Abstract
Objectives
To examine the rate of osteoporosis (OP) undertreatment and the association between gastrointestinal (GI) events and OP treatment initiation among elderly osteoporotic women with Medicare Part D drug coverage.
Methods
This retrospective cohort study utilized a 20% random sample of Medicare beneficiaries. Included were women ≥66 years old with Medicare Part D drug coverage, newly diagnosed with OP in 2007–2008 (first diagnosis date as the index date), and with no prior OP treatment. GI event was defined as a diagnosis or procedure for a GI condition between OP diagnosis and treatment initiation or at the end of a 12-month follow-up, whichever occurred first. OP treatment initiation was defined as the use of any bisphosphonate (BIS) or non-BIS within 1 year postindex. Logistic regression, adjusted for patient characteristics, was used to model the association between 1) GI events and OP treatment initiation (treated versus nontreated); and 2) GI events and type of initial therapy (BIS versus non-BIS) among treated patients only.
Results
A total of 126,188 women met the inclusion criteria: 72.1% did not receive OP medication within 1 year of diagnosis and 27.9% had GI events. Patients with a GI event were 75.7% less likely to start OP treatment (odds ratio [OR]=0.243; P<0.001); among treated patients, patients with a GI event had 11.3% lower odds of starting with BIS versus non-BIS (OR=0.887; P<0.001).
Conclusion
Among elderly women newly diagnosed with OP, only 28% initiated OP treatment. GI events were associated with a higher likelihood of not being treated and, among treated patients, a lower likelihood of being treated with BIS versus non-BIS.
Keywords: gastrointestinal, osteoporosis, postmenopausal women, treatment initiation
Introduction
Over one-quarter of women aged 70 or older in the US are estimated to have osteoporosis (OP) and women account for nearly 90% of all OP cases in this age group.1 Fragility fractures are the most serious consequence of OP and are more common in older patients than in their younger counterparts,2,3 affecting as many as 40% of those who are 85 years and older.2 OP-related fractures result in significant health care costs4 and they have been shown to adversely affect health-related quality of life.5
Available treatments shown to reduce fracture risk include bisphosphonates (BIS) (alendronate, ibandronate, risedronate, zoledronic acid), polypeptide hormone (calcitonin), a selective estrogen receptor modulator (raloxifene), a receptor activator of nuclear factor kappa-B ligand inhibitor (denosumab), and parathyroid hormone 1–34 (teriparatide).6 Based on recommendations from the National Osteoporosis Foundation, an estimated 30% of American women aged 50 years and older should be considered for pharmacologic treatment of OP to reduce the risk of fracture.7,8 Despite this recommendation, previous studies have demonstrated substantial undertreatment,9–17 including among patients who have previously experienced a fracture.9,15,18–20 The reasons for undertreatment are not fully understood.
There may be multiple barriers to treatment initiation, including concerns over side effects of OP medication.21 Some observational studies have cited gastrointestinal (GI) events as contributing to therapy discontinuation of oral BIS.22–24 However, GI conditions are prevalent in elderly patients, as is the chronic use of non-OP medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs),25 which can cause symptoms in the upper GI tract.26,27 The cost of therapy, patient frailty, and the presence of cognitive impairment – not uncommon in elderly fracture patients28,29 – have also been identified as potential barriers to treatment.29,30
Given the frequency and seriousness of fractures in an elderly population, understanding the extent of treatment penetration in this population, as well as the risk factors for undertreatment, are essential to improving clinical outcomes. The objective of this study was to estimate the rate of pharmacologic treatment for OP among women with Medicare Part D coverage during the 1-year period after OP diagnosis, and to examine the association between GI events and OP treatment initiation.
Methods
Study design and data source
Review board approval was not sought as all data were de-identified and accessed in compliance with US Health Insurance Portability and Accountability Act privacy guidelines. This study used data from a random 20% sample of Medicare beneficiaries from 2006 to 2009 to identify a retrospective cohort of women diagnosed with OP during 2007–2008. The data source contains demographic data and medical (Medicare Parts A and B) and pharmacy (Medicare Part D) claims. Medicare Parts A and B claims include primary and secondary International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes, Current Procedural Terminology Version 4 (CPT-4) procedure codes, type of service (including J-codes for physician-administered drugs), and setting of care (eg, physician’s office, emergency room). Medicare Part D claims include National Drug Code identifiers, days’ supply, and quantity dispensed.
Patient identification and cohort definition
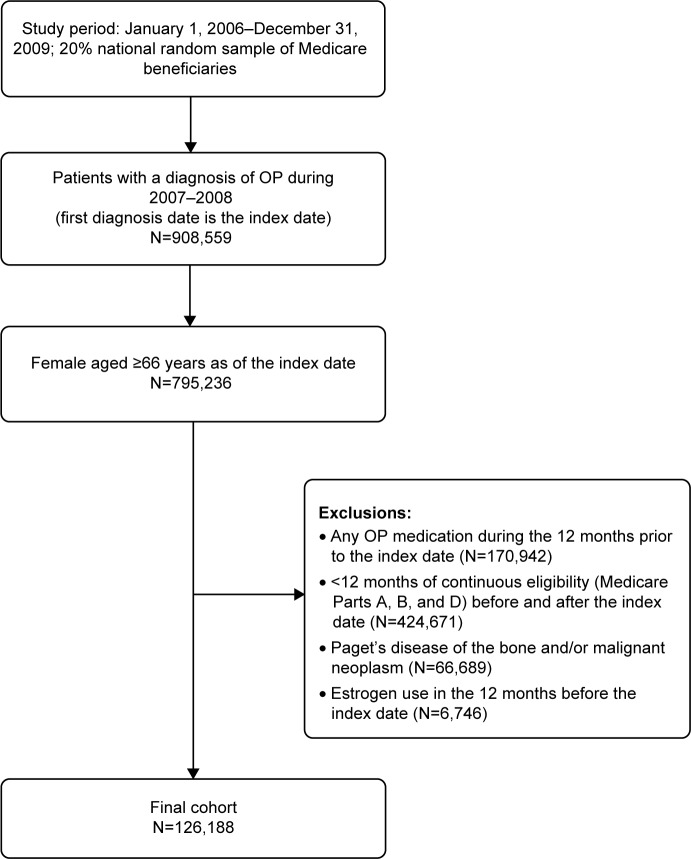
Patients newly diagnosed with OP (ICD-9-CM 733.0x) between January 1, 2007 and December 31, 2008 were identified. The index date was the first date of OP diagnosis. The baseline period (ie, preindex period) was the 12 months prior to the first OP diagnosis and patients were followed for up to 1 year after the index date (ie, postindex period). The sample was limited to women aged at least 66 years as of the index date who had continuous Medicare enrollment (with Medicare as the primary payer) in Parts A, B, and D during the entire study period (baseline and follow-up). To be included in the study, patients were also required to be naïve to OP pharmacotherapy during the baseline period, meaning that they could not have any claims for oral or intravenous BIS (alendronate, ibandronate, risedronate, zoledronic acid) or non-BIS (calcitonin, raloxifene, teriparatide). Denosumab was not included as a pharmacotherapy because it was approved in the USA in 2010, after the last index date for this study. Patients were excluded if they had a diagnosis of malignant neoplasm (ICD-9-CM codes 141–171, 173–208, and 230–239) or Paget’s disease of the bone (ICD-9-CM code 731.0) at any time during the study interval, or pharmacy claims for estrogen use during the baseline period. Treated patients were defined as those having received at least one pharmacologic OP medication identified on either Part B or Part D claims during the follow-up period. Patients who suffered a fracture and subsequently received and filled a prescription for an OP medication were considered as treated patients, but only if they had a diagnosis of OP.
