Abstract
In this review, we highlight recent re-evaluations of the classical cell sorting models and their application to understanding embryonic morphogenesis. Modern genetic and biophysical techniques reveal that tissue self-assembly is not solely a result of differential adhesion, but rather incorporates dynamic cytoskeletal tension and extracellular matrix assembly. There is growing evidence that these biomechanical modules cooperate to organize developing tissues. We describe the contributions of Cadherins and Integrins to tissue assembly and propose a model in which these very different adhesive regimes affect the same outcome through separate but convergent mechanisms.
Adhesive sorting
Pioneering work during the last half of the 20th century showed that when cells from different embryonic germ layers are mixed they spontaneously separate into distinct populations. Townes and Holtfreter found that these populations recapitulate the spatial orientation of their parent tissues, with ectodermal cells surrounding mesodermal cells, which in turn surround endodermal cells. This work showed that gastrulating cells contain a cell-autonomous sorting ability [1]. These experiments predicted that cells sort due to differential expression of adhesion proteins (Figure 1A). Eventually, Cadherins were identified as effectors of cell sorting, with cells expressing different recombinant Cadherins selectively adhering to cells expressing like Cadherins [2].
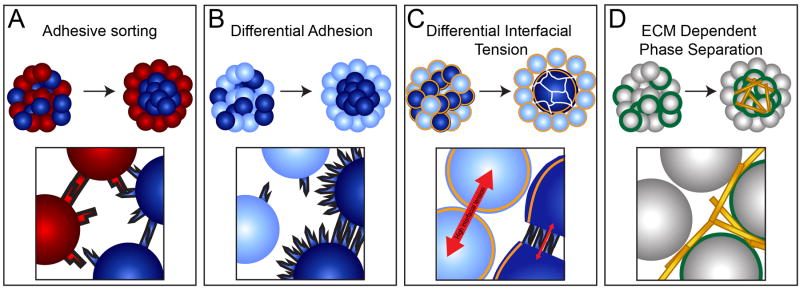
Figure 1. Models for cell sorting.
(A) Cells expressing different Cadherins will sort into distinct populations. (B) The DAH accurately predicts that cells expressing different levels of the same Cadherin will effectively sort. (C) The DITH predicts that changes in cortical tension largely mediated by the cytoskeleton drives cell sorting. Cadherins affect sorting by both reducing cytoskeletal tension and by mediating cell-cell adhesion. (D) Integrin-ECM interactions also mediate cell sorting by indirectly linking neighboring cells.
The Differential Adhesion Hypothesis (DAH)
These in vitro cell sorting experiments were put into a physical context by the Differential Adhesion Hypothesis (DAH) [3]. The DAH conceptualizes populations of cells as immiscible fluids and states that cell sorting is driven by surface tension to minimize the overall energy of the system. Thus, more strongly adhering cells will be surrounded by less strongly adhering cells. An important prediction of the DAH is that cells need not express different Cadherins in order to sort but rather will effectively segregate into distinct populations when expressing different levels of the same Cadherin (Figure 1B). This prediction was later experimentally verified [4].
The Differential Interfacial Tension Hypothesis (DITH)
The DAH is elegantly simple and became the textbook model explaining cell sorting [5]. However, mathematical modeling led to a reconsideration of the experimental literature, and the recognition that the DAH could not fully explain the mechanism of cell sorting [6]. More recent work leveraged advances in atomic force microscopy technology to introduce quantitative measurements of cell level biophysics into models of cell sorting [7]. As predicted by the DAH, superficial ectodermal cells were less adhesive than deeper mesodermal and endodermal cells. Surprisingly however, in aggregates made from these cells, strongly adhering mesodermal cells surrounded the loosely adhering ectodermal cells, in direct contradiction to the DAH. Further, the sorting patterns of the cells correlated with their cortical tension, rather than their adhesion strength, with softer mesodermal and endodermal cells surrounding stiff ectodermal cells.
