Abstract
Purpose of developing the guidelines: The first guidelines for diagnosis and treatment of 21-hydroxylase deficiency (21-OHD) were published as a diagnostic handbook in Japan in 1989, with a focus on patients with severe disease. The “Guidelines for Treatment of Congenital Adrenal Hyperplasia (21-Hydroxylase Deficiency) Found in Neonatal Mass Screening (1999 revision)” published in 1999 were revised to include 21-OHD patients with very mild or no clinical symptoms. Accumulation of cases and experience has subsequently improved diagnosis and treatment of the disease. Based on these findings, the Mass Screening Committee of the Japanese Society for Pediatric Endocrinology further revised the guidelines for diagnosis and treatment. Target disease/conditions: 21-hydroxylase deficiency. Users of the guidelines: Physician specialists in pediatric endocrinology, pediatric specialists, referring pediatric practitioners, general physicians; and patients.
Keywords: 21-hydroxylase deficiency, mass screening, guideline
Introduction
Mass screening for 21-hydroxylase deficiency (21-OHD) started in Japan in January 1989, and one per 18,000 to 19,000 infants are found to have 21-OHD (1,2,3). Many patients with 21-OHD have skin pigmentation, virilization of the external genitalia (females), poor suckling and poor weight gain, but others have 21-OHD with only very mild clinical symptoms. 21-OHD requires continuous treatment and it is recommended that specialized medical facilities make a differential diagnosis and treat subjects found to be positive in mass screening to avoid unnecessary treatment. The first guidelines for diagnosis and treatment of 21-OHD were published as a diagnostic handbook in Japan in 1989, and were developed for severe patients (4, 5). The “Guidelines for Treatment of Congenital Adrenal Hyperplasia (21-Hydroxylase Deficiency) Found in Neonatal Mass Screening (1999 revision)” published in 1999 were revised to include 21-OHD patients with very mild or no clinical symptoms (6, 7). However, problems in endocrine tests, new specialized endocrine tests, and new diseases to be differentiated have arisen. Furthermore, long-term courses of 21-OHD patients have been reported, and problems in the adult stage have emerged. The Lawson Wilkins Pediatric Endocrine Society (the present Pediatric Endocrine Society) and the European Society for Paediatric Endocrinology proposed a consensus statement in 2002 (8), and the Endocrine Society (USA) also published new clinical practice guidelines in 2010 (9). Therefore, we revised the previous diagnosis and treatment guidelines and developed the current guidelines (hereinafter referred to as the Guidelines) based on newly emerging information.
A “grade” and “evidence level” are given for each recommendation in the guidelines. The grade indicates the recommendation level based on published studies, and the evidence level reflects the level of the study used as a rationale. Expert opinions are used in the Guidelines if there no appropriate findings in published articles. Surgery for external genitalia; and complications and prognosis are not described in the Guidelines. However, other guidelines are under development in cooperation with the Sex Differentiation Committee of the Japanese Society for Pediatric Endocrinology.
Grade level
-
1.
Major recommendation: Most patients receive benefits.
-
2.
Minor recommendation: Many patients receive benefits. Requires consideration and selection based on the patient’s conditions.
Evidence level
●○○ Low: Evaluation of case reports without controls
●●○ Medium: Cohort study without controls
●●● Cohort study with controls, nonrandomized comparative study
Consensus: Widely recognized ideas, even if a study has not been performed
Pathophysiology of 21-OHD
Congenital adrenal hyperplasia (CAH) refers to a range of autosomal recessive diseases resulting from deficiency of cortisol secretion. The incidence is 1/10,000–20,000 globally and in Japan (1, 2, 9). 21-OHD is the most frequent disease among CAH cases, and develops due to mutation or deficit of the CTYP21A2 gene, which encodes the steroid 21-hydroxylase (p450c21) (9,10,11). This enzyme converts 17-hydroxyprogesterone (17-OHP) into 11-deoxycortisol, and progesterone into 11-deoxycorticosterone, which are then converted into cortisol and aldosterone, respectively. Therefore, production of cortisol and aldosterone is disturbed in patients with severe 21-OHD.
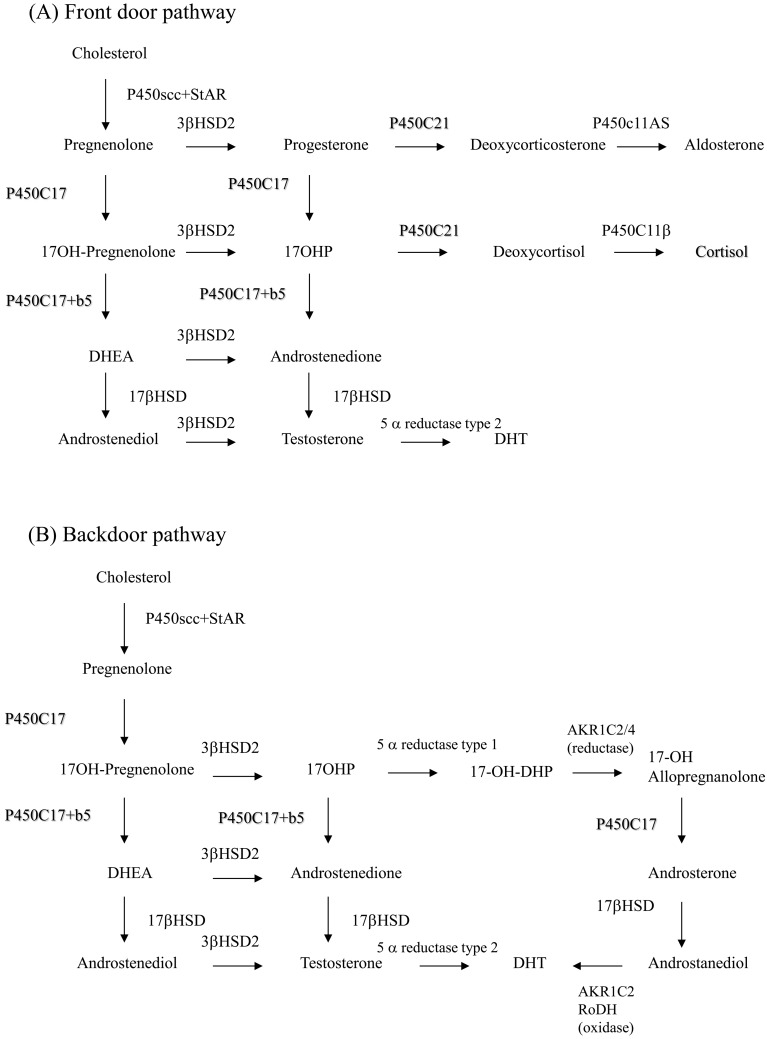
Disturbance of cortisol production causes accumulation of precursors of cortisol by stimulation of ACTH, and these precursors lead to a pathway for adrenal androgen (Fig. 1). Virilization of the external genitalia is one of the most important symptoms in female neonates with salt-wasting (SW) or simple virilizing (SV) disease. Rapid accelerated growth is stimulated, and sexual precocity develops in both male and female patients who are not treated after the neonatal stage; and serious SW may be fatal. Approximately 75% of 21-OHD cases have the serious SW type (1, 9, 10). In addition to the classical SW and SV types, a nonclassical (NC) type is also found (12,13,14,15). NC patients develop excessive adrenal androgen after birth; however, the severity differs, and there are asymptomatic patients. Steroid synthesis pathways are shown in Fig. 1. Backdoor pathways have been implicated in virilization of the external genitalia of girls with 21-OHD (refer to the Fig. 1 legend) (16,17,18).
Fig. 1.
(A) Front door pathway. Steroid production in the normal fetus. 3β-HSD activity is low in the fetal adrenal gland, and the most produced adrenal steroid is DHEA (and DHEA-S). However, small amounts of steroids go into the aldosterone and cortisol pathways. 21-hydroxylase in the adrenal gland is required for both pathways. The adrenal gland can produce small amounts of testosterone through the activity of 17β-HSD. (B) Backdoor pathway. If 21-hydroxylase (P450c21) is deficient, three pathways produce androgen. The first is a pathway from cholesterol to DHEA. Large amounts of DHEA are converted to DHEA-S and inactivated, while some DHEA is converted to testosterone and dihydrotestosterone (DHT). In the second pathway of the normal adrenal gland, a tiny amount of 17-OHP is converted to androstenedione, small amounts of 17-OHP produced in CAH are converted to androstenedione, and testosterone is produced. The third pathway is a backdoor pathway in which 17-OHP undergoes by 5α- and 3β-reduction and is converted to 17-OH allopregnanolone. This steroid is converted to androstanediol, oxidized by 3α-HSD oxidoreductase and converted to DHT. This pathway was first found in Marsupialia and mammals and is also present in humans based on urine steroid analysis using mass spectrometry. This pathway is thought to be involved in virilization of the external genitalia in girls with 21-OHD.
21-OHD results from genetic abnormalities of CYP21A2, and the disease severity is likely to correlate with the CYP21A2 genotype (9, 19, 20). The 21-OHD genotype requires careful determination because genetic results can be complicated due to duplication, deletion and recombination of CYP21A2 in the 6q21.3 region. More than 100 CYP21A2 gene mutations have been found, and mutation of intron 2 (mutation from C/A to G between the splice acceptor site and –13 bp upstream) causing a deletion and splicing variant is found in one allele in about 50% of SW patients. An Ile172Asn mutation in exon 4 giving an enzyme with 1–2% activity is common in SV patients. A Val281Leu mutation in exon 7 giving an enzyme with 20–50% activity is found in an allele in about 70% of Caucasian NC patients (12, 13), and a Pro30Leu mutation is common in Japanese NC patients (14). Many patients have heterozygosity of at least two mutations and variable remaining enzyme activity; thus, the phenotype can be diverse, and the CYP21A2 genotype (extent of loss of enzyme activity) does not always correspond to the phenotype (13, 21).
1. Neonatal Mass Screening
Recommendation
-
1.
We recommend that 21-OHD mass screening be conducted as part of a series of neonatal mass screenings. 1 (Consensus)
-
2.
We recommend that an immunological measurement (e.g., ELISA) be used for 21-OHD mass screening, with direct determination in the first tier test and use of an extraction procedure in the second tier test. To decrease the false-positive rate and increase the positive predictive value, steroid profiles should be measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) in the second tier test. 1 (●○○)
-
3.
We recommend that to provide rapid and appropriate treatment for neonates found to be positive in mass screening, prefectural and major city governments that conduct mass screening establish procedures for screening inborn errors of metabolism and develop a practical treatment protocol. 1 (Consensus)
Explanation
1-1. Outcomes of neonatal mass screening
Neonatal mass screening of 21-OHD started in 1989 in Japan. The incidence of 21-OHD before mass screening was conducted was estimated to be 1/43,674, based on the patient survey by Suwa et al. (22). However, in mass screening from 1981 to the end of 1987 in about 500,000 subjects in Sapporo, Tokyo (partial), Kanagawa and Shizuoka (western region), 16 SW, 7 SV and 2 unknown type cases (25 in total) were found, indicating a rate of 1/20,570 (23). Suwa et al. reported the findings of a survey in 51 testing institutions nationwide (1, 3). Of 4,085,448 mass screening cases over 10 yr from April 1982 to March 1992, 271 were found to have 21-OHD, i.e., an incidence of 1/18,827. In this survey, there were more SW cases than SV cases regardless of sex, and the male-female ratio was 1:1 for both types. Other studies show that the incidence of clinical diagnosis before mass screening is higher in girls, but that SW 21-OHD has a similar incidence in males and females (24, 25). Therefore, males with SV21-OHD seem to have been missed prior to mass screening, while patients with severe SW 21-OHD were incorrectly diagnosed as cases of idiopathic sudden death.
In a collaborative study in 15 medical institutions from 1990 to 1995, 70 patients who were identified after the start of mass screening were followed up (26). For 49 patients identified in mass screening and 21 who visited a hospital due to symptoms, the mean ages at the first visit were 17.6 and 7.4 d, respectively. Many patients (about 30%) who visited the hospital before receiving mass screening results were girls with a chief complaint of external genitalia anomaly. Clitoromegaly was found in all girls, but half of these patients did not visit a hospital until the results of mass screening were obtained.
The results of mass screening show a national incidence of 21-OHD of 1/19,000–20,000, which is similar to those in other countries (27,28,29,30). For neonatal mass screening performed by maternal and child health services of prefectural and major city governments, the cost-benefit ratio is also important. In Japan, the cost includes expenses for mass screening tests and treatment and management of patients who are found to have 21-OHD, but the benefit is the reduced expenses of facilities, child rearing and special education facilitated by early diagnosis (31). The net benefit of screening for congenital hypothyroidism is the highest among diseases (3.1 billion Yen), and the net benefit of 21-OHD screening is 0.2 billion Yen.
The major problem of 21-OHD mass screening is the high false-positive rate [low positive predictive value (PPV)] (27, 32,33,34,35,36,37,38,39,40). In the prescribed cost-benefit analysis, the costs of testing false-positive subjects for diagnosis and nursing costs of in-patients are not considered. Parents also have psychological anxiety that their child has a lifelong, life-threatening chronic disease (41). Improvement of this problem may require two-step mass screening in which the second tier test has a high PPV in ELISA-positive subjects in the first tier test (9, 42,43,44,45).
1-2. Current status of mass screening
In December 2010, 17-hydroxyprogesterone (17-OHP) was determined in 45 test facilities in Japan using ELISA kits produced by two Japanese manufacturers. In the first tier test, all facilities used a direct method. In the second tier test, only two used the direct method, and one of the two used kits produced by the two manufacturers. Extraction was used in 42 testing facilities, and 3 set a cutoff value in combination with the direct method. The remaining test facility used high performance liquid chromatography (HPLC) (46).
