Abstract
The effects of dietary polyphenols on human health have mainly been discussed in the context of preventing degenerative diseases, particularly cardiovascular diseases and cancer. The antioxidant properties of polyphenols have been widely studied, but it has become clear that the mechanism of action of polyphenols extends beyond the modulation of oxidative stress, as they are poorly absorbed from the digestive tract. The purpose of this study was to clarify the effects of polyphenols on the colonic environment, intestinal barrier function, and gut microbiota. We demonstrated that dietary polyphenols derived from aronia, haskap, and bilberry, markedly elevated the amount of fecal mucin and immunoglobulin A (IgA) as an intestinal barrier function and ameliorated the disturbance in gut microbiota caused by a high fat diet in rats. These results suggest that dietary polyphenols play a significant role in the prevention of degenerative diseases through improvement of the colonic environment without any absorption from the digestive tract.
Keywords: polyphenol, mucin, immunoglobulin A, gut microbiota
Introduction
The effects of dietary polyphenols on human health have mainly been discussed in the context of preventing degenerative diseases, particularly cardiovascular diseases and cancers. The antioxidant properties of polyphenols have been widely studied, but it has become clear that the mechanisms of action of polyphenols go beyond the modulation of oxidative stress, as they are poorly absorbed from the digestive tract.(1) Axling et al.(2) showed that green tea powder and Lactbacillus plantarum affect gut microbiota, lipid metabolism, and inflammation in mice fed a high-fat diet.
There is accumulating evidence to support the notion that intestinal microbiota play a significant role in the maintenance of health.(3–5) Recent data from both epidemiological and clinical studies, suggests a relationship between intestinal microbiota and the onset of obesity and the metabolic syndrome.(5) The gut microbiota is composed of billions of bacteria and archaea. Firmicutes and Bacteroidetes are the two dominant bacteria, which consist of more than 90% of all phylogenetic types. A significantly greater proportion of Firmicutes were identified in obese animals than in lean controls whilst a significant reduction in the relative abundance of Bacteroidetes was also found.(6–8) Moreover, Cani et al.(9) have shown that bacterial lipopolysaccharide (LPS) is a triggering factor for the onset of insulin resistance, obesity, and diabetes, a process termed metabolic endotoxemia. However, the underlying cellular and molecular mechanisms remain unclear. Recently, we showed that adipokine secretion from visceral adipocytes was regulated according to the presence of cell wall components from Gram-positive or Gram-negative bacteria. Our data suggest that cell wall components derived from intestinal microbiota may affect the pathogenesis of metabolic syndrome by mediating the secretion of adipokines, such as adiponectin, leptin, and resistin, from visceral adipose tissue. Furthermore, our in vivo data suggest that metabolic endotoxemia is not due to the increasing dominance of Gram-negative bacteria but a reduction of mucin affecting the intestinal barrier function.(10) On the other hand, Takahashi et al.(11) have shown that dietary polyphenols inhibit postprandial hyperlipidemia and hyperglycemia in rats.
We report here the effect of dietary polyphenols on fecal mucin, IgA content, and the micobiota in the colonic environment. Our results demonstrate that dietary polyphenols significantly increased fecal mucin and IgA content as an intestinal barrier function, in conjunction with ameliorating the disturbance in the gut microbiota caused by a high fat diet in rats. These results suggest that dietary polyphenols may play a significant role in the prevention of degenerative diseases via improvement in the colonic environment, without any absorption from the digestive tract.
Materials and Methods
Materials
Four week-old Sprague Dawley rats were purchased from Sankyo Labo service Corporation Inc. (Tokyo, Japan). Fecal Mucin Assay kit (FFA-MU-K01) was obtained from Cosmobio, Co., Ltd. (Tokyo, Japan).
Purification of polyphenols
Anthocyanin-rich polyphenols were extracted with 50% ethanol (v/v) from aronia and haskap fruits. After concentration of the extract in vacuo, the extracts were partially purified using styrene divinylbenzene synthetic adsorbent (Dia-ion HP-20). Bilberry polyphenol was purchased from Nippon Shinyaku Co., Ltd. (Kyoto, Japan). The contents of anthocyanins and other polyphenols were analyzed with an ODS (4.6 × 150 mm) column. 0.05% phosphoric acid solution was used for the mobile phase A whilst a methanol: 5% phosphoric (99:1) acid solution was used the mobile phase B at a flow rate of 1 ml/min using high-performance liquid chromatography (Shimadzu CLASS-VP V5.032, Shimadzu, Kyoto, Japan). Measurement was done with the column oven temperature set at 40°C and the UV detector set at 280 nm, over a period of the 80 min analysis time. These analyses were done in the Japan Food Research Laboratories Inc. (Tokyo, Japan). The polyphenol content of each sample is shown in Table 1.