Measures
Patient characteristics identified during the baseline period included age as of the index date, dual Medicare and Medicaid status, common OP-related comorbidities (from ICD-9-CM diagnosis codes: celiac disease; chronic inflammatory bowel disease; chronic inflammatory joint disease; diabetes [type 1 or type 2]; chronic kidney disease; hypertension; hyperparathyroidism; or vitamin D deficiency), bone mineral density (BMD) test status (yes/no) in the last 6 months of the baseline period, and a history of osteoporotic fractures (hip, vertebral, and nonvertebral). The count of comorbid conditions was computed using the Charlson comorbidity index (CCI), which represents 17 conditions and serves as a proxy for comorbid disease burden.31 A score of 0 indicates the absence of any of the conditions, and scores >0 correspond to the number of comorbid conditions present. Medication use assessed during baseline included gastroprotective agents (proton pump inhibitors, H2-receptor blockers, and cytoprotectants), NSAIDs, and glucocorticoids. The baseline total pill burden was defined as the count of distinct oral drug molecules in the baseline period. The occurrence of GI events was also assessed during the baseline period and included acute gastritis, duodenal ulcer, duodenitis, dysphagia, gastric ulcer, esophagitis, gastroesophageal reflux disease, GI hemorrhage, nausea/vomiting, peptic ulcer, and ulcer/stricture/perforation/hemorrhage of the esophagus. Codes used to identify these events appear in Table S1. Total health care costs during the baseline period were determined from the sum of all patient-and health-plan paid amounts.
During the postindex period (follow-up), patients were considered to initiate OP treatment if they had a claim for either a BIS or a non-BIS within 12 months after the index date. Patients who initiated treatment were classified as “treated” patients, while all others were considered to be “untreated”. Among treated patients, the type of medication initiated was recorded and classified as BIS or non-BIS. The observation period for postindex GI events was 12 months for untreated patients. For treated patients, the postindex GI events were identified only up until the date of treatment initiation. Outcomes were OP treatment initiation (yes/no) and, among patients who initiated OP treatment, whether they initiated with a BIS versus a non-BIS.
Statistical analysis
Summary measures of baseline characteristics were calculated, including mean and standard deviation (SD) for continuous variables and number and percent for categorical variables. Differences in baseline characteristics between treated and untreated patients were compared using Wilcoxon rank-sum tests for continuous variables and chi-squared tests for categorical variables. Treatment outcomes (whether or not treatment was initiated and the type of treatment initiated [BIS versus non-BIS]) were modeled using logistic regression; the primary independent variable of interest was the presence or absence of postindex GI events. The model was stratified by the presence or absence of baseline GI events and also included the following adjustment variables: age (grouped in years: 66–74, 75–84, and ≥85); baseline count of CCI comorbid conditions; baseline health care costs; baseline pill burden; and binary variables indicating medication use (gastroprotective agents, glucocorticoids, and NSAIDs), dual eligibility status, baseline BMD test, and OP-related comorbidities. Two sensitivity analyses were also conducted. Because of the varying length of time between OP diagnosis and OP treatment initiation, the association between GI events that occurred after OP diagnosis and before OP treatment initiation was examined with Cox proportional hazards regression with the same adjustment variables used in the logistic regression model. The second analysis assessed the relationship between post-OP diagnosis GI events and BIS versus non-BIS treatment among patients who initiated treatment with a discrete choice conditional logit model and the same adjustment variables used in the logistic regression model. This analysis examined multiple mutually exclusive alternative choices, while the primary logistic regression analysis examined binary outcomes.
Results
Patient sample and treatment initiation patterns
A total of 126,188 patients met the study inclusion criteria (Figure 1). Overall, a majority of patients (72.1%) did not initiate OP treatment in the year following their OP diagnosis; 21.6% of patients initiated BIS and 6.3% started with non-BIS (Table 1). Alendronate was the most common BIS (12.0% of patients) and calcitonin was the most common non-BIS (4.3% of patients). For those receiving OP treatment, initiation occurred, on average (SD), 75.4 (88.9) days after the index OP diagnosis.
Figure 1.
Identification of the study cohort.
Abbreviations: N, total number; OP, osteoporosis.
Table 1.
OP treatment patterns after diagnosis
| Treatment pattern | N (%) |
|---|---|
| All patients | 126,188 (100) |
| No OP treatment within 1 year of OP diagnosis | 91,021 (72.1) |
| First OP treatment within 1 year of OP diagnosis | |
| Bisphosphonate | 27,267 (21.6) |
| Alendronate | 15,194 (12.0) |
| Ibandronate | 4,702 (3.7) |
| Risedronate | 6,144 (4.9) |
| Zoledronic acid | 1,227 (1.0) |
| Nonbisphosphonate | 7,900 (6.3) |
| Calcitonin | 5,438 (4.3) |
| Raloxifene | 1,317 (1.0) |
| Teriparatide | 1,145 (0.9) |
| Average time from OP diagnosis to treatment initiation, days | Mean (SD) |
| Any treatment (N=35,167) | 75.4 (88.9) |
| Bisphosphonate (N=27,267) | 79.4 (91.8) |
| Nonbisphosphonate (N=7,900) | 61.6 (76.3) |
Abbreviations: OP, osteoporosis; SD, standard deviation; N, total number.
Patient baseline characteristics
Baseline patient characteristics for all patients and by postindex OP treatment status are shown in Table 2. The mean age (SD) at the date of the first OP diagnosis was 78.5 (7.9) years. About one in five patients were dually eligible for Medicare and Medicaid. The mean (SD) count of CCI comorbid conditions was 1.53 (1.60), and the most common comorbidities identified were hypertension (76%), chronic inflammatory joint disease (59%), and diabetes (30%). Prior to the index date, 42% experienced a GI event and 12% had at least one OP-related fracture. During the baseline period, gastroprotective agents were used by 32% of the cohort, while 20% and 24% used glucocorticoids and NSAIDs, respectively.
Table 2.