The cutoff value for 17-OHP is not standardized in Japan because local governments that implement mass screening, consultant physicians and liaison councils have different purposes for mass screening (34,35,36,37, 46, 47). The contact procedures for positive subjects and detailed examinations depend on the region, and particularly on the pediatric endocrinologists in each region. Therefore, local governments should establish procedures for mass screening that permit neonates who are positive in mass screening to be appropriately treated in any region. If a neonate has an extremely high 17-OHP level or clinical symptoms of adrenal insufficiency, the neonate should be seen by a pediatric endocrinologist as soon as possible.
Premature and low birth weight infants are likely to give false positive 17-OHP findings. This is because abundant steroids are secreted from the adrenal gland of neonates and cross-react with antibodies to 17-OHP; and because 17-OHP secretion is enhanced by stress in premature and low birth weight infants (34,35,36,37,38,39,40,41,42,43,44,45,46,47). Therefore, in Europe and the United States, cutoff values by birth weight or gestational week have been established, and this has decreased the rate of recall and improved the PPV (48,49,50,51). Cutoff values for low birth weight infants based on modified gestational weeks have also reduced the rates of recall and detailed examinations in Japan (36). Cutoff values by birth weight and gestational week determined in Tokyo and Niigata also reduced false-positive rates (30, 34). However, test facilities for mass screening do not always use this approach (46).
Standard procedures for low birth weight infants include blood sampling at 30 d after birth, at discharge; or when the body weight reaches 2,500 g; based on guidelines on secondary blood sampling in low birth weight infants (52). It is difficult to determine if 21-OHD is present in low birth weight infants with a high 17-OHP level in blood sampling 4 to 6 d after birth. Therefore, it is important for the physician in charge of the results of the first test to be informed about the possibility of CAH and to request careful follow-up of clinical symptoms (46, 52). Many infants with a birth weight ≤ 1,500 g are cared for in a neonatal intensive care unit (NICU) for a long period, and continuous blood sampling and tests may be requested by the physician in charge, although the guidelines for the second blood sampling are used as a general rule. It is also important to perform a detailed examination immediately after this is determined to be necessary (46, 52).
Steroid hormones can be accurately determined by LC-MS/MS, and many studies worldwide have shown decreased rates of recall and an increased PPV using LC-MS/MS as a second tier test after mass screening (42,43,44). However, a study in the US showed that false-positive rates increased when using LC-MS/MS as a second tier test (53), suggesting the difficulty of setting suitable cutoff values. Fujikura et al. reported the results of secondary tests after mass screening in Japan; and suggested that setting of appropriate cutoff values is important because LS-MS/MS may also be used in Japan (45). CYP21A2 mutation can also be examined by DNA extraction from filter paper blood; this method is technically feasible (54, 55) and has been validated in Japan (56, 57). However, there are many difficulties with routine genetic testing, and no large-scale studies have evaluated the utility of this procedure as a secondary test after mass screening.
2. Diagnosis of 21-OHD
Recommendation
-
1.
We recommend that if the 17-OHP level is high in neonatal mass screening (beyond the reference value in detailed tests at a mass screening facility), a detailed examination should be conducted regardless of external genitalia anomaly, pigmentation and adrenal insufficiency symptoms. 1 (Consensus)
-
2.
We recommend that if the 17-OHP level is high in neonatal mass screening and does not return to normal at recall (beyond the upper limit in a mass screening facility), a detailed examination should be conducted regardless of external genitalia anomaly, pigmentation and adrenal insufficiency symptoms. 1 (Consensus)
-
3.
We recommend that 21-OHD be diagnosed with utmost care based on adrenal insufficiency symptoms including poor suckling, body weight loss and vomiting and that treatment start immediately after these symptoms and hyponatremia, hypokalemia and metabolic acidosis are found, even if endocrinological test results are not available. 1 (Consensus)
Explanation
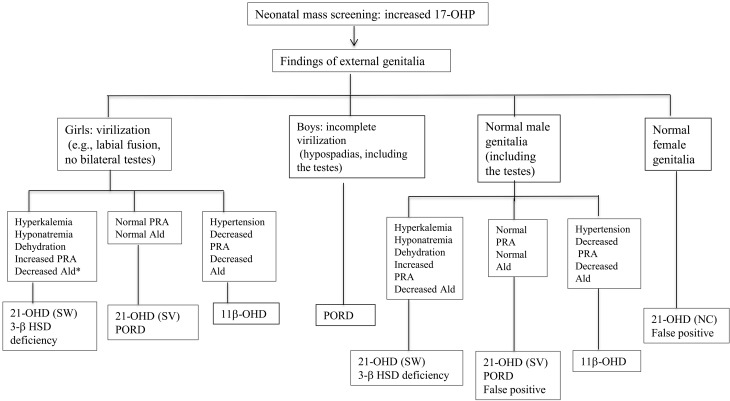
A summary of disease diagnosis (Table 1) and a flow chart (Fig. 2) are provided.
Table 1. Handbook for diagnosis of 21-hydroxylase deficiency in the neonatal stage.
Fig. 2.
Flow chart of diagnosis of individuals with increased 17-OHP levels detected by neonatal mass screening. *Ald, aldosterone. (Amano N, Hasegawa T. Diagnostic algorithm for adrenocortical insufficiency. Jpn J Pediatr Med 2012;44:588-92).
2-1. Clinical symptoms
If the 17-OHP level is extremely high, patients should be examined for hyperpigmentation, virilization of female external genitalia and signs of adrenal insufficiency including poor suckling and dehydration. Testes should not be palpable in virilizing girls with 21-OHD. Patients should also be examined for the presence or absence of a common urogenital sinus including the vaginal and urethral opening. Those with virilization of the external genitalia including clitoromegaly and labial fusion should undergo a detailed examination prior to mass screening, since such patients are suspected to have 21-OHD. Therefore, filter paper blood should be drawn for 17-OHP testing at a mass screening facility. About half of girls with 21-OHD undergo a detailed examination prior to mass screening due to an external genitalia anomaly (24, 25, 30, 58). Boys with normal external genitalia and a high 17-OHP level should be similarly examined for adrenal insufficiency and pigmentation to diagnose 21-OHD. It is difficult to evaluate increased penile length, a virilizing symptom, in boys with SW and SV 21-OHD (24, 25, 27, 30).
2-2. Biochemical and endocrine tests and imaging
In addition to the above examinations, biochemical and endocrine tests can be conducted. If virilization of the external genitalia in girls or under masculinization of the external genitalia in boys is suspected, karyotype analysis is required, in addition to differential diagnosis of other diseases. Blood 17-OHP is the most useful test item for diagnosis, and determination of serum or filter paper blood 17-OHP using approved kits is covered by insurance. Therefore, it is preferable to use kits at a test institution or mass screening facility. Repeated determination of the 17-OHP level is required. 21-Deoxycortisol (21-DOF) is an 11-hydroxyl derivative of 17-OHP without a 21-hydroxyl group and is useful for diagnosis (see note 3 in Table 1, not covered by insurance). Urine pregnanetriol (PT) is a direct urine metabolite of 17-OHP, and increased PT may also be helpful for diagnosis of 21-OHD. However, overlap with normal infants, transient hyper-17-hydroxyprogesteronemia, and increased 17-OHP in premature infants have been shown, which makes PT less useful for diagnosis (59).
Diagnosis can be confirmed using gas chromatography-mass spectrometry, in which urine steroid metabolites are determined by selective ion monitoring (urine steroid profile) (note 2 in Table 1). Homma et al. determined the urine pregnanetriolone (PTL)/Cr ratio in 59 patients with 21-OHD, 83 patients with transient hyper-17-hydroxyprogesteronemia; and 62 normal subjects, and found that 21-OHD was distinguishable in full-term and preterm infants (59, 60). An increased ratio of urine metabolites of androstenedione and pregnenolone, 11β-hydroxyandrosterone/pregnanediol, was also useful for diagnosis. This test is not covered by insurance; but is useful for diagnosis of 21-OHD and is described in the “Handbook of Diagnosis of 21-Hydroxylase Deficiency of the Study Group for Adrenal Hormone Production Abnormality” prepared by the Ministry of Health, Labour and Welfare Project on Intractable Disease (61).
Tests for diagnosis and understanding of the pathological condition include plasma ACTH, serum electrolytes, plasma glucose, plasma aldosterone, plasma renin activity or concentration, and blood gas (6, 9). Findings in these tests can identify SW and SV cases and improve diagnostic reliability. It has been suggested that all 21-OHD patients are unresponsive to mineralocorticoids (62) and mineralocorticoid deficiency in the neonatal stage (63). The flow chart in Fig. 2 shows the differentiation of SW and SV types, but the type is sometimes indistinguishable. If 21-OHD is suspected due to clinical symptoms and abnormal test results, and a manifestation of adrenal insufficiency is found, treatment must be the priority.
Imaging by ultrasonography can rapidly detect enlargement of adrenal glands, but experience is required to determine adrenal enlargement accurately. However, endocrine tests and karyotype analysis are time-consuming, and imaging findings can be used to suggest the presence of 21-OHD in 46,XX girls if the uterus is visualized in addition to the adrenal gland.
2-3. Genetic diagnosis
Genetic diagnosis is currently performed in commercial laboratories and not covered by insurance. A genetic abnormality can be detected in about 90% of 21-OHD patients (9), and analysis of parents improves the accuracy of diagnosis of 21-OHD. However, 21-OHD can be diagnosed based on the above clinical symptoms and test findings, and evaluation of the CYP21A2 gene is not always necessary. However, genetic analysis can assist diagnosis of patients without typical clinical symptoms, including girls without marked virilizing symptoms, boys with mild SV21-OHD, and NC patients. Genetic diagnosis also provides important information for genetic counseling. However, the higher rates of de novo mutation in comparison with other autosomal recessive diseases require attention (64,65,66). The structures of the CYP21A2 gene and its pseudogene CYP21A1P are also complicated, and deletion or point mutations may not be detected. Southern blotting and restriction fragment length polymorphism (RFLP) analysis are sometimes needed (67, 68). Also, as described above, the genotype does not always correspond to the phenotype.
2-4. Differential diagnosis
Other CAH conditions with high 17-OHP levels in mass screening include P450 oxidoreductase (POR) deficiency, 3β-hydroxysteroid dehydrogenase (3β HSD) deficiency, and 11β-hydroxylase deficiency (11β OHD). Virilization of the external genitalia develops in 46,XX girls with POR deficiency, while incomplete virilization in 46,XY boys with POR deficiency is frequently associated with early craniosynostosis, characteristic facies, humeral-radial synostosis, and joint contracture (69, 70). Electrolytes are normal in POR deficiency, and neonatal adrenal insufficiency is rarely found. POR deficiency and 21-OHD can be differentiated by analyzing urinary steroid profiles (60). 3β HSD deficiency causes virilization of the external genitalia in 46,XX girls and incomplete virilization in 46,XY boys, resulting in adrenal insufficiency, and may increase the 17-OHP level (71, 72). Serum steroid metabolites should be determined, and 3β HSD deficiency is diagnosable from the ratios of pregnenolone/progesterone, 17-OH pregnenolone/17-OHP, and dehydroepiandrosterone (DHEA)/androstenedione (11, 61). 11β OHD is characterized by hypertension, but some neonates do not have hypertension (11, 73, 74). In contrast to 21-OHD, 11β OHD is endocrinologically diagnosed by a decreased plasma renin concentration or activity, decreased level of plasma aldosterone and increased baseline and ACTH-stimulated levels of deoxycorticosterone and 11-deoxycortisol (61). Diagnosis of 3β HSD deficiency and 11β OHD by urinary steroid profile analysis, in contrast to 21-OHD and POR deficiency, should be made based on several test results until 3 to 4 mo after birth. Genetic diagnosis is also useful for differentiation of POR deficiency, 3β HSD deficiency and 11β OHD. These genetic tests are conducted by research institutions.
Cases of adrenal tumor due to high 17-OHP levels have also been reported (75, 76). As described above, false-positive results in mass screening are common in preterm and low birth weight infants, and the urinary steroid profile is useful for differentiation regardless of gestational week and days old (59, 60).
2-5. Diagnosis of NC and very mild SV types found in mass screening
The major purpose of mass screening is early detection of severe SW and SV 21-OHD. Very mild SV and NC types with unremarkable increased levels of androgen are also found in mass screening (69, 77,78,79,80,81). Ishii et al. estimated that the prevalence of the NC type was 1/2,000,000 (81). A total of 15 patients were identified in a national survey, including 11 found in mass screening, 3 diagnosed due to virilizing symptoms or accelerated growth, and 1 identified due to an affected sibling (80).
These diagnoses require confirmation based on a high 17-OHP level using various methods. If the baseline 17-OHP level is not high in blood sampling, the test should be repeated (6). For very mild SV and NC type patients, increased 17-OHP levels should be confirmed in an ACTH stimulation test. The NC type sometimes shows a normal 17-OHP level in random tests, and the baseline 17-OHP level should be determined before 8 a.m. (9, 82, 83). The ACTH stimulation test is then performed for differentiation from other CAHs. Data for 17-OHP before and after an ACTH stimulation test have been reported in overseas studies (82, 83). In a test that is not performed for diagnosis of 21-OHD alone, Cortrosyn®.
(ACTH 1-24) at 0.25 mg/m2 is intravenously administered for 90 min at intervals of 30 min. In addition to 17-OHP, cortisol, deoxycorticosterone, 11-deoxycortisol, 17-OH pregnenolone, DHEA and androstenedione are determined after stimulation with Cortrosyn® to differentiate 21-OHD from other CAH conditions (some test items are not covered by insurance).
