Abstract
Neuroplasticity is key to the operation of brain machine interfaces (BMIs)—a direct communication pathway between the brain and a man-made computing device. Whereas exogenous BMIs that associate volitional control of brain activity with neurofeedback have been shown to induce long lasting plasticity, endogenous BMIs that use prolonged activity-dependent stimulation – and thus may curtail the time scale that governs natural sensorimotor integration loops – have been shown to induce short lasting plasticity. Here we summarize recent findings from studies using both categories of BMIs, and discuss the fundamental principles that may underlie their operation and the longevity of the plasticity they induce. We draw comparison to plasticity mechanisms known to mediate natural sensorimotor skill learning and discuss principles of homeostatic regulation that may constrain endogenous BMI effects in the adult mammalian brain. We propose that BMIs could be designed to facilitate structural and functional plasticity for the purpose of re-organization of target brain regions and directed augmentation of sensorimotor maps, and suggest possible avenues for future work to maximize their efficacy and viability in clinical applications.
INTRODUCTION
An estimated 1.11 million individuals are diagnosed in the United States each year with some loss of sensorimotor function due to amputation, spinal cord injury (SCI), stroke, amyotrophic lateral sclerosis (ALS), to name just a few—40% of which survive but with moderate to severe motor disability [1, 2]. In addition, an estimated 11-12 million individuals continue to live with these conditions [3, 4]. BMI technology promises to have a major impact on the health of many of these individuals, particularly those whose recovery of sensorimotor function is inherently limited but otherwise remain cognitively intact.
Broadly defined, BMIs – also known as Brain Computer Interfaces (BCIs) –include any form of a direct interface between the brain and an artificial device equipped with some form of computations. In sensorimotor control, BMIs rely on the fundamental concept of causation between volitional modulation of neural activity and movement of an end effector, or between targeted stimulation of ascending sensory pathways and perception of artificial sensory feedback. Efferent BMIs read out neural activity from descending neural pathways using a sensing device – typically an array of electrodes – by extracting spike event rates from well isolated neurons [5], or from multiple single units [6, 7], that are subsequently “decoded” to generate control signals that actuate the end effector. This could be the natural impaired limb [8, 9], or an artificial limb [10, 11]. Likewise, afferent BMIs, such as cochlear implants [12, 13], sense signals from the surrounding and extract features from these signals using an “encoder” which subsequently modulates stimulation patterns in order to evoke artificial percepts.
While early efferent BMI work has focused on demonstrating the phenomenological aspects of neuroplasticity—the brain’s ability to change its structure and/or function during development, learning or recovery from injury, recent work has focused on the technical aspects of the system design such as device fabrication, control of multiple degrees of freedom (DOFs) with increasing dexterity in arm/hand control [10,11]. Reigniting the focus on potential mechanisms of neuroplasticity, particularly at the cellular and subcellular levels, remains equally important, in part, because it may provide guidance to BMI design and training protocols to harness neuroplasticity in ways that supersede traditional physical rehabilitation exercises [14]. This review examines the principles that govern BMI-mediated neuroplasticity, with particular emphasis on invasive BMI technology. We summarize the published data and draw comparisons with some potential mechanisms known to mediate natural sensorimotor learning at cellular and subcellular levels that could be at play during BMI operation.
BMI CAUSALITY PRINCIPLES
Learning the causality that underlie BMI operation involves a number of principles that are critical for improving performance. The first is to gather sensory information about the consequences of volitional modulation of neural activity that cause changes in the end effector state. Different sensory modalities, such as vision, touch and proprioception play an indispensable role in natural motor learning. But with the exception of a few studies [15-17], efferent BMIs thus far have relied almost exclusively on vision as the only sensory modality that provides feedback about the state of the volitionally modulated neurons. Importantly, changes in the end effector state have to be noticeable enough to enable the subject to infer the most critical parameters for task success, such as the number of inputs needed to cause instantaneous changes in that state, the magnitude of that change as a function of the depth of neural modulation, and most importantly, the latency at which the change occurs relative to the timing of volition. Some parameters are nonetheless hard to infer based on visual feedback alone, such as inferring the correct amount of digit force needed to successfully grasp an object without crushing it or letting it slip from the hand.
The second principle is coping with the likely mismatch between the number of input neural channels – which is typically of higher dimension – and the number of independently controlled DOFs in the task space, which is typically of lower dimension. This creates an avenue to involve redundancy because it allows subjects to explore different combinations of neural inputs to a muscle (or group of muscles)—or to a decoded motor variable during BMI control—to optimize the effort and increase control accuracy [18-20]. Furthermore, it may allow the interface to be more robust against potential neural coding errors and noise in the biological system [21].
The third principle is the extent of plasticity in the neural circuits mediating BMI control that is needed to improve performance. Neuroplasticity in adulthood refers to a multitude of different processes of reorganization that occur at multiple temporal and spatial scales for the purpose of increasing efficacy of information transfer in the nervous system to serve control efficiency and accuracy, or to cope with injury. These processes may include changes in existing synaptic strengths, formation of new synapses, or even formation of new neurons [22-24]. Neural circuits must undergo some change for motor memory to be formed, consolidated and easily recalled [25-28]. And it has been consistently shown that changes in neural activity correlates with BMI performance improvement [29-33].
The fourth causality principle is the extent to which BMI skills learned in one task cause generalization (i.e. transfer) to other BMI – or even non-BMI – tasks, and whether the extent of this generalization decreases as tasks become increasingly different from those in which the initial skill was learned, akin to natural motor skill learning [34]. It has been reported that increasing the population size for a given level of task complexity does not necessarily lead to improving performance, as it plateaus after a certain level [35]. The link between the small size of the populations typically used in BMI control (few tens to hundreds of cells) compared to the many orders of magnitude larger size used in natural limb control is not entirely clear, and published studies have been mostly focused on improving performance through extensive practice and less so on addressing the generalization question.
PLASTICITY ASSOCIATED WITH UNI-DIRECTIONAL BMIs
With the exception of human studies [36] [10, 11, 37] and one recent animal study [38-40], demonstrations of BMIs have been primarily carried out in able-bodied subjects. During manual control (MC) in instructed delay tasks, a monkey is trained to make real arm movements from one starting position to a target – or continuously pursue a randomly appearing target – after which the arm returns to the starting position before a new trial starts [41, 42]. During Brain Control (BC), neural activity is “decoded” as the monkey attempts to volitionally modulate the firing pattern of those neurons recorded during the MC mode. The parameters of the decoder are calculated by minimizing the error between the measured arm movement parameters and their linear estimates from the concurrently recorded neural signals [41, 43, 44]. The population vector algorithm (PVA)[41] is a special case of this class of estimators when the neuronal tuning functions are cosine shaped and the preferred directions are uniformly distributed [45]. The uniformity may not be present during early training sessions [46], but may arise with continued practice of the BMI task. This biomimetic approach is assumed to mimic what downstream motoneurons would presumably do to “decode” the intended movement represented by M1 activity. It should be noted however that these downstream circuits are also innervated by numerous feedforward and feedback cortico-cortical as well as cortico-spinal tracts that carry information from and back to cortex where these neurons are sampled [47, 48].
It is noteworthy that during the MC mode, the arm movement is obscured from the monkey’s vision and rendered to a computer screen in which a cursor indicates the arm’s endpoint. Thus, the proprioceptive feedback about the arm state is present but decoupled from the visual feedback. This is to ensure that proprioceptive feedback influence on MI activity is minimized so that neural data gathered to train decoders for the BC mode provide a good representation of visually guided modulation of neural firing (for more details, [49] summarizes studies comparing arm restraint to no restraint). Motor imagery/action observation has been used to train decoders in paralyzed humans (Dushanova and Donoghue 2010, Vigneswaran, Philipp et al. 2013, Brunner, Skouen et al. 2014) based on the observation that rehearsal of movements or motor imagery appears to engage M1 [27] [48], though the source of this activation remains unclear [50]. Similar approaches have been adopted in other studies, with differences primarily in assumptions related to movement parameters that neurons encode (e.g. position [51], velocity [44], acceleration [43], endpoint [52]), or the area of recording (e.g. dorsal premotor cortex (PMd) [52], posterior parietal cortex (PPC) [53], or M1 [41, 54, 55]), or the number of Degrees of Freedom (DOF) controlled [11, 36].
In the BC mode, subjects have shown a remarkable ability to rapidly adapt their neural activity patterns to the task context, as suggested by the marked differences between M1 activities in the MC and BC modes [21, 56]. There are likely multiple explanations. The primary somatosensory cortex (SI), for example, is anatomically and functionally connected to multiple motor cortical areas [57], and evidence suggests that it exercises significant influence on motor cortex during natural movement planning and execution [58-62]. Further support for SI involvement in adaptation is provided by studies in which congruence of visual and proprioceptive feedback improved BMI performance [16]. Other sources of adaptation may come from other brain areas, particularly the posterior parietal cortex (PPC) that was shown to mediate visually-guided, on-line corrections of movement trajectories [63, 64]. Given the abstract nature of BMI learning, some degree of cognitive control flexibility given the altered form of feedback is required, which may favor executive control areas known to mediate such processes such as the prefrontal cortex [65-70].