Baseline patient characteristics for all patients and by postindex OP treatment status
| Characteristic | All patients(N=126,188) | Treatment status
|
BIS treatment status among those being treated
|
||||
|---|---|---|---|---|---|---|---|
| No treatment (N=91,021) | Initiated any treatment (N=35,167) | P-value | Initiated BIS treatment (N=27,267) | Initiated non-BIS treatment (N=7,900) | P-value | ||
| Age, mean (SD) | 78.5 (7.9) | 78.7 (8.0) | 77.9 (7.4) | <0.0001 | 77.2 (7.1) | 80.2 (8.1) | <0.0001 |
| Age distribution, N (%) | <0.0001 | <0.0001 | |||||
| 66–74 years | 43,156 (34.2) | 30,662 (33.7) | 12,494 (35.5) | 10,393 (38.1) | 2,101 (26.6) | ||
| 75–84 years | 50,882 (40.3) | 36,010 (39.6) | 14,872 (42.3) | 11,696 (42.9) | 3,176 (40.2) | ||
| >85 years | 32,150 (25.5) | 24,349 (26.8) | 7,801 (22.2) | 5,178 (19.0) | 2,623 (33.2) | ||
| Dual eligibility,a N (%) | 23,606 (18.7) | 16,416 (18.0) | 7,190 (20.5) | <0.0001 | 5,250 (19.3) | 1,940 (24.6) | <0.0001 |
| Charlson comorbidity index, mean (SD) | 1.53 (1.60) | 1.57 (1.63) | 1.42 (1.52) | <0.0001 | 1.35 (1.47) | 1.64 (1.63) | <0.0001 |
| Common OP-related comorbidities, N (%) | |||||||
| Chronic inflammatory bowel disease | 1,080 (0.9) | 771 (0.9) | 309 (0.9) | 0.58 | 238 (0.9) | 71 (0.9) | 0.83 |
| Chronic inflammatory joint disease | 74,323 (58.9) | 54,159 (59.6) | 20,164 (57.3) | <0.0001 | 15,326 (56.2) | 4,838 (61.2) | <0.0001 |
| Celiac disease | 206 (0.2) | 141 (0.2) | 65 (0.2) | 0.24 | 40 (0.2) | 25 (0.3) | 0.002 |
| Diabetes (type 1 or 2) | 36,565 (29.0) | 27,159 (29.8) | 9,406 (26.8) | <0.0001 | 7,387 (27.1) | 2,019 (25.6) | 0.007 |
| Chronic kidney disease | 13,399 (10.6) | 10,029 (11.0) | 3,370 (9.6) | <0.0001 | 2,433 (8.9) | 937 (11.9) | <0.0001 |
| Hypertension | 96,178 (76.2) | 69,802 (76.7) | 26,376 (75.0) | <0.0001 | 20,467 (75.1) | 5,909 (74.8) | 0.63 |
| Hyperparathyroidism | 1,224 (1.0) | 902 (1.0) | 322 (0.9) | 0.22 | 245 (0.9) | 77 (1.0) | 0.53 |
| Vitamin D deficiency | 1,934 (1.5) | 1,450 (1.6) | 484 (1.4) | 0.01 | 366 (1.3) | 118 (1.5) | 0.31 |
| OP-related fractures, N (%) | |||||||
| Any fracture | 15,231 (12.1) | 10,263 (11.3) | 4,968 (14.1) | <0.0001 | 3,514 (12.9) | 1,454 (18.4) | <0.0001 |
| Hip | 4,278 (3.4) | 3,014 (3.3) | 1,264 (3.6) | 0.01 | 894 (3.3) | 370 (4.7) | <0.0001 |
| Vertebral | 5,367 (4.3) | 3,354 (3.7) | 2,013 (5.7) | <0.0001 | 1,287 (4.7) | 726 (9.2) | <0.0001 |
| Nonvertebral | 8,542 (6.8) | 5,915 (6.5) | 2,627 (7.5) | <0.0001 | 1,966 (7.2) | 661 (8.4) | 0.001 |
| GI-related drug use, N (%) | |||||||
| Glucocorticoids | 25,601 (20.3) | 18,000 (19.8) | 7,601 (21.6) | <0.0001 | 5,811 (21.3) | 1,790 (22.7) | 0.01 |
| NSAIDs | 30,251 (24.0) | 21,237 (23.3) | 9,014 (25.6) | <0.0001 | 7,176 (26.3) | 1,838 (23.3) | <0.0001 |
| Gastroprotective agents | |||||||
| Any agent | 40,748 (32.3) | 28,779 (31.6) | 11,969 (34.0) | <0.0001 | 8,495 (31.2) | 3,474 (44.0) | <0.0001 |
| Proton pump inhibitors | 34,055 (27.0) | 23,919 (26.3) | 10,136 (28.2) | <0.0001 | 7,163 (26.3) | 2,973 (37.6) | <0.0001 |
| H2 antagonist | 8,881 (7.0) | 6,367 (7.0) | 2,514 (7.2) | 0.34 | 1,800 (6.6) | 714 (9.0) | <0.0001 |
| Cytoprotectants | 2,461 (2.0) | 1,730 (1.9) | 731 (2.1) | 0.04 | 482 (1.8) | 249 (3.2) | <0.0001 |
| Total pill burden,b mean (SD) | 7.8 (5.7) | 7.79 (5.71) | 7.83 (5.64) | 0.07 | 7.53 (5.52) | 8.85 (5.93) | <0.0001 |
| Total annual health care costs, mean USD (SD) | 24,999 (54,394) | 25,595 (55,954) | 23,456 (50,101) | 0.60 | 21,885 (47,736) | 28,882 (57,193) | <0.0001 |
Notes:
Eligible for Medicaid and Medicare;
count of distinct oral drug molecules in the baseline period.
Abbreviations: OP, osteoporosis; BIS, bisphosphonates; N, total number; SD, standard deviation; GI, gastrointestinal; NSAIDs, nonsteroidal anti-inflammatory drugs.
Patients initiating any OP treatment or BIS treatment were slightly younger than the patients in the respective comparator cohorts, and the proportion of patients in the OP treatment initiation group with dual eligibility was slightly higher than the cohort that did not initiate any OP treatment. A lesser percentage of patients who initiated with a BIS had dual eligibility compared to non-BIS patients. Patients initiating any OP treatment, as well as those initiating BIS, had slightly lower mean counts of CCI comorbid conditions than patients who did not initiate any OP treatment or who initiated non-BIS. During baseline, the proportion of patients with OP-related fractures was higher in both the OP treatment initiation and non-BIS treatment cohorts. The proportion of patients with gastroprotective agent use was higher in the OP treatment initiation and non-BIS treatment initiation cohorts.
Rate of GI events during baseline and follow-up periods
The distribution of patients by the presence or absence of GI events during baseline and after OP diagnosis is shown in Table 3. Overall, 41.6% of patients had a baseline GI event and 27.9% of patients had a GI event between their OP diagnosis and OP treatment initiation or end of follow-up, whichever occurred first. Among patients who did not initiate any OP treatment, 41.5% had baseline GI events; of these patients, 69.2% also experienced a GI event in the follow-up period. The rate of GI events during follow-up among all patients who did not initiate any OP treatment was 47.9%. For patients who initiated BIS, 40.0% had baseline GI events and 30.7% of these patients continued to experience GI events during follow-up. The rate of GI events during follow-up in the cohort of patients who initiated BIS was 18.9%. Among patients who initiated non-BIS, 48.7% had baseline GI events and 38.1% of these patients experienced a GI event during follow-up. The rate of GI events during follow-up was 27.0% among all patients who initiated non-BIS.
Table 3.
Distribution of patients by GI events before and after OP diagnosis
| Cohort | Pre-OP diagnosis of GI events, N (%) | Post-OP diagnosis GI events, N (%)
|
Total | |
|---|---|---|---|---|
| No | Yes | |||
| All patients (N=126,188) | No | 61,100 (82.9) | 12,577 (17.1) | 73,677 (58.4) |
| Yes | 29,831 (56.8) | 22,680 (43.2) | 52,511 (41.6) | |
| Total | 90,931 (72.1) | 35,257 (27.9) | 126,188 (100.0) | |
| Patients who did not initiate | No | 35,767 (67.2) | 17,497 (32.9) | 53,264 (58.5) |
| OP treatment (N=91,021) | Yes | 11,614 (30.8) | 26,143 (69.2) | 37,757 (41.5) |
| Total | 47,381 (52.1) | 43,640 (47.9) | 91,021 (100.0) | |
| Patients initiated on BIS (N=27,267) | No | 14,558 (89.0) | 1,803 (11.0) | 16,361 (60.0) |
| Yes | 7,554 (69.3) | 3,352 (30.7) | 10,906 (40.0) | |
| Total | 22,112 (81.1) | 5,155 (18.9) | 27,267 (100.0) | |
| Patients initiated on non-BIS (N=7,900) | No | 3,389 (83.6) | 663 (16.4) | 4,052 (51.3) |
| Yes | 2,379 (61.8) | 1,469 (38.2) | 3,848 (48.7) | |
| Total | 5,768 (73.0) | 2,132 (27.0) | 7,900 (100.0) | |
Abbreviations: GI, gastrointestinal; OP, osteoporosis; N, total number; BIS, bisphosphonates.