Boys with classical (and particularly very mild SV) 21-OHD are difficult to differentiate from NC cases using endocrinological and genetic tests alone, and follow-up may be required. Patients with virilizing symptoms (e.g. , accelerated growth, accelerated bone maturation) require treatment. Those who are followed up without treatment should be monitored for adrenal insufficiency symptoms and SW findings (78, 80).
3. Initial Treatment in the Neonatal Stage and Maintenance Therapy in the Childhood Stage (Table 2)
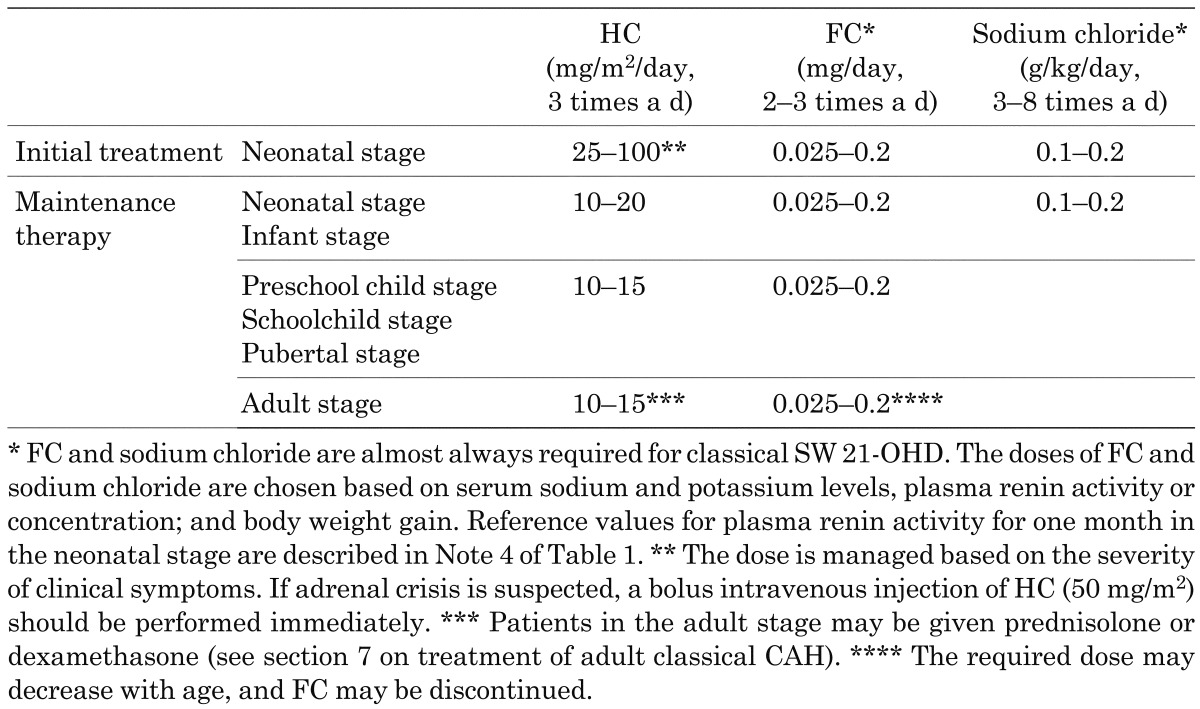
Table 2. Glucocorticoid and mineralocorticoid dosages for initial treatment and maintenance therapy.
Recommendation
Glucocorticoids:
-
1.
We suggest that initial treatment of classical 21-OHD in the neonatal stage requires administration of glucocorticoids at a dose higher than that of maintenance therapy to rapidly reduce enhanced adrenal androgen production. 2 (●○○)
-
2.
We recommend use of hydrocortisone (HC) for maintenance therapy in growing children with classical 21-OHD. 1 (●○○)
-
3.
We recommend against use of long-acting glucocorticoids for maintenance therapy in growing children with classical 21-OHD. 1 (●○○)
-
4.
We recommend that the dose of glucocorticoids during maintenance therapy be carefully chosen to prevent overdose and underdose. 1 (Consensus)
Mineralocorticoids:
-
5.
Administration of fludrocortisone (FC) and sodium chloride is recommended for SW neonates and infants. 1 (●●●)
Explanation
3-1. Treatment principles
The principles of treatment of 21-OHD are to supplement insufficient glucocorticoid and mineralocorticoid levels, inhibit enhanced adrenal androgen production, and maintain growth and maturation similar to those of healthy children. Treatment continues for life. Insufficient treatment causes adrenal crisis (acute and severe adrenal insufficiency) due to decreased tolerance to physical stress and short stature due to bone age advancement. Excessive treatment causes iatrogenic Cushing’s syndrome, including short stature, obesity and hypertension; 21-OHD is managed by pediatric endocrinologists when possible.
3-2. Glucocorticoids: initial treatment in the neonatal stage
In the neonatal stage of classical 21-OHD, adrenal androgen production is markedly enhanced. The previous Japanese treatment guidelines recommended initial treatment with high-dose HC (100–200 mg/m2/d) to inhibit adrenal androgen production (7). In contrast, the European and American guidelines include initial treatment with up HC 25 mg/m2/d, typically with a low-dose of HC of 10–15 mg/m2/d (8,9,10). The 17-OHP level remains high until 3 mo after birth in females and 6 mo after birth in males treated with HC at the initial dose in Europe and the US (84). This suggests that this dose does not sufficiently inhibit adrenal androgen production. However, the target height SDS was reached at 3 yr old without bone age advancement, and adrenal insufficiency occurred in only one subject with gastroenteritis (84). Therefore, low-dose HC does not rapidly reduce adrenal androgen production, but there is no clear evidence that initial low-dose HC is disadvantageous.
In children with classical 21-OHD, height SDS decreases from birth to 1–2 yr old, and height SDS at 2–3 yr old significantly correlates with adult height (85,86,87,88,89). The decrease in height SDS during this period is also significantly correlated with the dose of glucocorticoids (85,86,87,88,89). However, in studies using initial treatment with low-dose HC (9–15 mg/m2/d) (84) and a high dose in accordance with the Japanese treatment guidelines (90, 91), the height at 1 yr old corresponded to –1 SD. There was also no significant difference in height SDS at 1, 2 and 3 yr old between groups initially treated with > 150 and 100 mg/m2/d HC (92). Therefore, the relationship of decreased height SDS early after birth with the dose of glucocorticoids in initial treatment is uncertain, and there is no clear evidence that initial treatment at a high dose worsens the prognosis for height.
There is also no clear evidence for the optimal dose of glucocorticoids in initial treatment. Therefore, the initial dose in neonatal treatment is defined as HC 25–100 mg/m2/d in the Guidelines based on a survey (hereinafter referred to as the JSPE survey) of councilors of the Japanese Society for Pediatric Endocrinology (Table 2). A patient with adrenal crisis or a similar condition should be treated with 100 mg/m2/d HC after bolus administration. This treatment is used by many pediatric endocrinologists. A patient with no adrenal crisis may start treatment at a lower dose. Since a patient with no manifestation of adrenal insufficiency may have NC 21-OHD, treatment does not start immediately, but symptoms and biochemical data should be carefully evaluated. After adrenal androgen production is reduced by the treatment, the dose is immediately decreased at intervals of 5–7 d, and maintenance therapy begins 3–4 wk after birth. These doses and modes of administration are considered to be targets, and practical administration is dependent on the individual patient and clinical experience.
3-3. Glucocorticoids: maintenance therapy in growing children
HC is also used as a glucocorticoid for maintenance therapy in growing children with 21-OHD. HC causes fewer adverse reactions than more potent long-acting glucocorticoids, particularly risks for growth retardation, because of its short half-life. Prednisolone and dexamethasone produce growth suppression that is 15-fold (93) and 70- to 80-fold (94) higher than that with HC. Thus, long-acting glucocorticoids should not be used for maintenance therapy in growing children. HC is often administered three times a day, and there is no clear advantage of increasing the dose in the morning or evening (95).
Physiological cortisol production is estimated to be 5–6 mg/m2/d as HC (96,97,98). Adult height decreased in patients given >20 mg/m2/d in the infant stage and > 15–17 mg/m2/d in the pubertal stage (85,86,87,88,89, 99). Adult height of patients with 21-OHD is also negatively correlated with the dose of glucocorticoids in the early pubertal stage (87,88,89). However, in a meta-analysis of patients with 21-OHD, adult height SDS adjusted for parents’ height had no significant correlation with the total dose of glucocorticoids (100). Thus, there is no clear correlation between the dose of glucocorticoids in maintenance therapy and the height prognosis; however, it is reasonable to treat children before the pubertal stage with as low a dose as possible.
Based on the JSPE survey, the Guidelines recommend a dose of HC for maintenance therapy similar to that in the European and American guidelines (Table 2). The appropriate dose in maintenance therapy differs among individuals for unknown reasons. In the pubertal stage, patient control is sometimes insufficient because cortisol clearance increases, even if replacement therapy is performed and compliance is good (101). The JSPE survey showed that some patients require a dose exceeding the recommended dose for HC maintenance therapy; and that some patients are controlled well for a short to medium period with long-acting glucocorticoids, including dexamethasone.
As described above, underdosing causes adrenal insufficiency and excessive adrenal androgen and inhibits growth, while an overdose causes Cushing’s syndrome and inhibits growth. Therefore, it is important to control the dose balance in treatment of patients. The dose and mode of administration are targets, and practical administration is dependent on the individual patient and the patient’s age.
3-4. Mineralocorticoids
Classical SW 21-OHD is not sufficiently treated with HC alone and requires FC (7, 9, 63, 102). Sodium intake from breast milk and bottle formula is insufficient for treatment in the infant stage, and sodium chloride replacement is necessary (7,8,9). Aldosterone resistance may occur in the neonatal stage (62). Aldosterone deficiency is found in SW cases; and may also occur in SV cases (63). Sodium balance is appropriately maintained by decreasing vasopressin and the ACTH level, reducing the HC dose, and improving adult height (103). The results of a meta-analysis of patients with CAH showed that adult height SDS adjusted for parents’ height was significantly higher in patients treated with FC than in those without FC (100).
The Guidelines recommend a dose of FC in maintenance therapy based on the previous Japanese guidelines, American guidelines; and the results of the JSPE survey (Table 2) (7, 9, 104). The European and American guidelines recommend FC administration to all patients (8, 9), but there is no clear evidence for a benefit of FC in all patients. A patient with poor body weight gain, high plasma renin activity or concentration; and electrolyte imbalance (hyponatremia and hyperkalemia) should be diagnosed with SW 21-OHD and treated with FC, even if FC was not administered earlier. The FC dose is determined based on the plasma renin activity or concentration, electrolyte levels; and body weight gain. Adverse reactions including increased blood pressure and edema should be monitored. If glucocorticoid treatment starts at a high dose (100 mg/m2/d HC), mineralocorticoid deficiency sometimes appears when reducing the dose of glucocorticoids for replacement with maintenance therapy (7). The proposed dose and mode of administration are targets, and practical administration is dependent on the individual patient and the patient’s age.
4. Stress Dosing During Maintenance Therapy (Table 3)
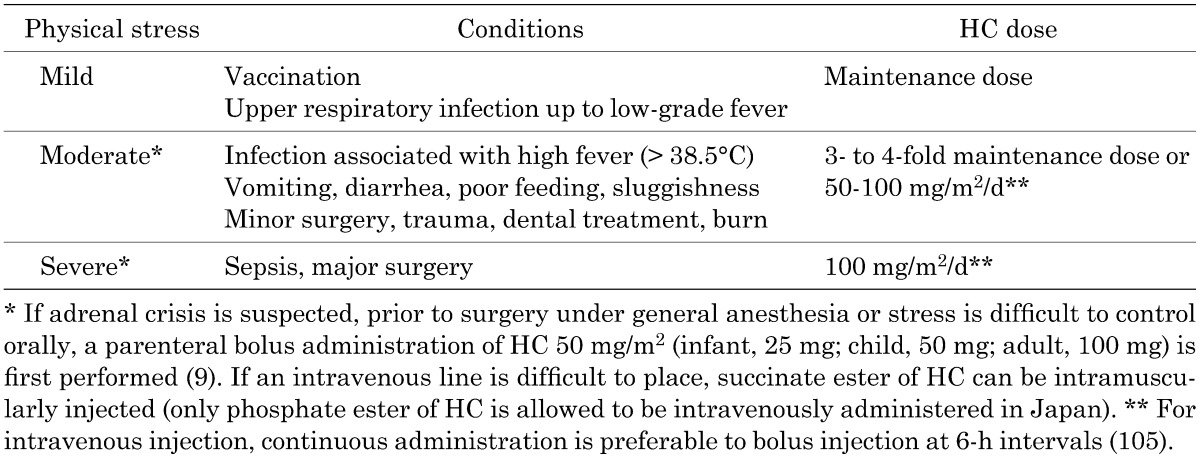
Table 3. Stress dosing.
Recommendation
-
1.
We recommend that the dose of glucocorticoids be increased for febrile illness (>38.5°C), gastroenteritis with dehydration, surgery under general anesthesia, and major trauma. 1 (●●○)
-
2.
We suggest that a patient have a medical identification tag indicating adrenal insufficiency. 2 (●○○)
-
3.
