when injected into squamous cell carcinomas in sheep, an effect enhanced by combination therapy with cyclophosphamide [18]. Intralesional injection of gamma inulin into equine sarcoids or spontaneous tumours in dogs similarly resulted in tumour regression (P. Cooper, personal communication). Gamma inulin also potentiated anti-cancer effects of photodynamic therapy in an ACP-dependent manner [20].
4. Development of microcrystalline inulin as an immune adjuvant
Complement also plays an important role in generation of adaptive immunity. This effect of complement has been exploited in vaccine design. For example, covalent coupling of antigens to complement factor C3d was shown to enhance their immunogenicity [21]. Given its potent ability to activate complement, we asked whether co-formulation of antigens with gamma inulin might similarly enhance their immunogenicity. When tested in this way, gamma inulin proved to have a beneficial effect on adaptive immune responses. Many of the early studies were performed with keyhole limpet hemocyanin (KLH) and when KLH was co-administered with gamma inulin this resulted in enhanced anti-KLH antibody responses in mice with similar results in guinea pigs and rabbits [22]. Gamma inulin was subsequently shown to improve the immunogenicity of a range of vaccine antigens (reviewed in [23]).
The mechanism of adjuvant action of gamma inulin was shown to involve increased C3 deposition on the surface of macrophages leading to enhanced T-cell activation [24]. Although it did not induce macrophage activation as measured by chemiluminescent respiratory burst, gamma inulin primed macrophages for a greater respiratory burst in response to phorbol ester. Incubation of human peripheral blood mononuclear cells with tetanus toxoid in the presence of gamma inulin resulted in enhanced IL-2 secretion when compared to toxoid alone, consistent with augmented antigen processing and presentation [18]. Cobra venom depletion of serum complement in mice prior to immunization blocked gamma inulin's adjuvant effects, consistent with adjuvant activity being dependent upon ACP activation [24]. In an attempt to enhance its adjuvant potency gamma inulin was co-crystallized with aluminium hydroxide to form Algammulin, resulting in further enhancement of antibody responses [17]. Nevertheless, Algammulin had significant drawbacks that prevented its development as a human vaccine adjuvant including potential toxicity of the aluminium adjuvant component [25], [26], Th2-immune bias [27], [28], and high batch-to-batch variability in potency. To overcome these problems, studies were undertaken into the structure and function of inulin with the aim of developing a more stable and consistent inulin adjuvant platform. This led to development of the delta inulin isoform that not only had much greater and more consistent adjuvant activity than gamma inulin but, most importantly, was able to be manufactured as a highly consistent cGMP product [29]. Delta inulin subsequently formed the basis of the Advax™ adjuvant platform.
5. Structural basis of delta inulin
Inulin has a hydrophobic polyoxyethylene-like backbone that is critical to its structure in solution and when crystallized. Inulin particles obtained by precipitation from cold water are referred to as alpha inulin and the particles obtained by precipitation from ethanol as beta inulin; dried commercial inulin is almost all in alpha or beta forms. Both alpha and beta isoforms are highly soluble and readily dissolved in water at 25 °C, gamma inulin starts to become soluble in water at 37 °C, and delta inulin is insoluble at temperatures below 40 °C, an important distinction as this means that delta inulin particles are insoluble at body temperatures. Hence, the key factor behind understanding of delta inulin is the fact that inulin polymers can be crystallized into discrete isoforms characterized by different solubility rates in aqueous media [29], [30]. In essence, each inulin particle is made up of individual inulin polymers each formed into an antiparallel double helix, with these helixes then being aggregated together through lateral hydrogen bonding to form lamellar sheets [29], [31], [32]. The greater stability of delta inulin when compared to earlier inulin isoforms reflects greater aggregate strength of H-bonding between each of the individual inulin polymers forming the crystal structure [30]. Transmission and scanning electron microscopy and atomic force microscopy studies of delta inulin particles show consistent spherulite-like discoid particles 1–2 μm in diameter made up of a series of lamellar sheets [33]. Better knowledge of the structural basis of delta inulin crystallization assisted design of a cGMP production process, thereby ensuring ability to produce Advax™ adjuvant particles with a highly consistent size and reproducible adjuvant properties, as shown in Fig. 2 . This was in marked contrast to the highly irregular shape, size and inconsistent adjuvant properties of gamma inulin and initial non-GMP delta inulin formulations.
Fig. 2.