Association of GI events with treatment initiation and choice of treatment
The results of the logistic regression model for the association between postindex GI events and OP treatment initiation, adjusted for patient baseline characteristics, are shown in Table 4. Patients with GI events post-OP diagnosis had lower odds of initiating any OP treatment; patients who experienced both baseline and follow-up GI events were 74.0% less likely to initiate OP treatment (odds ratio [OR]=0.260; 95% confidence interval [CI]=0.250–0.270), and patients who had only follow-up GI events (ie, no baseline events) were 75.7% less likely to initiate OP treatment (OR=0.243; 95% CI=0.232–0.254). Other risk factors for the reduced likelihood of treatment initiation included baseline comorbidities of diabetes, hypertension, chronic kidney disease, and vitamin D deficiency. Patient characteristics associated with greater odds of treatment initiation were older age, the presence of baseline BMD testing, and the baseline use of gastroprotective agents, NSAIDs, and glucocorticoids. In the sensitivity analysis, using Cox proportional hazards regression to account for the varying time from OP diagnosis to treatment initiation, the results were similar (data not shown): patients with GI events during follow-up were 70.9% less likely to initiate OP treatment (hazard ratio [HR]=0.291; 95% CI=0.284–0.299); the same pattern was evident in the strata of patients who also experienced baseline GI events (HR=0.254; 95% CI=0.246–0.264) and among those who did not have baseline GI events (HR=0.312; 95% CI=0.299–0.325).
Table 4.
Logistic regression analysis of the association of postdiagnosis GI events and OP treatment initiation
| Independent variable | OP treatment initiation Any OP treatment versus no OP treatment
|
|||
|---|---|---|---|---|
| Odds ratioa | 95% CI | P-value | ||
| Presence of postdiagnosis GI event (ref: absence of postdiagnosis GI events) | ||||
| Patients without a prediagnosis GI event | 0.243 | 0.232 | 0.254 | <0.0001 |
| Patients with a prediagnosis GI event | 0.260 | 0.250 | 0.270 | <0.0001 |
| Baseline characteristics | ||||
| Age at diagnosis, years (ref: 66–74 years) | ||||
| 75–84 years | 1.142 | 1.108 | 1.177 | <0.0001 |
| ≥85 years | 1.097 | 1.057 | 1.138 | <0.0001 |
| Pill burden | 1.014 | 1.011 | 1.018 | <0.0001 |
| Medication use | ||||
| Gastroprotective agents | 1.577 | 1.527 | 1.628 | <0.0001 |
| Glucocorticoids | 1.078 | 1.044 | 1.113 | <0.0001 |
| NSAID | 1.122 | 1.085 | 1.161 | <0.0001 |
| Dual eligibility (yes) | 1.379 | 1.333 | 1.427 | <0.0001 |
| Baseline bone mineral density testing (yes) | 2.097 | 2.040 | 2.156 | <0.0001 |
| Charlson comorbidity index | 0.988 | 0.975 | 1.000 | 0.059 |
| OP-related comorbidities | ||||
| Chronic inflammatory bowel disease | 1.082 | 0.940 | 1.246 | 0.273 |
| Chronic inflammatory joint disease | 0.996 | 0.968 | 1.025 | 0.795 |
| Celiac disease | 1.247 | 0.911 | 1.707 | 0.167 |
| Diabetes | 0.817 | 0.789 | 0.847 | <0.0001 |
| Chronic kidney disease | 0.949 | 0.904 | 0.996 | 0.034 |
| Hypertension | 0.900 | 0.872 | 0.930 | <0.0001 |
| Hyperparathyroidism | 0.918 | 0.801 | 1.051 | 0.215 |
| Vitamin D deficiency | 0.869 | 0.779 | 0.970 | 0.012 |
| 12-month preindex health care costs | 1.001 | 1.000 | 1.001 | <0.001 |
Note:
Adjusted for patient baseline characteristics.
Abbreviations: GI, gastrointestinal; OP, osteoporosis; CI, confidence interval; ref, reference group; NSAID, nonsteroidal anti-inflammatory drug.
The logistic regression analysis examining the association between postindex GI events and the choice of OP treatment (BIS versus non-BIS) among patients who initiated treatment, and adjusted for patient baseline characteristics, is shown in Table 5. Patients with follow-up GI events were less likely to initiate treatment with a BIS compared with a non-BIS. Patients who experienced GI events during both baseline and follow-up were 14.1% less likely to receive a BIS (OR=0.859; 95% CI=0.797–0.926), and patients with GI events only during follow-up were 11.3% less likely to receive a BIS (OR=0.887; 95% CI=0.804–0.979). Other risk factors for the reduced likelihood of receiving a BIS included older age, higher pill burden, greater number of CCI comorbid conditions, and gastroprotective agent use. Patient characteristics associated with a greater likelihood of initiating with a BIS versus a non-BIS included the presence of a baseline BMD test and the baseline use of glucocorticoids and NSAIDs. The results of the sensitivity analysis employing a discrete choice model showed that patients with GI events during follow-up (irrespective of baseline GI events) had 11.6% lower odds of initiating BIS versus non-BIS (OR=0.884; 95% CI=0.829–0.942), and that patients with baseline GI events (irrespective of follow-up GI events) were 9.0% less likely to receive BIS versus non-BIS (OR=0.910; 95% CI=0.856–0.967) (data not shown).
Table 5.
Logistic regression of the association of postdiagnosis GI events and the type of treatment initiated among those patients who initiated treatment
| Independent variable | Bisphosphonate treatment initiation Bisphosphonate versus nonbisphosphonate
|
|||
|---|---|---|---|---|
| Odds ratioa | 95% CI | P-value | ||
| Presence of postdiagnosis GI event (ref: absence of postdiagnosis GI events) | ||||
| Patients without a prediagnosis GI event | 0.887 | 0.804 | 0.979 | 0.0171 |
| Patients with a prediagnosis GI event | 0.859 | 0.797 | 0.926 | <0.0001 |
| Baseline characteristics | ||||
| Age at diagnosis (ref: 66–74 years) | ||||
| 75–84 years | 0.801 | 0.752 | 0.854 | <0.0001 |
| ≥85 years | 0.521 | 0.485 | 0.559 | <0.0001 |
| Pill burden | 0.970 | 0.964 | 0.976 | <0.0001 |
| Medication use | ||||
| Gastroprotective agents | 0.682 | 0.642 | 0.724 | <0.0001 |
| Glucocorticoids | 1.291 | 1.211 | 1.377 | <0.0001 |
| NSAID | 1.102 | 1.031 | 1.178 | 0.0043 |
| Dual eligibility (yes) | 0.818 | 0.767 | 0.871 | <0.0001 |
| Baseline bone mineral density testing (yes) in 6 months before the OP diagnosis | 2.446 | 2.318 | 2.581 | <0.0001 |
| Charlson comorbidity index | 0.956 | 0.933 | 0.981 | 0.0005 |
| OP-related comorbidities | ||||
| Chronic inflammatory bowel disease | 1.030 | 0.779 | 1.360 | 0.8369 |
| Chronic inflammatory joint disease | 0.966 | 0.912 | 1.024 | 0.2453 |
| Celiac disease | 0.461 | 0.273 | 0.780 | 0.0039 |
| Diabetes | 1.285 | 1.195 | 1.381 | <0.0001 |
| Chronic kidney disease | 0.933 | 0.850 | 1.023 | 0.1390 |
| Hypertension | 1.227 | 1.151 | 1.309 | <0.0001 |
| Hyperparathyroidism | 0.914 | 0.697 | 1.198 | 0.5137 |
| Vitamin D deficiency | 0.881 | 0.707 | 1.097 | 0.2562 |
| 12-month health care costs | 1.000 | 0.999 | 1.000 | 0.8289 |
Note:
Adjusted for patient baseline characteristics.