We suggest not routinely increasing the dose of glucocorticoids in patients with mental and emotional stress or slight disease or before mild exercise. 2 (●○○)
Explanation
Cortisol does not respond sufficiently to physical stress in patients with 21-OHD, resulting in adrenal crisis. Adrenal crisis frequently occurs in children < 10 yr old and particularly in those < 1 yr old; and is likely to be associated with gastroenteritis (106). Therefore, if a patient has febrile illness, gastroenteritis with dehydration, surgery or trauma, it is necessary to increase the dose of glucocorticoids transiently. A sufficient dosage of HC has the effect of a mineralocorticoid; therefore, FC administration is unnecessary. Maintenance therapy is resumed immediately after conditions are stabilized. A child at an early age has a risk for hypoglycemia and electrolyte imbalance; therefore, long-term fasting conditions should be avoided, and intravenous administration of glucose and sodium should be performed as required. For rapid and appropriate treatment of adrenal crisis, the patient should have a medical identification tag indicating adrenal insufficiency.
Mild exercise and mental stress (e.g., anxiety and tests) do not routinely require an increased dose of glucocorticoids (107), but based on the JSPE survey, the dose should be increased in patients before strenuous exercise (e.g., a marathon) associated with wasting. It is important to understand the level of physical distress for each patient to determine the appropriate dose increase. The relationships of the type of physical stress with the dose and mode of administration are under discussion. The Guidelines recommend stress-based HC doses based on the JSPE survey and European and American guidelines (Table 3). The proposed doses and modes of administration should be used for each patient based on clinical experience. A large-scale comparative study is required to determine the appropriate doses and modes of administration.
5. Treatment Monitoring for Growing Children
Recommendation
-
1.
We recommend that monitoring of treatment in children be determined with consideration of changes in growth rate and bone age, in addition to test findings. 1 (Consensus)
-
2.
We suggest that routine evaluation of height, body weight and blood pressure be performed at all ages in growing children and that bone age be determined after one year old. 2 (●○○)
-
3.
We suggest that treatment be evaluated by endocrine test at a constant time (before administration of glucocorticoids early in the morning). 2 (●○○)
-
4.
We recommend that monitoring of adverse events (Cushing’s syndrome) due to excessive glucocorticoids and avoidance of complete inhibition of intrinsic adrenal steroid secretion are required. 1 (●○○)
Explanation
It is not easy to monitor treatment of 21-OHD (7,8,9,10). The JSPE survey suggested that there is no endocrinological indicator for complete monitoring and determination of the optimal dose of glucocorticoids. Thus, the appropriateness of treatment has to be judged based on clinical symptoms and test findings. Therefore, the Guidelines recommend comprehensive monitoring of treatment in children based on changes in growth rate and bone age, in addition to test findings.
Excessive glucocorticoid levels cause a reduced growth rate and obesity, while insufficient levels enhance the growth rate and bone age. Insufficient mineralocorticoids reduce the growth rate and cause poor body weight gain. Therefore, height and body weight should be routinely evaluated. Untreated patients with classical 21-OHD do not show increased bone age until 1–1.5 yr old; therefore, bone age is evaluated after one year old. Evaluation once a year is usually sufficient (7, 9, 108), but bone age should be determined twice a year when the growth rate is rapidly changing or the patient is in the pubertal stage. Growth and maturation that proceed with age are important as long-term indicators.
Endocrinological tests should be conducted using precise methods and evaluated based on appropriate references. Adrenal steroid hormone levels depend on the measurement method, and it is desirable to determine adrenal steroid hormones by extracted immunological assays and LC-MS/MS. The best indicator for treatment with glucocorticoids is serum 17-OHP (7, 9, 109, 110). Determination of serum 17-OHP using approved kits is covered by insurance, and it is preferable to use a kit at a test institution or mass screening facility. Serum androstenedione and testosterone (prepubertal males and females, pubertal females) can be used for treatment monitoring (7, 9, 10, 111), but measurement of the serum androstenedione level is not covered by insurance in Japan, and standard references by sex and age have not been established for androstenedione.
Plasma ACTH has a robust circadian rhythm and is difficult to use for monitoring. Serum 17-OHP also has a circadian rhythm and daily variance, and thus it is preferable to determine serum 17-OHP before administration of glucocorticoids early in the morning (9. 10, 109, 110). However, in the JSPE survey, most institutions determined casual 17-OHP in outpatient consultations. The target for serum 17-OHP is 400–1200 ng/dL before administration early in the morning in both the child and adult stages (10, 110) and < 590 ng/dL in the pubertal stage (84). Normalized 17-OHP suggests an overdose of glucocorticoids. Monitoring using pregnanetriol (PT), a urinary metabolite of 17-OHP, by urine collection has also been proposed (7, 112,113,114). In a study of 21-OHD in Japanese children, excluding the neonatal and pubertal stages, a PT range of 1.2–2.1 mg/m2/d was shown to indicate good control (113). If 21-OHD cannot be determined before administration of glucocorticoids early in the morning, determination of the 17-OHP level and monitoring of PT by urine collection may be useful, while keeping the circadian rhythm and daily variance of 17-OHP in mind.
If treatment to control of 21-OHD is difficult, determination of the 17-OHP level before administration of glucocorticoids and monitoring of PT by urine collection may be considered.
Endocrinological tests include short-term indicators that may be changed by sampling conditions; therefore, tests should be performed several times and comprehensively evaluated. Indicators for monitoring mineralocorticoid treatment include blood pressure, serum electrolytes and plasma renin. Excessive mineralocorticoids increase systolic blood pressure (115). Plasma renin should be close to the average level for each age, but it is also frequently high in healthy children in the neonatal and infant stages; therefore, it should be used only as a reference. Decreased plasma renin suggests an overdose of FC or sodium chloride. Ten of 134 infants who were given FC were found to have hypertension and leg edema (104). The FC dose was 0.025–0.05 mg/d in 7 of the 10 patients. These patients were neonates who were given FC due to immature sodium reabsorption in the renal tubule, and required subsequent reduction of the FC dose. These findings reflect individual differences in sensitivity to FC and changes in sensitivity with age.
6. Treatment of NC 21-OHD
Recommendation
-
1.
We suggest that NC 21-OHD associated with excessive adrenal androgen symptoms including enhanced growth rate and bone age and virilization of the external genitalia in females be treated with maintenance therapy similar to that for classical 21-OHD. 2 (●○○)
-
2.
We recommend against treatment of subclinical NC 21-OHD. 1 (●○○)
-
3.
We recommend that the dose of glucocorticoids for NC 21-OHD under glucocorticoid treatment be increased for febrile illness (>38.5°C), gastroenteritis with dehydration, surgery under general anesthesia; and major trauma. 1 (●○○)
Explanation
NC 21-OHD associated with characteristics of 21-OHD in endocrinological tests, but without symptoms of glucocorticoid or mineralocorticoid deficiency; requires routine evaluation of physical findings, height, body weight and bone age to determine the timing of treatment (7, 9, 80, 81). If excessive adrenal androgen symptoms including enhanced growth rate and bone age and virilization of the external genitalia are found in females, maintenance therapy similar to treatment of classical 21-OHD should be started. There are no large-scale studies showing benefits of treatment of subclinical NC 21-OHD. Female NC 21-OHD patients who were diagnosed in the child stage in Japan showed enhanced bone age and virilization of the external genitalia (80, 81); but no early pubic hair development, hirsutism, acne or menstrual irregularity, which have been found in Europe and the US. Some patients developed adrenal crisis after starting treatment, suggesting the importance of stress dosing in maintenance therapy (78). As in classical 21-OHD, the appropriate dose should be determined individually in each patient with NC 21-OHD to adjust the progression rates of height and bone age such that they correspond with age.
7. Treatment of Adult Classical CAH
Recommendation
-
1.
We suggest that patients with classical CAH be treated with short- or long-acting glucocorticoids. 2 (●○○)
-
2.
We suggest that a patient under treatment with glucocorticoids and mineralocorticoids have a consultation at least twice a year and monitoring based on hormone tests. 2 (●○○)
-
3.
We recommend that comprehensive evaluation of test findings and clinical symptoms are required for follow-up in the adult stage. 1 (●○○)
-
4.
We suggest that measurement of serum 17-OHP before administration of glucocorticoids early in the morning be used for monitoring and that the appropriate range is 400–1200 ng/dL. 2 (●○○)
-
5.
We suggest that determination of bone density should be considered if glucocorticoids exceed the recommended dose and Cushing’s syndrome is suspected. 2 (●○○)
Explanation
Treatment for adult CAH patients requires that (1) no corticosteroid deficiency symptom develops, (2) a female patient has no virilization or menstrual irregularity, (3) both male and female patients have no gonadotropin suppression and (4) the risk of adrenal rest tumor in the testis is reduced. However, an overdose of glucocorticoids causes iatrogenic Cushing’s syndrome, whereas an underdose causes chronic adrenal insufficiency symptoms, including general fatigue. Overdose of mineralocorticoids causes hypertension. A large-scale randomized controlled study to determine the optimal dose of glucocorticoids and mineralocorticoids in the adult stage has not been performed.
The results of a survey in Japanese adults showed that HC was administered in 47% of male patients (mean 22.5 mg/d) and 61% of female patients (mean 20.0 mg/d). Other patients were treated with a single administration of synthetic glucocorticoids or combined administration with HC. Monotherapy with dexamethasone was administered in 29% of males (mean 0.5 mg/d) and 15% of females (0.5 mg/d). Monotherapy with prednisolone was used in 5% of males (5 mg/d) and 5% of females (7.5 mg/d) (116). In Europe, CAH patients in the adult stage receive replacement therapy with HC (mean 13.75 mg/m2) in 36% of patients, prednisolone in 14% of patients (4.74 mg/d), and dexamethasone in 33% of patients (0.5 mg/d) (117).
In a nationwide study of 203 patients in the United Kingdom (UK), HC was administered to 26% of patients (mean daily dose 25 mg, range 10–60 mg), prednisolone was administered to 43% of patients (7.6 mg, 2.5–10 mg), dexamethasone was administered to 19% of patients (0.5 mg, 0.25–0.75 mg), and a combination of HC with prednisolone or dexamethasone was administered to 10% of patients (118). In a study in 224 CAH patients in the US, about 80% of pediatric patients received HC (daily dose 15.0±5.9 mg/m2), and about 30% each of adult patients received HC (daily dose 17.9 ± 7.6 mg/m2), prednisolone, and dexamethasone (119). Therefore, a patient with no negative reaction to HC in the child stage can receive similar treatment in the adult stage. HC can be replaced with prednisolone or dexamethasone, but the dose should be carefully determined. Long-acting glucocorticoids were more likely to be used in adult patients than in pediatric patients in all studies.
Several studies have examined methods to find the optimal dose of glucocorticoids (9, 120). A study in the US proposed a target range of serum 17-OHP of 100–1200 ng/dL before administration of glucocorticoid early in the morning for monitoring of glucocorticoid treatment, similarly to that in the child stage; however, the dose and range should be decided individually. Another study suggested a target 17-OHP level of < 800 ng/dL before administration of glucocorticoids early in the morning in females of childbearing age; and < 2500 ng/dL for males without adrenal rest tumor in the testis (120). However, these were not large-scale clinical studies. In a study in adult patients in the US, about 30% had a serum 17-OHP level of 100–1200 ng/dL before administration of glucocorticoid early in the morning (119). In a UK study, the target 17-OHP level was achieved in 10% of patients (118). Therefore, appropriate treatment with glucocorticoids is difficult in the adult stage.
A recent study in the UK showed that the quality of life (QOL) of adult patients given prednisolone or dexamethasone was lower than that of patients treated with HC (121). However, it is uncertain if prednisolone and dexamethasone reduce QOL or are used for patients in which CAH is difficult to control. Testosterone in males reflects reproductive function more than adrenal function and is not useful for monitoring of treatment. If a male patient with a large adrenal rest tumor in the testis has a low level of testosterone early in the morning, Leydig cell dysfunction is suspected (9).
The optimal dose of FC in adult patients has not been examined. The results of a nationwide study in the UK showed that FC was administered to 72% of patients at a mean dose of 0.125 mg (0.01–0.5 mg/day) (118). The requirement for FC decreases with age (5), and patients given FC in the child stage often do not need FC in the adult stage. Therefore, adjustment of administration of FC while monitoring the patient’s blood pressure and plasma renin activity or concentration is required. If renin activity increases after discontinuation, readministration of FC should be considered. However, prednisolone has less effect on mineralocorticoids in comparison with HC, and dexamethasone has no effect. Therefore, if HC is replaced with these drugs, the dose of FC may require adjustment.
In the adult stage, 21-OHD can be accompanied by metabolic abnormalities (118,119,120,121,122,123,124,125,126,127). Bone mineral density (BMD) may decrease, and fracture and osteoporosis may occur, but there are conflicting studies (123, 124). BMD decreased in adults ≥ 30 yr old and in postmenopausal females with 21-OHD (123), but BMD did not decrease in comparison with controls in pubertal children and young adults with 21-OHD (124). There are reciprocal studies of the correlation of the total dose of glucocorticoids with BMD (123,124,125, 127). Thus, there is currently no evidence to support routine monitoring of BMD; however, if the dose of glucocorticoids is higher than the recommended dose and obesity and Cushing’s syndrome are present, a BMD test should be assessed
A study of metabolic syndrome in 203 adult patients with 21-OHD in the UK found obesity in 41% of the patients, complication with hypercholesterolemia in 46% of the patients, insulin resistance in 29% of the patients, osteopenia in 40% of the patients, and osteoporosis in 7% of the patients (118). The same study group showed that insulin resistance and increased fat were related to decreased QOL (121). The results of a study in American adult patients with 21-OHD showed obesity in 30% of the patients, hypertension in 60% the patients, metabolic syndrome in 18% the patients, and decreased BMD in 50% the patients (119). In a study by the adrenal group in Japan, the rates of high-grade obesity (BMI ≥ 30) were 23% in males with 21-OHD and 16% in females with 21-OHD, with no difference between the types of glucocorticoids; however, the HC dose in the high obesity group was significantly lower (116). No definite relationship of obesity with administration of glucocorticoids can be established from these results due to the small number of subjects.