The importance of cortical tension, rather than solely adhesion, supports an alternative model to the DAH called the Differential Interfacial Tension hypothesis (DITH) [8]. This model was originally proposed as the Differential Surface Contraction Hypothesis [6,9]. Similar to the DAH, the DITH postulates that cells sort in order to minimize the interfacial tension of the system. The interfacial tension as defined in the DITH is similar to surface tension in imiscible liquids but more specifically incorporates the roles of cortical tension and adhesive tension. Most importantly, interfacial tension is increased by higher cortical tension but is decreased by higher adhesive tension [8]. In other words, cortical tension generated principally by the cytoskeleton minimizes the contact area between cells while adhesive tension mediated by Cadherins increases the cell-cell contact area. Because adhesive tension opposes cortical tension, it decreases the overall tension in the system, and thus cells sort in such a way as to maximize their adhesion tension while minimizing their cortical tension. Cadherins are believed to mediate sorting by both increasing adhesion energy and, via rearrangement of the actin cytoskeleton to link cortices on neighboring cells, by decreasing cortical tension [10••,11]. Indeed, the decrease in cortical tension mediated by Cadherins may be more important than their actual adhesive function [7]. This model is consistent with the observation that the tissue tension in cell aggregates is four orders of magnitude greater than that predicted to be caused by Cadherin adhesion energy [12](Figure 1C).
The DITH effectively describes cell sorting in vitro. However, cell sorting in vitro does not necessarily recapitulate cell sorting in embryos, as zebrafish ectodermal cells sort internally in aggregates and externally in embryos [7]. Further, artificial mixing of cell populations is not the same as developmentally regulated changes in cell mechanics, and the resulting in vitro cell sorting may occur several fold more slowly than in vivo [7,13]. It is clear that the in vivo context influences patterns and rates of cell sorting. Computer simulations suggest that both extra-embryonic tissues and the interface between cells and interstitial fluid impact cell sorting [7,8]. Charged glycoproteins on the cell surface may also direct cell sorting as has been implicated in opening of some vascular lumens [14]. Another component of the in vivo context is cell-ECM interactions which play an important role in embryonic morphogenesis. Dimensionalty and physical context strongly influence cell-ECM interactions and may be very different in cell aggregates compared to an embryo [15].
ECM assembly stimulates cell-cell cohesivity in cultured tissue aggregates independent of Cadherin
Cell-cell and cell-ECM adhesion seem to be fundamentally different processes. Nonetheless, ECM assembly by Integrin α5β1 has been found to confer strong tissue cohesion to 3D aggregates of CHO cells lacking endogenous Cadherins. This Integrin generated increase in cohesion is more than twice as strong as that conferred by Cadherins [16]. Moreover, Integrin α5β1 dependent increased cohesion does not occur in 2D culture, further underscoring the importance of the physical context in understanding morphogenesis [17].
The DAH and DITH predict that any factors that produce differential cohesion should induce cell sorting. Accordingly, differential Integrin α5β1 expression is sufficient to drive cell sorting similar to differential Cadherin expression. Integrin α5β1 expressing cells are “glued” together by the forming ECM, causing them to undergo a phase transition from a viscoelastic fluid to viscoelastic solid in which Integrin α5β1 deficient cells and Integrin α5β1 expressing cells segregate (Figure 1D) [18•]. This finding that Integrin-ECM interactions are able to mediate cell sorting raises important questions about how cells integrate Cadherin and Integrin activity to effect proper tissue morphogenesis
Cadherin/Integrin interactions are context dependent
Crosstalk between Integrins and Cadherins is bidirectional and likely varies between cell types and physical contexts. Engagement with Fibronectin coated beads strongly increases the amount of force required to separate S180 murine sarcoma cell doublets linked by Cadherin 1 [19]. In direct contrast, plating S180 murine sarcoma cells on micropatterned Fibronectin coated 2D surfaces weakens interactions between the cell and a Cadherin 1 coated bead [20]. A number of technical factors could contribute to this discrepancy, including the stiffness of the substrates [21], the 2D/3D nature of the culture setup [22], or the fact that static Cadherin 1 coated beads were used in one study while cells capable of dynamic cytoskeletal rearrangements were used in the other. Whatever the reason, the starkly opposite nature of these findings underscores the complexity of Integrin/Cadherin crosstalk and highlights the necessity of understanding these interactions in vivo. A complete discussion of the multiple complex and often contradictory molecular interactions between Cadherins and Integrins in assorted culture systems is beyond the scope of this review, thus we refer to reader to recent excellent reviews on this topic [23,24]. We instead focus on the tissue level consequences of Cadherin/Integrin crosstalk during early embryonic development.