The pioneering work of Fetz et al. [5] provides a foundational basis for the emergence of this adaptation mechanism, since it established the causal relationship between the volitional control of neuronal activity and reward when no overt movement is produced. Able-bodied monkeys could condition the firing rate of single neurons in precentral “motor” cortex to earn food reinforcement [71]. Because the monkeys did not know beforehand what the conditioning rule is, neurofeedback—the transformation of the neural state into sensory feedback through an engineered decoding rule—was used to shape the monkeys’ behavior. It was further suggested that selectively dissociating patterns of neural activity by reinforcing one activity while simultaneously suppressing another could be used to study causality between the underlying brain structures where these neurons reside. In essence, the simple integrator of spiking activity from single units reported by Fetz et al. constitutes the first “closed loop decoder” according to modern BMI terminology. This simple—yet powerful—neurofeedback idea capitalizes on intrinsic and instantaneous adaptation mechanisms that are not entirely clear. Unlike the biomimetic approach reported in subsequent BMI studies [41], knowledge of the tuning properties of the neurons being conditioned is not necessary. Instead, making the state of neural activity observable to the subject likely engages cognitive control mechanisms that are critical to rapid learning of the non-biomimetic decoding rule.
The neurofeedback concept has been recently extended to condition spatiotemporal patterns of activity from multiple neurons simultaneously recorded in the hand and forearm areas of the primary motor cortex of two non-human primates with chronic transradial and transhumeral amputation – the first BMI study with an animal having a chronic form of disability [38, 40]. Using recordings from ‘stable’ populations of M1 neurons [39], units were divided into a number of non-overlapping clusters of functionally connected neurons – as measured by spike train correlations during periods of spontaneous activity [72]. An unsupervised, non-biomimetic decoder was built from offline analysis of spontaneous neural activity to enable the monkeys to control multiple DOFs [40]. Monkeys learned to control Cartesian and hand grasp velocities in a self-paced reach-to-grasp task. Furthermore, they mastered control of this BMI (unpublished data) to a level that is comparable to a supervised, biomimetic-based decoding approach that was tested earlier in the same subjects [38]. They also demonstrated the ability to learn to coordinate the reach and the grasp components associated with the reach-to-grasp task with increasing task difficulty [73]. As these BMI-assigned neurons have been arguably de-afferented for several years prior to chronic implantation of the recording electrodes, this study is the first to shed some light on BMI-induced plasticity in local neural populations while being minimally confounded by other potential non-BMI related mechanisms engaged during natural behavior.
Scale and speed of adaptation
Learning a particular task can take anywhere from a few minutes to multiple days [10, 11, 41, 43]. Early BMI studies have reported significant changes in tuning quality and preferred directions of BMI-assigned neurons over multiple trials [41, 74]. Most of these studies employed decoder recalibration strategies within each BMI session primarily to try to optimize these decoders and not to study how the animals adapt to various decoder designs. Such decoder recalibration practice essentially defines a new mathematical transformation between brain signals and the movement of the end effector that subjects had to relearn within each session [75]. In particular, it was shown that, after proficient control with one fixed biomimetic decoder, training another biomimetic decoder (which was still capable of reconstructing the offline kinematics as accurately as the first one) and using it online triggers a new learning cycle that was initially rapid but slowed down later on [76] [77]. Interestingly, while decoders were calibrated almost daily, subjects slowly became more proficient in learning a new decoder mapping in every new session. This learning trend results in larger performance gains within daily sessions [56, 78]. A more recent study reported that a two-stage decoder calibration process – in which the first stage initializes the decoder while the other recalibrates it based on closed loop brain control data – results in a reduction in the observed changes in neuronal tuning between training and test data [79].
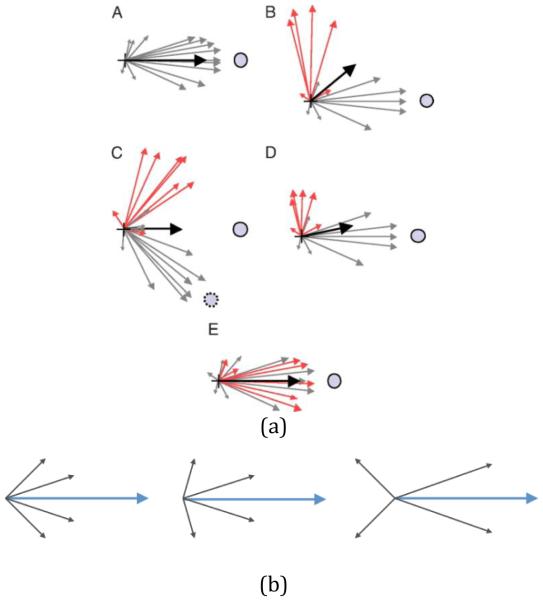
Since the decoder defines a causal link between the neural activity and the state of the end effector, a few studies have examined the extent to which subjects could learn to adapt to perturbations to a given decoding rule [29, 32, 33, 76, 80]. Changes in the statistics of neural activity were reported as changes in directional tuning and modulation depth. In particular, Jarosiewicz et al. [29, 32] reported that a large percentage (84%) of total error reduction was attributed to what seemed to be a global adaptation strategy by the entire population to the visuomotor rotation to adjust the error between the desired cursor movement and the decoded movement, with the remaining error (16%) explained by a local adaptation strategy that appears as changes in the tuning curves of only the perturbed subpopulation. In addition, the non-uniqueness of the solution to the vector summation as illustrated in Figure 1 implies that multiple scenarios could be possible. Thus, a mix of adaptation strategies could be at play, depending on the local connectivity and the degree of influence that other areas could exercise on each of the recorded cells.
Figure 1.
(a) Three possible compensation mechanisms following perturbation to population vector decoders (©2008 by National Academy of Sciences, adapted from Jarosiewicz B et al. PNAS 2008;105:19486-19491). A. Population vector before perturbation (black vector) pointing straight toward the target. B. After perturbation, some units will contribute differently to the population vector because of their rotated decoder coefficients (red vectors) and the resulting population vector does not point at the target. C. One possible re-aiming strategy based on a virtual target (dotted circle) that recruits a set of neurons with preferred directions that point toward the virtual target. The net vector sum is a population vector pointing toward the actual target. D. A re-weighting strategy. Down-modulating the firing rate of the rotated units makes the population vector straighten toward the target. E. A possible re-mapping strategy based on recruiting only unperturbed cells that point toward the target and perturbed cells that point 90° from the target to make the population vector point directly at the target.
(b) Conceptual illustration of redundancy using population vector addition where three of infinitely many solutions are depicted that give rise to the same population vector (blue vector).
Another possible explanation for such changes is that subjects attempt to match their neural activity patterns to the transformation imposed by the decoder and the end effector dynamics, which together represent the external environment to be learned by the subject. In other words, subjects could be learning an internal model of the external environment in which the plant state is observed [81-83]. Evidence of such model in natural motor behavior dates back to von Helmholtz [84] and later in the 1950’s [85] who proposed that the CNS generates efference copy signals in parallel with descending motor commands that both interact with reafferent signals generated by the actual self-generated movements [86]. These signals may also interact at the spinal cord level as recent evidence suggests [87]. Other evidence of changes in representation as learning progresses comes from functional brain imaging studies in which a shift from prefrontal regions of the cortex to premotor, posterior parietal, and cerebellar cortex structures was observed a few hours after completion of practice while performance remains unchanged [88]. This shift was specific to the recall of an established motor skill and suggests that with the passage of time, the change in the neural representation of the task may underlie its increased functional stability. These gradual shifts in the representation could be a viable means to optimize the spatiotemporal patterns of neural activity that drive the decoder in order to produce desired dynamics in the task space.
In another series of studies [56, 76], the authors contended that a “stable” fixed decoder mapping is needed to facilitate proficient control and skill learning in efferent BMIs. Stability here refers to stationarity of unit waveform shapes and underscores the fact that keeping the decoder fixed across days necessitates the stability of the decoded units across these days. As such, a stable transformation not only requires fixing the decoder mapping, but also assigning units to the BMI that have stationary spike waveform shapes that does not alter the day-to-day spike sorting outcome. Only a few neurons (on the order of tens), however, can be stably isolated across a period of days to weeks in typical chronic microelectrode recordings [39, 41, 76, 89-91], although Taylor et al. empirically reported maintaining stability of neurons—as determined by their tuning properties to overt movement kinematics—for ~3 years (Taylor, Tillery et al. 2002). Consequently, with few exceptions [92], most BMI studies to date rely on identification of new units and decoder recalibration before each session. Novel techniques that track units across days could be useful to stabilize the mapping as learning progresses [39, 93].
