Abstract
Background. Mass vaccination campaigns of the population aged 1–29 years with 1 dose of group A meningococcal (MenA) conjugate vaccine (PsA-TT, MenAfriVac) in African meningitis belt countries has resulted in the near-disappearance of MenA. The vaccine was tested in clinical trials in Africa and in India and found to be safe and highly immunogenic compared with the group A component of the licensed quadrivalent polysaccharide vaccine (PsACWY). Antibody persistence in Africa and in India was investigated.
Methods. A total of 900 subjects aged 2–29 years were followed up for 4 years in Senegal, Mali, and The Gambia (study A). A total of 340 subjects aged 2–10 years were followed up for 1 year in India (study B). In study A, subjects were randomized in a 2:1 ratio, and in study B a 1:1 ratio to receive either PsA-TT or PsACWY. Immunogenicity was evaluated by measuring MenA serum bactericidal antibody (SBA) with rabbit complement and by a group A–specific immunoglobulin G (IgG) enzyme-linked immunosorbent assay.
Results. In both studies, substantial SBA decay was observed at 6 months postvaccination in both vaccine groups, although more marked in the PsACWY group. At 1 year and 4 years (only for study A) postvaccination, SBA titers were relatively sustained in the PsA-TT group, whereas a slight increasing trend, more pronounced among the youngest, was observed in the participants aged <18 years in the PsACWY groups. The SBA titers were significantly higher in the PsA-TT group than in the PsACWY group at any time point, and the majority of subjects in the PsA-TT group had SBA titers ≥128 and group A–specific IgG concentrations ≥2 µg/mL at any point in time in both the African and Indian study populations.
Conclusions. Four years after vaccination with a single dose of PsA-TT vaccine in Africa, most subjects are considered protected from MenA disease.
Clinical Trials Registration. PsA-TT-003 (ISRCTN87739946); PsA-TT-003a (ISRCTN46335400).
Keywords: MenA conjugate vaccine, antibody persistence, African meningitis belt, India
For almost a century, recurrent epidemics of meningitis due to group A meningococci (MenA) represent one of the major public health problems for the African meningitis belt countries [1–3]. The MenA conjugate vaccine, MenAfriVac (PsA-TT), developed within the framework of the Meningitis Vaccine Project, and manufactured by the Serum Institute of India, Ltd [3–6], was found to be safe and immunogenic in 2- to 29-year-old subjects in Mali, The Gambia, and Senegal [7], as well as in adults and children aged 2–10 years in India [8, 9]. In children aged 12–23 months, the ability of PsA-TT to prime for immunologic memory was also shown [7]. Four weeks after vaccination, PsA-TT was able to elicit serum bactericidal antibody (SBA) titers 16-fold higher than the meningococcal ACWY quadrivalent polysaccharide vaccine (PsACWY) in 12- to 23-month-old children, and almost all vaccine recipients achieved SBA titers ≥128 [7], deemed to be a discriminatory titer [10, 11]. On these grounds, and together with an excellent safety profile, the vaccine was licensed in India and prequalified by the World Health Organization (WHO). At the end of 2010, the vaccine was introduced at large scale in Burkina Faso, Mali, and Niger [12–14], the most endemic countries of the African meningitis belt, by mass vaccination campaigns targeting the 1- to 29-year-old population. Meningitis surveillance data show a dramatic reduction, or near-disappearance, of MenA in those countries where MenAfriVac has been introduced [13, 14], in concordance with the low level of MenA carriage [15, 16]. Persistence of sustained levels of antibody titers is considered to represent a key element to predict individual protection against carriage and disease. We report here antibody persistence data for 4 years after vaccination with PsA-TT in Africa and for 1 year in India.
METHODS
The studies were designed and conducted in accordance with the Good Clinical Practice guidelines established by the International Conference on Harmonisation and with the Declaration of Helsinki and by respective country-specific regulatory authorities. Studies were approved by the competent institutional review boards as previously reported [7, 9, 17]. The International Standard randomized controlled trial number was ISRCTN87739946 for the African study (study A) and ISRCTN46335400 for the Indian study (study B).
Study A
Subjects were recruited at the Centre pour le Développement des Vaccins, Bamako, Mali; at the Medical Research Council Laboratories, Basse, The Gambia; and at the Institut de Recherche pour le Développement, Niakhar, Senegal. The detailed study methodology has been described elsewhere [7]. In brief, subjects aged 2–29 years were recruited, stratified into 3 age groups of 2–10, 11–17, and 18–29 years, and randomized in a 2:1 ratio to receive either 1 dose of PsA-TT or a licensed PsACWY vaccine (Mencevax ACWY, GSK). Blood draws for immunogenicity evaluation were performed at baseline, 1 month (results described elsewhere) [7], 6 months, and 1 year after vaccination. Four years postvaccination, a follow-up study on immune persistence was conducted on subjects who were traced and willing to participate in the study. One blood draw was collected 4 years after vaccination.
Study B
In study B, subjects aged 2–10 years were recruited at Shirdi Saibaba Rural Hospital, Vadu Budruk, India, and randomized in a 1:1 ratio to receive 1 dose of either PsA-TT or PsACWY. The detailed study methodology has been described elsewhere [9]. Blood draws for immunogenicity evaluation were performed at baseline, 1 month (results described elsewhere) [9], 6 months, and 1 year after vaccination.