Positive reciprocal stimulation of Cadherin and Integrin activity during tissue assembly
While cell-ECM interactions can increase tissue stiffness independently of Cadherins [18•], they can also stimulate Cadherin activity and thereby tissue cohesion in vivo. Blocking cell-ECM interactions during Xenopus gastrulation impairs Cadherin dependent cell intercalation and convergent extension [25,26]. Thus, cell-ECM interactions can affect tissue stiffening through two distinct molecular mechanisms. Cell-ECM interactions are not the only regulators of tissue stifferening in vivo, however, as a 60% reduction in fibrillar Fibronectin assembly does not detectably affect stiffening of the Xenopus paraxial mesoderm [27].
Just as cell-ECM interactions can promote cell-cell adhesion, Cadherin-Cadherin interactions can also promote cell-ECM adhesion. During Xenopus gastrulation ectopic overexpression of various Cadherins induces precocious Fibronectin matrix assembly, while dominant-negative Cadherins impair Fibronectin fibrillogenesis. cell-cell interactions also increase traction stress in primary cultures of early Xenopus embryos, though it is not clear if this increase in traction stress is Cadherin dependent. Notably, Fibronectin matrix forms at the boundary of Cadherin expressing and Cadherin deficient cells, though it is difficult to physiologically interpret this result as Fibronectin normally only forms along the blastopore roof at these stages, and the Cadherin that causes this effect is not normally expressed in these tissues [28].
Cadherin dependent stimulation of cell-ECM interactions has also been studied in vitro. Engagement of Cadherins organizes traction force to the periphery of primary mouse keratinocyte cell colonies [29•], and this force increase scales with clone size [30]. Given that cell generated traction force is necessary for ECM assembly [31], these findings suggest a potential mechanism for Cadherin dependent ECM assembly.
Physical association and regulation of Integrin α5β1 by Cadherin 2
Recently, Integrins and Cadherins expressed on adjacent cells were shown to physically associate and regulate tissue boundary formation. During the early development of the vertebrate musculoskeletal axis, the paraxial mesoderm is assembled from motile mesodermal progenitors and subsequently segmented into somites. The surface of the zebrafish paraxial mesoderm is coated in Fibronectin matrix as the tissue forms while Fibronectin fibrils are absent from the mesenchymal core of the tissue (Figure 2A). All cells in the zebrafish paraxial mesoderm transcribe Fibronectin and express the Fibronectin receptor Integrin α5β1, thus the question arises: why is ECM assembly restricted to the tissue surface?
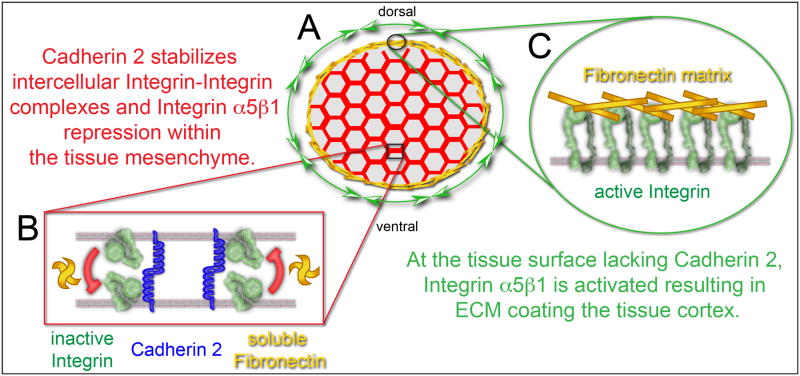
Figure 2. Cadherin 2 stabilizes intercellular Integrin α5 association and Integrin repression.