Table 1.
Composition of experimental diets
| Ingredient (g) | LF | HF | HF + Aronia | HF + Haskap | HF + Bilberry |
|---|---|---|---|---|---|
| Casein | 250.00 | 250.00 | 250.00 | 250.00 | 250.00 |
| Mineral mixture | 35.00 | 35.00 | 35.00 | 35.00 | 35.00 |
| Vitamin mixture | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Choline bitartrate | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Corn oil | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 |
| Lard | — | 200.00 | 200.00 | 200.00 | 200.00 |
| Cellulose | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 |
| Sucrose | 602.50 | 402.50 | 385.11 | 372.42 | 391.92 |
| Aronia pigment powder | — | — | 17.39 | — | — |
| Haskap pigment powder | — | — | — | 30.08 | — |
| Bilberry pigment powder | — | — | — | — | 10.58 |
| Total | 1,000.00 | 1,000.00 | 1,000.00 | 1,000.00 | 1,000.00 |
LF, low fat diet; HF, high fat diet; HF + Aronia, high fat diet supplemented with polyphenol derived from aronia; HF + Haskap, high fat diet supplemented with polyphenol derived from hascup; HF + Bilberry, high fat diet supplemented with polyphenol derived from bilberry.
Animals and feeding condition
The animals were maintained in accordance with the Fuji Women’s University guidelines for the care and use of laboratory animals. Four week-old rats were housed 1 rat/cage in a controlled environment (12 h light cycle). After one week of acclimatization, the rats were randomly divided into 5 groups (n = 6), and thereafter fed the experimental diets ad libitum with free access to drinking water. One group was fed a low fat diet (LF) for 8 weeks. The other 4 groups were fed high-fat diets (HF, 60% fat energy) for 4 weeks to induce an obese condition. The 4 experimental diets were placed in either a HF control, HF containing aronia polyphenol (anthocyanin content was 0.4%), HF containing haskap polyphenol (anthocyanin content was 0.4%), and HF containing bilberry polyphenol (anthocyanin content was 0.4%) group. These high fat groups were further fed for 4 weeks. Each diet composition is shown in Table 2.
Table 2.
Anthocyanin content
| Anthocyanin (%) | |
|---|---|
| Aronia pigment powder | 23.0 |
| Haskap pigment powder | 13.3 |
| Bilberry pigment powder | 37.8 |
Body weight and fecal samples
Body weight was measured 4 weeks later and at the end of study. After 55 days, rats were placed in clean cages with bedding for 3 days. Total feces from each cage were collected and ground in a mortar and stored at –20°C until further analysis.
DNA extraction from fecal samples and real-time polymerase chain reaction analysis
For DNA extraction, 100 mg of ground fecal samples were weighed. DNA was extracted using QIAamp DNA Stool Mini Kit (Qiagen, Venlo, Netherlands) according to the manufacturer’s instructions, and the DNA concentration was determined using a NanoDrop (Scrum, Tokyo, Japan). Phylum-level analysis was performed on 3 phyla of intestinal bacteria: Bacteroidetes, Firmicutes, and all bacteria combined using a slight modification of the method described by Guo et al.(12) The relative population of Bacteroidetes and Firmicutes to all bacteria was quantified on the basis on amplification of genomic DNA coding 16S ribosomal RNA (rRNA) using polymerase chain reaction (PCR) with group-specific primers.(12) For the analysis of Firmicutes, the primers Firm934F (5'-GGAGYATGTGGTTTAATTCGAAGCA) and Firm1060R (5'-AGCTGACGACAACCATGCAC) were used. For the analysis of Bacteroidetes, we used Bact934F (5'-GGARCATGTGGTTTAATTCGATGAT) and Bact1060R (5'-AGCTGACGACAACCATGCAG). For the analysis of all bacteria, forward (5'-ACTCCTACGGGAGGCAGCAGT) and reverse (5'-AGTATTACCGCGGCTGCTGGCAC) primers were used. Real-time PCR was conducted using a LightCycler 480 (Roche Applied Science, Mannheim, Germany) according to the LightCycler 480 SYBR Green I Master protocol (Roche Applied Science).