Immunogenicity Evaluation
The immunogenicity of PsA-TT and the group A component of PsACWY were assessed by measuring MenA SBA assay with baby rabbit complement and MenA-specific immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA). Measurement of SBA titers was performed at the Health Protection Agency (now Public Health England), Manchester, United Kingdom; and the ELISA was performed at the Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, with the use of the standard reference serum CDC1992 [18]. The SBA reference strain was F8238, and titers were expressed as the reciprocal of the final serum dilution, resulting in a colony-count reduction of at least 50% after 60 minutes of incubation [19].
Statistical Analysis
The difference in SBA geometric mean titers (GMTs) and group A–specific IgG geometric mean concentrations (GMCs) between the vaccine groups at 6 months and 1 year after vaccination were compared by a mixed-effects model adjusted for baseline titers or concentrations, age, sex, time, and interaction effects of interest with log2-transformed titers and log10-transformed concentrations as an outcome; the comparison was also adjusted for study site in study A. Four years postvaccination, the comparison was carried out by analysis of covariance (ANCOVA) adjusted for baseline value, sex, time, and study site. For other endpoints, the Cochran–Mantel–Haenszel test was used to compare the proportions between vaccine groups, adjusting for age in study A, and Fisher exact test was employed to compare the vaccine groups as appropriate. All immunogenicity analyses were performed in the intention-to-treat population. Missing values were treated as missing at random. Data were analyzed with SAS software, version 9.1.3.
RESULTS
Study A
Study Population
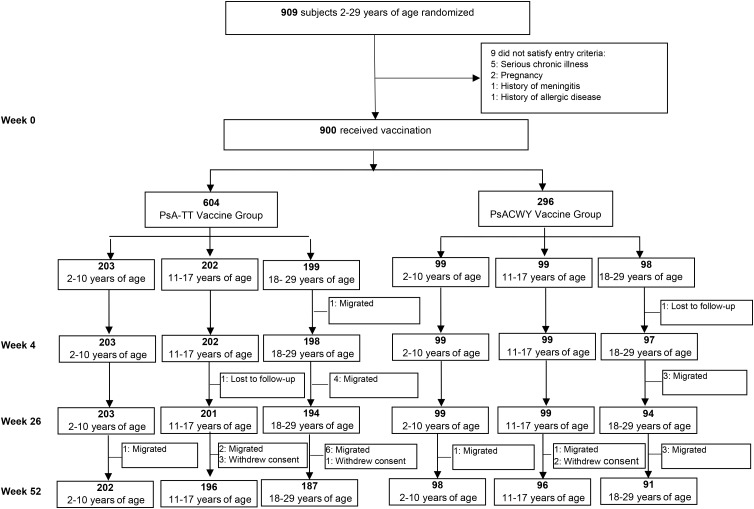
Subjects' disposition during 1-year follow-up is reported in Figure 1. At 1-year postvaccination, among 900 subjects who were randomized and vaccinated at baseline, a total of 30 subjects (3.3%) were discontinued from the study. After 4 years, a total of 482 subjects (53.6% of those initially vaccinated) were available for immunogenicity—that is, with evaluable samples and who did not report, when actively questioned, additional MenA vaccination or meningococcal disease: 175 subjects aged 2–10 years (118 PsA-TT and 57 PsACWY); 149 subjects aged 11–17 years (100 PsA-TT and 49 PsACWY); and 158 subjects aged 18–29 years (110 PsA-TT and 48 PsACWY).
Figure 1.
Disposition of subjects in study A conducted in healthy 2- to 29-year-olds.
Serum Bactericidal Antibody Titers
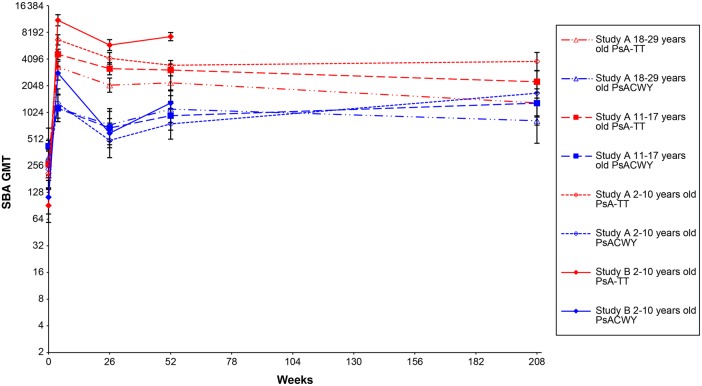
SBA titers are reported in Table 1 and Figure 2.
Table 1.