(A) A schematic transverse cross-section of paraxial mesoderm with adherent mesenchymal cells (red hexagons) and Fibronectin matrix (yellow) on the tissue surface. (B) Within the mesenchyme, Integrins and Cadherins on adjacent cell membranes associate and repress Integrin activity. (C) On the tissue surface, there is no Cadherin 2 and Integrin is activated resulting in Fibronectin fibril formation (green arrows).
A recent study elucidated at least part of the mechanism responsible for establishing this tissue topology [32••]. Within adherent mesenchymal cells, Integrin α5β1 expressed on adjacent cells physically associate in a protein complex that includes Cadherin 2 (Figure 2B). The Cadherin 2 stabilizes both Integrin-Integrin association as well as the bent, inactive conformation of the Integrin. On the tissue surface and somite borders, which lack Cadherin 2, Integrin α5 adopts the extended, active conformation and Fibronectin fibers are formed (Figure 2C). Thus, Cadherin 2 localization is anti-correlated with sites of ECM assembly. These data suggest a mechanism in which an adherent aggregate of cells (e.g. a tissue) may intrinsically bias ECM assembly to the surface of the aggregate via reciprocal repression of Integrin activity within the cell aggregate and de-repression of Integrin activity along the surface of the cell aggregate.
Eph/Ephrin interactions regulate both Cadherin and Integrin activity during morphogenesis
In addition to regulating one another, Cadherins and Integrins also share upstream regulatory pathways. The Eph/Ephrin juxtacrine signaling pathway in particular is notable for regulating both Cadherins and Integrins, and for its importance during a number of early morphogenic events. In Xenopus, Eph/Ephrin signaling drives paraxial mesoderm/notochord boundary formation by destabilizing Cadherin-Cadherin interactions [33••]. In zebrafish, the Fibronectin matrix that forms along this boundary mediates inter-tissue adhesion between the notochord and paraxial mesoderm [34•]. Eph/Ephrin also induces somite boundary formation by activating Integrins and thereby establishing Fibronectin matrix [35-37].
While the full molecular mechanisms of these interactions have not yet been decribed, the Rho family GTPases are believed to be key intermediates. These proteins reorganize the cytoskeleton and have been shown to mediate various positive and negative interactions between Cadherins and Integrins in a number of systems [24]. During notochord formation Rho activity is necessary for the Eph/Ephrin dependent disruption of Cadherins [33••]. During somitogenesis, Eph/Ephrin signaling reduces Cdc42 levels which in turn induces somite epithelialization in chick [38]. Disruption of Rac1 and RhoA also impairs chick somite morphogenesis, however these GTPases have not been shown to be downstream of Eph/Ephrin signaling [39]. Similarly, codepletion of integrin α5 and either ephrinb2a or rap1b prevents zebrafish somite border formation, but there is no evidence that rap1b is mediates Eph/Ephrin activation of Integrin α5 [40,41].