These results suggest that metabolic abnormalities may be associated with 21-OHD, but it is unclear if these abnormalities are related to overdose of glucocorticoids. No definite monitoring method to find the optimal dose of glucocorticoids is available in adults, which is similar to the pediatric field. Therefore, a large-scale systematic clinical study is required to examine administration of glucocorticoids and mineralocorticoids, monitoring methods, and metabolic abnormalities in the adult stage of 21-OHD.
8. Prenatal Diagnosis and Treatment
Recommendation
1. Prenatal diagnosis and treatment have yet to be established. We suggest that experienced physicians conduct diagnosis and treatment in institutions with genetic counseling after approval of the ethics committees of their institutions. 2 (●○○)
Explanation
8-1. Prenatal diagnosis and treatment
Administration of dexamethasone through placental transfer from the maternal body can reduce adrenal androgen production in a fetus with 21-OHD (128,129,130). The purposes of prenatal treatment are to block virilization of the external genitalia in female patients and avoid the need for surgery, and to relieve the emotional distress and anxiety of parents that may be caused by an external genitalia anomaly in their child (128,129,130,131,132). However, the disease cannot be completely remitted by prenatal treatment, and routine and careful follow-up and treatment are required. The risk of adrenal crisis also does not disappear.
Prenatal treatment is currently conducted based on previous reports (128, 131), but the dose and administration period for dexamethasone have not been fully established. The fetal cortisol concentration is usually very low in the first trimester (133) and increases from weeks 8 to 12 of gestation, but it is still about 10% of the maternal cortisol concentration in the second trimester (134, 135). Therefore, administration of dexamethasone from the first trimester can result in a 60-fold fetal physiological concentration in the second trimester (136). A method to decrease dexamethasone in fetal treatment after the completion of formation of the external genitalia has been proposed (136, 137).
21-OHD is an autosomal recessive disease, and the probability of the disease in a fetus of a woman who has already delivered a child with 21-OHD is 25% with the same partner. Virilization of the external genitalia in girls with 21-OHD occurs until 6 wk after conception; therefore, treatment should be performed as soon as possible if the woman recognizes pregnancy. Dexamethasone does not inactivate the placenta and can be used as a therapeutic drug (138). Treatment should start from weeks 6–7 of gestation, but genetic diagnosis using chorionic villus sampling cannot be conducted until weeks 10–12 of gestation. Therefore, the probability of CAH in the fetus is 25%, but dexamethasone should be administered to the pregnant woman in the first trimester. A new test method to determine sex using maternal blood within wk 6 of gestation has been applied to prenatal treatment of 21-OHD to shorten the administration period of dexamethasone (139, 140). Detection of a Y chromosome allows earlier discontinuation of unnecessary treatment. Only girls with 21-OHD receive the benefit of treatment, and only one-eighth of all pregnancies really require treatment. This results in unnecessary dexamethasone administration, and the related ethical issues are under discussion in Japan and other countries (103, 132, 141, 142).
8-2. Fetal safety and long-term prognosis
A survey of children given dexamethasone for prenatal treatment of 21-OHD showed that the children were significantly more introverted in comparison with the general population (143). However, in another survey that compared 174 patients who received prenatal treatment and 313 untreated controls, there were no differences in nine social and developmental indicators (144). In a study of Swedish children treated before birth with a constant therapy protocol and control children of matching sex and age, a survey and a standard neuropsychological test conducted by a clinical psychologist (145) indicated no differences in intelligence, proficiency and long-term memory. However, children who were given dexamethasone for a short period, but did not have 21-OHD; had a reduced verbal working memory, less self-recognition in academic achievement; and increased subjective social phobia (145, 146). Simultaneously, no difference in behavioral and adaptive performance was found, but 7 boys who did not have 21-OHD but were treated with dexamethasone for a short period had greater androgynous behavior in comparison with control boys (147). The authors concluded that it cannot be completely ruled out that the differences in children without disease were due to the small number of patients with 21-OHD who were continuously treated. In contrast, a survey and neuropsychological tests performed by Meyer-Bahlburg et al. showed that children who were given dexamethasone for a short period but did not have 21-OHD did not have a reduced verbal working memory, but 21-OHD female patients who were treated with dexamethasone for a long time had slightly reduced recognition (148).
8-3. Studies in Japan
Prenatal diagnosis and treatment of 21-OHD have also been examined in Japan (141, 142). In a survey of members of the Japanese Society for Pediatric Endocrinology, Kinoshita et al. identified 13 children who underwent prenatal treatment from 1995 to 2002, of whom 2 girls had 21-OHD and external and internal genitalia that were completely female. Nine of these patients discontinued treatment, including 8 boys and one fetus that was aborted (141). None of the 8 boys and their mothers had adverse reactions, but the results of follow-up tests are not available. In a survey from 2002 to 2007, detailed responses were obtained for only 7 patients (142). Four girls with 21-OHD were treated until birth, of whom 3 had completely female external genitalia and one had slight clitoromegaly, despite being treated with dexamethasone from wk 8 of gestation. Of the patients who discontinued treatment, 2 were determined to be normal girls by genetic diagnosis, and 1 was diagnosed as a boy in a sex check. Two of the 7 cases were spontaneous abortions, and 1 was an induced abortion, but the details were unknown. Three cases underwent genetic analysis and a sex check using chorionic villus sampling, and 2 underwent amniocentesis. Mild symptoms of Cushing’s syndrome were found in 2 mothers, and dyspepsia was present in 1 mother. There is no Japanese study in which children who were given dexamethasone for a short time or until birth were subsequently evaluated by neuropsychological tests. In the survey, 15% of respondents considered that this treatment had ethical problems.
8-4. Current status
The results of a meta-analysis of prenatal diagnosis of 21-OHD were reported in 2010 (149). In this analysis, treatment effects and fetal and maternal adverse reactions were examined in 4 studies and a total of 323 pregnancies. Dexamethasone was effective for prevention of virilization and had no adverse effects on the fetus, but it significantly increased stria and edema in mothers, but with no serious adverse effects. However, the authors concluded that there were too few studies for analysis and that the evidence was insufficient.
The American Endocrine Society has recently published clinical practice guidelines for CAH that indicate that unnecessary maternal and fetal exposure to dexamethasone should be avoided in prenatal treatment because the potential adverse events caused by dexamethasone are more important than the mental distress of parents and patients caused by virilization of the external genitalia (9).
There are no data on long-term prognosis in Japan. Therefore, the Mass Screening Committee of the Japanese Society for Pediatric Endocrinology concluded that the appropriateness of prenatal diagnosis and treatment based on the Guidelines has not been established and should be considered further.
Acknowledgments
Funds for development of the Guidelines were provided by the Japanese Society for Pediatric Endocrinology.
We are grateful to Prof. Keiko Homma, Clinical Laboratory, Keio University Hospital, and Prof. Tomonobu Hasegawa, Pediatrics, Keio University School of Medicine, for providing an analysis of urinary steroid profiles in advance.
Conflict of interest of the working committee members: None of the committee members have a conflict of interest regarding development of the Guidelines, based on the criteria for conflict of interest of the Japan Pediatric Society, in accordance with the rules of the Japanese Society for Pediatric Endocrinology.
Appendix 1
1. Development process
1-1. Understanding current conditions
A survey was conducted via email sent to 156 councilors of the Japanese Society for Pediatric Endocrinology. Initial treatment was examined from August 28 to September 28, 2012, and maintenance therapy and treatment in the pubertal stage were examined from January 10 to February 28, 2013. The response rates were 25.6% (40/156) and 19.8% (31/156), respectively. For the initial treatment in neonatal cases without SW symptoms, the HC dose was indicated to be 100–200, 50–100; and 20–50 mg/m2/d by 20, 13; and 6 of the 40 respondents, respectively.
1-2. External evaluation
The draft guidelines were published on a website for members of the Japanese Society for Pediatric Endocrinology from March 11 to April 10, 2013, to solicit opinions, and a revised draft was developed on June 7, 2013, with consideration of the received opinions. The validity of the guidelines was discussed in the Guidelines Committee, including external members, and revisions were made based on the proposal of the Guidelines Committee (April 7, 2014). This revision was approved by the Board of the Society on April 26, 2014, and published.
1-3. Consultation with relevant societies
The draft guidelines were sent to the councilors of the Japanese Society for Mass Screening by email from March 11 to April 10, 2013 to request opinions. A revised draft was developed on June 7, 2013, based on replies to this request.
1-4. Hearing of opinions of patient groups
There is no relevant patient group; therefore, no hearing was conducted.
2. Revision schedule
The Guidelines are planned to be revised within 4 yr after disclosure. The committee for revision will be organized by the board of the Japanese Society for Pediatric Endocrinology. If new developments occur that may have critical effects on the Guidelines, the board of the Japanese Society for Pediatric Endocrinology may decide to revise the Guidelines immediately as “Recommendations.”
References
- 1.Suwa S. Nationwide survey of neonatal mass-screening for congenital adrenal hyperplasia in Japan. Screening 1994;3: 141–51. doi: 10.1016/0925-6164(94)90022-1 [DOI] [Google Scholar]
- 2.Pang SY, Wallace MA, Hofman L, Thuline HC, Dorche C, Lyon IC, et al. Worldwide experience in newborn screening for classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics 1988;81: 866–74. [PubMed] [Google Scholar]
- 3.Suwa S. Congenital adrenal hyperplasia. Jpn J Pediatr Med 1994;26: 1967–72 (in Japanese). [Google Scholar]
- 4.Fujieda K. History and current status of neonatal mass screening for congenital adrenal hyperplasia. Jpn J Pediatr Med 2001;33: 1674–8 (in Japanese). [Google Scholar]
- 5.Suwa S, Igarashi Y, Kitagawa T, Shimozawa K, Tsuruhara T, Matsuura N, et al. Diagnostic handbook of congetal adrenal hyperplasia (21-hydroxylase deficiency) identified by neonatal mass screening. J Jpn Pediatr Soc 1989;93: 1632–3 (in Japanese). [Google Scholar]
- 6.Saisho S, Yokota I, Kusuda S, Tachibana K, Igarashi Y, Suwa S, et al. Japanese Society for Pediatric Endocrinology, Mass Screening Committee, and Japanese Society for Mass Screening. Guidelines for diagnosis of 21-hydroxylase deficiency. J Jpn Pediatr Soc 1999;103: 69–71 (in Japanese). [Google Scholar]
- 7.Kusuda S, Tachibana K, Saisho S, Yokota I, Igarashi Y, Suwa S, et al. Japanese Society for Pediatric Endocrinology, Mass Screening Committee, and Japanese Society for Mass Screening. Guidelines for treatment of 21-hydroxylase deficiency. J Jpn Pediatr Soc 1999;103: 72–5 (in Japanese). [Google Scholar]
- 8.Clayton PE, Miller WL, Oberfield SE, Ritzen EM, Sippell WG, Speiser PW, Joint LWPES/ESPE CAH Working Group.Consensus statement on 21-hydroxylase deficiency from the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. J Clin Endocrinol Metab 2002;87: 4048–53. doi: 10.1210/jc.2002-020611 [DOI] [PubMed] [Google Scholar]
- 9.Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, et al. Endocrine SocietyCongenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95: 4133–60. doi: 10.1210/jc.2009-2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merke DP, Bornstein SR, Avila NA, Chrousos GP. NIH conference. Future directions in the study and management of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Ann Intern Med 2002;136: 320–34. doi: 10.7326/0003-4819-136-4-200202190-00012 [DOI] [PubMed] [Google Scholar]
- 11.Fujieda K. Adrenal Insufficiency. In: Japanese Society for Japanese Pediatric Endocrinology editors. Tokyo: SHINDAN-TO-CHIRYOSHA; 2000.p338-61 (in Japanese). [Google Scholar]
- 12.New MI. Nonclassic 21-hydroxylase deficiency. Fertil Steril 2006;86(Suppl 1): S2. doi: 10.1016/j.fertnstert.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 13.Wilson RC, Mercado AB, Cheng KC, New MI. Steroid 21-hydroxylase deficiency: genotype may not predict phenotype. J Clin Endocrinol Metab 1995;80: 2322–9. [DOI] [PubMed] [Google Scholar]
- 14.Tajima T, Fujieda K, Nakae J, Toyoura T, Shimozawa K, Kusuda S, et al. Molecular basis of nonclassical steroid 21-hydroxylase deficiency detected by neonatal mass screening in Japan. J Clin Endocrinol Metab 1997;82: 2350–6. doi: 10.1210/jcem.82.7.4094 [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa Y, Kashima K, Ono M, Tomohiro I, Tajima T, Nagasaki K, et al. The Annual Report of Non-classic 21-hydroxylae deficiency in Japan. Report of Labour and Welfare Project on Intractable Disease. 2012. (in Japanese).