Outcomes of Meningococcal Group A Rabbit Complement Serum Bactericidal Antibody Titers for Study A Conducted in Healthy 2- to 29-Year-Olds
| Age Group | Vaccine Group | Week 0 (Preimmunization) |
Week 4 (28 d Postimmunization) |
Week 26 (6 mo Postimmunization) |
Week 52 (1 y Postimmunization) |
Long-term Follow-up Study (4 y Postimmunization) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | GMT (95% CI) | No. | GMT (95% CI) | No. | GMT (95% CI) | No. | GMT (95% CI) | No. | GMT (95% CI) | ||
| rSBA GMTs | |||||||||||
| 2–10 y | PsA-TT | 202 | 209.8 (144.4–304.7) | 203 | 6743.2 (5900.7–7705.9) | 203 | 4166.5 (3583.5–4844.4) | 202 | 3485.9 (3086.4–3937.2) | 118 | 3839.7 (3037.9–4853.2) |
| PsACWY | 97 | 233.3 (140.3–388.0) | 99 | 1290.2 (882.4–1886.4) | 98 | 494.2 (315.1–775.0) | 98 | 760.8 (510.7–1133.4) | 57 | 1685.9 (936.8–3033.9) | |
| 11–17 y | PsA-TT | 202 | 264.9 (187.7–374.0) | 202 | 4618.7 (4029.4–5294.2) | 196 | 3186.5 (2718.0–3735.8) | 196 | 3075.8 (2641.6–3581.3) | 100 | 2272.4 (1711.4–3017.3) |
| PsACWY | 99 | 426.8 (267.7–680.4) | 99 | 1137.4 (803.6–1609.8) | 99 | 682.2 (442.6–1051.6) | 95 | 938.2 (646.3–1361.7) | 49 | 1302.4 (735.6–2305.7) | |
| 18–29 y | PsA-TT | 199 | 199.9 (138.5–288.6) | 198 | 3331.6 (2872.3–3864.4) | 192 | 2077.8 (1727.7–2498.8) | 186 | 2206.5 (1837.6–2649.4) | 110 | 1309.3 (907.6–1888.8) |
| PsACWY | 97 | 314.9 (200.3–495.3) | 95 | 1150.8 (805.7–1643.8) | 93 | 737.7 (482.1–1128.9) | 90 | 1114.5 (785.3–1581.8) | 48 | 824.6 (458.9–1481.5) | |
| Total | PsA-TT | 603 | 223.3 (181.3–274.9) | 603 | 4712.6 (4336.0–5122.0) | 591 | 3040.8 (2758.6–3351.8) | 584 | 2889.4 (2643.3–3158.5) | 328 | 2281.1 (1910.5–2723.5) |
| PsACWY | 293 | 316.0 (240.4–415.3) | 293 | 1191.4 (969.1–1464.6) | 290 | 627.3 (488.6–805.4) | 283 | 921.6 (743.2–1142.9) | 154 | 1242.7 (890.7–1733.6) | |
| no./No. | % (95% CI) | no./No. | % (95% CI) | no./No. | % (95% CI) | no./No. | % (95% CI) | no./No. | % (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| rSBA titer ≥128 | |||||||||||
| 2–10 y | PsA-TT | 155/202 | 76.7 (70.3–82.4) | 203/203 | 100.0 (98.2–100.0) | 202/203 | 99.5 (97.3–100.0) | 202/202 | 100.0 (98.2–100.0) | 117/118 | 99.2 (95.4–100.0) |
| PsACWY | 77/97 | 79.4 (70.0–86.9) | 93/99 | 93.9 (87.3–97.7) | 84/98 | 85.7 (77.2–92.0) | 90/98 | 91.8 (84.5–96.4) | 53/57 | 93.0 (83.0–98.1) | |
| 11–17 y | PsA-TT | 155/202 | 76.7 (70.3–82.4) | 202/202 | 100.0 (98.2–100.0) | 195/196 | 99.5 (97.2–100.0) | 195/196 | 99.5 (97.2–100.0) | 98/100 | 98.0 (93.0–99.8) |
| PsACWY | 85/99 | 85.9 (77.4–92.0) | 93/99 | 93.9 (87.3–97.7) | 89/99 | 89.9 (82.2–95.0) | 88/95 | 92.6 (85.4–97.0) | 46/49 | 93.9 (83.1–98.7) | |
| 18–29 y | PsA-TT | 150/199 | 75.4 (68.8–81.2) | 197/198 | 99.5 (97.2–100.0) | 188/192 | 97.9 (94.8–99.4) | 183/186 | 98.4 (95.4–99.7) | 103/110 | 93.6 (87.3–97.4) |
| PsACWY | 81/97 | 83.5 (74.6–90.3) | 89/95 | 93.7 (86.8–97.6) | 85/93 | 91.4 (83.8–96.2) | 86/90 | 95.6 (89.0–98.8) | 44/48 | 91.7 (80.0–97.7) | |
| Total | PsA-TT | 460/603 | 76.3 (72.7–79.6) | 602/603 | 99.8 (99.1–100.0) | 585/591 | 99.0 (97.8–99.6) | 580/584 | 99.3 (98.3–99.8) | 318/328 | 97 (94.5–98.5) |
| PsACWY | 243/293 | 82.9 (78.1–87.1) | 275/293 | 93.9 (90.5–96.3) | 258/290 | 89.0 (84.8–92.3) | 264/283 | 93.3 (89.7–95.9) | 143/154 | 92.9 (87.6–96.4) | |
For the comparison of SBA GMTs between PsACWY and PsA-TT groups: P < .0001 at week 26 and week 52 after vaccination by mixed-effects model; P < .0001 for different age groups at week 26 and week 52. Four years after vaccination: P = .0002 for the comparison of SBA GMTs between PsACWY and PsA-TT groups by analysis of covariance; P < .0001 for age group of 2–10 years old and P > .05 for age groups of 11–17 years and 18–29 years. For the comparison of SBA titers ≥1:128 between PsACWY and PsA-TT groups: P < .0001 at week 26 and week 52 and P = .0350 four years after vaccination by Cochran–Mantel–Haenszel test; P < .05 for different age groups at week 26, week 52, and 4 years after vaccination by Fisher exact test with an exception of P > .05 for age groups of 11–17 years and 18–29 years 4 years after vaccination.
Abbreviations: CI, confidence interval; GMT, geometric mean titer; rSBA, rabbit complement serum bactericidal antibody.
Figure 2.
Serum bactericidal antibody (SBA) geometric mean titers (GMTs) over time for study A and study B.