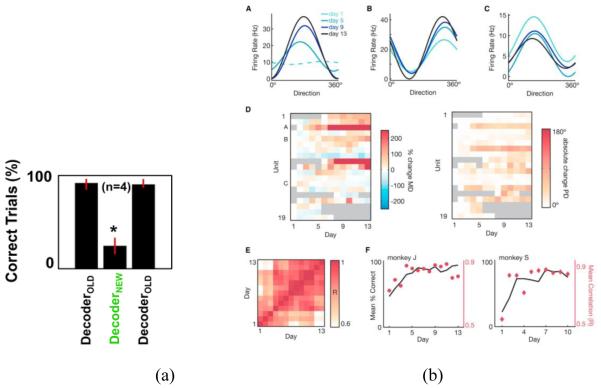
Evidence that late proficient control with initially unwieldy but later seamless switching between an “old” and “new” decoder rules could be attained is illustrated in Figure 2 from [76, 77]. In particular, subjects attained proficient control using one decoder over 8 days after which the decoder coefficients were shuffled such that weights corresponding to one neuron were randomly re-assigned to other neurons within the population that drive the decoder. Attaining comparable BMI control performance within a few days suggests that subjects not only learned a specific decoding rule per se, but they also learned to transfer experience learned under one rule to another [34], although the reported time scale for learning such skill (3-4 days) was different from the one reported during visuomotor perturbations (300 trials-same day [80]). Nonetheless, judicious comparison is necessary given that different decoding algorithms, control variables, and perturbation schemes were used. A more recent study suggested the existence of neural constraints on learning BMI skills [33]. In particular, when the high-dimensional neural activity was projected onto a low-dimensional “intrinsic manifold” that captures the statistics of naturally occurring patterns in the neural data, perturbations to decoders of this low dimensional representation—labeled within-manifold perturbations and hence termed “more intuitive” —were learnable on a scale of hours. In contrast, perturbations to the projections of the high-dimensional representation—labeled outside-manifold perturbations and hence “less intuitive”—were harder to learn within the same session. Notably, the “more intuitive mapping” contended to exist in the within manifold perturbation included both biomimetic and non-biomimetic decoder mappings that could be learned by the monkeys, as long as the non-biomimetic decoder was constrained by the span of the neural co-modulation patterns, which has been suggested by an earlier study [40]. Nonetheless, the study did not test across-session learning – a critical element in the comprehensive assessment of the true extent of skill learning as opposed to mere sensorimotor adaptation that is known to occur within single sessions.
Figure 2.
Neural adaptation to the decoder. (a) Changes in performance for similar trials with substitution of newly trained decoders (adapted from [76])
(b) (A-C) Sample units’ tuning curves across days within a decoder series (13-day). Color indicates the day (light to dark progression); dashed lines represent non-statistically significant tuning fits. (D) Changes in tuning modulation depth and preferred directions for all BMI-assigned units across the decoder series (relative to the first day). Grey squares indicate units that were not part of the ensemble, or were not significantly tuned. (E) Pairwise correlations of the ensemble tuning maps across the decoder series. (F) Average map correlation for each day (red) overlaid onto task percent correct (black) (adapted from [77])
PLASTICITY ASSOCIATED WITH BI-DIRECTIONAL BMIs
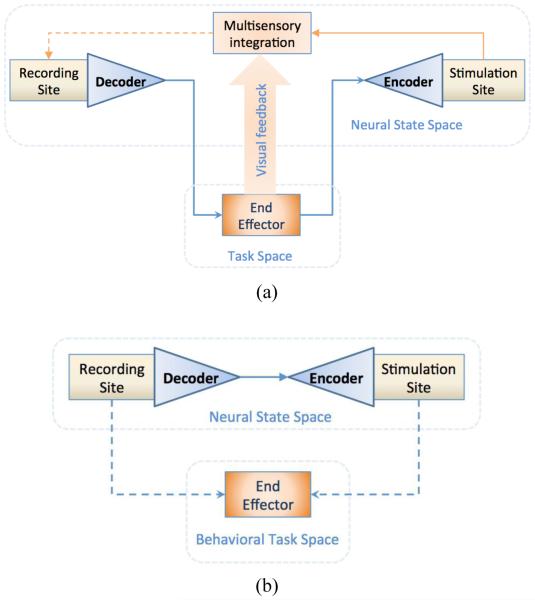
Bidirectional BMIs (BBMIs) are systems designed to combine recording and stimulation of neural activity, either within the same region or across different regions. A critical element in their operation is the nature of events that autonomously trigger stimulation in target areas. In particular, we categorize BBMIs as either being exogenous or endogenous, as illustrated in Figure 3. Exogenous BBMIs rely on stimulating the nervous system based on explicit events that occur in the task space such as limb movement or object touch by the end effector when it is under brain control. In contrast, endogenous BBMIs, rely on implicit events that occur in the recorded neural activity without necessarily being mapped in the task space in the form of neurofeedback. In both categories, a recurrent loop is formed, and the time scale that governs this loop could be a primary factor that distinguishes the extent and longevity of plasticity that each system may induce.
Figure 3.
(a) Exogenous BMIs: Decoding motor intent signals involves reducing the dimensionality of the sampled neural state space to a small number of control variables (Degrees of Freedom) in the task space. The converse is true in the encoding of sensory feedback where stimulation through a small number of channels likely activates a larger number of functionally heterogeneous cells. Stimulation is triggered based on the dynamics of the end effector state. The congruence between the artificial somatosensory feedback and other forms of feedback (e.g. visual) could facilitate BMI sensorimotor learning.
(b) Endogenous BMIs: Stimulation is triggered based on events in the neural state space independent of those in the task space. Neurons in the recording and stimulation sites are functionally distinct before conditioning, but become functionally similar after conditioning, as demonstrated in the behavioral task space. The time scale of the closed loop operation is critical to induce Hebbian-like plasticity that is consistent with strengthening synapses between the recording and stimulation sites.
Plasticity induced through activity-dependent stimulation
Endogenous BBMIs rely on plasticity mechanisms triggered by activity-dependent stimulation (ADS) that operate over a very short time scale (< 50 ms) and could take effect in just a few trials. This choice of time scale has roots in Hebbian plasticity [94] that triggers Long Term Potentiation (LTP) and long Term Depression (LTD) between pre- and postsynaptic neurons [95-97]. A proof of concept was demonstrated by Jackson and co-workers who delivered ADS to the wrist area of M1 over multiple days (1.6 days on average)[98]. For every action potential recorded from a trigger electrode implanted in this area, a single pulse was delivered after a pre-determined delay of 0-50ms to a target electrode implanted in an adjacent region. Thus, stimulation patterns were triggered based on spiking events from neurons that became active on the trigger electrode while monkeys engaged in natural behavior. The ensuing changes were assessed using an ICMS protocol, where two days of ADS were sufficient to shift the function of the neurons at the trigger site to resemble those on the target site, whereas no changes were observed in neighboring control sites that were not targeted by the ADS paradigm. This effect was observed for delays up to 50ms, whereas delays of 100 ms or more did not induce significant changes. Importantly, this effect developed gradually and lasted for one week. The authors suggested that these effects could be explained by the potentiation of synapses within the horizontal connectivity in cortex, although facilitation through indirect routes in the more extensive network that involve peripheral muscle groups innervated by the target site may still be possible [99].
Using a similar ADS paradigm, strengthening of neural connections between motor cortex and spinal cord was demonstrated [100], albeit the pairing was done between a motor cortex site and a spinal postsynaptic site to demonstrate the negative direction of the STDP rule, i.e., depression of synapses when post- before pre-synaptic neuron firing occurs. In particular, the finite conduction time from motor cortex to the spinal cord was argued to enable the stimulation of the target site to occur before spikes from the trigger area arrived at the target area and thus is likely to weaken the synaptic connectivity, consistent with a bi-directional STDP rule. The stimulation paradigm continued for 1-2 days, interleaved with recordings of neural and EMG activity during the behavioral task to assess the synaptic strength. It was shown that a brief stimulation period of 3.5 hours was enough to strengthen the synaptic connections – as measured by an increase in the magnitude of the rectified EMGs. With prolonged 1-2 days of conditioning, synaptic connections were strengthened with delays of 12-25ms, weakened with delays of 0ms, and not significantly changed with delays of 50ms or longer. These effects persisted for 1-2 days after cessation of the ADS paradigm.