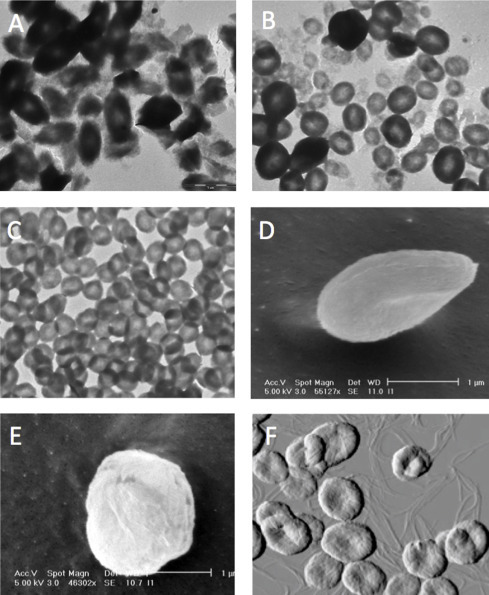
Microscopic structures of crystalline inulin particles. Transmission electron microscopy (TEM) reveals gamma inulin particles to have highly variable shape, size and structure (A), potentially explaining their large batch-to-batch variation in adjuvant activity. Initial delta inulin particles had better defined structure on TEM but still exhibited considerable size variation (B). Optimization and control of crystallization parameters allowed reproducible production of delta inulin particles of consistent size and shape (C) that subsequently formed the basis of Advax™ adjuvant. Freeze-fracture scanning EM of Advax™ particles suspended in water reveals their discoid shape and formation from lamellar sheets (D and E). Atomic force microscopy of Advax™ particles during their formation confirms their discoid character and also reveals fine filament precursors from which the final delta inulin particles are assembled (F).
6. Alternative complement pathway activation
Gamma and delta inulin both activate the ACP [29]. It is not known exactly why these inulin structures are such potent complement activators, although they do have a high number of surface hydroxyl groups recognized as important to complement activation. Virtually any biological surface can activate the ACP provided no sialic acid moieties are present with sialic acid functioning as a discriminator to protect vertebrate surfaces from self-complement attack [34]. Complement factor C3b has only a moderate preference for spontaneous reaction with glucose or fructose moieties [35]. Hence, inulin polymers themselves have no particular features to suggest high C3 convertase activation activity. Indeed, soluble inulin polymers have no ability to activate complement. For ACP initiation to lead to the rapid amplification of complement activation, the labile C3 convertase products produced by ACP activation must be localized on a surface site relatively protected from circulating complement inhibitors and degrading enzymes [35]. Therefore, it is possible that the surface of gamma and delta inulin crystal packing structures is able to protect activated complement components. Despite potent ACP activation capability, both gamma and delta inulin are extremely well tolerated even when injected at high doses. This suggests that the complement activation they induce occurs in a controlled fashion that avoids production of toxic downstream mediators. We hypothesize that delta inulin may activate complement regulators such as clusterin thereby blocking production of downstream anaphylatoxins and complement membrane attack complexes [36]. If so, this could explain how delta inulin is able to separate the beneficial effects on adaptive immunity from the harmful downstream effects of complement membrane attack complexes.
7. Preclinical vaccine studies
Delta inulin has multiple advantages as an adjuvant over gamma inulin including greater temperature stability, ability to be produced as a monodisperse particle population under cGMP (Fig. 2) and improved adjuvant potency in animal models. Delta inulin is also resistant to damage from sterilizing doses of gamma irradiation with gamma inulin being highly susceptible [37]. Consequently, development of gamma inulin was ceased more than decade ago and efforts instead refocused on delta inulin, in the form of Advax™ adjuvant. Whilst Advax™ adjuvant is comprised of delta inulin, the terms Advax™ and delta inulin are not synonymous, as Advax™ adjuvant comprises delta inulin of highly specific particle size and morphology, thereby distinguishing it from delta inulin more generally.
Early studies showed that immunization of mice with hepatitis B surface antigen (HBsAg) plus Advax™ adjuvant provided at least 4-fold antigen-dose sparing when compared to a standard alum-adjuvanted vaccine [29]. The Advax™-immunized mice also showed a more balanced Th1 and Th2 immune response by contrast to the extreme Th2-bais seen in mice immunized with alum adjuvant. Use of Advax™ adjuvant was associated with increased CD4+ and CD8+ T-cell proliferative responses and increased antigen-stimulated production of IFNγ, TNFα, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17 and GM-CSF [38], consistent with broad based enhancement of all T-cell subsets, rather than the strong Th2- or Th1-bias of alum or CpG adjuvants, respectively [39]. IL-1α production was not increased by Advax™ adjuvant [38] suggesting that, unlike alum [40], Advax™ does not induce inflammasome activation. Its adjuvant effect was not dependent on antigen absorption as antibody responses were enhanced even when Advax™ was injected 1 day prior to the antigen [38].