Abbreviations: GI, gastrointestinal; CI, confidence interval; ref, reference group; NSAID, nonsteroidal anti-inflammatory drug; OP, osteoporosis.
Discussion
This study demonstrates significant undertreatment among osteoporotic women with Medicare Part D coverage. Only 27.9% of patients started OP treatment in the first year after their diagnosis. Patients who experienced GI events following their OP diagnosis were 74%–76% less likely to initiate OP treatment than their counterparts who did not experience a postdiagnosis GI event. Among patients who did begin treatment, GI events were associated with 11%–14% lower likelihood of receiving BIS versus non-BIS treatment. The association between GI events and both OP treatment initiation and choice of OP treatment were robust in the sensitivity analyses.
The significant undertreatment of OP we found in this study is consistent with previous research. In studies of elderly patients with low-impact fractures or surgical repair of fracture, over 70% lacked treatment with OP medications other than estrogen prior to experiencing a fracture.9,10,13,14 In a retrospective analysis of claims data that identified patients based on an OP diagnosis or low bone mass density during 2000–2007 (mean age: 67 years), 42% received treatment within 90 days32 and, in a study that utilized data from the 2007 National Health and Wellness Survey, only 55% of those who self-reported a diagnosis of OP (mean age: 64 years) also reported using prescription medication to treat OP.33
The literature examining the contribution of GI events to OP treatment initiation and choice of OP treatment is sparse. However, some studies have suggested a potential relationship. In a study of elderly women who had experienced a wrist or hip fracture, a prior history of GI disease was associated with slightly lower, although not significantly lower, odds of receiving either OP treatment or BMD testing within 6 months of the fracture.34 However, this study was not limited to patients who were naïve to OP treatment, and prior OP treatment was by far the strongest predictor of receipt of OP treatment postfracture. Our population included only women who were OP treatment naïve, and our outcome was OP treatment initiation, not the combination of OP treatment initiation or BMD testing; thus, our study likely provides a more refined assessment of the association between pretreatment GI events and OP treatment initiation. Among patients who initiated OP treatment, we also noted slightly higher odds of receiving non-BIS treatment among patients who experienced a pretreatment GI event. Foster et al35 examined the relationship between patient characteristics and the receipt of raloxifene versus BIS; in their Medicare/commercially-insured cohort, patients with a pretreatment gastric ulcer, but not other GI conditions, had greater odds of receiving raloxifene compared with BIS, but the association was not apparent in the Medicaid cohort. There were several differences between the two studies, including the pretreatment rate of GI events. The baseline rate of GI events was 41.6% in our study versus less than 10% of patients in either the Medicare/commercial or Medicaid cohort in the study by Foster et al.35 We also used a more comprehensive series of codes to define a GI event and examined the collective impact of all GI events on choice of OP treatment, rather than individual categories of GI events (eg, peptic ulcer, gastric ulcer). Further, our non-BIS cohort was not limited to only patients receiving raloxifene, but it also included patients initiating treatment with calcitonin or teraparatide.
There were other characteristics, in addition to GI events, that were predictive of OP treatment initiation in our study. The strongest predictor of treatment initiation was the presence of a BMD test during baseline. Patients with a baseline BMD test were more than twice as likely to initiate OP treatment when compared with patients who did not have a baseline BMD test and, among patients who initiated OP treatment, those with a baseline BMD test were 2.4 times more likely to receive BIS versus non-BIS. There was also a modest, positive association between older age and OP treatment initiation. Some, but not all, previous studies have shown a positive link between older age and treatment initiation.32,36–38 The use of gastroprotective agents, NSAIDS, and glucocorticoids at baseline was also positively correlated with treatment initiation. Previous studies have noted a similar association between glucocorticoid or corticosteroid use and treatment initiation.32,34,37 Chronic kidney disease was also associated with a slightly lower likelihood of treatment initiation. Alendronate, the most commonly prescribed treatment in this study, is contraindicated for patients with severe renal impairment.
Our findings advance the understanding of the patient characteristics that contribute to undertreatment, an essential step in improving the management of OP patients. GI events are prevalent in older women diagnosed with OP, and our results suggest that GI events that occur following OP diagnosis are a significant risk factor for failure to initiate OP treatment. Elderly women have a heightened risk of fracture, and the clinical management of these patients should integrate characteristics that promote treatment and address risk factors for undertreatment. Future research is needed to distinguish between characteristics driven by clinician prescribing patterns versus patient choice to appropriately target interventions that promote treatment.
Limitations
There are several limitations to our study, and the results should be considered in this context. We included only patients with a claim for an OP diagnosis. BMD test results were not available to confirm the diagnosis, and the presence of a diagnosis code may indicate that a patient was simply screened for OP, potentially inflating our estimate of under-treatment. We also used Medicare prescription drug claims data to determine OP treatment initiation. If a patient had non-Medicare prescription drug coverage, claims for OP pharmacotherapy would not be captured in this analysis. We do not know the rationale for not initiating OP treatment, which may reflect a clinician’s decision not to prescribe treatment or a patient decision not to fill a prescription. Only GI events that resulted in medical service utilization were captured in this analysis. The potential association between less severe events (ie, that did not result in a medical encounter) and treatment initiation is unknown. Claims data do not provide information regarding the severity of symptoms (other than the medical classification) associated with each GI event, which could influence treatment initiation. Further, our analysis examining the relationship between baseline medication use and treatment initiation is limited to prescription drug claims and would not include over-the-counter drugs such as low-dose NSAIDs and aspirin. There may have been other unmeasured patient and clinician characteristics that influenced OP treatment initiation and the receipt of BIS versus non-BIS treatment. Finally, our results are based on a sample of women aged 66 years and older with Medicare Parts A, B, and D coverage, and they may not be applicable to younger patients or to patients with other forms of insurance.
Conclusion
Among women with Medicare Part D coverage, only 28% of those diagnosed with OP received pharmacologic treatment for OP in the first year following the diagnosis. A total of 28% experienced a GI event between their diagnosis and OP treatment initiation during the first year of OP diagnosis. The presence of a GI event following OP diagnosis was associated with 76% lower odds of initiating OP treatment. Among patients who did initiate OP treatment, a postdiagnosis GI event was associated with 11% lower odds of initiating BIS versus non-BIS treatment.
Supplementary material
Table S1.