A considerable decline in SBA GMTs was observed 6 months after vaccination in both vaccine groups, which was more marked in the PsACWY than in the PsA-TT group (approximate 62% drop in PsACWY group and 38% drop in PsA-TT group from 1 month to 6 months after vaccination). Subjects of any age group in the PSA-TT group had statistically significantly higher SBA GMTs than subjects in the PsACWY groups at any time point (P < .05), except at 4 years after vaccination. At 4 years postvaccination in those aged 11–17 years and 18–29 years, SBA GMTs did not significantly differ between the 2 vaccine groups. (Table 1 and Figure 2). Overall, at 1 year and 4 years after vaccination, a modest antibody decay was observed in the PsA-TT group, with a 4-year GMT of 2281.1 (95% confidence interval [CI], 1910.5–2723.5) with respect to the 1-year SBA GMT of 2889.4 (95% CI, 2643.3–3158.5) and the 6-month GMT of 3040.8 (95% CI, 2758.6–3351.8). In the PsACWY group, the SBA GMT 4 years after vaccination (1242.7 [95% CI, 890.7–1733.6]), was overall higher than the SBA GMT 1 year after vaccination (921.6 [95% CI, 743.2–1142.9]), which was also slightly higher than the GMT 6 months after vaccination (627.3 [95% CI, 488.6–805.4]). A similar trend was observed in subjects in the younger age groups of 2–10 and 11–17 years in the PsACWY group (Table 1 and Figure 2).
Six months after vaccination, the total proportion of subjects with SBA titers ≥128 was 99.0% (95% CI, 97.8%–99.6%) in the PsA-TT group and 89.0% (95% CI, 84.8%–92.3%) in the PsACWY group. In all age groups and at any time point, the proportion of subjects with SBA titer ≥128 was significantly higher in the PsA-TT group than in the PsACWY group (P < .05), except at 4 years after vaccination for the 2 older age groups of 11–17 years and 18–29 years. One year and 4 years after vaccination, >90% of subjects had an SBA titer ≥128 in both vaccine groups, with the significantly highest proportions observed in the PsA-TT group with 99.3% (95% CI, 98.3%–99.8%) and 97.0% (95% CI, 94.5%–98.5%), compared with 93.3% (95% CI, 89.7%–95.9%) and 92.9% (95% CI, 87.6%–96.4%) in the PsACWY vaccine group, respectively (P < .0001 at 1 year and P = .0350 at 4 years after vaccination) (Table 1).
GroupA–Specific IgG ELISA
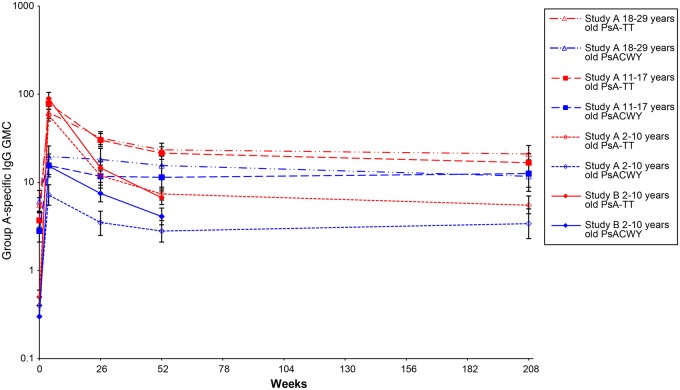
Group A–specific IgG concentrations are reported in Table 2 and Figure 3.
Table 2.
Outcomes of Meningococcal Group A-Specific Immunoglobulin G Concentrations for Study A Conducted in Healthy 2- to 29-Year-Olds
| Age Group | Vaccine Group | Week 0 (Preimmunization) |
Week 4 (28 d Postimmunization) |
Week 26 (6 mo Postimmunization) |
Week 52 (1 y Postimmunization) |
Long-term Follow-up Study (4 y Postimmunization) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | GMC (95% CI) | No. | GMC (95% CI) | No. | GMC (95% CI) | No. | GMC (95% CI) | No. | GMC (95% CI) | ||
| Group A–specific IgG GMCs | |||||||||||
| 2–10 y | PsA-TT | 202 | 0.5 (.4–.6) | 200 | 57.7 (49.7–67.1) | 203 | 11.9 (10.0–14.1) | 202 | 7.4 (6.3–8.8) | 118 | 5.5 (4.4–7.0) |
| PsACWY | 97 | 0.4 (.3–.5) | 98 | 7.2 (5.5–9.4) | 98 | 3.5 (2.5–4.7) | 98 | 2.8 (2.1–3.7) | 57 | 3.4 (2.3–5.0) | |
| 11–17 y | PsA-TT | 202 | 3.7 (3.1–4.5) | 202 | 78.4 (67.5–91.1) | 196 | 30.2 (25.4–35.9) | 195 | 21.4 (18.2–25.3) | 100 | 16.7 (13.1–21.2) |
| PsACWY | 99 | 2.8 (2.1–3.8) | 99 | 15.5 (11.5–21.0) | 99 | 11.7 (8.6–15.7) | 94 | 11.4 (8.4–15.3) | 49 | 12.6 (8.8–18.0) | |
| 18–29 y | PsA-TT | 199 | 5.6 (4.7–6.7) | 198 | 62.1 (53.1–72.7) | 192 | 31.7 (26.8–37.6) | 186 | 23.4 (19.8–27.8) | 110 | 21.0 (16.9–26.2) |
| PsACWY | 97 | 6.1 (4.6–8.1) | 95 | 19.6 (14.8–25.9) | 93 | 18.2 (13.5–24.3) | 90 | 15.5 (11.7–20.7) | 48 | 11.7 (7.9–17.5) | |
| Total | PsA-TT | 603 | 2.1 (1.9–2.5) | 600 | 65.6 (60.0–71.6) | 591 | 22.3 (20.1–24.7) | 583 | 15.3 (13.7–17.0) | 328 | 12.1 (10.5–14.1) |