Advax's effects on influenza vaccines have been extensively studied. Formulation of inactivated influenza vaccine with Advax™ enhanced serum neutralizing antibody titres in mice and provided up to 100-fold antigen sparing [41]. This correlated with a higher frequency of influenza-specific IgM- and IgG-secreting B cells in the bone marrow and spleen of immunized mice. As seen for HBsAg immunization, use of Advax™ was associated with an increase in influenza-specific CD4+ and CD8+ T-cell proliferation and increased IFN-γ, IL-2, IL-5, IL-6, and GM-CSF production, which translated into enhanced protection against lethal influenza challenge [41]. Immunity was long-lived with high antibody titres, T-cell responses and influenza protection still evident 1 year post-immunization with Advax™. Injection of mice with Advax™ alone in the absence of antigen provided no protection against influenza infection, indicating that it does not induce non-specific innate immune protection. Advax™ has also been shown to boost the immunogenicity of inactivated influenza vaccine even when delivered via the intrapulmonary route [42]. Advax™ adjuvant was also tested for its ability to help overcome the effects of pregnancy-associated immune suppression on influenza vaccine responses. Advax™ adjuvant was well tolerated by pregnant dams and was not associated with any adverse reproductive or developmental effects in mothers or pups [43]. Pregnant dams that received a single intramuscular injection of inactivated influenza antigen with Advax™ adjuvant had increased serum anti-influenza IgG titres and this translated into higher anti-influenza IgG titres in their breast milk. This in turn resulted in higher anti-influenza IgG titres in the serum of their suckling pups and these pups were completely protected when challenged with influenza at 4 weeks-of-age. By contrast, no survival was seen in pups of mothers immunized with a standard unadjuvanted influenza vaccine [43]. In a ferret challenge model Advax™ adjuvant enhanced protection conferred by a licensed vaccine against high-pathogenicity avian (H5N1) influenza. Two immunizations with inactivated H5N1 antigen plus Advax™ adjuvant with or without the addition of CpG oligonucleotide induced high serum H5N1 neutralizing antibody titres whereas antibody was barely detectable in ferrets that received standard vaccine [44]. Enhanced survival, reduced clinical disease and absence of brain invasion by virus was seen in the Advax™-adjuvanted groups with the virus being completely cleared from the nasal wash by day 4 post-challenge.
Advax™ adjuvant was similarly shown to enhance vaccine protection against pulmonary anthrax. Advax™ enhanced antibody responses to recombinant protective antigen (rPA) resulting in enhanced protection against a lethal anthrax challenge even after just a single immunization [45]. A synergistic effect was seen when murabutide, a NOD2 agonist, was co-formulated with the Advax™ adjuvant. As shown by in vivo imaging of cathepsin cleavage of ProSense 750, Advax™ plus murabutide induced significantly less local injection site inflammation than the alum adjuvant.
Flaviviruses represent another major vaccine challenge. Although there are human vaccines against Japanese encephalitis virus (JEV) [46], there are currently no licensed vaccines against West Nile virus or dengue. Inclusion of Advax™ adjuvant in an inactivated JEV antigen not only enhanced neutralizing antibody responses in immunized mice and horses but was also associated with induction of cross-neutralizing antibody responses against Murray Valley encephalitis virus (MVEV) and West Nile virus (WNV) [47]. It was subsequently shown that the Advax™ adjuvant enhanced production of memory B cells able to transfer protection against JEV and related flaviviruses to naïve mice [48]. Cross-protection against WNV was achieved after just a single dose of JEV vaccine formulated with Advax™ adjuvant and was maintained out to at least 1 year post-immunization [49]. Advax™-adjuvanted JEV vaccine was found to be safe and effective when administered to pregnant horses and newborn foals in which it similarly induced broadly cross-protective anti-flavivirus antibodies [50].
Advax™ adjuvant enhanced neutralizing antibody responses and protection in a murine model of severe acute respiratory syndrome (SARS) coronavirus. Formulations of either inactivated SARS virus or recombinant spike protein with Advax™ adjuvant protected mice and reduced lung virus titres when compared to immunization with either antigen alone [51]. One year post-immunization, animals immunized with Advax™-adjuvanted vaccine had higher SARS-specific CD4+ and CD8+ T-cell proliferation responses and IFNγ, IL2, IL4, IL6, IL10, IL17 and TNF α production. Whereas alum-formulated SARS antigens were associated with increased eosinophilic lung immunopathology in response to challenge with SARS virus, lung immunopathology was absent in mice immunized with delta inulin-adjuvanted vaccine. The absence of lung immunopathology in the Advax™ adjuvant groups correlated with a higher frequency of SARS-specific IFNγ-producing T cells, consistent with Advax™ adjuvant driving a more balanced Th1 and Th2 response. Inhibition of eosinophilic lung immunopathology was further assisted by co-formulation of Advax™ adjuvant with CpG oligonucleotide [51].