Fecal mucin and immunoglobulin A analysis
Fecal mucin contents were determined using a fluorometric assay kit (Fecal Mucin assay kit) that discriminates O-linked glycoproteins (mucins) from N-linked glycoproteins.(13,14) Fecal IgA contents were determined by enzyme-linked immunosorbent assay (ELISA) using a rat IgA ELISA Quantitation Set (Bethyl Laboratories, Inc., Montgomery, TX) as specified in the manufacturer’s instructions.
Statistical analysis
The calculated values for the in vivo experiments are expressed as means ± SE (n = 6). The calculated values for the in vitro experiments are expressed as means ± SD (n = 4). Tukey’s test was used for statistical analysis.
Results
Body weight gain and stool output
After four weeks of polyphenol-administration, body weight gain was inhibited but there was no significance to HF diet control (Table 3). Stool output significantly enhanced by the administration of aronia, haskap polyphenols conpared to HF diet control (Table 4, p<0.05). Bilberry polyphenol also enhanced the stool output but there was no significance to HF diet control.
Table 3.
Body weight and body weight gain
| LF | HF | HF-Aronia | HF-Haskap | HF-Bilberry | |
|---|---|---|---|---|---|
| Final body weight (g) | 497.62 ± 13.90 | 540.61 ± 13.98 | 535.5 ± 14.27 | 519.23 ± 11.69 | 537.42 ± 20.03 |
| Body weight gain (4–8 weeks, g) | 141.06 ± 6.61 | 160.21 ± 4.51 | 152.1 ± 9.57 | 136.95 ± 3.30 | 156.96 ± 9.47 |
n = 6, mean ± SE. LF, low fat diet; HF, high fat diet; HF + Aronia, high fat diet supplemented with polyphenol derived from aronia HF + Haskap, high fat diet supplemented with polyphenol derived from hascup; HF + Bilberry, high fat diet supplemented with polyphenol derived from bilberry.
Table 4.
Stool output
| LF | HF | HF-Aronia | HF-Haskap | HF-Bilberry | |
|---|---|---|---|---|---|
| Stool output (for 3 days, g) | 4.81 ± 0.13 | 4.33 ± 0.22b | 5.19 ± 0.19a | 5.37 ± 0.15a | 5.30 ± 0.13ab |
n = 6, mean ± SE, values with different superscripts are significantly different (p<0.05). LF, low fat diet; HF, high fat diet; HF + Aronia, high fat diet supplemented with polyphenol derived from aronia HF + Haskap, high fat diet supplemented with polyphenol derived from hascup; HF + Bilberry, high fat diet supplemented with polyphenol derived from bilberry.
Fecal mucin
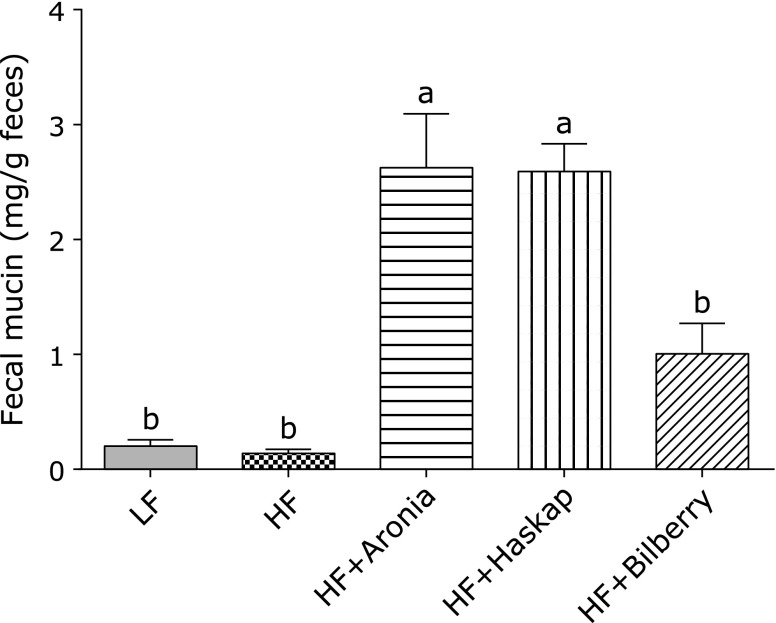
No difference was observed in fecal mucin content between the LF diet and the HF diet group. Dietary polyphenols derived from aronia and haskap markedly elevated the fecal mucin content with significance to HF diet control (p<0.05). Bilberry polyphenol also enhanced the fecal mucin content, but there was no significance to HF diet control.