| PsACWY | 293 | 1.9 (1.5–2.3) | 292 | 12.9 (10.9–15.3) | 290 | 8.9 (7.4–10.8) | 282 | 7.7 (6.4–9.3) | 154 | 7.6 (5.9–9.6) | |
| no./No. | % (95% CI) | no./No. | % (95% CI) | no./No. | % (95% CI) | no./No. | % (95% CI) | no./No. | % (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A–specific IgG concentration ≥2 µg/mL | |||||||||||
| 2–10 y | PsA-TT | 39/202 | 19.3 (14.1–25.4) | 200/200 | 100.0 (98.2–100.0) | 188/203 | 92.6 (88.1–95.8) | 174/202 | 86.1 (80.6–90.6) | 90/118 | 76.3 (67.6–83.6) |
| PsACWY | 16/97 | 16.5 (9.7–25.4) | 77/98 | 78.6 (69.1–86. 2) | 65/98 | 66.3 (56.1–75.6) | 58/98 | 59.2 (48.8–69.0) | 37/57 | 64.9 (51.1–77.1) | |
| 11–17 y | PsA-TT | 136/202 | 67.3 (60.4–73.7) | 202/202 | 100.0 (98.2–100.0) | 195/196 | 99.5 (97.2–100.0) | 192/195 | 98.5 (95.6–99.7) | 97/100 | 97.0 (91.5–99.4) |
| PsACWY | 56/99 | 56.6 (46.2–66.5) | 89/99 | 89.9 (82.2–95.0) | 87/99 | 87.9 (79.8–93.6) | 80/94 | 85.1 (76.3–91.6) | 45/49 | 91.8 (80.4–97.7) | |
| 18–29 y | PsA-TT | 151/199 | 75.9 (69.3–81.6) | 198/198 | 100.0 (98.2–100.0) | 190/192 | 99.0 (96.3–99.9) | 183/186 | 98.4 (95.4–99.7) | 109/110 | 99.1 (95.0–100.0) |
| PsACWY | 77/97 | 79.4 (70.0–86.9) | 91/95 | 95.8 (89.6–98.8) | 87/93 | 93.5 (86.5–97.6) | 83/90 | 92.2 (84.6–96.8) | 42/48 | 87.5 (74.8–95.3) | |
| Total | PsA-TT | 326/603 | 54.1 (50.0–58.1) | 600/600 | 100.0 (99.4–100.0) | 573/591 | 97.0 (95.2–98.2) | 549/583 | 94.2 (91.9–95.9) | 296/328 | 90.2 (86.5–93.2) |
| PsACWY | 149/293 | 50.9 (45.0–56.7) | 257/292 | 88.0 (83.7–91.5) | 239/290 | 82.4 (77.5–86.6) | 221/282 | 78.4 (73.1–83.0) | 124/154 | 80.5 (73.4–86.5) | |
For the comparison of group A–specific IgG GMCs between PsACWY and PsA-TT groups: P < .0001 at week 26 and week 52 after vaccination by mixed-effects model; P < .0001 for different age groups at week 26 and week 52. Four years after vaccination, P < .0001 for the comparison of SBA GMTs between PsACWY and PsA-TT groups by analysis of covariance: the P < .05 for age groups of 2–10 years and 18–29 years and P > .05 for ages 11–17 years. For the comparison of group A–specific IgG concentration ≥2 µg/mL between PsACWY and PsA-TT groups: P < .0001 at week 26 and week 52 and P = .0023 four years after vaccination by Cochran–Mantel–Haenszel test; P < .05 for different age groups at week 26, week 52, and 4 years after vaccination by Fisher exact test with an exception of P > .05 for age groups of 2–10 years and 11–17 years 4 years after vaccination.
Abbreviations: CI, confidence interval; GMC, geometric mean concentration; IgG, immunoglobulin G.
Figure 3.
Group A–specific immunoglobulin G (IgG) geometric mean concentrations (GMCs) over time for study A and study B.
Group A–specific IgG GMCs at 6 months, 1 year, and 4 years after vaccination in the PsA-TT group were 22.3 (95% CI, 20.1–24.7), 15.3 (95% CI, 13.7–17.0), and 12.1 (95% CI, 10.5–14.1) µg/mL, respectively, compared with 8.9 (95% CI, 7.4–10.8), 7.7 (95% CI, 6.4–9.3), and 7.6 (95% CI, 5.9–9.6) µg/mL in the PsACWY group, respectively. GMCs were significantly higher in the PsA-TT group than in the PsACWY group at any time point in any of the 3 age groups (P < .05), with an exception those aged 11–17 years 4 years after vaccination (Table 2 and Figure 3).
At 6 months, 1 year, and 4 years after vaccination, the proportions of subjects in the PsA-TT group with group A–specific IgG concentrations ≥2 µg/mL were 97.0% (95% CI, 95.2%–98.2%), 94.2% (95% CI, 91.9%–95.9%), and 90.2% (95% CI, 86.5%–93.2%), respectively, compared with 82.4% (95% CI, 77.5%–86.6%), 78.4% (95% CI, 73.1%–83.0%), and 80.5% (95% CI, 73.4%–86.5%), respectively, in the PsACWY group. The proportion of subjects with group A–specific IgG concentrations ≥2 µg/mL was significantly higher in the PsA-TT group than in the PsACWY group at any time point in any of the 3 age groups (P < .05), with an exception of those aged 2–10 years and 11–17 years, 4 years postvaccination (Table 2).