Advax™ adjuvant alone or in combination with CpG oligonucleotide enhanced the immunogenicity in mice of an HIV envelope protein boost following a DNA plasmid intramuscular prime, thereby inducing persistent gp120-specific mucosal IgA, serum IgG and memory T- and B-cell responses [52]. Similarly, in rabbits and nonhuman primates Advax™ adjuvant enhanced production of simian immunodeficiency virus cross-neutralizing antibodies when combined with an envelope protein boost following a DNA prime [53].
Advax™ adjuvant enhanced protection afforded by a Listeria vaccine based on T-cell peptide epitopes conjugated to gold nanoparticles [54], [55]. Enhanced protection by the Advax™ adjuvant correlated with an increased frequency of splenic CD4+ and CD8+ T cells, NK cells, CD8α+ DC and Th1 cytokine production (IL12, IFNγ, TNFα and MCP1) and was associated with increased T-cell epitope spreading following live Listeria challenge [56].
Examples of veterinary vaccines where Advax™ adjuvant has been shown to have beneficial effects on neutralizing antibody responses and/or protection include vaccines against Peste de petit ruminants in mice and goats [57] and against African Horse Sickness and Melioidosis in camels [58].
8. Administration routes, species-specificity and mechanism of action
In most of the above-described preclinical studies, Advax™ adjuvant was administered intramuscularly or subcutaneously to mimic its intended human use. However, Advax™ adjuvant is also effective if given via other routes, including intradermal, intraperitoneal or intranasal delivery (unpublished data). It was recently shown to have adjuvant activity even when administered directly into the murine lung. Intratracheal administration of a dry powder inactivated influenza vaccine together with Advax™ adjuvant induced higher influenza-specific intranasal IgA titres and a more balanced Th1/Th2 antibody isotype response with no observed adverse effects [42].
Whilst the vast majority of immunogenicity studies of Advax™ adjuvant have been undertaken in mice, adjuvant activity has been demonstrated across a broad range of other animal species including guinea pigs [38], ferrets [44], goats [57], horses [47] and camels [58]. To date, no species limitation in Advax™ adjuvant action has been seen with adjuvant activity also seen in rats, rabbits, dogs, cats and sheep (unpublished data). It is also effective across different life stages, being efficacious and safe when administered in combination with inactivated influenza vaccine to pregnant mice [43] or 7-day-old mouse pups [59] or in combination with inactivated Japanese encephalitis vaccine to pregnant mares or newborn foals [50].
Most adjuvants have been shown to work via danger signals. For example, aluminium salts inducing cell necrosis resulting in inflammasome activation [60] and also triggering toll-like receptor (TLR)-9 activation through DNA released from lysed cells [61]. Similarly, monophosphoryl lipid A activates TLR4, flagellin activates TLR5 and CpG oligonucleotides activate TLR9. The final common pathways for all these adjuvants working via danger signals is activation of the transcription factor, nuclear factor-kappa B (NFkB), the master regulator of inflammatory responses [62]. Advax™ adjuvant is an exception as it fails to induce NFkB activation in human monocytes or murine splenocytes and similarly does not activate the inflammasome or induce inflammatory cytokine production (unpublished data). The mechanism underlying the unique ability of Advax™ adjuvant to separate innate immune activation and inflammation from enhancement of adaptive immunity is the subject of intense ongoing study by our group.
9. Clinical development
A first-in-man Phase 1 clinical trial was conducted to asses the safety and tolerability of three intramuscular doses of HBsAg formulated with Advax™ adjuvant in healthy adult subjects was undertaken following successful preclinical acute and repeat dose toxicology studies in which no safety problems were identified [63]. Advax™ adjuvant was well tolerated by the trial subjects with injection site pain scores not significantly different to subjects receiving HBsAg alone. Advax™ enhanced anti-HBsAg antibody titres and seroprotection rates when compared to administration of HBsAg alone. The proportion of subjects with positive anti-HBsAg CD4+ T-cell responses was also significantly higher in subjects that received Advax™ adjuvant [63].
A Phase 1/2 study was undertaken in adult subjects to assess the ability of Advax™ adjuvant to enhance the immunogenicity of a pandemic influenza A/H1N1/2009pdm vaccine made from recombinant hemagglutinin [64]. Advax™ increased seroprotection rates by 1.9 times after the first, and 2.5 times after the second, immunization, when compared to immunization with the recombinant hemagglutinin alone. Advax™ adjuvant was well tolerated with no adjuvant-associated adverse reactions observed.