Fecal Immunoglobulin A
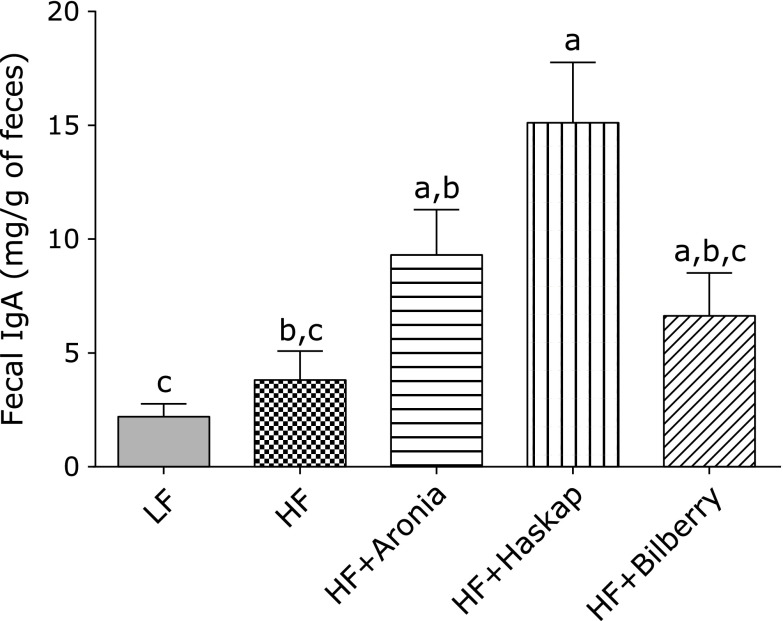
Moreover, no difference was observed in fecal IgA content between the LF diet and HF diet groups. Dietary polyphenols derived from haskap also markedly elevated the fecal IgA content (p<0.05). Aronia and bilberry polyphenol also elevated the fecal IgA content, but there were not statistically significant (p = 0.055, p = 0.053) compared to the HF diet.
Fecal microbiota
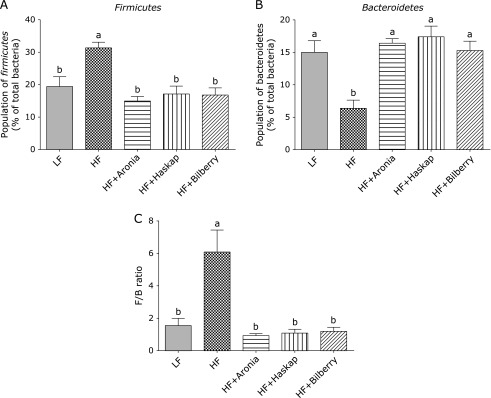
The HF diet significantly increased the population of Firmicutes and decreased the population of Bacteroidetes (p<0.05; Fig. 1 and 2), whilst the three dietary polyphenols promoted beneficial change in the population of both Firmicutes and Bacteroidetes with some approaching LF levels (p<0.05; Fig. 1 and 2). Fig. 3 shows the Firmicutes/Bacteroidetes ratio (F/B ratio) calculated in each fecal sample. The fecal F/B ratio was significantly increased in the groups fed the HF diet (p<0.05), whilst the three dietary polyphenols also ameliorated the disturbance in fecal microbiota.
Fig. 1.
Fecal mucin content. Concentration of fecal mucin was determined by using a specific fluorimetric assay. Values are presented as means ± SE (n = 6). Means which differ significantly by the Tukey’s test (p<0.05) do not share a common letter. LF, low fat diet; HF, high fat diet; HF + Aronia, high fat diet supplemented with polyphenol derived from aronia; HF + Haskap, high fat diet supplemented with polyphenol derived from hascup; HF + Bilberry, high fat diet supplemented with polyphenol derived from bilberry.