Longevity of plasticity and homeostatic regulation
In endogenous BMIs, the functional re-organization induced by the artificially synchronized populations of neurons seems to be localized to the recording and stimulation sites, and thus other distal areas that perhaps have access to information about the target activity rates of neurons in the conditioned sites are likely unaffected by the ADS paradigm. O’Donnell and Nolan [101] review evidence that, among neurons of a single type, integration of synaptic responses is tuned according to the particular function that individual neurons carry out. This tuning may not be restricted to sensory pathways, but could extend to cognitive and motor circuits [102]. In fact, neurons in V1 show decreased firing within hours of sensory deprivation followed by a rebound to baseline levels 1-6 days post deprivation, with similar patterns in sleeping and awake states [103,104]. Our recent data suggest that this also occurs in primary somatosensory cortex (S1), and that population – but not single neuron – dynamics may provide the necessary sensing mechanism at milliseconds time scale to detect perturbations to individual neurons’ function, and consequently adjust connectivity strength to restore the “old” function of these neurons [105], or adapt them to the “new” function. With the likely continued participation of neurons near stimulation sites in other behavioral functions after cessation of the ADS paradigm, populations in distal areas could provide the necessary readout to ensure that the activity level of the artificially synchronized neurons returns to the homeostatic state prior to the induction of the paradigm, and an efficient way to carry out this function could depend on the dynamics of coherent oscillations among these areas that spikes are phase locked to.
We then ask: what are the intrinsic mechanisms that allow neurons to change their firing patterns to execute reward-based goal-directed behavior such as BMI control while constraining their activity to allow them to perform their original function? Some clues that could help address this complicated question may come from one of the most prominent forms of plasticity: homeostatic regulation of individual cells and cell assemblies [106-109]. Turrigiano [102] defines a homeostatic form of plasticity as one “that acts to stabilize the activity of a neuron or neuronal circuit in the face of perturbations, such as changes in cell size or in synapse number or strength, that alter excitability”. Homeostatic regulation involves changes in intrinsic excitability that arise from various combinations of ion channel densities in a cell membrane [110]. Indeed, some BCI and motor neurophysiology studies have hinted at such homeostatic regulatory mechanisms - albeit being referred to as "retention”[111, 112]. Other potential mechanisms act to adjust synaptic strength (Turrigiano and Nelson, 2004) or synapse number (Kirov et al., 2004) through changes in postsynaptic receptors and GABA (γ-aminobutyric acid)[107-109]. The process depends critically, however, on neurons having a way to sense their own activity rate and constantly modify their intrinsic parameters as a function of the sensed values. This sensing mechanism could be localized within an area, or globally distributed across multiple areas. Coordination between multiple brain areas could be key to integrating information between these subprocesses, as has been suggested using widespread surveillance of cortical activity during an ECoG-based BMI experiment documenting the engagement of prefrontal cortex, premotor cortex, and posterior parietal cortex in the early phase of BCI training [113].
DISCUSSION
What is the extent to which rapid neural adaptation contributes to BMI learning? When no overt movements are produced, it is critical to bind the volitional motor intent with neurofeedback within a short time scale to establish causality. This process is likely subserved by a number of sub-processes that encode: the volition, the feedback and the transformation between the two, as well as the attentional demands to the causal evidence resulting from this transformation. Information integration must occur between these sub-processes, and thus requires an effective way to be communicated – both in space and time – between the brain areas involved.
Our first proposal is that this communication is mostly carried out using coherent oscillatory brain activity. While evidence from BMI studies has been scarce, oscillations have been shown to play a prominent role in natural sensorimotor learning [114, 115]. For example, fast oscillations in the gamma-range (30-80 Hz) emerge during attentional selection [116], perception of spatial saliency maps [117, 118], and volitional scaling of movements [119-121]. In addition, enhanced power in the beta frequency range (15 to 25 Hz) has been observed during learning skilled movements, reflecting interregional coherence in large-scale sensorimotor networks [122-125]. Widespread surveillance of cortical activity suggests that oscillations gradually decrease as motor skill learning progresses over a single training session [126-128].
Our second proposal is that learning exogenous BMI control engages brain areas similar to those that mediate natural sensorimotor learning that involve thalamo-cortical, cortico-striatal, fronto-parietal loops [88, 129-133] as well as the cerebellum [58, 134]. In these loops, development of a motor skill with extended practice results in a profound reduction in the synaptic activity required to produce internally generated commands but not visually guided movement sequences [135]. The cellular mechanisms at play behind this learning-related plasticity may involve brain-derived neurotrophic factors (BDNF) that influence synaptic plasticity [136-138], and if one considers that local field potentials (LFPs) – a class of oscillatory brain activity – to represent the spatial summation of coherent dendro-synaptic membrane potentials [139], changes in synaptic strength would translate into changes in the overall magnitude of LFP oscillations as well as their time constants.
Our third proposal is that coherence between distant neuronal assemblies may not be a mere signature of learning but rather a potential mechanism to induce cortical re-organization that is essential for learning to progress. Evidence that support this proposal is that mere induction of coherent response patterns to presentation of specific stimuli via a neurofeedback approach (and without repeated stimulus presentation) results in significant behavioral performance improvement upon testing with these stimuli, but not for other stimuli [140]. Thus, coherent oscillatory activity could be critical to promote cortical re-organization to enable consolidation of BMI skills while preserving the overall stability of the sensorimotor network that regulate the excitation/inhibition balance in the local population as learning progresses.
One experimental design to test the hypotheses we put forward here is to perform widespread surveillance of cortical and subcortical activity when small populations of BMI-assigned neurons are used for BMI control and test the extent to which these areas become less (or more) involved in the task as subjects become proficient. One could then use cell-type-specific optical perturbation through optogenetic means to precisely control the entrainment of oscillations in one or more of these areas at various epochs of the task. This will effectively create “information lesions” that can be used to test the extent to which this manipulation influences the coherence of the oscillations (or their unit phase locking) where BMI-assigned neurons are recorded, and whether this correlates with changes in within-session performance in BMI tasks, or in the subject’s ability to exercise consistent BMI control across sessions.
It is certainly plausible that oscillations may serve other roles in exogenous BMIs, such as volitional control. Indeed, a recent study suggested that subjects can volitionally control the oscillation dynamics in motor cortex – as measured by an overall decrease in gamma power, unit phase locking and depth of entrainment – as a function of distance from conditioning sites [141]. The distribution of correlated sites appeared to be widespread within sensorimotor cortex, confirming previous studies that documented elevated levels of inter-areal coherence between somatosensory and motor areas that may be a signature of acquiring novel sensorimotor associations [142] during the planning and execution of instructed delay tasks [143], as well as during exploratory behavior [70, 92, 127, 141, 144-150]. A few BMI studies have demonstrated the ability of subjects to simultaneously control two DOFs via online LFP decoding [92][151][152]. Nevertheless, the efficacy of oscillations as independent signals for use as a source for multiple (>2) DOF BMI control remains to be shown.
To summarize, we propose that BMIs induce long-term plasticity that consolidates motor BMI memory through the involvement of multiple cortical and subcortical areas during the early phase of learning. During that phase, it is critical that volitional control of neural activity is transformed from being primarily sensory guided to more anticipatory planning with some level of control automation. The initial phase seems to be important to explore the ability to coordinate the activity needed to drive the populations controlling the end effector, depending on the complexity of the task. Redundancy in information representation in space and time among neurons contributing – directly or indirectly - to BMI control could be a strategy to determine the most flexible and efficient way for inter-areal communication in order to generate neuronal activity that is optimal for the decoding rule while minimizing metabolic cost. The late phase, on the other hand, may be critical to ensure an internal model of the plant that includes the transformation of motor intent is stable enough to enable the transfer of skills learned in one control setting to other types of tasks without significant decoder recalibration. Further refinement of that model could be served by plasticity mechanisms associated with learning-induced changes in gray matter, similar to those seen in individuals learning a particular skill over relatively long time periods [153]. With continued practice, the volitional control of neural activity could be more efficiently achieved within smaller and more focused areas akin to that observed in sensory and motor cortices of professional musicians compared to amateurs or non-musicians [154, 155]. It is necessary that future BMI studies be carried out over prolonged periods enough to provide support for these hypotheses, and to further assess whether changes in structural and/or functional connectivity between areas, if any, following termination of extensive BMI training are paralleled with changes in BMI control skills.
CONCLUSIONS
We have reviewed a substantial body of literature documenting multiple forms of neuroplasticity in BMI studies and variable rates of learning that could explain the large variability in measured performance. We compared potential BMI learning mechanisms to those known to mediate natural sensorimotor skill learning. In particular, we emphasized the causality principles that govern BMI operation as well as findings that document the involvement of multiple brain areas in learning these principles, even though only a small population of neurons is typically selected to control the end effector. We also highlighted the potential role that oscillations might play during this process and their contribution to binding volitional motor intent signals with the ensuing sensory feedback. This body of evidence strongly support the idea that formation, consolidation and recall of BMI memory relies on binding operant intentional actions with congruent sensory feedback in ways that facilitate the cognitive control of the plant. We have categorized BBMIs that include artificial stimulation based on the information they provide, as well as the type and longevity of plasticity they induce depending on the occurrence of events in the task space or in the neural space. In this realm, the time scale that governs the integration of information from multiple subprocesses in exogenous BBMIs seems to be the most critical; the perceived lack of such integration in endogenous BBMIs could strip those brain areas involved in BMI learning from the ability to maintain the artificially induced plasticity for prolonged periods of time.