Advax™ adjuvant has also been found to be safe and effective when combined with a bee venom-based immunotherapy administered to human subjects with bee-sting anaphylaxis. It induced more rapid and higher titres of venom-specific IgG4, a marker of successful immunotherapy [65]. Other clinical trials where Advax™ adjuvant has been successfully tested include in combination with seasonal trivalent inactivated influenza vaccines and in combination with a universal T-cell vaccine against influenza based on synthetic peptide epitopes (unpublished data).
10. Conclusions and future prospects
Advax™ delta inulin adjuvant successfully enhanced vaccine immunogenicity across a broad range of antigen types including whole inactivated viruses, recombinant proteins, synthetic peptides, toxins and venoms. It has been shown effective across all animal species tested, and during pregnancy and early neonatal life. It consistently enhances serum antibody titres and CD4+ and CD8+ T-cell immunity and acts synergistically with traditional innate immune activators such as murabutide or CpG oligonucleotide. Early promise in preclinical studies has been supported by successful human clinical trials confirming its safety, tolerability and efficacy. Ongoing priorities are to further characterize delta inulin's mechanism of action, develop it as a mucosal adjuvant, explore synergistic combinations with other immune activators and extend human clinical studies to paediatric populations.
Acknowledgments
NP and PC are associates of Vaxine Pty Ltd, which owns the Advax™ adjuvant platform. Work described in this paper was funded by grants to Vaxine from AusIndustry through its Biotechnology Innovation Fund (BIF), START, Commercial Ready and Researcher-in-Business programs and by funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health under Contracts No. HHSN272200800039C and U01AI061142.
References
- 1.Barclay T., Ginic-Markovic M., Cooper P., Petrovsky N. The chemistry and sources of fructose and their effect on functionality and health implications. J Excipients Food Chem. 2012;3:67–82. [Google Scholar]
- 2.Leroux X. Commercial Publication; 1996. La vie c’est bon comme tout; p. 5. [Google Scholar]
- 3.Robertfroid M. CRC Press; 2004. Inulin-type fructans functional food ingredients. [DOI] [PubMed] [Google Scholar]
- 4.Franck A., De Leenheer L. Wiley-VCH Verlag; 2005. Inulin polysaccharides and polyamides in the food industry properties, production and patents; pp. 281–322. [Google Scholar]
- 5.Jackson K.G., Taylor G.R., Clohessy A.M., Williams C.M. The effect of the daily intake of inulin on fasting lipid, insulin and glucose concentrations in middle-aged men and women. Br J Nutr. 1999;82:23–30. doi: 10.1017/s0007114599001087. [DOI] [PubMed] [Google Scholar]
- 6.Daubioul C., Rousseau N., Demeure R., Gallez B., Taper H., Declerck B. Dietary fructans, but not cellulose, decrease triglyceride accumulation in the liver of obese Zucker fa/fa rats. J Nutr. 2002;132:967–973. doi: 10.1093/jn/132.5.967. [DOI] [PubMed] [Google Scholar]
- 7.Rault-Nania M.H., Gueux E., Demougeot C., Demigne C., Rock E., Mazur A. Inulin attenuates atherosclerosis in apolipoprotein E-deficient mice. Br J Nutr. 2006;96:840–844. doi: 10.1017/bjn20061913. [DOI] [PubMed] [Google Scholar]
- 8.Johnson I.T. New approaches to the role of diet in the prevention of cancers of the alimentary tract. Mutat Res. 2004;551:9–28. doi: 10.1016/j.mrfmmm.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Femia A.P., Luceri C., Dolara P., Giannini A., Biggeri A., Salvadori M. Antitumorigenic activity of the prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis on azoxymethane-induced colon carcinogenesis in rats. Carcinogenesis. 2002;23:1953–1960. doi: 10.1093/carcin/23.11.1953. [DOI] [PubMed] [Google Scholar]
- 10.Petrovsky N. Immunomodulation with microbial vaccines to prevent type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6:131–138. doi: 10.1038/nrendo.2009.273. [DOI] [PubMed] [Google Scholar]
- 11.Shannon J.A., Smith H.W. The excretion of inulin, xylose and urea by normal and phlorizinized man. J Clin Invest. 1935;14:393–401. doi: 10.1172/JCI100690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.British Pharmaceutical codex 1979.