Fig. 2.
Fecal immunoglobulin A content. Fecal IgA content was determined by using an enzyme linked immunosorbent assay kit. Values are presented as means ± SE (n = 6). Means which differ significantly by the Tukey’s test (p<0.05) do not share a common letter. LF, low fat diet; HF, high fat diet; HF + Aronia, high fat diet supplemented with polyphenol derived from aronia; HF + Haskap, high fat diet supplemented with polyphenol derived from hascup; HF + Bilberry, high fat diet supplemented with polyphenol derived from bilberry.
Fig. 3.
Population of Firmicutes and Bacteroidetes in relation to the total bacteria. Population of the Firmicutes (A) and Bacteroidetes (B) division to the total bacteria was determined by group-specific primer with quantitative polymerase chain reaction method. Ratio of Firmicutes and Bacteroidetes was shown as an F/B ratio (C). Values are presented as means ± SE (n = 6). Means which differ significantly by the Tukey’s test (p<0.05) do not share a common letter. LF, low fat diet; HF, high fat diet; HF + Aronia, high fat diet supplemented with polyphenol derived from aronia; HF + Haskap, high fat diet supplemented with polyphenol derived from hascup; HF + Bilberry, high fat diet supplemented with polyphenol derived from bilberry.
Discussion
Takahashi et al.(11) have shown that dietary haskap pigment inhibits postprandial hyperlipidemia and hyperglycemia in a short-term administration. Furthermore, long-term administration of haskap pigment showed that the increase in serum triglyceride, total cholesterol and blood glucose were significantly suppressed in the rats fed with high fat diet. The purpose of this study was to clarify the effects of polyphenols on the colonic environment, intestinal barrier function, and gut microbiota. In this study, we demonstrated that dietary polyphenols derived from northern fruits, aronia, haskap, and bilberry, markedly elevated the amount of fecal mucin and IgA as an intestinal barrier function, in conjunction with ameliorating disturbance in gut microbiota caused by an HF diet in rats. Moreover, addition of the aronia haskap polyphenol enhanced the stool output with significance to HF diet control. Administration of these three polyphenols inhibited weight gain but there was no significance to HF diet control. Administration of haskap polyphenol enhanced fecal mucin and IgA content the most with significance to HF diet control (p<0.05), and also inhibited weight gain to a greater extent than the other polyphenols. However, haskap pigment powder was added significant weight to the experiment diet as an ingredient, because of the anthocyanin content of haskap pigment powder was lowest of the others (Table 1). Polyphenols except for anthocyanin may raise these effects. Anthocyanin of bilberry pigment is the highest content of all three pigments, therefore these effects of bilberry pigment may be lower than others. These effects may be dependent on the amount of pigment powder.
Bacteria form symbiotic relationships with many organisms, including humans. The bacteria live inside the human digestive system. Advancements in molecular biology have paved the way for analysis of intestinal microbiota without any cultivation of bacteria. Another advantage of this genome analysis is not only to analyze the increase and decrease of single strains but also to make it possible to analyze the flora in all taxonomic levels, Kingdom, Phylum, Class, Order, Family, Genus, and Species. It has been reported that 80–90% of bacterial phylotypes are a member of two phyla, namely Bacteroidetes (including the genera Bacteroides and Prevotella) and Firmicutes (including genera Clostridium, Enterococcus, Lactobacillus, and Ruminococcus). A significant extent of phylum level analysis of Firmicutes and Bacteroidetes using specific primers has shown associations between obesity and the reduced proportion of the phylum Bacteroidetes which were also accompanied by increased proportion of Firmicutes.(6–8,12) Therefore, the relative proportion of Firmicutes and Bacteroidetes (F/B ratio) is considered a good indicator of major changes in intestinal microbiota constitution. Our study also showed that the F/B ratio correlated highly with diet-induced obesity. Indeed, the fecal F/B ratio was significantly increased by feeding an HF diet compared with a LF diet, and the three dietary polyphenols ameliorated the disturbance in fecal microbiota caused by an HF diet.
Okazaki et al.(15–17) have shown that dietary polyphenols increase fecal mucin and IgA, on the other hand, Parkar et al.(18) have shown that dietary polyphenols directly alter the gut microbiota balance. It is not clear whether the effects of dietary polyphenols on host cells is first, or alteration of microbiota balance is first.