Codes for the identification of gastrointestinal events
| Description | |
|---|---|
| ICD-9-CM code | |
| 456.0 | Esophageal varices with hemorrhage |
| 456.1 | Esophageal varices without hemorrhage |
| 530.0x | Achalasia and cardiospasm |
| 530.1x | Esophagitis |
| 530.2 | Ulcer of esophagus |
| 530.2 | Ulcer of esophagus without bleeding |
| 530.21 | Ulcer of esophagus with bleeding |
| 530.3 | Stricture of esophagus |
| 530.4 | Perforation of esophagus |
| 530.5 | Dyskinesia of esophagus |
| 530.7 | Mallory–Weiss syndrome |
| 530.81 | Esophageal reflux (GERD) |
| 530.82 | Esophageal hemorrhage |
| 530.84 | Tracheoesophageal fistula |
| 530.89 | Other disorders of the esophagus |
| 531.xx | Gastric ulcer |
| 531 | Gastric ulcer, acute with hemorrhage |
| 531.1 | Gastric ulcer, acute with perforation |
| 531.2 | Gastric ulcer, acute with hemorrhage and perforation |
| 531.3 | Gastric ulcer, acute without hemorrhage or perforation |
| 531.4 | Gastric ulcer, chronic or unspecified with hemorrhage |
| 531.5 | Gastric ulcer, chronic or unspecified with perforation |
| 531.6 | Gastric ulcer, chronic or unspecified with hemorrhage and perforation |
| 531.7 | Gastric ulcer, chronic without hemorrhage or perforation |
| 531.9 | Gastric ulcer, unspecified as acute or chronic, without hemorrhage or perforation |
| 532.xx | Duodenal ulcer |
| 532 | Duodenal ulcer, acute with perforation |
| 532.1 | Duodenal ulcer, acute with hemorrhage |
| 532.2 | Duodenal ulcer, acute with hemorrhage and perforation |
| 532.3 | Duodenal ulcer, acute without hemorrhage or perforation |
| 532.4 | Duodenal ulcer, chronic or unspecified with hemorrhage |
| 532.5 | Duodenal ulcer, chronic or unspecified with perforation |
| 532.6 | Duodenal ulcer, chronic or unspecified with hemorrhage and perforation |
| 532.7 | Duodenal ulcer, chronic without hemorrhage or perforation |
| 532.9 | Duodenal ulcer, unspecified as acute or chronic, without hemorrhage or perforation |
| 533.xx | Peptic ulcer, site NOS |
| 533 | Peptic ulcer, acute with hemorrhage |
| 533.1 | Peptic ulcer, acute with perforation |
| 533.2 | Peptic ulcer, acute with perforation and hemorrhage |
| 533.3 | Peptic ulcer, acute without hemorrhage or perforation |
| 533.4 | Peptic ulcer, chronic or unspecified with hemorrhage |
| 533.5 | Peptic ulcer, chronic or unspecified with perforation |
| 533.6 | Peptic ulcer, chronic or unspecified with hemorrhage and perforation |
| 533.7 | Peptic ulcer, chronic without hemorrhage or perforation |
| 533.9 | Peptic ulcer, unspecified as acute or chronic, without hemorrhage or perforation |
| 534 | Gastrojejunal ulcer |
| 534.1 | Gastrojejunal ulcer |
| 534.2 | Gastrojejunal ulcer |
| 534.3 | Gastrojejunal ulcer |
| 535.0x | Acute gastritis |
| 535.11 | Atrophic gastritis with hemorrhage |
| 535.21 | Gastric mucosal hypertrophy with hemorrhage |
| 535.4x | Gastritis NEC |
| 535.5x | Gastritis/duodenitis NOS |
| 535.6x | Duodenitis |
| 536.2 | Persistent vomiting |
| 536.8 | Dyspepsia and other specified disorders of function of stomach |
| 536.9 | Stomach function disorders NOS |
| 537.4 | Gastric/duodenal fistula |
| 537.8x | Gastroduodenal disorders NEC |
| 537.9 | Gastroduodenal disorders NOS |
| 569.83 | Perforation of intestine |
| 578.xx | GI hemorrhage |
| 787.0x | Nausea and vomiting |
| 787.1 | Heartburn |
| 787.2 | Dysphagia |
| 789.0x | Abdominal pain |
| 792.1 | Abnormal stool/occult blood |
| 793.4 | Abnormal exam GI tract |
| CPT-4 code | |
| 43200 | Endoscopy, rigid or flexible; diagnostic, with or without collection of specimen(s) by brushing or washing (separate procedure) |
| 43202 | Esophagoscopy, rigid or flexible; diagnostic, with or without collection of specimen(s) by brushing or washing (separate procedure) with biopsy, single or multiple |
| 43227 | Esophagoscopy, rigid or flexible; diagnostic, with or without collection of specimen(s) by brushing or washing (separate procedure) with control of bleeding, any method |
| 43235 | Endoscopy, rigid or flexible; diagnostic, with or without collection of specimen(s) by brushing or washing (separate procedure) |
| 43239 | Upper GI endoscopy including esophagus, stomach, and either the duodenum and/or jejunum as appropriate; diagnostic with or without collection of specimen(s) by brushing or washing (separate procedure) with biopsy, single or multiple |
| 43255 | Upper GI endoscopy including esophagus, stomach, and either the duodenum and/or jejunum as appropriate; diagnostic with or without collection of specimen(s) by brushing or washing (separate procedure), with control of bleeding, any method |
| 44602 | Suture of small intestine (enterorrhaphy) for perforated ulcer |
| 44603 | Suture of small intestine (enterorrhaphy) for perforated ulcer |
| 44605 | Suture of large intestine (colorrhaphy) for perforated ulcer |
| 74240 | Radiologic examination, GI tract, upper; with or without delayed film, without KUB |
| 74241 | Radiologic examination, GI tract, upper; with or without delayed films, with KUB |
| 74245 | Radiologic examination, GI tract, upper; with small bowel, includes multiple serial films |
| 74246 | Radiologic examination, GI tract, upper, air contrast, with specific high-density barium, effervescent agent, with or without glycagon; with or without delayed films, with KUB |
| 74247 | Radiologic examination, GI tract, upper, air contrast, with specific high-density barium, effervescent agent, with or without glycagon; with or without delayed films, without KUB |
| 74249 | Radiologic examination, GI tract, upper, air contrast, with specific high-density barium, effervescent agent, with or without glycagon; with small-bowel follow-through |
Abbreviations: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; GERD, gastroesophageal reflux disease; NOS, not otherwise specified; NEC, not elsewhere classifiable; GI, gastrointestinal; CPT, Current Procedural Terminology; KUB, kidney, ureter, and bladder X-ray.
Acknowledgments
This study was funded by Merck & Co., Inc. Medical writing support was provided by Optum (Eden Prairie, MN) and funded by Merck & Co., Inc.
Footnotes
Disclosure
Ethel S Siris has done consulting for Amgen, Eli Lilly, Merck, Novartis, AgNovos and Radius. Katalin Bognar and Anshu Shrestha are employees of Precision Health Economics, LLC which provides consulting services to Merck. Mitch DeKoven is an employee of IMS Health which provides consulting services to Merck. John A Romley provided consulting services to Precision Health Economics in support of this study. Ankita Modi is an employee of Merck & Co., Inc. Jingbo Yu was an employee of Merck & Co., Inc. at the time of the study and during manuscript development.