- 16.Homma K, Hasegawa T, Nagai T, Adachi M, Horikawa R, Fujiwara I, et al. Urine steroid hormone profile analysis in cytochrome P450 oxidoreductase deficiency: implication for the backdoor pathway to dihydrotestosterone. J Clin Endocrinol Metab 2006;91: 2643–9. doi: 10.1210/jc.2005-2460 [DOI] [PubMed] [Google Scholar]
- 17.Flück CE, Meyer-Böni M, Pandey AV, Kempná P, Miller WL, Schoenle EJ, et al. Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. Am J Hum Genet 2011;89: 201–18. doi: 10.1016/j.ajhg.2011.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamrath C, Hochberg Z, Hartmann MF, Remer T, Wudy SA. Increased activation of the alternative “backdoor” pathway in patients with 21-hydroxylase deficiency: evidence from urinary steroid hormone analysis. J Clin Endocrinol Metab 2012;97: E367–75. doi: 10.1210/jc.2011-1997 [DOI] [PubMed] [Google Scholar]
- 19.Tajima T, Fujieda K, Nakayama K, Fujii-Kuriyama Y. Molecular analysis of patient and carrier genes with congenital steroid 21-hydroxylase deficiency by using polymerase chain reaction and single strand conformation polymorphism. J Clin Invest 1993;92: 2182–90. doi: 10.1172/JCI116820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.New MI, Abraham M, Gonzalez B, Dumic M, Razzaghy-Azar M, Chitayat D, et al. Genotype-phenotype correlation in 1,507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Proc Natl Acad Sci USA 2013;110: 2611–6. doi: 10.1073/pnas.1300057110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koyama S, Toyoura T, Saisho S, Shimozawa K, Yata J. Genetic analysis of Japanese patients with 21-hydroxylase deficiency: identification of a patient with a new mutation of a homozygous deletion of adenine at codon 246 and patients without demonstrable mutations within the structural gene for CYP21. J Clin Endocrinol Metab 2002;87: 2668–73. doi: 10.1210/jcem.87.6.8522 [DOI] [PubMed] [Google Scholar]
- 22.Suwa S, Igarashi Y, Katoh S, Kusunoki T, Tanae A, Niimi H, et al. Questionnaire survey of congenital adrenal hyperplasia. Part 1. J Jpn Pediatr Soc 1981;85: 204–10 (in Japanese). [Google Scholar]
- 23.Suwa S, Igarashi Y, Katoh S, Kusunoki T, Tanae A, Niimi H, et al. Questionnaire survey of congenital adrenal hyperplasia. Part 4. Analysis of symptoms. J Jpn Pediatr Soc 1982;86: 2162-7 (in Japanese). [Google Scholar]
- 24.Balsamo A, Cacciari E, Piazzi S, Cassio A, Bozza D, Pirazzoli P, et al. Congenital adrenal hyperplasia: neonatal mass screening compared with clinical diagnosis only in the Emilia-Romagna region of Italy, 1980-1995. Pediatrics 1996;98: 362–7. [PubMed] [Google Scholar]
- 25.Brosnan PG, Brosnan CA, Kemp SF, Domek DB, Jelley DH, Blackett PR, et al. Effect of newborn screening for congenital adrenal hyperplasia. Arch Pediatr Adolesc Med 1999;153: 1272–8. doi: 10.1001/archpedi.153.12.1272 [DOI] [PubMed] [Google Scholar]
- 26.Suwa S, Kusuda S, Toyoura T, Fujieda K, Koda N, Nishiyama S, et al. Follow up study of severity cases with 1-hysdroxylase deficiency detected in neonatal mass screening. Part 1. Clinical findings before treatment. J Jpn Pediatr Soc 1997;101: 1149–57 (in Japanese). [Google Scholar]
- 27.Tajima T, Fujikura K, Fukushi M, Hostubo T, Mitsuhashi Y. Neonatal screening for congenital adrenal hyperplasia in Japan. Pediatr Endocr Rev 2012;10:72–8. [PubMed] [Google Scholar]
- 28.Kuyo M, Yoneda Y, Igarashi N. Neonatal screening for 21-hydroxylase deficiency in Toyama, Japan: 10 years experience and results. Jpn J Mass Screening 2009;19: 233–42 (in Japanese). [Google Scholar]
- 29.Konishi K, Hasegawa T, Anazawa A, Kashimada K, Kitagawa T. Neonatal screening for 21-hydroxylase deficiency in Tokyo, Japan: 23 years experience and results. Folia Endocrinol Jpn 2013;89: 256 (in Japanese). [Google Scholar]
- 30.Nagasaki K, Asami N, Nomura M, Hokari K, Otabe N. Neonatal screening for 21-hydroxylase deficiency in Niigata Japan: 20 years experience and results. Jpn J Mass Screening 2010;20: 223–7 (in Japanese). [Google Scholar]
- 31.Hisashige T. Heisei fifth The Minister Welfare Research for mental and physical disorder of children 1994. p 63 (in Japanese).
- 32.Fukushi M, Arai O, Mizushima Y, Takasugi N, Fujieda K, Matsuura N. Development of enzyme linked immunosorbent assay for dried blood cortisol and its application to neonatal screening for congenital adrenbal hyperplasia due to 21-hydroxylase deficiency. Part 4. Folia. Endocrinol Jpn 1987;63: 205–14 (in Japanese). [DOI] [PubMed] [Google Scholar]
- 33.Mikami A, Fukushi M, Oda H, Fujita K, Fujieda K. Newborn screening for congenital adrenal hyperplasia in Sapporo City: sixteen years experience. Southeast Asian J Trop Med Public Health 1999;30(Suppl 2): 100–2. [PubMed] [Google Scholar]
- 34.Konishi K, Hara A, Sakurai K, Anazawa A, Suzuki T, Toyoura T. Age-related change in blood spot 17α−hydroxyprogesterone in low birth weight infants. Jpn J Mass Screening 2005;15: 63–8 (in Japanese). [Google Scholar]
- 35.Yamagami Y, Yamada Y, Majima K, Haruki E, Tachibana K, Sugawara T, et al. Problem of neonatal mass screening for congenital adrenal hyperplasia. Prev Med 2005;47: 65–9 (in Japanese). [Google Scholar]
- 36.Yasukata K, Inomata H, Minagawa M, Uetaki K, Hirota M, Inada Y, et al. Usefulness of cut off value of low birth weight infants in the neonatal mass screening for congenital adrenal hyperplasia. Jpn J Mass Screening 2006;16: 57–61 (in Japanese). [Google Scholar]
- 37.Yamano K, Ichihara T, Harada S, Arai J, Fujieda K, Kudo T, et al. Problem of neonatal mass scrennoing for congenital adrenal hyperplasia in Hokkaido. Jpn J Mass Screening 1996;6: 5–10 (in Japanese). [Google Scholar]
- 38.Tachibana K, Yamagami Y. Neonatal mass screening for congenital adrenal hyperplasia in low birth weight infants. Jpn J Mass Screening 2005;15: 19–22 (in Japanese). [Google Scholar]
- 39.Adachi M. Follow-up for infants with elevated 17-OHP in neonatal mass screening for congenital adrenal hyperplasia. Jpn J Pediatr Med 2004;36: 1913–6 (in Japanese). [Google Scholar]
- 40.Coulm B, Coste J, Tardy V, Ecosse E, Roussey M, Morel Y, et al. DHCSF Study GroupEfficiency of neonatal screening for congenital adrenal hyperplasia due to 21-hydroxylase deficiency in children born in mainland France between 1996 and 2003. Arch Pediatr Adolesc Med 2012;166: 113–20. doi: 10.1001/archpediatrics.2011.774 [DOI] [PubMed] [Google Scholar]
- 41.Gurian EA, Kinnamon DD, Henry JJ, Waisbren SE. Expanded newborn screening for biochemical disorders: the effect of a false-positive result. Pediatrics 2006;117: 1915–21. doi: 10.1542/peds.2005-2294 [DOI] [PubMed] [Google Scholar]
- 42.Matern D, Tortorelli S, Oglesbee D, Gavrilov D, Rinaldo P. Reduction of the false-positive rate in newborn screening by implementation of MS/MS-based second-tier tests: the Mayo Clinic experience (2004-2007). J Inherit Metab Dis 2007;30: 585–92. doi: 10.1007/s10545-007-0691-y [DOI] [PubMed] [Google Scholar]
- 43.Janzen N, Peter M, Sander S, Steuerwald U, Terhardt M, Holtkamp U, et al. Newborn screening for congenital adrenal hyperplasia: additional steroid profile using liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab 2007;92: 2581–9. doi: 10.1210/jc.2006-2890 [DOI] [PubMed] [Google Scholar]
- 44.Schwarz E, Liu A, Randall H, Haslip C, Keune F, Murray M, et al. Use of steroid profiling by UPLC-MS/MS as a second tier test in newborn screening for congenital adrenal hyperplasia: the Utah experience. Pediatr Res 2009;66: 230–5. doi: 10.1203/PDR.0b013e3181aa3777 [DOI] [PubMed] [Google Scholar]
- 45.Fujikura K, Yamagishi T, Tagami Y, Nomachi S, Hanai J, Misumi Y, et al. Second-tier testing of neonatal screening for congenital adrenal hyperplasia using liquid chromatography-tandem mass spectrometry. Jpn J Mass Screening 2013;23: 85–92 (in Japanese). [Google Scholar]
- 46.Fukushi M. Cut off value of 17-OHP in neonatal mass screening for congenital adrenal hyperplasia. Textbook of congenital metabolic disease for laboratory science. 2011. p 4-15 (in Japanese).
- 47.Tachibana K, Inomata H, Aoki K, Kuroda Y, Yamagami Y, Ichijima M. Nation wide survey for cases with 21-hydroxylase deficiency undetected by neonatal mass screening. Jpn J Mass Screening 2001;11: 47–52 (in Japanese). [Google Scholar]
- 48.Allen DB, Hoffman GL, Fitzpatrick P, Laessig R, Maby S, Slyper A. Improved precision of newborn screening for congenital adrenal hyperplasia using weight-adjusted criteria for 17-hydroxyprogesterone levels. J Pediatr 1997;130: 128–33. doi: 10.1016/S0022-3476(97)70321-4 [DOI] [PubMed] [Google Scholar]
- 49.Olgemöller B, Roscher AA, Liebl B, Fingerhut R. Screening for congenital adrenal hyperplasia: adjustment of 17-hydroxyprogesterone cut-off values to both age and birth weight markedly improves the predictive value. J Clin Endocrinol Metab 2003;88: 5790–4. doi: 10.1210/jc.2002-021732 [DOI] [PubMed] [Google Scholar]
- 50.van der Kamp HJ, Oudshoorn CG, Elvers BH, van Baarle M, Otten BJ, Wit JM, et al. Cutoff levels of 17-alpha-hydroxyprogesterone in neonatal screening for congenital adrenal hyperplasia should be based on gestational age rather than on birth weight. J Clin Endocrinol Metab 2005;90: 3904–7. doi: 10.1210/jc.2004-2136 [DOI] [PubMed] [Google Scholar]
- 51.Steigert M, Schoenle EJ, Biason-Lauber A, Torresani T. High reliability of neonatal screening for congenital adrenal hyperplasia in Switzerland. J Clin Endocrinol Metab 2002;87: 4106–10. doi: 10.1210/jc.2002-012093 [DOI] [PubMed] [Google Scholar]
- 52.Togari S, Kusuda S. Guideline for sampling of blood for neonatal mass screening in low birth weight infants. Journal of Japanese Society for Premature and Newborn Medicine 2004;16: 108 (in Japanese). [Google Scholar]
- 53.Sarafoglou K, Banks K, Gaviglio A, Hietala A, McCann M, Thomas W. Comparison of one-tier and two-tier newborn screening metrics for congenital adrenal hyperplasia. Pediatrics 2012;130: e1261–8. doi: 10.1542/peds.2012-1219 [DOI] [PubMed] [Google Scholar]
- 54.Nordenström A, Thilén A, Hagenfeldt L, Larsson A, Wedell A. Genotyping is a valuable diagnostic complement to neonatal screening for congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab 1999;84: 1505–9. [DOI] [PubMed] [Google Scholar]
- 55.Kösel S, Burggraf S, Fingerhut R, Dörr HG, Roscher AA, Olgemöller B. Rapid second-tier molecular genetic analysis for congenital adrenal hyperplasia attributable to steroid 21-hydroxylase deficiency. Clin Chem 2005;51: 298–304. doi: 10.1373/clinchem.2004.042416 [DOI] [PubMed] [Google Scholar]
- 56.Mikami A, Tajima T, Yamaguchi A, Sato Y, Fukushi M, Kikuchi Y, et al. Molecular diagnosis for steroid 21-hydroxylase deficiency by polymerase chain reaction with dried blood spots. Clin Pediatr Endocrinol 1997;6: 15–22. doi: 10.1297/cpe.6.15 [DOI] [Google Scholar]
- 57.Mikami A, Fukushi M, Fujita K, Fujieda K. Rapid genetic diagnosis of 21-hydroxylase deficiency using dried blood spot. Jpn J Mass Screening 2000;10: 29–34 (in Japanese). [Google Scholar]
- 58.Votava F, Török D, Kovács J, Möslinger D, Baumgartner-Parzer SM, Sólyom J, et al. Middle European Society for Paediatric Endocrinology -- Congenital Adrenal Hyperplasia (MESPE-CAH) Study Group.Estimation of the false-negative rate in newborn screening for congenital adrenal hyperplasia. Eur J Endocrinol 2005;152: 869–74. doi: 10.1530/eje.1.01929 [DOI] [PubMed] [Google Scholar]
- 59.Homma K, Hasegawa T, Takeshita E, Watanabe K, Anzo M, Toyoura T, et al. Elevated urine pregnanetriolone definitively establishes the diagnosis of classical 21-hydroxylase deficiency in term and preterm neonates. J Clin Endocrinol Metab 2004;89: 6087–91. doi: 10.1210/jc.2004-0473 [DOI] [PubMed] [Google Scholar]