- 13.Gotze O., Muller-Eberhard H.J. The c3-activator system: an alternate pathway of complement activation. J Exp Med. 1971;134:90–108. [PMC free article] [PubMed] [Google Scholar]
- 14.Terman D.S. Protein A and staphylococcal products in neoplastic disease. Crit Rev Oncol Hematol. 1985;4:103–124. doi: 10.1016/s1040-8428(85)80012-3. [DOI] [PubMed] [Google Scholar]
- 15.Cooper P.D. Complement and cancer: activation of the alternative pathway as a theoretical base for immunotherapy. Adv Immun Cancer Ther. 1985;1:125–166. doi: 10.1007/978-1-4612-5068-5_4. [DOI] [PubMed] [Google Scholar]
- 16.Phelps C.F. The physical properties of inulin solutions. Biochem J. 1965;95:41–47. doi: 10.1042/bj0950041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper P.D., Steele E.J. Algammulin, a new vaccine adjuvant comprising gamma inulin particles containing alum: preparation and in vitro properties. Vaccine. 1991;9:351–357. doi: 10.1016/0264-410x(91)90063-c. [DOI] [PubMed] [Google Scholar]
- 18.Cooper P.D. In: Activators and inhibitors of complement. Sim R.B., editor. Kluwer Academic Publishers; Dordrecht, the Netherlands: 1993. pp. 69–106. [Google Scholar]
- 19.Cooper P.D., Carter M. The anti-melanoma activity of inulin in mice. Mol Immunol. 1986;23:903–908. doi: 10.1016/0161-5890(86)90076-3. [DOI] [PubMed] [Google Scholar]
- 20.Korbelik M., Cooper P.D. Potentiation of photodynamic therapy of cancer by complement: the effect of gamma-inulin. Br J Cancer. 2007;96:67–72. doi: 10.1038/sj.bjc.6603508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dempsey P.W., Allison M.E., Akkaraju S., Goodnow C.C., Fearon D.T. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 22.Cooper P.D., Steele E.J. The adjuvanticity of gamma inulin. Immunol Cell Biol. 1988;66(Pt 5–6):345–352. doi: 10.1038/icb.1988.45. [DOI] [PubMed] [Google Scholar]
- 23.Silva D.G., Cooper P.D., Petrovsky N. Inulin-derived adjuvants efficiently promote both Th1 and Th2 immune responses. Immunol Cell Biol. 2004;82:611–616. doi: 10.1111/j.1440-1711.2004.01290.x. [DOI] [PubMed] [Google Scholar]
- 24.Kerekes K., Cooper P.D., Prechl J., Jozsi M., Bajtay Z., Erdei A. Adjuvant effect of gamma-inulin is mediated by C3 fragments deposited on antigen-presenting cells. J Leukoc Biol. 2001;69:69–74. [PubMed] [Google Scholar]
- 25.Fanni D., Ambu R., Gerosa C., Nemolato S., Iacovidou N., Van Eyken P. Aluminum exposure and toxicity in neonates: a practical guide to halt aluminum overload in the prenatal and perinatal periods. World J Pediatr. 2014;10:101–107. doi: 10.1007/s12519-014-0477-x. [DOI] [PubMed] [Google Scholar]
- 26.Gherardi R.K., Authier F.J. Aluminum inclusion macrophagic myofasciitis: a recently identified condition. Immunol Allergy Clin N Am. 2003;23:699–712. doi: 10.1016/s0889-8561(03)00095-x. [DOI] [PubMed] [Google Scholar]
- 27.Leventhal J.S., Berger E.M., Brauer J.A., Cohen D.E. Hypersensitivity reactions to vaccine constituents: a case series and review of the literature. Dermatitis. 2012;23:102–109. doi: 10.1097/DER.0b013e31825228cf. [DOI] [PubMed] [Google Scholar]
- 28.Tseng C.T., Sbrana E., Iwata-Yoshikawa N., Newman P.C., Garron T., Atmar R.L. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012;7:e35421. doi: 10.1371/journal.pone.0035421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper P.D., Petrovsky N. Delta inulin: a novel, immunologically active, stable packing structure comprising beta-d-[2 → 1] poly(fructo-furanosyl) alpha-d-glucose polymers. Glycobiology. 2011;21:595–606. doi: 10.1093/glycob/cwq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper P.D., Barclay T.G., Ginic-Markovic M., Petrovsky N. The polysaccharide inulin is characterized by an extensive series of periodic isoforms with varying biological actions. Glycobiology. 2013;23:1164–1174. doi: 10.1093/glycob/cwt053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andre I., Putaux J.L., Chanzy H., Taravel F.R., Timmermans J.W., de Wit D. Single crystals of inulin. Int J Biol Macromol. 1996;18:195–204. doi: 10.1016/0141-8130(95)01075-0. [DOI] [PubMed] [Google Scholar]