These results suggest that dietary polyphenols play a significant role in the prevention of degenerative diseases via the improvement in colonic environment without any absorption from digestive tract. Or Antioxidant property of polyphenols may play a role to maintain the anaerobic condition in the large intestine.
Acknowledgments
Some parts of this study were conducted with the grant of the Grant-in-Aid for Scientific Research B (Grant number 25292017).
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Scalbert A, Johnson IT, Saltmarsh M.Polyphenols: antioxidants and beyond. Am J Clin Nutr 2005; 81 (Suppl): 215S–217S. [DOI] [PubMed] [Google Scholar]
- 2.Axling U, Olsson C, Xu J, et al. Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr Metab (Lond) 2012;9:105. doi: 10.1186/1743-7075-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitsuoka T. The effect of nutrition on intestinal flora. Nahrung. 1984;28:619–625. doi: 10.1002/food.19840280616. (in German) [DOI] [PubMed] [Google Scholar]
- 4.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 5.Membrez M, Blancher F, Jaquet M, et al. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 2008;22:2416–2426. doi: 10.1096/fj.07-102723. [DOI] [PubMed] [Google Scholar]
- 6.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ley RE, Bäckhed F, Turnbaugh PJ, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:1107–1175. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cani PD, Amar J, Iqlesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 10.Taira R, Yamaguchi S, Shimizu K, Nakamura K, Ayabe T, Taira T. Bacterial cell wall components regulate adipokine secretion from visceral adipocytes. J Clin Biochem Nutr. 2015;56:149–154. doi: 10.3164/jcbn.14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi A, Okazaki Y, Nakamoto A, et al. Dietary anthocyanin-rich Haskap phytochemicals inhibit postprandial hyperlipidemia and hyperglycemia in rats. J Oleo Sci. 2014;63:201–209. doi: 10.5650/jos.ess13196. [DOI] [PubMed] [Google Scholar]
- 12.Guo X, Xia X, Tang R, Zhou J, Zhao H, Wang K. Development of a real-time PCR method for Firmicutes and Bacteroidetes in feces and its application to quantify intestinal population of obese and lean pig. Lett Appl Microbiol. 2008;47:367–373. doi: 10.1111/j.1472-765X.2008.02408.x. [DOI] [PubMed] [Google Scholar]
- 13.Bovee-Oudenhoven IM, Termont DS, Heidt PJ, Van der Meer R. Increasing the intestinal resistance of rats to the invasive pathogen Salmonella enteritidis: additive effects of dietary lactulose and calcium. Gut. 1997;40:497–504. doi: 10.1136/gut.40.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowther RS, Wetmore RF. Fluorometric assay of O-linked glycoproteins by reaction with 2-cyanoacetamide. Anal Biochem. 1987;163:170–174. doi: 10.1016/0003-2697(87)90108-4. [DOI] [PubMed] [Google Scholar]
- 15.Okazaki Y, Han Y, Kayahara M, Watanabe T, Arishige H, Kato N. Consumption of curcumin elevates fecal immunoglobulin A, an index of intestinal immune function, in rats fed a high-fat diet. J Nutr Sci Vitaminol (Tokyo) 2010;56:68–71. doi: 10.3177/jnsv.56.68. [DOI] [PubMed] [Google Scholar]
- 16.Utama Z, Okazaki Y, Tomotake H, Kato N. Tempe consumption modulate fecal secondary bile acids, mucins, immunoglobulin A, enzyme activities, and cecal microflora and organic acid in rats. Plant Foods Hum Nutr. 2013;68:177–183. doi: 10.1007/s11130-013-0357-x. [DOI] [PubMed] [Google Scholar]
- 17.Okazaki Y, Tomotake H, Tsujimoto K, Sasaki M, Kato N. Consumption of a resistant protein, sericin, elevates fecal immunoglobulin A, mucin, and cecal organoic acids in rats fed a high-fat diet. J Nutr. 2011;141:1975–1981. doi: 10.3945/jn.111.144246. [DOI] [PubMed] [Google Scholar]
- 18.Parkar SG, Trower TM, Stevenson DE. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe. 2013;23:12–19. doi: 10.1016/j.anaerobe.2013.07.009. [DOI] [PubMed] [Google Scholar]