Hypothesis: Mechanical convergence
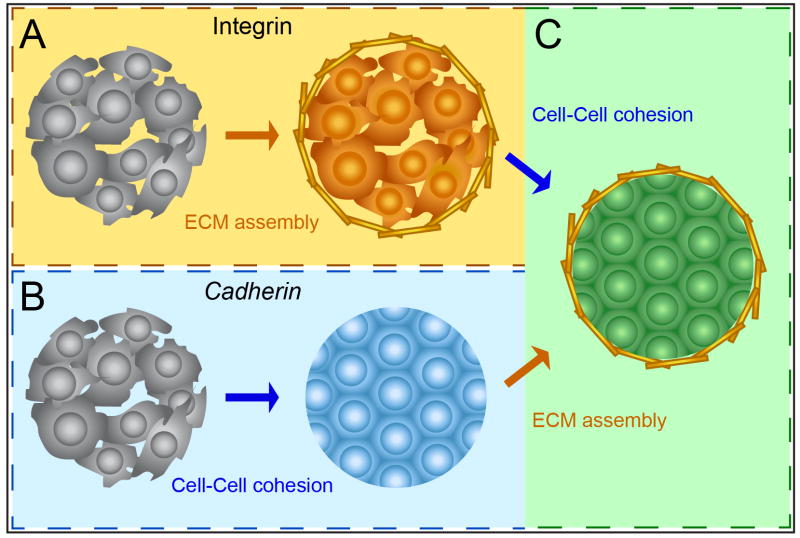
Cadherins canonically mediate cell-cell cohesion, while Integrins canonically mediate cell-ECM adhesion. As we have discussed, however, Cadherins also promote tissue self-assembly by redistributing contractile forces along tissue boundaries and thereby stimulating boundary ECM assembly (Figure 3B). Conversely, Integrin dependent ECM assembly stimulates Cell-cell cohesion via Cadherin dependent and Cadherin independent mechanisms (Figure 3A). In this way, these two fundamentally distinct adhesion pathways converge to a single result: self-assembly of a collection of individual cells into a cohesive, ECM-bound tissue.
Figure 3. Mechanical convergence of cell-cell and cell-ECM adhesion in tissue assembly.
(A) Integrins canonically mediate ECM assembly, but also promote cellular cohesion as a secondary effect. (B) Cadherins canonically mediate cell-cell cohesion, but stimulate ECM assembly as a secondary effect. (C) The convergence of these secondary effects suggests that Integrins and Cadherins, via very different mechanisms, act semi-redundantly to effect the same outcome.
Importantly, this model relies largely on data collected in vitro. However, artificial mixing and sorting of cell populations in vitro does not recapitulate the dynamically regulated changes in cell mechanics during morphogenesis. Thus, sorting events that are completed in a few hours in vivo may take a day or more in vitro. Furthermore, different cellular and molecular processes implicated in cell sorting happen at different time-scales. Initial trans Cadherin binding occurs in minutes while Cadherin and cytoskeletal remodeling happens over tens of minutes [12,13,42]. in vivo activation of Integrins, Fibronectin matrix assembly and activation of Integrin signaling through Focal Adhesion Kinase occurs in minutes along somite borders, but these initial cell-ECM adhesions can differ from more mature adhesion that are under greater mechanical tension [32,43,44]. Future in vivo studies leveraging improved genetic and biophysical techniques will elucidate the relative mechanical contributions of Cadherins and Integrins in tissue self-assembly along physiological time-scales. Moreover, our understanding of the underlying molecular mechanisms of these interactions is far from complete. A better understanding of these mechanisms will be essential in establishing a complete, integrated model of tissue assembly during embryonic development and organogenesis.