References
- 1.Wright NC, Looker AC, Saag KG, et al. The Recent Prevalence of Osteoporosis and Low Bone Mass in the United States Based on Bone Mineral Density at the Femoral Neck or Lumbar Spine. J Bone Miner Res. 2014;29(11):2520–2526. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ettinger B, Black DM, Dawson-Hughes B, Pressman AR, Melton LJ., 3rd Updated fracture incidence rates for the US version of FRAX. Osteoporos Int. 2010;21(1):25–33. doi: 10.1007/s00198-009-1032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfeilschifter J, Cooper C, Watts NB, et al. Regional and age-related variations in the proportions of hip fractures and major fractures among postmenopausal women: the Global Longitudinal Study of Osteoporosis in Women. Osteoporos Int. 2012;23(8):2179–2188. doi: 10.1007/s00198-011-1840-6. [DOI] [PubMed] [Google Scholar]
- 4.Blume SW, Curtis JR. Medical costs of osteoporosis in the elderly Medicare population. Osteoporos Int. 2011;22(6):1835–1844. doi: 10.1007/s00198-010-1419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roux C, Wyman A, Hooven FH, et al. GLOW investigators Burden of non-hip, non-vertebral fractures on quality of life in postmenopausal women: the Global Longitudinal study of Osteoporosis in Women (GLOW) Osteoporos Int. 2012;23(12):2863–2871. doi: 10.1007/s00198-012-1935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watts NB, Bilezikian JP, Camacho PM, et al. AACE Osteoporosis Task Force American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16(Suppl 3):1–37. doi: 10.4158/ep.16.s3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Osteoporosis Foundation . The Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2014. [Accessed January 12, 2015]. Available from: http://nof.org/files/nof/public/content/file/2791/upload/919.pdf. [Google Scholar]
- 8.Dawson-Hughes B, Looker AC, Tosteson AN, Johansson H, Kanis JA, Melton LJ. The potential impact of the National Osteoporosis Foundation guidance on treatment eligibility in the USA: an update in NHANES 2005–2008. Osteoporos Int. 2012;23(3):811–820. doi: 10.1007/s00198-011-1694-y. [DOI] [PubMed] [Google Scholar]
- 9.Andrade SE, Majumdar SR, Chan KA, et al. Low frequency of treatment of osteoporosis among postmenopausal women following a fracture. Arch Intern Med. 2003;163(17):2052–2057. doi: 10.1001/archinte.163.17.2052. [DOI] [PubMed] [Google Scholar]
- 10.Gleason LJ, Menzies IB, Mendelson DA, Kates SL, Friedman SM. Diagnosis and treatment of osteoporosis in high-risk patients prior to hip fracture. Geriatr Orthop Surg Rehabil. 2012;3(2):79–83. doi: 10.1177/2151458512454878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenspan SL, Wyman A, Hooven FH, et al. Predictors of treatment with osteoporosis medications after recent fragility fractures in a multinational cohort of postmenopausal women. J Am Geriatr Soc. 2012;60(3):455–461. doi: 10.1111/j.1532-5415.2011.03854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guggina P, Flahive J, Hooven FH, et al. GLOW Investigators Characteristics associated with anti-osteoporosis medication use: data from the Global Longitudinal Study of Osteoporosis in Women (GLOW) USA cohort. Bone. 2012;51(6):975–980. doi: 10.1016/j.bone.2012.08.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrella RJ, Jones TJ. Do patients receive recommended treatment of osteoporosis following hip fracture in primary care? BMC Fam Pract. 2006;7:31. doi: 10.1186/1471-2296-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Premaor MO, Pilbrow L, Tonkin C, Adams M, Parker RA, Compston J. Low rates of treatment in postmenopausal women with a history of low trauma fractures: results of audit in a Fracture Liaison Service. QJM. 2010;103(1):33–40. doi: 10.1093/qjmed/hcp154. [DOI] [PubMed] [Google Scholar]
- 15.Simonelli C, Chen YT, Morancey J, Lewis AF, Abbott TA. Evaluation and management of osteoporosis following hospitalization for low-impact fracture. J Gen Intern Med. 2003;18(1):17–22. doi: 10.1046/j.1525-1497.2003.20387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh S, Foster R, Khan KM. Accident or osteoporosis?: Survey of community follow-up after low-trauma fracture. Can Fam Physician. 2011;57(4):e128–e133. [PMC free article] [PubMed] [Google Scholar]
- 17.Teede HJ, Jayasuriya IA, Gilfillan CP. Fracture prevention strategies in patients presenting to Australian hospitals with minimal-trauma fractures: a major treatment gap. Intern Med J. 2007;37(10):674–679. doi: 10.1111/j.1445-5994.2007.01503.x. [DOI] [PubMed] [Google Scholar]
- 18.Kroth PJ, Murray MD, McDonald CJ. Undertreatment of osteoporosis in women, based on detection of vertebral compression fractures on chest radiography. Am J Geriatr Pharmacother. 2004;2(2):112–118. doi: 10.1016/s1543-5946(04)90016-5. [DOI] [PubMed] [Google Scholar]
- 19.Lüthje P, Nurmi-Lüthje I, Kaukonen JP, Kuurne S, Naboulsi H, Kataja M. Undertreatment of osteoporosis following hip fracture in the elderly. Arch Gerontol Geriatr. 2009;49(1):153–157. doi: 10.1016/j.archger.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Panneman MJ, Lips P, Sen SS, Herings RM. Undertreatment with anti-osteoporotic drugs after hospitalization for fracture. Osteoporos Int. 2004;15(2):120–124. doi: 10.1007/s00198-003-1544-7. [DOI] [PubMed] [Google Scholar]
- 21.National Osteoporosis Foundation [webpage on the Internet] Types of osteoporosis medications. Washington, DC: National Osteoporosis Foundation; 2014. [Accessed July 21,2014]. Available from: http://nof.org/articles/22. [Google Scholar]
- 22.Ettinger B. Alendronate use among 812 women: prevalence of gastrointestinal complaints, noncompliance with patient instructions, and discontinuation. J Manage Care Spec Pharm. 1998;4(5):488–492. [Google Scholar]
- 23.Hamilton B, McCoy K, Taggart H. Tolerability and compliance with risedronate in clinical practice. Osteoporos Int. 2003;14(3):259–262. doi: 10.1007/s00198-002-1370-3. [DOI] [PubMed] [Google Scholar]
- 24.Turbí C, Herrero-Beaumont G, Acebes JC, et al. Compliance and satisfaction with raloxifene versus alendronate for the treatment of postmenopausal osteoporosis in clinical practice: An open-label, prospective, nonrandomized, observational study. Clin Ther. 2004;26(2):245–256. doi: 10.1016/s0149-2918(04)90023-9. [DOI] [PubMed] [Google Scholar]
- 25.Hall KE, Proctor DD, Fisher L, Rose S. American gastroenterological association future trends committee report: effects of aging of the population on gastroenterology practice, education, and research. Gastroenterology. 2005;129(4):1305–1338. doi: 10.1053/j.gastro.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Knopp-Sihota JA, Cummings GG, Homik J, Voaklander D. The association between serious upper gastrointestinal bleeding and incident bisphosphonate use: a population-based nested cohort study. BMC Geriatr. 2013;13:36. doi: 10.1186/1471-2318-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vestergaard P, Schwartz K, Pinholt EM, Rejnmark L, Mosekilde L. Gastric and esophagus events before and during treatment of osteoporosis. Calcif Tissue Int. 2010;86(2):110–115. doi: 10.1007/s00223-009-9323-x. [DOI] [PubMed] [Google Scholar]
- 28.Daniels AH, Daiello LA, Lareau CR, et al. Preoperative cognitive impairment and psychological distress in hospitalized elderly hip fracture patients. Am J Orthop (Belle Mead NJ) 2014;43(7):E146–E152. [PubMed] [Google Scholar]
- 29.Switzer JA, Jaglal S, Bogoch ER. Overcoming barriers to osteoporosis care in vulnerable elderly patients with hip fractures. J Orthop Trauma. 2009;23(6):454–459. doi: 10.1097/BOT.0b013e31815e92d2. [DOI] [PubMed] [Google Scholar]
- 30.Simonelli C, Killeen K, Mehle S, Swanson L. Barriers to osteoporosis identification and treatment among primary care physicians and orthopedic surgeons. Mayo Clin Proc. 2002;77(4):334–338. doi: 10.4065/77.4.334. [DOI] [PubMed] [Google Scholar]
