Abstract
Botulinum toxin injection is an accepted treatment modality for esophageal achalasia in western countries. This pilot study aimed to clarify the effectiveness of botulinum toxin injection for esophageal achalasia in Japanese patients. We enrolled 10 patients diagnosed with esophageal achalasia between 2008 and 2014. A total of 100 U botulinum toxin A was divided into eight aliquots and injected around the esophagogastric junction. We compared the lower esophageal sphincter pressure before and 1 week after treatment. Scores of subjective symptoms for esophageal achalasia were assessed using a visual analog scale (VAS) before and after 1 week of follow-up of treatment. Barium passage was improved in barium esophagography and passage of contrast agent was also improved. Mean Eckardt score was reduced from 5.5 to 1.6 after treatment (p<0.001). By esophageal manometric study, mean lower esophageal sphincter pressure was reduced from 46.9 to 29.1 mmHg after treatment (p = 0.002). One week after treatment, mean VAS score was reduced from 10 to 3.9 (p<0.001). There were no side effects in any cases. Botulinum toxin injection for esophageal achalasia was safe and effective with few complications. Therefore, botulinum toxin could be used as minimally invasive therapy for esophageal achalasia in Japan.
Keywords: esophageal achalasia, botulinum toxins, injections, endoscopy, esophageal sphincter
Introduction
Esophageal achalasia is a primary esophageal motility disorder characterized by lack of peristalsis and failure of the lower esophageal sphincter (LES) to relax in response to swallowing. The etiology of achalasia has not yet been clarified. However, degeneration and loss of ganglion cells in the Auerbach’s nerve plexus have been reported and result in esophageal motor dysfunction.(1)
The diagnosis of achalasia is based on a careful clinical history and the results of barium esophagography, endoscopy, and esophageal manometry. Dysphagia, regurgitation, chest pain, and weight loss are among the most recognized clinical features of the disease.(2) Achalasia is a rare disease, with an estimated annual incidence of one case per 100,000 people.(3)
By esophagogastroscopy, we identified esophageal dilation, atony, chaotic spasticity of the esophageal body, food or fluid retention, or resistance to the passage of the endoscope into the stomach. The main reason to perform endoscopy was to exclude the presence of other pathologies, such as cancer. Patients with achalasia have an increased risk of developing stasis-related squamous cell cancer.(4)
Treatment for esophageal achalasia can be endoscopic or surgical. Botulinum toxin injection was developed in 1995,(5) and has been used for many patients in Europe and the United States. Botulinum toxin injection is the most common initial treatment for achalasia in the United States and is more efficacious than other treatments such as pneumatic dilation (PD) or laparoscopic Heller myotomy (LHM).(4) According to one report of 896 patients with achalasia in the United States, botulinum toxin injection was the most frequently used as the first-choice endoscopic therapy in 41%.(6)
In Japan, esophageal achalasia has been treated with oral drugs, endoscopic balloon dilation,(2,3) per-oral endoscopic myotomy (POEM),(7,8) and surgery. However, local injection of botulinum toxin for esophageal achalasia has not been approved as a treatment for medical insurance in Japan. As a result, botulinum toxin injection is rare in Japan. We have reported two cases of esophageal achalasia in Japan, in which symptoms were improved by localized botulinum toxin injections.(9)
In this study, we conducted a pilot trial to evaluate the effectiveness of local injection of botulinum toxin for esophageal achalasia, and to assess its safety and adverse effects among Japanese patients.
Materials and Methods
Between January 2008 and December 2014, 10 patients were diagnosed with esophageal achalasia in Saga Medical School Hospital, Japan. Informed consent was obtained from these patients to receive botulinum toxin injection therapy. Patients were clinically assessed before treatment, including barium esophagography, esophagogastroscopy, and esophageal manometry. The definition of achalasia by barium esophagography is dilatation of the esophagus and/or liquid or food retention in the esophagus with the lower esophagus having the appearance of a ‘‘bird’s beak.’’ The maximum diameter of the thoracic esophagus was measured using barium esophagography, and achalasia was classified into Grade I, diameter <3.5 cm; Grade II, diameter 3.5–6.0 cm; and Grade III, diameter >6.0 cm, in accordance with the descriptive rules.(10) Achalasia was classified as straight or sigmoid using barium esophagography.
Esophageal manometry analyzed each swallow for the morphology of the esophagogastric junction, the extent of esophagogastric junction relaxation, the contractile front velocity of peristalsis, vigor of the peristaltic contraction, and abnormality of intrabolus pressure. Esophageal manometry was performed in all patients with four-channel manometry (GMMS-600; Star Medical, Tokyo, Japan). All patients were measured in the standing position after fasting overnight. The manometric assembly was passed via an anesthetized nostril and positioned so that the most distal of the 4 cm interval side holes was 1 cm above the LES. The LES pressure was measured by the rapid pull-through method, with the subject in the standing position. Baseline recordings of the lower esophageal sphincter pressure (LESP) were made for 5 min, after which primary peristalsis was assessed. The manometric protocol included 5 min to assess basal sphincter pressure and 5-ml water swallows.