While we have emphasized studies that examined adaptation to perturbations in the decoding rule, a number of challenges encumber the ability to assess the full extent to which neuroplasticity subserves BMI skill acquisition. First, stability of neural recordings is critical for the formation, consolidation and recall of BMI experience. Yet, stability is not always predictable, particularly when using invasive microelectrodes, due to adverse biotic and abiotic factors beyond the experimenter’s control [156]. We have summarized evidence suggesting that the use of not-necessarily-stable neural ensembles and daily decoder recalibration schemes, as classic BMI experiments typically do, correlates with significant variability in day-to-day performance and possibly result in a directional bias in decoded trajectories [157], albeit subjects can still maintain an overall improvement in performance. Thus, it appears that the lack of stability of the interface and the need for subjects to frequently relearn decoder mapping is a major impediment to BMI skill acquisition and to the ability to fully assess the extent of plasticity accompanying BMI learning. Further controlled studies are needed to elucidate the mechanisms by which subjects adapt to recording instability or inaccuracy in front-end signal processing such as online spike sorting. Other neural recording techniques, such as calcium imaging of neurons engaged in BMI [31], may shed some light on how adaptation may be taking place within local populations without being confounded by limitations of microelectrode recording techniques.
Second, the assessment of changes in the level of excitability of BMI-assigned neurons remains a challenge in subjects unable to move their limbs. The availability of kinematic data from able-bodied subjects permits to some extent the assessment of such changes, but recent evidence suggests that these data may not be sufficient to single out these changes, given the engagement of animals in other unrelated behavior that may in by itself alter this excitability. Animal models that are closest to the actual human subjects who will ultimately benefit from this technology would be key to elucidate these mechanisms [38-40].
Third, neurons during BMI control may be susceptible to variations in attentional levels and degree of motivation and fatigue that are hard to measure in an awake behaving subject. Subjects are typically exposed to the BMI task for a few hours per day, after which they return to their normal activity in which they engage in behavior that may involve the recruitment of BMI-assigned neurons. As such, their tuning properties may change due to their participation in other non-BMI related motor and cognitive functions, making it hard to disentangle plastic changes solely caused by BMI practice from those that are not.
In summary, it would be important for future studies to shed some light on the potential involvement of higher order areas, particularly frontal lobes, in BMI learning to provide support for the number of hypotheses we put forward here. Ultimately, sensorimotor events during this learning process are instances of specific cognitive functions that the brain must engage in to optimize BMI control. An important element of these functions is the ability to predict sensory consequences of efferent control, whether it is natural (as in uni-directional BMIs) or artificial (as in exogenous BBMIs), to provide some form of integration between top down modulation of motor commands and bottom up sensory processing – that may be subconscious – to minimize prediction error. Perhaps when this prediction becomes highly reliable, the control may shift from being primarily sensory guided to being more internally generated and autonomous, much like the control of the natural motor repertoire. Future work will need to further characterize the interaction between training protocols and BMI-induced plasticity, and to provide a systematic approach to augment this training based on subject specific learning progress that is independent of task difficulty, so as to continue to promote plasticity that improves performance. These features would be critical for the widespread use of BMI technology and for broadening their application in other neurological diseases and disorders.
Acknowledgment
This work was supported by NIH-NINDS grant# NS062031 and DARPA grant# N66 001-12-1-4023.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009 Aug;8:741–54. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- [2].Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013 Jan 1;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Young J, Forster A. Review of stroke rehabilitation. BMJ. 2007 Jan 13;334:86–90. doi: 10.1136/bmj.39059.456794.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013 Jan 1;127:143–52. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- [5].Fetz EE. Operant conditioning of cortical unit activity. Science. 1969 Feb 28;163:955–8. doi: 10.1126/science.163.3870.955. [DOI] [PubMed] [Google Scholar]
- [6].Fetz EE, Baker MA. Operantly conditioned patterns on precentral unit activity and correlated responses in adjacent cells and contralateral muscles. J Neurophysiol. 1973 Mar;36:179–204. doi: 10.1152/jn.1973.36.2.179. [DOI] [PubMed] [Google Scholar]
- [7].Oweiss K. Statistical Signal Processing for Neuroscience and Neurotechnology. 1st edition Academic Press-an Elsevier Imprint; 2010. [Google Scholar]
- [8].Moritz CT, Perlmutter SI, Fetz EE. Direct control of paralysed muscles by cortical neurons. Nature. 2008 Dec 4;456:639–42. doi: 10.1038/nature07418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ethier C, Oby ER, Bauman MJ, Miller LE. Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature. 2012 May 17;485:368–71. doi: 10.1038/nature10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012 May 17;485:372–5. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2013 Feb 16;381:557–64. doi: 10.1016/S0140-6736(12)61816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Spelman FA. The cochlear prosthesis: a review of the design and evaluation of electrode implants for the profoundly deaf. Crit Rev Biomed Eng. 1982;8:223–52. [PubMed] [Google Scholar]
- [13].Harrison RV. Cochlear implants: a review of the principles and important physiological factors. J Otolaryngol. 1987 Oct;16:268–75. [PubMed] [Google Scholar]
- [14].Grosse-Wentrup M, Mattia D, Oweiss K. Using brain-computer interfaces to induce neural plasticity and restore function. J Neural Eng. 2011 Apr;8:025004. doi: 10.1088/1741-2560/8/2/025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Suminski AJ, Tkach DC, Hatsopoulos NG. Exploiting multiple sensory modalities in brain-machine interfaces. Neural Netw. 2009 Nov;22:1224–34. doi: 10.1016/j.neunet.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Suminski AJ, Tkach DC, Fagg AH, Hatsopoulos NG. Incorporating feedback from multiple sensory modalities enhances brain-machine interface control. J Neurosci. 2010 Dec 15;30:16777–87. doi: 10.1523/JNEUROSCI.3967-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].O'Doherty JE, Lebedev MA, Ifft PJ, Zhuang KZ, Shokur S, Bleuler H, et al. Active tactile exploration using a brain-machine-brain interface. Nature. 2011 doi: 10.1038/nature10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Orban G, Wolpert DM. Representations of uncertainty in sensorimotor control. Curr Opin Neurobiol. 2011 Aug;21:629–35. doi: 10.1016/j.conb.2011.05.026. [DOI] [PubMed] [Google Scholar]
- [19].Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nat Rev Neurosci. 2011 Dec;12:739–51. doi: 10.1038/nrn3112. [DOI] [PubMed] [Google Scholar]
- [20].Shadmehr R, Mussa-Ivaldi S. Biological Learning and Control: How the Brain Builds Representations, Predicts Events, and Makes Decisions. 2012 Mit Pr. [Google Scholar]
- [21].So K, Ganguly K, Jimenez J, Gastpar MC, Carmena JM. Redundant information encoding in primary motor cortex during natural and prosthetic motor control. J Comput Neurosci. 2012 Jun;32:555–61. doi: 10.1007/s10827-011-0369-1. [DOI] [PubMed] [Google Scholar]
- [22].Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: a possible role in learning. Trends in cognitive sciences. 1999;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- [23].Gazzaniga MS. The cognitive neurosciences. MIT press: 2004. [Google Scholar]
- [24].Sampaio-Baptista C, Khrapitchev AA, Foxley S, Schlagheck T, Scholz J, Jbabdi S, et al. Motor Skill Learning Induces Changes in White Matter Microstructure and Myelination. The Journal of Neuroscience. 2013;33:19499–19503. doi: 10.1523/JNEUROSCI.3048-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996 Jan 15;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996 Jun 21;272:1791–4. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- [27].Sanes JN, Donoghue JP. Static and dynamic organization of motor cortex. Brain Plasticity. 1997;73:277–296. [PubMed] [Google Scholar]