Study B
Study Population
Demographics of study population at enrollment were reported previously [9]. In brief, a total of 340 subjects aged 2–10 years were enrolled and vaccinated, of whom 169 received PsA-TT and 171 PsACWY. Before the 6-month follow-up, 1 subject in each group was excluded from the study (1 for protocol violation, 1 withdrew consent). Therefore, 168 subjects in the PsA-TT group and 170 in the PsACWY group completed the study 1 year after vaccination.
Serum Bactericidal Antibody
In both vaccine groups, SBA GMTs decreased during the 6 months after vaccination, but were found to increase at 1 year of follow-up. At both time points, SBA GMTs in the PsA-TT group were significantly higher than in the PsACWY group (P < .0001; Figure 2 and Table 3). Six months and 1 year after vaccination, all subjects in the PsA-TT group had SBA titers ≥128, compared with 85.0% (95% CI, 78.7%–90.1%) and 93.3% (95% CI, 88.4%–96.6%), respectively, in the PsACWY group. The difference between 2 vaccine groups was significant at both time points (P < .0001 at 6 months and P = .0008 at 1 year after vaccination; Table 3).
Table 3.
Outcomes of Meningococcal Group A Serum Bactericidal Titers for Study B Conducted in Healthy 2- to 10-Year-Olds
| Vaccine Group | Week 0 (Preimmunization) |
Week 4 (28 d Postimmunization) |
Week 26 (6 mo Postimmunization) |
Week 52 (1 y Postimmunization) |
||||
|---|---|---|---|---|---|---|---|---|
| No. | GMT (95% CI) | No. | GMT (95% CI) | No. | GMT (95% CI) | No. | GMT (95% CI) | |
| SBA GMTs | ||||||||
| PsA-TT | 169 | 91.1 (58.7–141.3) | 168 | 11209.1 (9708.2–12942.1) | 163 | 5879.5 (5105.7–6770.6) | 162 | 7329.5 (6589.2–8153.1) |
| PsACWY | 171 | 112.9 (73.1–174.4) | 170 | 2837.9 (2368.2–3400.7) | 167 | 597.0 (409.1–871.2) | 165 | 1306.5 (954.9–1787.6) |
| no./No. | % (95% CI) | no./No. | % (95% CI) | no./No. | % (95% CI) | no./No. | % (95% CI) | |
| SBA titer ≥128 | ||||||||
| PsA-TT | 104/169 | 61.5 (53.8–68.9) | 168/168 | 100.0 (97.8–100.0) | 163/163 | 100.0 (97.8–100.0) | 162/162 | 100.0 (97.7–100.0) |
| PsACWY | 111/171 | 64.9 (57.3–72.0) | 170/170 | 100.0 (97.9–100.0) | 142/167 | 85.0 (78.7–90.1) | 154/165 | 93.3 (88.4–96.6) |
For the comparison of SBA GMTs between PsACWY and PsA-TT groups: P < .0001 at week 26 and week 52 after vaccination by mixed-effects model. For the comparison of SBA titers ≥1:128 between PsACWY and PsA-TT groups: P < .0001 at week 26 and P = .0008 at week 52 by Fisher exact test.
Abbreviations: CI, confidence interval; GMT, geometric mean titer; SBA, serum bactericidal antibody.
Group A–Specific IgG
Six months after vaccination, GMCs had decreased steadily in both vaccine groups, whereas a more limited decrease was observed at 1 year. At both time points, GMCs were significantly higher in the PsA-TT group than the PsACWY group (P < .0001). The proportion of subjects with group A–specific IgG concentrations ≥2 µg/mL decreased over time, but was statistically significantly higher in the PsA-TT group than the PsACWY group at both time points (P < .0001 at 6 months and P = .0009 at 1 year after vaccination; Table 4 and Figure 3).
Table 4.
Outcomes of Meningococcal Group A–Specific Immunoglobulin G Concentrations for Study B Conducted in Healthy 2- to 10-Year-Olds
| Vaccine Group | Week 0 (Preimmunization) |
Week 4 (28 d Postimmunization) |
Week 26 (6 mo Postimmunization) |
Week 52 (1 y Postimmunization) |
||||
|---|---|---|---|---|---|---|---|---|
| No. | GMC (95% CI) | No. | GMC (95% CI) | No. | GMC (95% CI) | No. | GMC (95% CI) | |
| Group A–specific IgG GMCs | ||||||||
| PsA-TT | 169 | 0.3 (.3–0.4) | 168 | 89.1 (75.5–105.0) | 163 | 14.6 ( 12.3–17.4) | 161 | 6.7 ( 5.6–8.0) |
| PsACWY | 171 | 0.3 (.3–0.4) | 170 | 15.3 (12.3–19.2) | 166 | 7.5 ( 6.0–9.3) | 165 | 4.1 ( 3.3–5.1) |
| No. | % (95% CI) | No. | % (95% CI) | No. | % (95% CI) | No. | % (95% CI) | |
| Group A–specific IgG concentration ≥2 µg/mL | ||||||||
| PsA-TT | 19/169 | 11.2 (6.9–17.0) | 168/168 | 100.0 (97.8–100.0) | 160/163 | 98.2 (94.7–99.6) | 137/161 | 85.1 (78.6–90.2) |
| PsACWY | 20/171 | 11.7 (7.3–17.5) | 155/170 | 91.2 (85.9–95.0) | 142/166 | 85.5 (79.3–90.5) | 115/165 | 69.7 (62.1–76.6) |
For the comparison of IgG GMCs between PsACWY and PsA-TT groups: P < .0001 at week 26 and week 52 after vaccination by mixed-effects model. For the comparison of group A-specific IgG concentration ≥2 μg/ml between PsACWY and PsA-TT groups: P < .0001 at week 26 and P = .0009 at week 52 by Fisher exact test.