- 60.Koyama Y, Homma K, Fukami M, Miwa M, Ikeda K, Ogata T, et al. Two-step biochemical differential diagnosis of classic 21-hydroxylase deficiency and cytochrome P450 oxidoreductase deficiency in Japanese infants by GC-MS measurement of urinary pregnanetriolone/ tetrahydroxycortisone ratio and 11β-hydroxyandrosterone. Clin Chem 2012;58: 741–7. doi: 10.1373/clinchem.2011.173286 [DOI] [PubMed] [Google Scholar]
- 61.Handbook of Diagnosis of 21-Hydroxylase Deficiency of the Study Group for Adrenal Hormone Production Abnormality from the Ministry of Health, Labor and Welfare Project on Intractable Disease. 2006. p. 173-185. [Google Scholar]
- 62.Martinerie L, Viengchareun S, Delezoide AL, Jaubert F, Sinico M, Prevot S, et al. Low renal mineralocorticoid receptor expression at birth contributes to partial aldosterone resistance in neonates. Endocrinology 2009;150: 4414–24. doi: 10.1210/en.2008-1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nimkarn S, Lin-Su K, Berglind N, Wilson RC, New MI. Aldosterone-to-renin ratio as a marker for disease severity in 21-hydroxylase deficiency congenital adrenal hyperplasia. J Clin Endocrinol Metab 2007;92: 137–42. doi: 10.1210/jc.2006-0964 [DOI] [PubMed] [Google Scholar]
- 64.Asanuma A, Ohura T, Ogawa E, Sato S, Igarashi Y, Matsubara Y, et al. Molecular analysis of Japanese patients with steroid 21-hydroxylase deficiency. J Hum Genet 1999;44: 312–7. doi: 10.1007/s100380050167 [DOI] [PubMed] [Google Scholar]
- 65.Mao R, McDonald J, Cantwell M, Tang W, Ward K. The implication of de novo 21-hydroxylase mutation in clinical and prenatal molecular diagnoses. Genet Test 2005;9: 121–5. doi: 10.1089/gte.2005.9.121 [DOI] [PubMed] [Google Scholar]
- 66.Tusié-Luna MT, White PC. Gene conversions and unequal crossovers between CYP21 (steroid 21-hydroxylase gene) and CYP21P involve different mechanisms. Proc Natl Acad Sci USA 1995;92: 10796–800. doi: 10.1073/pnas.92.23.10796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, et al. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 2011;96: E161–72. doi: 10.1210/jc.2010-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Usui K, Kawashim Y, Tagami T, Naruse M, Shimatsu A. Genetic diagnosis of 21-hydroxylase deficiency in Japan. Folia Endocrinol Jpn 2010;86: 735 (in Japanese). [Google Scholar]
- 69.Flück CE, Tajima T, Pandey AV, Arlt W, Okuhara K, Verge CF, et al. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet 2004;36: 228–30. doi: 10.1038/ng1300 [DOI] [PubMed] [Google Scholar]
- 70.Fukami M, Nishimura G, Homma K, Nagai T, Hanaki K, Uematsu A, et al. Cytochrome P450 oxidoreductase deficiency: identification and characterization of biallelic mutations and genotype-phenotype correlations in 35 Japanese patients. J Clin Endocrinol Metab 2009;94: 1723–31. doi: 10.1210/jc.2008-2816 [DOI] [PubMed] [Google Scholar]
- 71.Jeandron DD, Sahakitrungruang T. A novel homozygous Q334X mutation in the HSD3B2 gene causing classic 3β-hydroxysteroid dehydrogenase deficiency: an unexpected diagnosis after a positive newborn screen for 21-hydroxylase deficiency. Horm Res Paediatr 2012;77: 334–8. doi: 10.1159/000336004 [DOI] [PubMed] [Google Scholar]
- 72.Nordenström A, Forest MG, Wedell A. A case of 3beta-hydroxysteroid dehydrogenase type II (HSD3B2) deficiency picked up by neonatal screening for 21-hydroxylase deficiency: difficulties and delay in etiologic diagnosis. Horm Res 2007;68: 204–8. doi: 10.1159/000102593 [DOI] [PubMed] [Google Scholar]
- 73.White PC. Neonatal screening for congenital adrenal hyperplasia. Nat Rev Endocrinol 2009;5: 490–8. doi: 10.1038/nrendo.2009.148 [DOI] [PubMed] [Google Scholar]
- 74.Valentino R, Tommaselli AP, Rossi R, Lombardi G, Varrone S. A pilot study for neonatal screening of congenital adrenal hyperplasia due to 21-hydroxylase and 11-beta-hydroxylase deficiency in Campania region. J Endocrinol Invest 1990;13: 221–5. doi: 10.1007/BF03349544 [DOI] [PubMed] [Google Scholar]
- 75.Hishiki T, Kazukawa I, Saito T, Terui K, Mitsunaga T, Nakata M, et al. Diagnosis of adrenocortical tumor in a neonate by detection of elevated blood 17-hydroxyprogesterone measured as a routine neonatal screening for congenital adrenal hyperplasia: a case report. J Pediatr Surg 2008;43: e19–22. doi: 10.1016/j.jpedsurg.2008.05.023 [DOI] [PubMed] [Google Scholar]
- 76.Sato T, Muroya K, Hanakawa J, Asakura Y, Matsui H, Maruo Y, et al. A case of adrenocortical tumor detected by neonatal mass screening for congenital adrenal hyperplasia. Jpn J Mass Screening 2012;22: 244–9 (in Japanese). [Google Scholar]
- 77.Therrell BL, Jr, Berenbaum SA, Manter-Kapanke V, Simmank J, Korman K, Prentice L, et al. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics 1998;101: 583–90. doi: 10.1542/peds.101.4.583 [DOI] [PubMed] [Google Scholar]
- 78.Kashimada K, Ono M, Onishi T, Koyama S, Toyoura T, Imai K, et al. Clinical course of patients with nonclassical 21-hydroxylase deficiency (21-OHD) diagnosed in infancy and childhood. Endocr J 2008;55: 397–404. doi: 10.1507/endocrj.K07E-057 [DOI] [PubMed] [Google Scholar]
- 79.Nagasaki K, Usui T, Asami T, Ogawa Y, Kikuchi T, Uchiyama M. H62L Mutation of CYP21A2 identified in the non-classical form of 21-hydroxylase deficiency. Clin Pediatr Endocrinol 2009;18: 111–3. doi: 10.1297/cpe.18.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ishi T, Kashimada K, Nagasaki K, Tajima T, Yokota I, Hasegawa Y. Non-classic 21-hydroxylase deficiency in Japan. Abstract 46th Annual meeting for Japanese Society for Pediatric Endocrinology. p.118, 201.
- 81.Kashimada K, Ishii T, Nagasaki K, Ono M, Tajima T, Yokota I, et al. Clinical, biochemical, and genetic features of non-classical 21-hydroxylase deficiency in Japanese children. Endocr J 2015;62: 277–82. doi: 10.1507/endocrj.EJ14-0377 [DOI] [PubMed] [Google Scholar]
- 82.Armengaud JB, Charkaluk ML, Trivin C, Tardy V, Bréart G, Brauner R, et al. Precocious pubarche: distinguishing late-onset congenital adrenal hyperplasia from premature adrenarche. J Clin Endocrinol Metab 2009;94: 2835–40. doi: 10.1210/jc.2009-0314 [DOI] [PubMed] [Google Scholar]
- 83.Bidet M, Bellanné-Chantelot C, Galand-Portier MB, Tardy V, Billaud L, Laborde K, et al. Clinical and molecular characterization of a cohort of 161 unrelated women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency and 330 family members. J Clin Endocrinol Metab 2009;94: 1570–8. doi: 10.1210/jc.2008-1582 [DOI] [PubMed] [Google Scholar]
- 84.Bonfig W, Schmidt H, Schwarz HP. Growth patterns in the first three years of life in children with classical congenital adrenal hyperplasia diagnosed by newborn screening and treated with low doses of hydrocortisone. Horm Res Paediatr 2011;75: 32–7. doi: 10.1159/000316973 [DOI] [PubMed] [Google Scholar]
- 85.Hargitai G, Sólyom J, Battelino T, Lebl J, Pribilincová Z, Hauspie R, et al. MEWPE-CAH Study GroupGrowth patterns and final height in congenital adrenal hyperplasia due to classical 21-hydroxylase deficiency. Results of a multicenter study. Horm Res 2001;55: 161–71. doi: 10.1159/000049990 [DOI] [PubMed] [Google Scholar]
- 86.Stikkelbroeck NM, Van’t Hof-Grootenboer BA, Hermus AR, Otten BJ, Van’t Hof MA. Growth inhibition by glucocorticoid treatment in salt wasting 21-hydroxylase deficiency: in early infancy and (pre)puberty. J Clin Endocrinol Metab 2003;88: 3525–30. doi: 10.1210/jc.2002-030011 [DOI] [PubMed] [Google Scholar]
- 87.Balsamo A, Cicognani A, Baldazzi L, Barbaro M, Baronio F, Gennari M, et al. CYP21 genotype, adult height, and pubertal development in 55 patients treated for 21-hydroxylase deficiency. J Clin Endocrinol Metab 2003;88: 5680–8. doi: 10.1210/jc.2003-030123 [DOI] [PubMed] [Google Scholar]
- 88.Grigorescu-Sido A, Bettendorf M, Schulze E, Duncea I, Heinrich U. Growth analysis in patients with 21-hydroxylase deficiency influence of glucocorticoid dosage, age at diagnosis, phenotype and genotype on growth and height outcome. Horm Res 2003;60: 84–90. doi: 10.1159/000071876 [DOI] [PubMed] [Google Scholar]
- 89.Van der Kamp HJ, Otten BJ, Buitenweg N, De Muinck Keizer-Schrama SM, Oostdijk W, Jansen M, et al. Longitudinal analysis of growth and puberty in 21-hydroxylase deficiency patients. Arch Dis Child 2002;87: 139–44. doi: 10.1136/adc.87.2.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jinno K. Growth of patients with 21-hydroxylase deficiency detected by neonatal mas screening for congenital adrenal; hyperplasia. Jpn J Mass Screening 2002;12: 21–6 (in Japanese). [Google Scholar]
- 91.Tachibana K, Adachi M, Asakura Y. Growth of 21-hydroxylase deficiency. The Annual Report of Foundation for Growth Science 2002;26: 255–8 (in Japanese). [Google Scholar]
- 92.Takasawa K, Ono M, Miyai K, Matsubara Y, Takizawa F, Onishi T, et al. Initial high dose hydrocortisone (HDC) treatment for 21-hydroxylase deficiency (21-OHD) does not affect linear growth during the first three years of life. Endocr J 2012;59: 1001–6. doi: 10.1507/endocrj.EJ12-0036 [DOI] [PubMed] [Google Scholar]
- 93.Punthakee Z, Legault L, Polychronakos C. Prednisolone in the treatment of adrenal insufficiency: a re-evaluation of relative potency. J Pediatr 2003;143: 402–5. doi: 10.1067/S0022-3476(03)00294-4 [DOI] [PubMed] [Google Scholar]
- 94.Rivkees SA, Crawford JD. Dexamethasone treatment of virilizing congenital adrenal hyperplasia: the ability to achieve normal growth. Pediatrics 2000;106: 767–73. doi: 10.1542/peds.106.4.767 [DOI] [PubMed] [Google Scholar]
- 95.German A, Suraiya S, Tenenbaum-Rakover Y, Koren I, Pillar G, Hochberg Z. Control of childhood congenital adrenal hyperplasia and sleep activity and quality with morning or evening glucocorticoid therapy. J Clin Endocrinol Metab 2008;93: 4707–10. doi: 10.1210/jc.2008-0519 [DOI] [PubMed] [Google Scholar]
- 96.Kerrigan JR, Veldhuis JD, Leyo SA, Iranmanesh A, Rogol AD. Estimation of daily cortisol production and clearance rates in normal pubertal males by deconvolution analysis. J Clin Endocrinol Metab 1993;76: 1505–10. [DOI] [PubMed] [Google Scholar]
- 97.Linder BL, Esteban NV, Yergey AL, Winterer JC, Loriaux DL, Cassorla F. Cortisol production rate in childhood and adolescence. J Pediatr 1990;117: 892–6. doi: 10.1016/S0022-3476(05)80128-3 [DOI] [PubMed] [Google Scholar]
- 98.Esteban NV, Loughlin T, Yergey AL, Zawadzki JK, Booth JD, Winterer JC, et al. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab 1991;72: 39–45. doi: 10.1210/jcem-72-1-39 [DOI] [PubMed] [Google Scholar]
- 99.Bonfig W, Pozza SB, Schmidt H, Pagel P, Knorr D, Schwarz HP. Hydrocortisone dosing during puberty in patients with classical congenital adrenal hyperplasia: an evidence-based recommendation. J Clin Endocrinol Metab 2009;94: 3882–8. doi: 10.1210/jc.2009-0942 [DOI] [PubMed] [Google Scholar]
- 100.Muthusamy K, Elamin MB, Smushkin G, Murad MH, Lampropulos JF, Elamin KB, et al. Clinical review: Adult height in patients with congenital adrenal hyperplasia: a systematic review and metaanalysis. J Clin Endocrinol Metab 2010;95: 4161–72. doi: 10.1210/jc.2009-2616 [DOI] [PubMed] [Google Scholar]
- 101.Charmandari E, Hindmarsh PC, Johnston A, Brook CG. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency: alterations in cortisol pharmacokinetics at puberty. J Clin Endocrinol Metab 2001;86: 2701–8. doi: 10.1210/jcem.86.6.7522 [DOI] [PubMed] [Google Scholar]
- 102.Frisch H, Battelino T, Schober E, Baumgartner-Parzer S, Nowotny P, Vierhapper H. Salt wasting in simple virilizing congenital adrenal hyperplasia. J Pediatr Endocrinol Metab 2001;14: 1649–55. [DOI] [PubMed] [Google Scholar]
- 103.Miller WL. Clinical review 54: Genetics, diagnosis and management of 21-hydroxylasae deficiency. J Clin Endocrinol Metab 1994;78: 241–6. [DOI] [PubMed] [Google Scholar]
- 104.Tachibana K, Suwa S. Evaluation on of prescribed dose of fludrocortisone acetate in neonate and infantile patients. J Jpn Pediatr Soc 1998;102: 880–4 (in Japanese). [Google Scholar]