- 31.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 32.Asche C, Nelson R, McAdam-Marx C, Jhaveri M, Ye X. Predictors of oral bisphosphonate prescriptions in post-menopausal women with osteoporosis in a real-world setting in the USA. Osteoporos Int. 2010;21(8):1427–1436. doi: 10.1007/s00198-009-1079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meadows ES, Mitchell BD, Bolge SC, Johnston JA, Col NF. Factors associated with treatment of women with osteoporosis or osteopenia from a national survey. BMC Womens Health. 2012;12:1. doi: 10.1186/1472-6874-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Block AE, Solomon DH, Cadarette SM, Mogun H, Choudhry NK. Patient and physician predictors of post-fracture osteoporosis management. J Gen Intern Med. 2008;23(9):1447–1451. doi: 10.1007/s11606-008-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster SA, Foley KA, Meadows ES, et al. Characteristics of patients initiating raloxifene compared to those initiating bisphosphonates. BMC Womens Health. 2008;8:24. doi: 10.1186/1472-6874-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cranney A, Tsang JF, Leslie WD. Factors predicting osteoporosis treatment initiation in a regionally based cohort. Osteoporos Int. 2009;20(9):1621–1625. doi: 10.1007/s00198-008-0823-8. [DOI] [PubMed] [Google Scholar]
- 37.Pressman A, Forsyth B, Ettinger B, Tosteson AN. Initiation of osteoporosis treatment after bone mineral density testing. Osteoporos Int. 2001;12(5):337–342. doi: 10.1007/s001980170099. [DOI] [PubMed] [Google Scholar]
- 38.Ryder KM, Shorr RI, Tylavsky FA, et al. Health ABC Study Correlates of use of antifracture therapy in older women with low bone mineral density. J Gen Intern Med. 2006;21(6):636–641. doi: 10.1111/j.1525-1497.2006.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Codes for the identification of gastrointestinal events
| Description | |
|---|---|
| ICD-9-CM code | |
| 456.0 | Esophageal varices with hemorrhage |
| 456.1 | Esophageal varices without hemorrhage |
| 530.0x | Achalasia and cardiospasm |
| 530.1x | Esophagitis |
| 530.2 | Ulcer of esophagus |
| 530.2 | Ulcer of esophagus without bleeding |
| 530.21 | Ulcer of esophagus with bleeding |
| 530.3 | Stricture of esophagus |
| 530.4 | Perforation of esophagus |
| 530.5 | Dyskinesia of esophagus |
| 530.7 | Mallory–Weiss syndrome |
| 530.81 | Esophageal reflux (GERD) |
| 530.82 | Esophageal hemorrhage |
| 530.84 | Tracheoesophageal fistula |
| 530.89 | Other disorders of the esophagus |
| 531.xx | Gastric ulcer |
| 531 | Gastric ulcer, acute with hemorrhage |
| 531.1 | Gastric ulcer, acute with perforation |
| 531.2 | Gastric ulcer, acute with hemorrhage and perforation |
| 531.3 | Gastric ulcer, acute without hemorrhage or perforation |
| 531.4 | Gastric ulcer, chronic or unspecified with hemorrhage |
| 531.5 | Gastric ulcer, chronic or unspecified with perforation |
| 531.6 | Gastric ulcer, chronic or unspecified with hemorrhage and perforation |
| 531.7 | Gastric ulcer, chronic without hemorrhage or perforation |
| 531.9 | Gastric ulcer, unspecified as acute or chronic, without hemorrhage or perforation |
| 532.xx | Duodenal ulcer |
| 532 | Duodenal ulcer, acute with perforation |
| 532.1 | Duodenal ulcer, acute with hemorrhage |
| 532.2 | Duodenal ulcer, acute with hemorrhage and perforation |
| 532.3 | Duodenal ulcer, acute without hemorrhage or perforation |
| 532.4 | Duodenal ulcer, chronic or unspecified with hemorrhage |
| 532.5 | Duodenal ulcer, chronic or unspecified with perforation |
| 532.6 | Duodenal ulcer, chronic or unspecified with hemorrhage and perforation |
| 532.7 | Duodenal ulcer, chronic without hemorrhage or perforation |
| 532.9 | Duodenal ulcer, unspecified as acute or chronic, without hemorrhage or perforation |
| 533.xx | Peptic ulcer, site NOS |
| 533 | Peptic ulcer, acute with hemorrhage |
| 533.1 | Peptic ulcer, acute with perforation |
| 533.2 | Peptic ulcer, acute with perforation and hemorrhage |
| 533.3 | Peptic ulcer, acute without hemorrhage or perforation |
| 533.4 | Peptic ulcer, chronic or unspecified with hemorrhage |
| 533.5 | Peptic ulcer, chronic or unspecified with perforation |
| 533.6 | Peptic ulcer, chronic or unspecified with hemorrhage and perforation |
| 533.7 | Peptic ulcer, chronic without hemorrhage or perforation |
| 533.9 | Peptic ulcer, unspecified as acute or chronic, without hemorrhage or perforation |
| 534 | Gastrojejunal ulcer |
| 534.1 | Gastrojejunal ulcer |
| 534.2 | Gastrojejunal ulcer |
| 534.3 | Gastrojejunal ulcer |
| 535.0x | Acute gastritis |
| 535.11 | Atrophic gastritis with hemorrhage |
| 535.21 | Gastric mucosal hypertrophy with hemorrhage |
| 535.4x | Gastritis NEC |
| 535.5x | Gastritis/duodenitis NOS |
| 535.6x | Duodenitis |
| 536.2 | Persistent vomiting |
| 536.8 | Dyspepsia and other specified disorders of function of stomach |
| 536.9 | Stomach function disorders NOS |
| 537.4 | Gastric/duodenal fistula |
| 537.8x | Gastroduodenal disorders NEC |
| 537.9 | Gastroduodenal disorders NOS |
| 569.83 | Perforation of intestine |
| 578.xx | GI hemorrhage |
| 787.0x | Nausea and vomiting |
| 787.1 | Heartburn |
| 787.2 | Dysphagia |
| 789.0x | Abdominal pain |
| 792.1 | Abnormal stool/occult blood |
| 793.4 | Abnormal exam GI tract |
| CPT-4 code | |
| 43200 | Endoscopy, rigid or flexible; diagnostic, with or without collection of specimen(s) by brushing or washing (separate procedure) |
| 43202 | Esophagoscopy, rigid or flexible; diagnostic, with or without collection of specimen(s) by brushing or washing (separate procedure) with biopsy, single or multiple |
| 43227 | Esophagoscopy, rigid or flexible; diagnostic, with or without collection of specimen(s) by brushing or washing (separate procedure) with control of bleeding, any method |
| 43235 | Endoscopy, rigid or flexible; diagnostic, with or without collection of specimen(s) by brushing or washing (separate procedure) |
| 43239 | Upper GI endoscopy including esophagus, stomach, and either the duodenum and/or jejunum as appropriate; diagnostic with or without collection of specimen(s) by brushing or washing (separate procedure) with biopsy, single or multiple |
| 43255 | Upper GI endoscopy including esophagus, stomach, and either the duodenum and/or jejunum as appropriate; diagnostic with or without collection of specimen(s) by brushing or washing (separate procedure), with control of bleeding, any method |
| 44602 | Suture of small intestine (enterorrhaphy) for perforated ulcer |
| 44603 | Suture of small intestine (enterorrhaphy) for perforated ulcer |
| 44605 | Suture of large intestine (colorrhaphy) for perforated ulcer |
| 74240 | Radiologic examination, GI tract, upper; with or without delayed film, without KUB |
| 74241 | Radiologic examination, GI tract, upper; with or without delayed films, with KUB |
| 74245 | Radiologic examination, GI tract, upper; with small bowel, includes multiple serial films |
| 74246 | Radiologic examination, GI tract, upper, air contrast, with specific high-density barium, effervescent agent, with or without glycagon; with or without delayed films, with KUB |
| 74247 | Radiologic examination, GI tract, upper, air contrast, with specific high-density barium, effervescent agent, with or without glycagon; with or without delayed films, without KUB |
| 74249 | Radiologic examination, GI tract, upper, air contrast, with specific high-density barium, effervescent agent, with or without glycagon; with small-bowel follow-through |
Abbreviations: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; GERD, gastroesophageal reflux disease; NOS, not otherwise specified; NEC, not elsewhere classifiable; GI, gastrointestinal; CPT, Current Procedural Terminology; KUB, kidney, ureter, and bladder X-ray.