Abbreviations: CI, confidence interval; GMC, geometric mean concentration; IgG, immunoglobulin G.
DISCUSSION
We have described 4-year antibody persistence in 2- to 29-year-old subjects in Africa and 1-year antibody persistence in 2- to 10-year-old subjects in India who had received a single dose of either PsA-TT or PsACWY. Four years after vaccination, African subjects who received PsA-TT had sustained SBA titers almost 10 times as high as the baseline values before vaccination, with the majority of subjects having SBA titers ≥128 and group A–specific IgG concentrations ≥2 µg/mL. As both these thresholds are considered potentially protective [10, 11, 20, 21], we can reasonably assume that 4 years after vaccination with the PsA-TT, the majority of subjects are still protected against MenA disease.
In both vaccine groups, SBA titers showed a substantial decline, but only during the first 6 months after vaccination. Subsequently, at 1 year and 4 years of follow-up, SBA GMTs reached a plateau, with only a modest decline compared with 6 months following PsA-TT, in those aged <18 years. The same pattern of antibody decline 6 months after vaccination was observed in the Indian subjects. However, a substantial increase in SBA titers was observed at 1 year compared with 6 months of follow-up. During the study period—4 years in The Gambia, Senegal, and Mali and 1 year in India—there was no outbreak of MenA in any of the study sites. However, the study population experienced some boosting of group A SBA, as we previously observed in a study performed in toddlers in The Gambia and Mali over approximately 1 year [7]. Exposure to cross-reacting bacteria [22–24] has been demonstrated and may explain the very high SBA titers against MenA in naive populations in the African meningitis belt [7]. In our study, exposure to cross-reacting bacteria may contribute in maintaining a sustained level of functional antibodies up to 4 years after vaccination with the MenA conjugate vaccine in Africa. The same circulating cross-reacting bacteria may well be the cause of the increase in SBA GMTs observed 1 year after vaccination in India.
SBA GMTs were higher in the PsA-TT group than in the PsACWY group, at any point in time and with any parameter considered and in both the African and Indian studies. In the African study, the age trend present in the PsA-TT group with higher SBA titers in younger subjects was conserved throughout the 4 years of follow-up. Persistence of group A IgG concentrations was also sustained over time. However, no boosting effect and no age trend were observed. Group A–specific IgG GMCs and the proportion of subjects with concentrations ≥2 µg/mL were higher in the PsA-TT group than the PsACWY group at any time point, and in both studies. A group A–specific IgG concentration ≥2 µg/mL is the only parameter validated in efficacy studies [20], and thus can be considered a putative correlate of protection against MenA. In the study in Africa, we found that 4 years after vaccination with PsA-TT, an overall 90% of subjects are still potentially protected, with the youngest age group, 2–10 years, showing the lowest level above the putative threshold of protection, with 76% of subjects having group A–specific IgG concentrations ≥2 µg/mL.
PsA-TT has already been introduced in several African meningitis belt countries, including Burkina Faso, Mali, Senegal, and The Gambia through large single-dose mass vaccination campaigns targeting the population from 1 to 29 years of age. The effect of these campaigns has been drastic, with near disappearance of cases of meningitis due to MenA as recorded by the WHO surveillance system [12–14]. Reduction of carriage of the organism is also an important parameter to demonstrate interruption of MenA transmission at the population level [15, 16].
In this context of a highly vaccinated population 4 years after vaccination with 1 dose of PsA-TT vaccine, no booster dose is required, as the majority of individuals still have the putative protective level of antibody titer [10, 11, 20, 21]. However, with time, the need for individual booster dose or revaccination of all or a portion of the population will be established by continuous monitoring of long-term antibody persistence, and by detecting any resurgence of meningitis due to group A meningococci in areas or countries where the disease was highly endemic or epidemic before introduction of PsA-TT vaccine [25].
Notes
Acknowledgments. The authors thank the subjects, their families, and communities for actively participating in both studies; field staff in Niakhar Institut de Recherche pour le Développement, Unité de recherche sur les maladies infectieuses et tropicales émergentes, Senegal; Medical Research Council Basse Station, The Gambia; Centre pour le Développement des Vaccins Bamako, Mali; and Shirdi Sai Baba Rural Hospital, Vadu Budruk, India, for the valuable work with their communities. The authors also thank the laboratories at the Public Health England (formerly Health Protection Agency) and the US Centers for Disease Control and Prevention (Julie Chatt-Soroka and Jeffrey Martin) for serum bactericidal antibody and enzyme-linked immunosorbent assay testing, respectively.
Disclaimers. 1) The authors and editors alone are responsible for the views expressed in this publication and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated; 2) The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of PATH or the World Health Organization (WHO) concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement; 3) The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by PATH or the WHO in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
Financial support. These studies were funded by the Meningitis Vaccine Project (PATH) through a grant from the Bill & Melinda Gates Foundation (BMGF).