- 105.Charmandari E, Lichtarowicz-Krynska EJ, Hindmarsh PC, Johnston A, Aynsley-Green A, Brook CG. Congenital adrenal hyperplasia: management during critical illness. Arch Dis Child 2001;85: 26–8. doi: 10.1136/adc.85.1.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reisch N, Willige M, Kohn D, Schwarz HP, Allolio B, Reincke M, et al. Frequency and causes of adrenal crises over lifetime in patients with 21-hydroxylase deficiency. Eur J Endocrinol 2012;167: 35–42. doi: 10.1530/EJE-12-0161 [DOI] [PubMed] [Google Scholar]
- 107.Weise M, Drinkard B, Mehlinger SL, Holzer SM, Eisenhofer G, Charmandari E, et al. Stress dose of hydrocortisone is not beneficial in patients with classic congenital adrenal hyperplasia undergoing short-term, high-intensity exercise. J Clin Endocrinol Metab 2004;89: 3679–84. doi: 10.1210/jc.2003-032051 [DOI] [PubMed] [Google Scholar]
- 108.Kaufman FR, Sy JP. Regular monitoring of bone age is useful in children treated with growth hormone. Pediatrics 1999;104: 1039–42. [PubMed] [Google Scholar]
- 109.Zerah M, Ueshiba H, Wood E, Speiser PW, Crawford C, McDonald T, et al. Prevalence of nonclassical steroid 21-hydroxylase deficiency based on a morning salivary 17-hydroxyprogesterone screening test: a small sample study. J Clin Endocrinol Metab 1990;70: 1662–7. doi: 10.1210/jcem-70-6-1662 [DOI] [PubMed] [Google Scholar]
- 110.Charmandari E, Matthews DR, Johnston A, Brook CG, Hindmarsh PC. Serum cortisol and 17-hydroxyprogesterone interrelation in classic 21-hydroxylase deficiency: is current replacement therapy satisfactory? J Clin Endocrinol Metab 2001;86: 4679–85. doi: 10.1210/jcem.86.10.7972 [DOI] [PubMed] [Google Scholar]
- 111.Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet 2005;365: 2125–36. doi: 10.1016/S0140-6736(05)66736-0 [DOI] [PubMed] [Google Scholar]
- 112.Erhardt E, Sólyom J, Homoki J, Juricskay S, Soltész G. Correlation of blood-spot 17-hydroxyprogesterone daily profiles and urinary steroid profiles in congenital adrenal hyperplasia. J Pediatr Endocrinol Metab 2000;13: 205–10. doi: 10.1515/JPEM.2000.13.2.205 [DOI] [PubMed] [Google Scholar]
- 113.Izawa M, Aso K, Higuchi A, Aruiyasu D, Hasegawa Y. Pregnanetriol in the range of 1.2-2.1 mg/m2/day as an index of optimal control in CYP21A2 deficiency. Clin Pediatr Endocrinol 2007;16: 45–52. doi: 10.1297/cpe.16.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Izawa M, Aso K, Higuchi A, Ariyasu D, Hasegawa Y. The range of 2.2-3.3 mg/gCr of pregnanetriol in the first morning urine sample as an index of optimal control on CYP21 deficiency. Clin Pediatr Endocrinol 2008;17: 75–80. doi: 10.1297/cpe.17.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roche EF, Charmandari E, Dattani MT, Hindmarsh PC. Blood pressure in children and adolescents with congenital adrenal hyperplasia (21-hydroxylase deficiency): a preliminary report. Clin Endocrinol (Oxf) 2003;58: 589–96. doi: 10.1046/j.1365-2265.2003.01757.x [DOI] [PubMed] [Google Scholar]
- 116.Tanahashi Y. Adult height of patients with 21-hydroxylase deficinecy. National survey. Research Committee on Disorders of Adrenal Hormone form Intractable Disease from the Ministry of Health, Labor and Welfare. 2012. p. 52-62 (in Japanese). [Google Scholar]
- 117.Riepe FG, Krone N, Viemann M, Partsch CJ, Sippell WG. Management of congenital adrenal hyperplasia: results of the ESPE questionnaire. Horm Res 2002;58: 196–205. doi: 10.1159/000065492 [DOI] [PubMed] [Google Scholar]
- 118.Arlt W, Willis DS, Wild SH, Krone N, Doherty EJ, Hahner S, et al. United Kingdom Congenital Adrenal Hyperplasia Adult Study Executive (CaHASE)Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab 2010;95: 5110–21. doi: 10.1210/jc.2010-0917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Finkielstain GP, Kim MS, Sinaii N, Nishitani M, Van Ryzin C, Hill SC, et al. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab 2012;97: 4429–38. doi: 10.1210/jc.2012-2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Merke DP. Approach to the adult with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 2008;93: 653–60. doi: 10.1210/jc.2007-2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Han TS, Krone N, Willis DS, Conway GS, Hahner S, Rees DA, et al. United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE)Quality of life in adults with congenital adrenal hyperplasia relates to glucocorticoid treatment, adiposity and insulin resistance: United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE). Eur J Endocrinol 2013;168: 887–93. doi: 10.1530/EJE-13-0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arlt W, Krone N. Adult consequences of congenital adrenal hyperplasia. Horm Res 2007;68(Suppl 5): 158–64. doi: 10.1159/000110615 [DOI] [PubMed] [Google Scholar]
- 123.Falhammar H, Filipsson H, Holmdahl G, Janson PO, Nordenskjöld A, Hagenfeldt K, et al. Fractures and bone mineral density in adult women with 21-hydroxylase deficiency. J Clin Endocrinol Metab 2007;92: 4643–9. doi: 10.1210/jc.2007-0744 [DOI] [PubMed] [Google Scholar]
- 124.Christiansen P, Mølgaard C, Müller J. Normal bone mineral content in young adults with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res 2004;61: 133–6. doi: 10.1159/000075588 [DOI] [PubMed] [Google Scholar]
- 125.Falhammar H, Filipsson H, Holmdahl G, Janson PO, Nordenskjöld A, Hagenfeldt K, et al. Metabolic profile and body composition in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 2007;92: 110–6. doi: 10.1210/jc.2006-1350 [DOI] [PubMed] [Google Scholar]
- 126.Sartorato P, Zulian E, Benedini S, Mariniello B, Schiavi F, Bilora F, et al. Cardiovascular risk factors and ultrasound evaluation of intima-media thickness at common carotids, carotid bulbs, and femoral and abdominal aorta arteries in patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 2007;92: 1015–8. doi: 10.1210/jc.2006-1711 [DOI] [PubMed] [Google Scholar]
- 127.Reisch N, Arlt W, Krone N. Health problems in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res Paediatr 2011;76: 73–85. doi: 10.1159/000327794 [DOI] [PubMed] [Google Scholar]
- 128.David M, Forest MG. Prenatal treatment of congenital adrenal hyperplasia resulting from 21-hydroxylase deficiency. J Pediatr 1984;105: 799–803. doi: 10.1016/S0022-3476(84)80310-8 [DOI] [PubMed] [Google Scholar]
- 129.Evans MI, Chrousos GP, Mann DW, Larsen JW, Jr, Green I, McCluskey J, et al. Pharmacologic suppression of the fetal adrenal gland in utero. Attempted prevention of abnormal external genital masculinization in suspected congenital adrenal hyperplasia. JAMA 1985;253: 1015–20. doi: 10.1001/jama.1985.03350310097034 [DOI] [PubMed] [Google Scholar]
- 130.Forest MG, David M, Morel Y. Prenatal diagnosis and treatment of 21-hydroxylase deficiency. J Steroid Biochem Mol Biol 1993;45: 75–82. doi: 10.1016/0960-0760(93)90125-G [DOI] [PubMed] [Google Scholar]
- 131.New MI, Carlson A, Obeid J, Marshall I, Cabrera MS, Goseco A, et al. Prenatal diagnosis for congenital adrenal hyperplasia in 532 pregnancies. J Clin Endocrinol Metab 2001;86: 5651–7. doi: 10.1210/jcem.86.12.8072 [DOI] [PubMed] [Google Scholar]
- 132.Tajima T, Fujieda K. Prenatal diagnosis and treatment of steroid 21-hydroxylase deficiency Clin Pedaitr Endcorinol 2008;17:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Goto M, Piper Hanley K, Marcos J, Wood PJ, Wright S, Postle AD, et al. In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. J Clin Invest 2006;116: 953–60. doi: 10.1172/JCI25091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kari MA, Raivio KO, Stenman UH, Voutilainen R. Serum cortisol, dehydroepiandrosterone sulfate, and steroid-binding globulins in preterm neonates: effect of gestational age and dexamethasone therapy. Pediatr Res 1996;40: 319–24. doi: 10.1203/00006450-199608000-00021 [DOI] [PubMed] [Google Scholar]
- 135.Partsch CJ, Sippell WG, MacKenzie IZ, Aynsley-Green A. The steroid hormonal milieu of the undisturbed human fetus and mother at 16-20 weeks gestation. J Clin Endocrinol Metab 1991;73: 969–74. doi: 10.1210/jcem-73-5-969 [DOI] [PubMed] [Google Scholar]
- 136.White PC. Ontogeny of adrenal steroid biosynthesis: why girls will be girls. J Clin Invest 2006;116: 872–4. doi: 10.1172/JCI28296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Coleman MA, Honour JW. Reduced maternal dexamethasone dosage for the prenatal treatment of congenital adrenal hyperplasia. BJOG 2004;111: 176–8. doi: 10.1046/j.1471-0528.2003.00040.x [DOI] [PubMed] [Google Scholar]
- 138.White PC, Mune T, Agarwal AK. 11 beta-Hydroxysteroid dehydrogenase dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev 1997;18: 135–56. [DOI] [PubMed] [Google Scholar]
- 139.Rijnders RJ, van der Schoot CE, Bossers B, de Vroede MA, Christiaens GC. Fetal sex determination from maternal plasma in pregnancies at risk for congenital adrenal hyperplasia. Obstet Gynecol 2001;98: 374–8. doi: 10.1016/S0029-7844(01)01480-6 [DOI] [PubMed] [Google Scholar]
- 140.Bartha JL, Finning K, Soothill PW. Fetal sex determination from maternal blood at 6 weeks of gestation when at risk for 21-hydroxylase deficiency. Obstet Gynecol 2003;101: 1135–6. doi: 10.1016/S0029-7844(03)00064-4 [DOI] [PubMed] [Google Scholar]
- 141.Kinoshita E, Inomata H, Okada T, Ogawa E, Kusuda S, Saisyo S, et al. Prenatal diagnosis and treatment of congenital adrenal hyperplasia in Japan. Questionaries’ survey on Japanese pediatric endocrinologist. Part 1. Clin Endocrinol (Oxf) 2002;50: 1157–63 (in Japanese). [Google Scholar]
- 142.Tajima T, Hasegawa T, Ogawa E, Horikawa R, Kinosita E, Harada S, et al. Questionaries’ survey on Japanese pediatric endocrinologist. Part 2. Clin Endocrinol (Oxf) 2009;57: 1021–3 (in Japanese). [Google Scholar]
- 143.Trautman PD, Meyer-Bahlburg HF, Postelnek J, New MI. Effects of early prenatal dexamethasone on the cognitive and behavioral development of young children: results of a pilot study. Psychoneuroendocrinology 1995;20: 439–49. doi: 10.1016/0306-4530(94)00070-0 [DOI] [PubMed] [Google Scholar]
- 144.Meyer-Bahlburg HF, Dolezal C, Baker SW, Carlson AD, Obeid JS, New MI. Cognitive and motor development of children with and without congenital adrenal hyperplasia after early-prenatal dexamethasone. J Clin Endocrinol Metab 2004;89: 610–4. doi: 10.1210/jc.2002-021129 [DOI] [PubMed] [Google Scholar]
- 145.Hirvikoski T, Nordenström A, Lindholm T, Lindblad F, Ritzén EM, Wedell A, et al. Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. J Clin Endocrinol Metab 2007;92: 542–8. doi: 10.1210/jc.2006-1340 [DOI] [PubMed] [Google Scholar]
- 146.Hirvikoski T, Nordenström A, Lindholm T, Lindblad F, Ritzén EM, Lajic S. Long-term follow-up of prenatally treated children at risk for congenital adrenal hyperplasia: does dexamethasone cause behavioural problems? Eur J Endocrinol 2008;159: 309–16. doi: 10.1530/EJE-08-0280 [DOI] [PubMed] [Google Scholar]
- 147.Hirvikoski T, Lindholm T, Lajic S, Nordenström A. Gender role behaviour in prenatally dexamethasone-treated children at risk for congenital adrenal hyperplasia—a pilot study. Acta Paediatr 2011;100: e112–9. doi: 10.1111/j.1651-2227.2011.02260.x [DOI] [PubMed] [Google Scholar]
- 148.Meyer-Bahlburg HF, Dolezal C, Haggerty R, Silverman M, New MI. Cognitive outcome of offspring from dexamethasone-treated pregnancies at risk for congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol 2012;167: 103–10. doi: 10.1530/EJE-11-0789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fernandez-Balsells MM, Muthusamy K, Murad MH, Smushkin G, Lampropulos JF, Elamin MB, et al. Prenatal dexamethasone use for the prevention of virilization in pregnancies at risk for classical congenital adrenal hyperplasia due to 21 hydroxylase (CYP21A2) deficiency: a systematic review and meta-analyses. Clin Endocrinol (Oxf) 2010;10:73 436-44. [DOI] [PubMed]