- 32.Cooper P.D., Barclay T.G., Ginic-Markovic M., Gerson A.R., Petrovsky N. Inulin isoforms differ by repeated additions of one crystal unit cell. Carbohydr Polym. 2014;103:392–397. doi: 10.1016/j.carbpol.2013.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper P.D., Rajapaksha K.H., Barclay T.G., Ginic-Markovic M., Gerson A.R., Petrovsky N. Inulin crystal initiation via a glucose–fructose cross-link of adjacent polymer chains: atomic force microscopy and static molecular modelling. Carbohydr Polym. 2015;117:964–972. doi: 10.1016/j.carbpol.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pangburn M.K., Ferreira V.P., Cortes C. Discrimination between host and pathogens by the complement system. Vaccine. 2008;26(Suppl. 8):I15–I21. doi: 10.1016/j.vaccine.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahu A., Kozel T.R., Pangburn M.K. Specificity of the thioester-containing reactive site of human C3 and its significance to complement activation. Biochem J. 1994;302(Pt 2):429–436. doi: 10.1042/bj3020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy B.F., Saunders J.R., O’Bryan M.K., Kirszbaum L., Walker I.D., d’Apice A.J. SP-40,40 is an inhibitor of C5b-6-initiated haemolysis. Int Immunol. 1989;1:551–554. doi: 10.1093/intimm/1.5.551. [DOI] [PubMed] [Google Scholar]
- 37.Cooper P.D., Barclay T.G., Ginic-Markovic M., Petrovsky N. Gamma ray sterilization of delta inulin adjuvant particles (Advax™) makes minor, partly reversible structural changes without affecting adjuvant activity. Vaccine. 2014;32:552–557. doi: 10.1016/j.vaccine.2013.11.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saade F., Honda-Okubo Y., Trec S., Petrovsky N. A novel hepatitis B vaccine containing Advax™, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine. 2013;31:1999–2007. doi: 10.1016/j.vaccine.2012.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weeratna R.D., Brazolot Millan C.L., McCluskie M.J., Davis H.L. CpG ODN can re-direct the Th bias of established Th2 immune responses in adult and young mice. FEMS Immunol Med Microbiol. 2001;32:65–71. doi: 10.1111/j.1574-695X.2001.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 40.Eisenbarth S.C., Colegio O.R., O’Connor W., Sutterwala F.S., Flavell R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honda-Okubo Y., Saade F., Petrovsky N. Advax™, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine. 2012;30:5373–5381. doi: 10.1016/j.vaccine.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murugappan S., Frijlink H.W., Petrovsky N., Hinrichs W.L. Enhanced pulmonary immunization with aerosolized inactivated influenza vaccine containing delta inulin adjuvant. Eur J Pharm Sci. 2014;66C:118–122. doi: 10.1016/j.ejps.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Honda-Okubo Y., Kolpe A., Li L., Petrovsky N. A single immunization with inactivated H1N1 influenza vaccine formulated with delta inulin adjuvant (Advax) overcomes pregnancy-associated immune suppression and enhances passive neonatal protection. Vaccine. 2014;32:4651–4659. doi: 10.1016/j.vaccine.2014.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Layton R.C., Petrovsky N., Gigliotti A.P., Pollock Z., Knight J., Donart N. Delta inulin polysaccharide adjuvant enhances the ability of split-virion H5N1 vaccine to protect against lethal challenge in ferrets. Vaccine. 2011;29:6242–6251. doi: 10.1016/j.vaccine.2011.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feinen B., Petrovsky N., Verma A., Merkel T.J. Advax™-adjuvanted recombinant protective antigen provides protection against inhalational anthrax that is further enhanced by addition of murabutide adjuvant. Clin Vaccin Immunol. 2014;21:580–586. doi: 10.1128/CVI.00019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.WHO Japanese encephalitis vaccines: WHO position paper, February 2015 – recommendations. Vaccine. 2015 doi: 10.1016/j.vaccine.2015.07.057. [DOI] [PubMed] [Google Scholar]
- 47.Lobigs M., Pavy M., Hall R.A., Lobigs P., Cooper P., Komiya T. An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax™, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses. J Gen Virol. 2010;91:1407–1417. doi: 10.1099/vir.0.019190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larena M., Prow N.A., Hall R.A., Petrovsky N., Lobigs M. JE-ADVAX vaccine protection against Japanese encephalitis virus mediated by memory B cells in the absence of CD8+ T cells and pre-exposure neutralizing antibody. J Virol. 2013;87:4395–4402. doi: 10.1128/JVI.03144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrovsky N., Larena M., Siddharthan V., Prow N.A., Hall R.A., Lobigs M. An inactivated cell culture Japanese encephalitis vaccine (JE-ADVAX) formulated with delta inulin adjuvant provides robust heterologous protection against West Nile encephalitis via cross-protective memory B cells and neutralizing antibody. J Virol. 