Supplement sponsorship. This article appears as part of the supplement “The Meningitis Vaccine Project: The Development, Licensure, Introduction and Impact of a New Group A Meningococcal Conjugate Vaccine for Africa,” sponsored by the Meningitis Vaccine Project through a grant from the Bill & Melinda Gates Foundation.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lapeyssonie L. La méningite cérébrospinale en Afrique. Bull World Health Organ 1963; 28(suppl):3–114. [PMC free article] [PubMed] [Google Scholar]
- 2.Greenwood B. Editorial: 100 years of epidemic meningitis in West Africa—has anything changed? Trop Med Int Health 2006; 11:773–80. [DOI] [PubMed] [Google Scholar]
- 3.LaForce FM, Ravenscroft N, Djingarey M, Viviani S. Epidemic meningitis due to group A Neisseria meningitidis in the African meningitis belt: a persistent problem with an imminent solution. Vaccine 2009; 27(suppl 2):B13–9. [DOI] [PubMed] [Google Scholar]
- 4.Jodar L, LaForce FM, Ceccarini C, Aguado T, Granoff DM. Meningococcal conjugate vaccine for Africa: a model for development of new vaccines for the poorest countries. Lancet 2003; 361:1902–4. [DOI] [PubMed] [Google Scholar]
- 5.LaForce FM, Konde K, Viviani S, Preziosi MP. The Meningitis Vaccine Project. Vaccine 2007; 25(suppl 1):A97–100. [DOI] [PubMed] [Google Scholar]
- 6.Frasch CE, Preziosi MP, LaForce FM. Development of a group A meningococcal conjugate vaccine, MenAfriVac. Hum Vaccin Immunother 2012; 8:715–24. [DOI] [PubMed] [Google Scholar]
- 7.Sow SO, Okoko BJ, Diallo A et al. . Immunogenicity and safety of a meningococcal A conjugate vaccine in Africans. N Engl J Med 2011; 364:2293–304. [DOI] [PubMed] [Google Scholar]
- 8.Kshirsagar N, Mur N, Thatte U et al. . Safety, immunogenicity, and antibody persistence of a new meningococcal group A conjugate vaccine in healthy Indian adults. Vaccine 2007; 25:A101–7. [DOI] [PubMed] [Google Scholar]
- 9.Hirve S, Bavdekar A, Pandit A et al. . Immunogenicity and safety of a new meningococcal A conjugate vaccine in Indian children aged 2–10 years: a phase II/III double-blind randomized controlled trial. Vaccine 2012; 30:6456–60. [DOI] [PubMed] [Google Scholar]
- 10.Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection—serum bactericidal antibody activity. Vaccine 2005; 23:2222–7. [DOI] [PubMed] [Google Scholar]
- 11.Balmer P, Borrow R. Serologic correlates of protection for evaluating the response to meningococcal vaccines. Expert Rev Vaccines 2004; 3:77–87. [DOI] [PubMed] [Google Scholar]
- 12.Djingarey MH, Barry R, Bonkoungou M et al. . Effectively introducing a new meningococcal A conjugate vaccine in Africa: the Burkina Faso experience. Vaccine 2012; 30:B40–5. [DOI] [PubMed] [Google Scholar]
- 13.Meningitis Vaccine Project. A world without meningitis. Available at: http://www.meningvax.org/. Accessed 16 January 2015.
- 14.World Health Organization. Meningitis. Available at: http://who.int/topics/meningitis/en/ Accessed 16 January 2015.
- 15.Kristiansen PA, Ba AK, Ouédraogo AS, Sanou I et al. . Persistent low carriage of serogroup A Neisseria meningitidis two years after mass vaccination with the meningococcal conjugate vaccine, MenAfriVac. BMC Infect Dis 2014; 14:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daugla DM, Gami JP, Gamougam K et al. . Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study [corrected]. Lancet 2014; 383:40–7. Erratum: Lancet 2014; 383:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchetti E, Mazarin-Diop V, Chaumont J et al. . Conducting vaccine clinical trials in sub-Saharan Africa: operational challenges and lessons learned from the Meningitis Vaccine Project. Vaccine 2012; 30:6859–63. [DOI] [PubMed] [Google Scholar]
- 18.Carlone GM, Frasch CE, Siber GR et al. . Multicenter comparison of levels of antibody to the Neisseria meningitidis group A capsular polysaccharide measured by using an enzyme-linked immunosorbent assay. J Clin Microbiol 1992; 30:154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maslanka SE, Gheesling LL, Libutti DE et al. . Standardization and a multilaboratory comparison of Neisseria meningitidis A and C serum bactericidal assays. The Multilaboratory Study Group. Clin Diagn Lab Immunol 1997; 4:156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peltola H, Makela H, Kayhty H et al. . Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med 1977; 297:686–91. [DOI] [PubMed] [Google Scholar]
- 21.Makela PH, Kayhty H, Weckstrom P, Sivonen A, Renkonen OV. Effect of group-A meningococcal vaccine in army recruits in Finland. Lancet 1975; 2:883–6. [DOI] [PubMed] [Google Scholar]
- 22.Robbins JB, Myerowitz L, Whisnant JK et al. . Enteric bacteria cross-reactive with Neisseria meningitidis groups A and C and Diplococcus pneumoniae types I and 3. Infect Immun 1972; 6:651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vann WF, Liu TY, Robbins JB. Bacillus pumilus polysaccharide cross-reactive with meningococcal group A polysaccharide. Infect Immun 1976; 13:1654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guirguis N, Schneerson R, Bax A et al. . Escherichia coli K51 and K93 capsular polysaccharides are crossreactive with the group A capsular polysaccharide of Neisseria meningitidis. Immunochemical, biological, and epidemiological studies. J Exp Med 1985; 162:1837–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dakar Discussion Group on Priorities for Research on Epidemic Meningococcal Disease in Africa; Altmann D, Aseffa A, Bash M et al. . Priorities for research on meningococcal disease and the impact of serogroup A vaccination in the African meningitis belt. Vaccine 2013; 31:1453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]