- [28].Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annual Review of Neuroscience. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- [29].Jarosiewicz B, Chase SM, Fraser GW, Velliste M, Kass RE, Schwartz AB. Functional network reorganization during learning in a brain-computer interface paradigm. Proc Natl Acad Sci U S A. 2008 Dec 9;105:19486–91. doi: 10.1073/pnas.0808113105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Canolty RT, Cadieu CF, Koepsell K, Ganguly K, Knight RT, Carmena JM. Detecting event-related changes of multivariate phase coupling in dynamic brain networks. J Neurophysiol. 2012 Apr;107:2020–31. doi: 10.1152/jn.00610.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Clancy KB, Koralek AC, Costa RM, Feldman DE, Carmena JM. Volitional modulation of optically recorded calcium signals during neuroprosthetic learning. Nat Neurosci. 2014 Jun;17:807–9. doi: 10.1038/nn.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chase SM, Kass RE, Schwartz AB. Behavioral and neural correlates of visuomotor adaptation observed through a brain-computer interface in primary motor cortex. J Neurophysiol. 2012 Jul;108:624–44. doi: 10.1152/jn.00371.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sadtler PT, Quick KM, Golub MD, Chase SM, Ryu SI, Tyler-Kabara EC, et al. Neural constraints on learning. Nature. 2014 Aug 28;512:423–6. doi: 10.1038/nature13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Green AM, Kalaska JF. Learning to move machines with the mind. Trends Neurosci. 2011 Feb;34:61–75. doi: 10.1016/j.tins.2010.11.003. [DOI] [PubMed] [Google Scholar]
- [35].Carmena JM, Lebedev MA, Crist RE, O'Doherty JE, Santucci DM, Dimitrov DF, et al. Learning to control a brain-machine interface for reaching and grasping by primates. PLoS Biol. 2003 Nov;1:E42. doi: 10.1371/journal.pbio.0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- [37].Wodlinger B, Downey JE, Tyler-Kabara EC, Schwartz AB, Boninger ML, Collinger JL. Ten-dimensional anthropomorphic arm control in a human brain-machine interface: difficulties, solutions, and limitations. J Neural Eng. 2014 Dec 16;12:016011. doi: 10.1088/1741-2560/12/1/016011. [DOI] [PubMed] [Google Scholar]
- [38].Balasubramanian K, Southerland J, Vaidya M, Qian K, Eleryan A, Fagg AH, et al. Operant conditioning of a multiple degree-of-freedom brain-machine interface in a primate model of amputation. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:303–6. doi: 10.1109/EMBC.2013.6609497. [DOI] [PubMed] [Google Scholar]
- [39].Eleryan A, Vaidya M, Southerland J, Badreldin IS, Balasubramanian K, Fagg AH, et al. Tracking single units in chronic, large scale, neural recordings for brain machine interface applications. Front Neuroeng. 2014;7:23. doi: 10.3389/fneng.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Badreldin I, Sutherland J, Vaidya M, Elerya A, Balasubramanian K, Fagg A, et al. Unsupervised Decoder Initialization for Brain-Machine Interfaces Using Neural State Space Dynamics. Conference on Neural Engineering; San Diego, CA. 2013. presented at the IEEE Int. [Google Scholar]
- [41].Taylor DM, Tillery SIH, Schwartz AB. Direct Cortical Control of 3D Neuroprosthetic Devices. Science. 2002;296:1829. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- [42].Paninski L, Fellows MR, Hatsopoulos NG, Donoghue JP. Spatiotemporal tuning of motor cortical neurons for hand position and velocity. Journal of Neurophysiology. 2004 Jan;91:515–532. doi: 10.1152/jn.00587.2002. [DOI] [PubMed] [Google Scholar]
- [43].Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP. Brain-machine interface: Instant neural control of a movement signal. Nature. 2002;416:141–142. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- [44].Wessberg J, Nicolelis MA. Optimizing a linear algorithm for real-time robotic control using chronic cortical ensemble recordings in monkeys. Journal of cognitive neuroscience. 2004;16:1022–1035. doi: 10.1162/0898929041502652. [DOI] [PubMed] [Google Scholar]
- [45].Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986;233:1416. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- [46].Wang W, Chan SS, Heldman DA, Moran DW. Motor cortical representation of position and velocity during reaching. J Neurophysiol. 2007 Jun;97:4258–70. doi: 10.1152/jn.01180.2006. [DOI] [PubMed] [Google Scholar]
- [47].Cheney PD, Fetz EE, Mewes K. Neural Mechanisms Underlying Corticospinal and Rubrospinal Control of Limb Movements. Progress in Brain Research. 1991;87:213–252. doi: 10.1016/s0079-6123(08)63054-x. [DOI] [PubMed] [Google Scholar]
- [48].Kraskov A, Philipp R, Waldert S, Vigneswaran G, Quallo MM, Lemon RN. Corticospinal mirror neurons. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130174. doi: 10.1098/rstb.2013.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nuyujukian P, Fan JM, Gilja V, Kalanithi PS, Chestek CA, Shenoy KV. Monkey models for brain-machine interfaces: the need for maintaining diversity. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:1301–5. doi: 10.1109/IEMBS.2011.6090306. [DOI] [PubMed] [Google Scholar]
- [50].Kasai T, Kawai S, Kawanishi M, Yahagi S. Evidence for facilitation of motor evoked potentials (MEPs) induced by motor imagery. Brain Res. 1997 Jan 2;744:147–50. doi: 10.1016/s0006-8993(96)01101-8. [DOI] [PubMed] [Google Scholar]
- [51].Wessberg J, Stambaugh CR, Kralik JD, Beck PD, Laubach M, Chapin JK, et al. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature. 2000;408:361–365. doi: 10.1038/35042582. [DOI] [PubMed] [Google Scholar]
- [52].Santhanam G, Ryu SI, Yu BM, Afshar A, Shenoy KV. A high-performance brain-computer interface. Nature. 2006;442:195–198. doi: 10.1038/nature04968. [DOI] [PubMed] [Google Scholar]
- [53].Musallam S, Corneil BD, Greger B, Scherberger H, Andersen RA. Cognitive control signals for neural prosthetics. Science. 2004;305:258–262. doi: 10.1126/science.1097938. [DOI] [PubMed] [Google Scholar]
- [54].Nicolelis MA. Actions from thoughts. Nature. 2001 Jan 18;409:403–7. doi: 10.1038/35053191. [DOI] [PubMed] [Google Scholar]
- [55].Brockwell AE, Kass RE, Schwartz AB. Statistical Signal Processing and the Motor Cortex. Proceedings of the IEEE. 2007;95:881–898. doi: 10.1109/JPROC.2007.894703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ganguly K, Carmena JM. Neural correlates of skill acquisition with a cortical brain-machine interface. J Mot Behav. 2010 Nov;42:355–60. doi: 10.1080/00222895.2010.526457. [DOI] [PubMed] [Google Scholar]
- [57].Goldring S, Ratcheson R. Human motor cortex: sensory input data from single neuron recordings. Science. 1972 Mar 31;175:1493–5. doi: 10.1126/science.175.4029.1493. [DOI] [PubMed] [Google Scholar]
- [58].Avanzino L, Pelosin E, Abbruzzese G, Bassolino M, Pozzo T, Bove M. Shaping Motor Cortex Plasticity Through Proprioception. Cerebral Cortex. 2013:bht139. doi: 10.1093/cercor/bht139. [DOI] [PubMed] [Google Scholar]
- [59].Hatsopoulos NG, Suminski AJ. Sensing with the motor cortex. Neuron. 2011 Nov 3;72:477–87. doi: 10.1016/j.neuron.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Fogassi L, Gallese V, di Pellegrino G, Fadiga L, Gentilucci M, Luppino G, et al. Space coding by premotor cortex. Exp Brain Res. 1992;89:686–90. doi: 10.1007/BF00229894. [DOI] [PubMed] [Google Scholar]
- [61].di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res. 1992;91:176–80. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- [62].Shaikhouni A, Donoghue JP, Hochberg LR. Somatosensory responses in a human motor cortex. J Neurophysiol. 2013 Apr;109:2192–204. doi: 10.1152/jn.00368.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Andersen RA, Hwang EJ, Mulliken GH. Cognitive neural prosthetics. Annual review of psychology. 2010;61:169. doi: 10.1146/annurev.psych.093008.100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Cui H, Andersen RA. Posterior parietal cortex encodes autonomously selected motor plans. Neuron. 2007 Nov 8;56:552–9. doi: 10.1016/j.neuron.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotoxicity research. 2008;14:249–262. doi: 10.1007/BF03033814. [DOI] [PubMed] [Google Scholar]
- [66].Decety J. The neurophysiological basis of motor imagery. Behav Brain Res. 1996 May;77:45–52. doi: 10.1016/0166-4328(95)00225-1. [DOI] [PubMed] [Google Scholar]
- [67].Decety J. Neural representations for action. Rev Neurosci. 1996 Oct-Dec;7:285–97. doi: 10.1515/revneuro.1996.7.4.285. [DOI] [PubMed] [Google Scholar]
- [68].Stephan KM, Dettmers C, Frackowiak RS. Organization and reorganization of the human cortex. Arzneimittelforschung. 1995 Mar;45:390–3. [PubMed] [Google Scholar]
- [69].Gerardin E, Sirigu A, Lehericy S, Poline JB, Gaymard B, Marsault C, et al. Partially overlapping neural networks for real and imagined hand movements. Cereb Cortex. 2000 Nov;10:1093–104. doi: 10.1093/cercor/10.11.1093. [DOI] [PubMed] [Google Scholar]
- [70].Keizer AW, Verment RS, Hommel B. Enhancing cognitive control through neurofeedback: a role of gamma-band activity in managing episodic retrieval. Neuroimage. 2010 Feb 15;49:3404–13. doi: 10.1016/j.neuroimage.2009.11.023. [DOI] [PubMed] [Google Scholar]