2013;87:10324–10333. doi: 10.1128/JVI.00480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bielefeldt-Ohmann H., Prow N.A., Wang W., Tan C.S., Coyle M., Douma A. Safety and immunogenicity of a delta inulin-adjuvanted inactivated Japanese encephalitis virus vaccine in pregnant mares and foals. Vet Res. 2014;45:130. doi: 10.1186/s13567-014-0130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Honda-Okubo Y., Barnard D., Ong C.H., Peng B.H., Tseng C.T., Petrovsky N. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J Virol. 2015;89:2995–3007. doi: 10.1128/JVI.02980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cristillo A.D., Ferrari M.G., Hudacik L., Lewis B., Galmin L., Bowen B. Induction of mucosal and systemic antibody and T-cell responses following prime-boost immunization with novel adjuvanted human immunodeficiency virus-1-vaccine formulations. J Gen Virol. 2011;92:128–140. doi: 10.1099/vir.0.023242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menon V., Whitney S., Francis J., Ajayi L., Petrovsky N., Montefiori D. Characterization in rabbits & nonhuman primates of the neutralizing antibody response elicited by DNA & protein vaccination with SIVmac251 & SIVsmE660. Retrovirology. 2012;9(Suppl. 2):43. [Google Scholar]
- 54.Calderon-Gonzalez R., Marradi M., Garcia I., Petrovsky N., Alvarez-Dominguez C. Novel nanoparticle vaccines for listeriosis. Hum Vaccin Immunother. 2015 doi: 10.1080/21645515.2015.1063756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calderon-Gonzalez R., Tobes R., Pareja E., Frande-Cabanes E., Petrovsky N., Alvarez-Dominguez C. Identification and characterisation of T-cell epitopes for incorporation into dendritic cell-delivered Listeria vaccines. J Immunol Methods. 2015;424:111–119. doi: 10.1016/j.jim.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodriguez-Del Rio E., Marradi M., Calderon-Gonzalez R., Frande-Cabanes E., Penades S., Petrovsky N. A gold glyco-nanoparticle carrying a listeriolysin O peptide and formulated with Advax™ delta inulin adjuvant induces robust T-cell protection against listeria infection. Vaccine. 2015;33:1465–1473. doi: 10.1016/j.vaccine.2015.01.062. [DOI] [PubMed] [Google Scholar]
- 57.Cosseddu G.M., Polci A., Pinoni C., Capobianco Dondona A., Iapaolo F., Orsini G. Evaluation of humoral response and protective efficacy of an inactivated vaccine against peste des petits ruminants virus in goats. Transbound Emerg Dis. 2015 doi: 10.1111/tbed.12314. [DOI] [PubMed] [Google Scholar]
- 58.Eckersley A.M., Petrovsky N., Kinne J., Wernery R., Wernery U. Improving the dromedary antibody response: the hunt for the ideal camel adjuvant. J Camel Pract Res. 2011;18:35–46. [Google Scholar]
- 59.Honda-Okubo Y., Ong C.H., Petrovsky N. Advax delta inulin adjuvant overcomes immune immaturity in neonatal mice thereby allowing single-dose influenza vaccine protection. Vaccine. 2015 doi: 10.1016/j.vaccine.2015.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cullen S.P., Kearney C.J., Clancy D.M., Martin S.J. Diverse activators of the NLRP3 inflammasome promote IL-1beta secretion by triggering necrosis. Cell Rep. 2015;11:1535–1548. doi: 10.1016/j.celrep.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Marichal T., Ohata K., Bedoret D., Mesnil C., Sabatel C., Kobiyama K. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17:996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 62.Schuster J.M., Nelson P.S. Toll receptors: an expanding role in our understanding of human disease. J Leukoc Biol. 2000;67:767–773. [PubMed] [Google Scholar]
- 63.Gordon D., Kelley P., Heinzel S., Cooper P., Petrovsky N. Immunogenicity and safety of Advax™, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: a randomized controlled Phase 1 study. Vaccine. 2014;(September) doi: 10.1016/j.vaccine.2014.09.034. pii: S0264-410X(14)01293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gordon D.L., Sajkov D., Woodman R.J., Honda-Okubo Y., Cox M.M., Heinzel S. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax polysaccharide adjuvant. Vaccine. 2012;30:5407–5416. doi: 10.1016/j.vaccine.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heddle R., Russo P., Petrovsky N., Hanna R., Smith A. Immunotherapy – 2076. A controlled study of delta inulin-adjuvanted honey bee venom immunotherapy. World Allergy Organ J. 2013;6 P158. [Google Scholar]