Acknowledgments
We thank Dipjyoti Das for comments on the manuscript. SAH is supported by grants from the Eunice Kennedy Shriver National Institute of Child Health (1R21HD076173-01A1) and Human Development and the National Institute of General Medical Sciences (1R01GM107385-01A1 and 1R33GM114257-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steinberg MS, Gilbert SF. Townes and Holtfreter (1955): directed movements and selective adhesion of embryonic amphibian cells. J Exp Zool A Comp Exp Biol. 2004;301:701–706. doi: 10.1002/jez.a.114. [DOI] [PubMed] [Google Scholar]
- 2.Nose A, Nagafuchi A, Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988;54:993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg MS. Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J Exp Zool. 1970;173:395–433. doi: 10.1002/jez.1401730406. [DOI] [PubMed] [Google Scholar]
- 4.Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;278:255–263. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert SF. Developmental Biology. 8th. Sunderland, MA: Sinauer; 2006. [Google Scholar]
- 6.Brodland GW, Chen HH. The mechanics of cell sorting and envelopment. J Biomech. 2000;33:845–851. doi: 10.1016/s0021-9290(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 7.Krieg M, Arboleda-Estudillo Y, Puech PH, Kafer J, Graner F, Muller DJ, Heisenberg CP. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10:429–436. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 8.Brodland GW. The Differential Interfacial Tension Hypothesis (DITH): a comprehensive theory for the self-rearrangement of embryonic cells and tissues. J Biomech Eng. 2002;124:188–197. doi: 10.1115/1.1449491. [DOI] [PubMed] [Google Scholar]
- 9.Harris AK. Is Cell sorting caused by differences in the work of intercellular adhesion? A critique of the Steinberg hypothesis. J Theor Biol. 1976;61:267–285. doi: 10.1016/0022-5193(76)90019-9. [DOI] [PubMed] [Google Scholar]
- 10••.Maitre JL, Berthoumieux H, Krens SF, Salbreux G, Julicher F, Paluch E, Heisenberg CP. Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science. 2012;338:253–256. doi: 10.1126/science.1225399. This paper integrates physical and molecular apporaches to describe the mechanism by which Cadherins elicit germ layer segregation during zebrafish gastrulation. They find that sorting is largely mediated by cortical tension and that Cadherins primarily function to link the corticies of adjacent cells. [DOI] [PubMed] [Google Scholar]
- 11.Maitre JL, Heisenberg CP. Three functions of cadherins in cell adhesion. Curr Biol. 2013;23:R626–633. doi: 10.1016/j.cub.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amack JD, Manning ML. Knowing the boundaries: extending the differential adhesion hypothesis in embryonic cell sorting. Science. 2012;338:212–215. doi: 10.1126/science.1223953. [DOI] [PubMed] [Google Scholar]
- 13.Ninomiya H, David R, Damm EW, Fagotto F, Niessen CM, Winklbauer R. Cadherin-dependent differential cell adhesion in Xenopus causes cell sorting in vitro but not in the embryo. J Cell Sci. 2012;125:1877–1883. doi: 10.1242/jcs.095315. [DOI] [PubMed] [Google Scholar]
- 14.Strilic B, Eglinger J, Krieg M, Zeeb M, Axnick J, Babal P, Muller DJ, Lammert E. Electrostatic cell-surface repulsion initiates lumen formation in developing blood vessels. Curr Biol. 2010;20:2003–2009. doi: 10.1016/j.cub.2010.09.061. [DOI] [PubMed] [Google Scholar]
- 15.Baker BM, Chen CS. Deconstructing the third dimension - how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson EE, Zazzali KM, Corbett SA, Foty RA. Alpha5beta1 integrin mediates strong tissue cohesion. J Cell Sci. 2003;116:377–386. doi: 10.1242/jcs.00231. [DOI] [PubMed] [Google Scholar]