- [71].Fetz EB, Finocchi .Dv. Operant Conditioning of Specific Patterns of Neural and Muscular Activity. Science. 1971;174:431. doi: 10.1126/science.174.4007.431. [DOI] [PubMed] [Google Scholar]
- [72].Eldawlatly S, Jin R, Oweiss KG. Identifying functional connectivity in large-scale neural ensemble recordings: a multiscale data mining approach. Neural Comput. 2009 Feb;21:450–77. doi: 10.1162/neco.2008.09-07-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Vaidya M, Balasubramanian K, Southerland J, Eleryan A, Badreldin I, Fagg A, et al. Society for Neuroscience. Washington DC: 2014. Emergence of coordinated reach-to-grasp behavior in a long-term BMI experiment. [Google Scholar]
- [74].Carmena JM, Lebedev MA, Crist RE, O'Doherty JE, Santucci DM, Dimitrov DF, et al. Learning to control a brain-machine interface for reaching and grasping by primates. PLoS Biology. 2003;1:e2. doi: 10.1371/journal.pbio.0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ganguly K, Dimitrov DF, Wallis JD, Carmena JM. Reversible large-scale modification of cortical networks during neuroprosthetic control. Nat Neurosci. 2011 May;14:662–7. doi: 10.1038/nn.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ganguly K, Carmena JM. Emergence of a stable cortical map for neuroprosthetic control. PLoS Biol. 2009 Jul;7:e1000153. doi: 10.1371/journal.pbio.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Orsborn Amy L., Moorman Helene G., Overduin Simon A., Shanechi Maryam M., Dimitrov Dragan F., Carmena Jose M. Closed-Loop Decoder Adaptation Shapes Neural Plasticity for Skillful Neuroprosthetic Control. Neuron. 2014;82:1380–1393. doi: 10.1016/j.neuron.2014.04.048. [DOI] [PubMed] [Google Scholar]
- [78].Krakauer JW, Mazzoni P. Human sensorimotor learning: adaptation, skill, and beyond. Curr Opin Neurobiol. 2011 Aug;21:636–44. doi: 10.1016/j.conb.2011.06.012. [DOI] [PubMed] [Google Scholar]
- [79].Fan JM, Nuyujukian P, Kao JC, Chestek CA, Ryu SI, Shenoy KV. Intention estimation in brain-machine interfaces. J Neural Eng. 2014 Feb;11:016004. doi: 10.1088/1741-2560/11/1/016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Golub MD, Yu BM, Chase SM. Internal models engaged by brain-computer interface control. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:1327–30. doi: 10.1109/EMBC.2012.6346182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bhushan N, Shadmehr R. Computational nature of human adaptive control during learning of reaching movements in force fields. Biol Cybern. 1999 Jul;81:39–60. doi: 10.1007/s004220050543. [DOI] [PubMed] [Google Scholar]
- [82].Kawato M, Wolpert D. Internal models for motor control. Novartis Found Symp. 1998;218:291–304. doi: 10.1002/9780470515563.ch16. discussion 304-7. [DOI] [PubMed] [Google Scholar]
- [83].Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998 Sep 1;2:338–47. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- [84].Helmholtz H. v., Southall JPC. Treatise on physiological optics. III. The perceptions of vision. 1925 [Google Scholar]
- [85].Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol. 1950 Dec;43:482–9. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- [86].Crapse TB, Sommer MA. Corollary discharge circuits in the primate brain. Curr Opin Neurobiol. 2008 Dec;18:552–7. doi: 10.1016/j.conb.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Azim E, Jiang J, Alstermark B, Jessell TM. Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature. 2014 Apr 17;508:357–63. doi: 10.1038/nature13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science. 1997 Aug 8;277:821–5. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- [89].Oweiss KG, Anderson DJ. Spike sorting: a novel shift and amplitude invariant technique. Computational Neuroscience: Trends in Research 2002. 2002;44-46:1133–1139. [Google Scholar]
- [90].Chestek CA, Batista AP, Santhanam G, Yu BM, Afshar A, Cunningham JP, et al. Single-neuron stability during repeated reaching in macaque premotor cortex. J Neurosci. 2007 Oct 3;27:10742–50. doi: 10.1523/JNEUROSCI.0959-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Dickey AS, Suminski A, Amit Y, Hatsopoulos NG. Single-unit stability using chronically implanted multielectrode arrays. J Neurophysiol. 2009 Aug;102:1331–9. doi: 10.1152/jn.90920.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Flint RD, Wright ZA, Scheid MR, Slutzky MW. Long term, stable brain machine interface performance using local field potentials and multiunit spikes. Journal of neural engineering. 2013;10:056005. doi: 10.1088/1741-2560/10/5/056005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Aghagolzadeh M, Mohebi A, Oweiss K. Sorting and Tracking Neuronal Spikes via Simple Thresholding. IEEE Trans Neural Syst Rehabil Eng. 2013 doi: 10.1109/TNSRE.2013.2289918. [DOI] [PubMed] [Google Scholar]
- [94].Hebb DO. The Organization of Behavior; a Neuropsychological Theory. Wiley; New York: 1949. [Google Scholar]
- [95].Bi G.-q., Poo M.-m. Distributed synaptic modification in neural networks induced by patterned stimulation. Nature. 1999;401:792–796. doi: 10.1038/44573. [DOI] [PubMed] [Google Scholar]
- [96].Nudo R, Jenkins W, Merzenieh M. Repetitive microstimulation alters the cortical representation of movements in adult rats. Somatosensory & motor research. 1990;7:463–483. doi: 10.3109/08990229009144720. [DOI] [PubMed] [Google Scholar]
- [97].Teskey GC, Young NA, van Rooyen F, Larson SE, Flynn C, Monfils M-H, et al. Induction of neocortical long-term depression results in smaller movement representations, fewer excitatory perforated synapses, and more inhibitory synapses. Cerebral Cortex. 2007;17:434–442. doi: 10.1093/cercor/bhj160. [DOI] [PubMed] [Google Scholar]
- [98].Jackson A, Mavoori J, Fetz EE. Long-term motor cortex plasticity induced by an electronic neural implant. Nature. 2006;444:56–60. doi: 10.1038/nature05226. [DOI] [PubMed] [Google Scholar]
- [99].Schwartz AB. Neurobiology: crossed circuits. Nature. 2006;444:47–48. doi: 10.1038/444047a. [DOI] [PubMed] [Google Scholar]
- [100].Nishimura Y, Perlmutter SI, Fetz EE. Restoration of upper limb movement via artificial corticospinal and musculospinal connections in a monkey with spinal cord injury. Frontiers in neural circuits. 2013;7 doi: 10.3389/fncir.2013.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].O’Donnell C, Nolan MF. Tuning of synaptic responses: an organizing principle for optimization of neural circuits. Trends in neurosciences. 2011;34:51–60. doi: 10.1016/j.tins.2010.10.003. [DOI] [PubMed] [Google Scholar]
- [102].Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Keck T, Keller GB, Jacobsen RI, Eysel UT, Bonhoeffer T, Hubener M. Synaptic scaling and homeostatic plasticity in the mouse visual cortex in vivo. Neuron. 2013 Oct 16;80:327–34. doi: 10.1016/j.neuron.2013.08.018. [DOI] [PubMed] [Google Scholar]
- [104].Hengen KB, Lambo ME, Van Hooser SD, Katz DB, Turrigiano GG. Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron. 2013 Oct 16;80:335–42. doi: 10.1016/j.neuron.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Eldawlatly S, Oweiss K. Temporal precision in population - but not individual neuron - dynamics reveals rapid experience-dependent plasticity in the rat barrel cortex. Frontiers in Computational Neuroscience. 2014 doi: 10.3389/fncom.2014.00155. vol. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Destexhe A, Marder E. Plasticity in single neuron and circuit computations. Nature. 2004;431:789–795. doi: 10.1038/nature03011. [DOI] [PubMed] [Google Scholar]
- [107].Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Current opinion in neurobiology. 2000;10:358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- [108].Turrigiano GG, Nelson SB. Thinking globally, acting locally: AMPA receptor turnover and synaptic strength. Neuron. 1998;21:933–935. doi: 10.1016/s0896-6273(00)80607-8. [DOI] [PubMed] [Google Scholar]
- [109].Mody I. Aspects of the homeostaic plasticity of GABAA receptor-mediated inhibition. The Journal of physiology. 2005;562:37–46. doi: 10.1113/jphysiol.2004.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006 Jul;7:563–74. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- [111].Wolpaw JR. Brain-computer interface research comes of age: traditional assumptions meet emerging realities. J Mot Behav. 2010 Nov;42:351–3. doi: 10.1080/00222895.2010.526471. [DOI] [PubMed] [Google Scholar]
- [112].Ajemian R, D'Ausilio A, Moorman H, Bizzi E. A theory for how sensorimotor skills are learned and retained in noisy and nonstationary neural circuits. Proc Natl Acad Sci U S A. 2013 Dec 24;110:E5078–87. doi: 10.1073/pnas.1320116110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Wander JD, Blakely T, Miller KJ, Weaver KE, Johnson LA, Olson JD, et al. Distributed cortical adaptation during learning of a brain–computer interface task. Proceedings of the National Academy of Sciences. 2013;110:10818–10823. doi: 10.1073/pnas.1221127110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Yu S, Huang D, Singer W, Nikolic D. A Small World of Neuronal Synchrony. Cerebral Cortex. 2008 Dec;18:2891–2901. doi: 10.1093/cercor/bhn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–25. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Reynolds JH, Desimone R. The role of neural mechanisms of attention in solving the binding problem. Neuron. 1999 Sep;24:19–29. 111–25. doi: 10.1016/s0896-6273(00)80819-3. [DOI] [PubMed] [Google Scholar]
- [117].Lungarella M, Sporns O. Mapping information flow in sensorimotor networks. PLoS computational biology. 2006;2:e144. doi: 10.1371/journal.pcbi.0020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Itti L, Koch C. Computational modelling of visual attention. Nature reviews neuroscience. 2001;2:194–203. doi: 10.1038/35058500. [DOI] [PubMed] [Google Scholar]
- [119].Miller KJ, Schalk G, Fetz EE, den Nijs M, Ojemann JG, Rao RP. Cortical activity during motor execution, motor imagery, and imagery-based online feedback. Proceedings of the National Academy of Sciences. 2010;107:4430–4435. doi: 10.1073/pnas.0913697107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci. 2001 Jan 1;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- [121].Bullock D, Cisek P, Grossberg S. Cortical networks for control of voluntary arm movements under variable force conditions. Cerebral Cortex. 1998;8:48–62. doi: 10.1093/cercor/8.1.48. [DOI] [PubMed] [Google Scholar]
- [122].Murthy VN, Fetz EE. Coherent 25-to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proceedings of the National Academy of Sciences. 1992;89:5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL. Beta oscillations in a large-scale sensorimotor cortical network: directional influences revealed by Granger causality. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9849–9854. doi: 10.1073/pnas.0308538101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Andres FG, Gerloff C. Coherence of sequential movements and motor learning. J Clin Neurophysiol. 1999 Nov;16:520–7. doi: 10.1097/00004691-199911000-00004. [DOI] [PubMed] [Google Scholar]
- [125].Canolty RT, Ganguly K, Kennerley SW, Cadieu CF, Koepsell K, Wallis JD, et al. Oscillatory phase coupling coordinates anatomically dispersed functional cell assemblies. Proc Natl Acad Sci U S A. 2010 Oct 5;107:17356–61. doi: 10.1073/pnas.1008306107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995 Sep 14;377:155–8. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- [127].Ruiz S, Birbaumer N, Sitaram R. Brain-Computer Interface Research. Springer; 2014. Volitional Control of Neural Connectivity; pp. 63–74. [Google Scholar]
- [128].Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem. 2002 Nov;78:553–64. doi: 10.1006/nlme.2002.4091. [DOI] [PubMed] [Google Scholar]
- [129].Koralek AC, Jin X, Long JD, Costa RM, Carmena JM. Corticostriatal plasticity is necessary for learning intentional neuroprosthetic skills. Nature. 2012 Mar 15;483:331–5. doi: 10.1038/nature10845. 2nd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Chen H, Yang L, Xu Y, Wu G.-y., Yao J, Zhang J, et al. Prefrontal Control of Cerebellum-Dependent Associative Motor Learning. The Cerebellum. 2014;13:64–78. doi: 10.1007/s12311-013-0517-4. [DOI] [PubMed] [Google Scholar]
- [131].Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Current opinion in neurobiology. 2002;12:217–222. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- [132].Kantak SS, Sullivan KJ, Fisher BE, Knowlton BJ, Winstein CJ. Neural substrates of motor memory consolidation depend on practice structure. Nature neuroscience. 2010;13:923–925. doi: 10.1038/nn.2596. [DOI] [PubMed] [Google Scholar]
- [133].Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, et al. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- [134].Andrew D, Haavik H, Dancey E, Yielder P, Murphy B. Somatosensory evoked potentials show plastic changes following a novel motor training task with the thumb. Clinical Neurophysiology. 2014 doi: 10.1016/j.clinph.2014.05.020. [DOI] [PubMed] [Google Scholar]
- [135].Picard N, Matsuzaka Y, Strick PL. Extended practice of a motor skill is associated with reduced metabolic activity in M1. Nature neuroscience. 2013 doi: 10.1038/nn.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nature neuroscience. 2006;9:735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- [137].Akaneya Y, Tsumoto T, Kinoshita S, Hatanaka H. Brain-derived neurotrophic factor enhances long-term potentiation in rat visual cortex. The Journal of neuroscience. 1997;17:6707–6716. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Lu B. BDNF and activity-dependent synaptic modulation. Learning & Memory. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Buzsaki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012 Jun;13:407–20. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Shibata K, Watanabe T, Sasaki Y, Kawato M. Perceptual learning incepted by decoded fMRI neurofeedback without stimulus presentation. Science. 2011;334:1413–1415. doi: 10.1126/science.1212003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Engelhard B, Ozeri N, Israel Z, Bergman H, Vaadia E. Inducing gamma oscillations and precise spike synchrony by operant conditioning via brain-machine interface. Neuron. 2013 Jan 23;77:361–75. doi: 10.1016/j.neuron.2012.11.015. [DOI] [PubMed] [Google Scholar]
- [142].Andres FG, Mima T, Schulman AE, Dichgans J, Hallett M, Gerloff C. Functional coupling of human cortical sensorimotor areas during bimanual skill acquisition. Brain. 1999;122:855–870. doi: 10.1093/brain/122.5.855. [DOI] [PubMed] [Google Scholar]
- [143].Donoghue JP, Sanes JN, Hatsopoulos NG, Gaal G. Neural discharge and local field potential oscillations in primate motor cortex during voluntary movements. Journal of Neurophysiology. 1998 Jan;79:159–173. doi: 10.1152/jn.1998.79.1.159. [DOI] [PubMed] [Google Scholar]
- [144].Murthy VN, Fetz EE. Synchronization of neurons during local field potential oscillations in sensorimotor cortex of awake monkeys. Journal of Neurophysiology. 1996 Dec;76:3968–3982. doi: 10.1152/jn.1996.76.6.3968. [DOI] [PubMed] [Google Scholar]
- [145].Ziemann U, Iliać TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. The Journal of Neuroscience. 2004;24:1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Rosenkranz K, Kacar A, Rothwell JC. Differential modulation of motor cortical plasticity and excitability in early and late phases of human motor learning. J Neurosci. 2007 Oct 31;27:12058–66. doi: 10.1523/JNEUROSCI.2663-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Steriade M. Coherent oscillations and short-term plasticity in corticothalamic networks. Trends in neurosciences. 1999;22:337–345. doi: 10.1016/s0166-2236(99)01407-1. [DOI] [PubMed] [Google Scholar]
- [148].Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004 Jun 25;304:1926–9. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- [149].Hwang EJ, Andersen RA. The utility of multichannel local field potentials for brain–machine interfaces. Journal of neural engineering. 2013;10:046005. doi: 10.1088/1741-2560/10/4/046005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Williams JJ, Rouse AG, Thongpang S, Williams JC, Moran DW. Differentiating closed-loop cortical intention from rest: building an asynchronous electrocorticographic BCI. J Neural Eng. 2013 Aug;10:046001. doi: 10.1088/1741-2560/10/4/046001. [DOI] [PubMed] [Google Scholar]
- [151].So K, Dangi S, Orsborn AL, Gastpar MC, Carmena JM. Subject-specific modulation of local field potential spectral power during brain-machine interface control in primates. J Neural Eng. 2014 Apr;11:026002. doi: 10.1088/1741-2560/11/2/026002. [DOI] [PubMed] [Google Scholar]
- [152].Stavisky SD, Kao JC, Nuyujukian P, Ryu SI, Shenoy KV. A high performing brain-machine interface driven by low-frequency local field potentials alone and together with spikes. Journal of Neural Engineering. 2015:015750. doi: 10.1088/1741-2560/12/3/036009. vol. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004 Jan 22;427:311–2. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- [154].Amunts K, Schlaug G, Jancke L, Steinmetz H, Schleicher A, Dabringhaus A, et al. Motor cortex and hand motor skills: structural compliance in the human brain. Hum Brain Mapp. 1997;5:206–15. doi: 10.1002/(SICI)1097-0193(1997)5:3<206::AID-HBM5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- [155].Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. The Journal of Neuroscience. 2003;23:9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005 Oct 15;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- [157].Perge JA, Homer ML, Malik WQ, Cash S, Eskandar E, Friehs G, et al. Intra-day signal instabilities affect decoding performance in an intracortical neural interface system. J Neural Eng. 2013 Jun;10:036004. doi: 10.1088/1741-2560/10/3/036004. [DOI] [PMC free article] [PubMed] [Google Scholar]