- 17.Robinson EE, Foty RA, Corbett SA. Fibronectin matrix assembly regulates alpha5beta1-mediated cell cohesion. Mol Biol Cell. 2004;15:973–981. doi: 10.1091/mbc.E03-07-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Caicedo-Carvajal CE, Shinbrot T, Foty RA. Alpha5beta1 integrin-fibronectin interactions specify liquid to solid phase transition of 3D cellular aggregates. PLoS One. 2010;5:e11830. doi: 10.1371/journal.pone.0011830. This paper shows that Integrin dependent cell-ECM interactions can mediate cell sorting similar to Cadherin based cell-cell interactions. Increased α5β1 expression produced a linear increase in cohesion at a constant concentration of soluble Fibronectin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Rico C, Pincet F, Thiery JP, Dufour S. Integrins stimulate E-cadherin-mediated intercellular adhesion by regulating Src-kinase activation and actomyosin contractility. J Cell Sci. 2010;123:712–722. doi: 10.1242/jcs.047878. [DOI] [PubMed] [Google Scholar]
- 20.Al-Kilani A, de Freitas O, Dufour S, Gallet F. Negative feedback from integrins to cadherins: a micromechanical study. Biophys J. 2011;101:336–344. doi: 10.1016/j.bpj.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 22.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 23.Burute M, Thery M. Spatial segregation between cell-cell and cell-matrix adhesions. Curr Opin Cell Biol. 2012;24:628–636. doi: 10.1016/j.ceb.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Weber GF, Bjerke MA, DeSimone DW. Integrins and cadherins join forces to form adhesive networks. J Cell Sci. 2011;124:1183–1193. doi: 10.1242/jcs.064618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson LA, Hoffstrom BG, Keller R, DeSimone DW. Mesendoderm extension and mantle closure in Xenopus laevis gastrulation: combined roles for integrin alpha(5)beta(1), fibronectin, and tissue geometry. Dev Biol. 2002;242:109–129. doi: 10.1006/dbio.2002.0537. [DOI] [PubMed] [Google Scholar]
- 26.Marsden M, DeSimone DW. Integrin-ECM interactions regulate cadherin-dependent cell adhesion and are required for convergent extension in Xenopus. Curr Biol. 2003;13:1182–1191. doi: 10.1016/s0960-9822(03)00433-0. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Kim HY, Davidson LA. Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development. 2009;136:677–688. doi: 10.1242/dev.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dzamba BJ, Jakab KR, Marsden M, Schwartz MA, DeSimone DW. Cadherin adhesion, tissue tension, and noncanonical Wnt signaling regulate fibronectin matrix organization. Dev Cell. 2009;16:421–432. doi: 10.1016/j.devcel.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Mertz AF, Che Y, Banerjee S, Goldstein JM, Rosowski KA, Revilla SF, Niessen CM, Marchetti MC, Dufresne ER, Horsley V. Cadherin-based intercellular adhesions organize epithelial cell-matrix traction forces. Proc Natl Acad Sci U S A. 2013;110:842–847. doi: 10.1073/pnas.1217279110. Employing traction force microscopy, this study finds that cell-cell interactions affect the mechanics of cell-ECM interactions. A 2D colony of cells lacking cadherin exhibits traction force exerted on the underlying ECM throughout the colony. However, in the presence of Cadherin, the colony concentrates the traction force to the periphery of the colony. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mertz AF, Banerjee S, Che Y, German GK, Xu Y, Hyland C, Marchetti MC, Horsley V, Dufresne ER. Scaling of Traction Forces with the Size of Cohesive Cell Colonies. Physical review letters. 2012;108:198101. doi: 10.1103/PhysRevLett.108.198101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32••.Jülich D, Cobb G, Melo AM, McMillen P, Lawton AK, Mochrie SGJ, Rhoades E, Holley SA. Cross-scale Integrin regulation organizes ECM and tissue topology. Dev Cell. 2015 doi: 10.1016/j.devcel.2015.05.005. in press. Using fluorescence cross-correlation spectroscopy, this study reveals a physical association between Integrin α5 expressed on adjacent cells in live zebrafish embryos. Cadherin 2 stabilizes the interaction between Integrins. In the absence of Cadherin 2, the Integrins adopt the active conformation and assemble Fibronectin matrix. Thus, ECM assembly is repressed among adherent cells within the tissue, but is de-repressed along tissue boundaries lacking cell-cell adhesions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Fagotto F, Rohani N, Touret AS, Li R. A molecular base for cell sorting at embryonic boundaries: contact inhibition of cadherin adhesion by ephrin/Eph-dependent contractility. Dev Cell. 2013;27:72–87. doi: 10.1016/j.devcel.2013.09.004. In this paper, the authors show that Eph/Ephrin drives cells sorting along the Xenopus notochord-presomitic mesoderm boundary. Eph/Ephrin signaling increases cytoskeletal contractility, cellular blebbing and decreases Cadherin clustering along the boundary, thus facilitating separation of the tissues. [DOI] [PubMed] [Google Scholar]
- 34•.Dray N, Lawton AK, Nandi A, Jülich D, Emonet T, Holley SA. Cell-Fibronectin interactions propel vertebrate trunk elongation via tissue mechanics. Curr Biol. 2013;23:1335–1341. doi: 10.1016/j.cub.2013.05.052. In line with the observations of Fagotto et al., this studies shows that inter-tissue adhesion between the zebrafish notochord and presomitic mesoderm is mediated by cell-Fibronectin interactions rather than cell-cell adhesion. Loss of the two main Fibronectin receptors, Integrin α5 and αV, reveals the cellular blebbing observed along this boundary in Xenopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrios A, Poole RJ, Durbin L, Brennan C, Holder N, Wilson SW. Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Curr Biol. 2003;13:1571–1582. doi: 10.1016/j.cub.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 36.Durbin L, Sordino P, Barrios A, Gering M, Thisse C, Thisse B, Brennan C, Green A, Wilson S, Holder N. Anteriorposterior patterning is required within segments for somite boundary formation in developing zebrafish. Development. 2000;127:1703–1713. doi: 10.1242/dev.127.8.1703. [DOI] [PubMed] [Google Scholar]
- 37.Jülich D, Mould AP, Koper E, Holley SA. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development. 2009;136:2913–2921. doi: 10.1242/dev.038935. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe T, Sato Y, Saito D, Tadokoro R, Takahashi Y. EphrinB2 coordinates the formation of a morphological boundary and cell epithelialization during somite segmentation. Proc Natl Acad Sci U S A. 2009;106:7467–7472. doi: 10.1073/pnas.0902859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakaya Y, Kuroda S, Katagiri YT, Kaibuchi K, Takahashi Y. Mesenchymal-epithelial transition during somitic segmentation is regulated by differential roles of Cdc42 and Rac1. Dev Cell. 2004;7:425–438. doi: 10.1016/j.devcel.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Koshida S, Kishimoto Y, Ustumi H, Shimizu T, Furutani-Seiki M, Kondoh H, Takada S. Integrinalpha5-Dependent Fibronectin Accumulation for Maintenance of Somite Boundaries in Zebrafish Embryos. Dev Cell. 2005;8:587–598. doi: 10.1016/j.devcel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Lackner S, Schwendinger-Schreck J, Jülich D, Holley SA. Segmental assembly of Fibronectin matrix requires rap1b and integrin α5. Dev Dyn. 2013;242:122–131. doi: 10.1002/dvdy.23909. [DOI] [PubMed] [Google Scholar]
- 42.Borghi N, James Nelson W. Intercellular adhesion in morphogenesis: molecular and biophysical considerations. Curr Top Dev Biol. 2009;89:1–32. doi: 10.1016/S0070-2153(09)89001-7. [DOI] [PubMed] [Google Scholar]
- 43.Schiller HB, Hermann MR, Polleux J, Vignaud T, Zanivan S, Friedel CC, Sun Z, Raducanu A, Gottschalk KE, Thery M, et al. beta1- and alphav-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat Cell Biol. 2013;15:625–636. doi: 10.1038/ncb2747. [DOI] [PubMed] [Google Scholar]
- 44.Rossier O, Octeau V, Sibarita JB, Leduc C, Tessier B, Nair D, Gatterdam V, Destaing O, Albiges-Rizo C, Tampe R, et al. Integrins beta1 and beta3 exhibit distinct dynamic nanoscale organizations inside focal adhesions. Nat Cell Biol. 2012;14:1057–1067. doi: 10.1038/ncb2588. [DOI] [PubMed] [Google Scholar]