Achalasia was classified into two types by LESP. Vigorous achalasia was defined by amplitude ≥37 mmHg, and classic achalasia as amplitude <37 mmHg.(11)
Symptoms were evaluated with the Eckardt score.(8) Eckardt score was the sum of the symptom scores for dysphagia, regurgitation, and chest pain (with 0 indicating absence of symptoms, 1 occasional symptoms, 2 daily symptoms, and 3 symptoms at each meal), and weight loss (with 0 indicating no weight loss, 1 loss of <5 kg, 2 loss of 5–10 kg, and 3 indicating loss of >10 kg). Thus, the maximum score on the Eckardt scale, indicating the most pronounced symptoms, was 12. Patients were considered to have reached clinical remission if the total symptom score was ≤3. Recurrence was defined as Eckardt score >3.
In this study, a total of 100 U botulinum toxin A (BOTOX; Allergan, Irvine, CA) was divided into eight aliquots (25 U per quadrant), with four injections every 90 degree at the esophagogastric junction and four injections every 90 degree 1 cm above the esophagogastric junction. After 1 week, we re-measured the shape of the esophagus using barium esophagography and LESP by esophageal manometry. We compared the shape and LESP of achalasia before and after treatment.
After 1 week of follow-up of the local injection, the subjective symptoms for esophageal achalasia were assessed using a visual analog scale (VAS; no symptom 0, maximum symptoms 10). Patients were periodically followed up at our outpatient clinic. When symptoms of esophageal achalasia relapsed, local injection of botulinum toxin was repeated according to the patients’ request.
The Eckardt score, LESP and subjective symptom score data were analyzed using Student’s t test. Differences were considered to be statistically significant at the p<0.05 level. All analyses were performed using SAS ver. 9.1 (SAS Institute, Cary, NC).
The study was conducted after the protocol had been approved by the Ethics Review Committee of Saga Medical School.
Results
Table 1 summarizes the characteristics of 10 cases of esophageal achalasia. There were five men and five women, with a mean age of 55 (33–72) years. Six cases were straight type and four were sigmoid type. Two cases had Grade I achalasia (diameter 4 cm) and eight were Grade II (diameter 4–6 cm). Esophageal manometry showed that seven cases were classic type and three were vigorous type. The mean duration of symptoms was 8.1 (1–20) years. Four cases had comorbidity of hypertension, diabetes mellitus, and chronic hepatitis C.
Table 1.
Characteristics of 10 cases of esophageal achalasia
| Age (years) | Gender | Form | Size (Grade) | Type | Duration of symptoms (years) | Comorbidities | |
|---|---|---|---|---|---|---|---|
| Case 1 | 64 | M | Straight | II | vigorous | 10 | none |
| Case 2 | 57 | F | Straight | II | vigorous | 3 | none |
| Case 3 | 33 | F | Straight | II | vigorous | 18 | none |
| Case 4 | 42 | M | Straight | II | classic | 3 | none |
| Case 5 | 66 | M | Straight | II | vigorous | 1 | none |
| Case 6 | 35 | F | Sigmoid | I | vigorous | 7 | none |
| Case 7 | 64 | M | Straight | I | vigorous | 20 | chronic hepatitis C |
| Case 8 | 72 | F | Sigmoid | II | classic | 2 | diabetes mellitus |
| Case 9 | 63 | M | Sigmoid | II | classic | 10 | hypertension |
| Case 10 | 54 | F | Sigmoid | II | vigorous | 7 | hypertension |
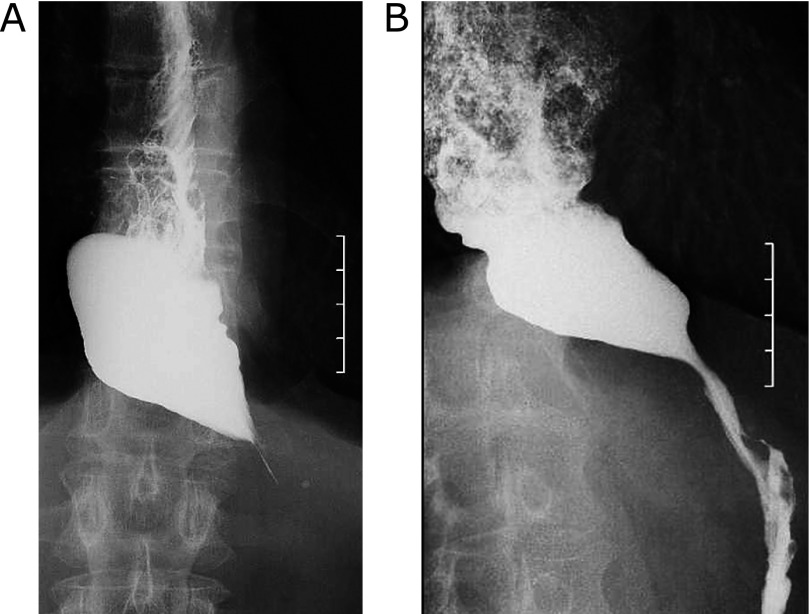
Cases that were treated effectively with botulinum toxin are shown in Fig. 1. Fig. 1 shows barium esophagography in esophageal achalasia. Before botulinum toxin injection, the lower esophagus had a bird’s beak appearance and barium did not pass through the esophagogastric junction for more than a few minutes (Fig. 1A). After botulinum toxin injection, passage of contrast agent was improved (Fig. 1B). Barium passage was improved in barium esophagography and passage of contrast agent was also improved in all cases.
Fig. 1.
Barium esophagography in esophageal achalasia before (A) and after (B) botulinum toxin injection. The lower esophagus with a bird’s beak appearance was observed and barium did not pass through the esophagogastric junction for more than a few minutes (A). Passage of contrast agent was improved (B).
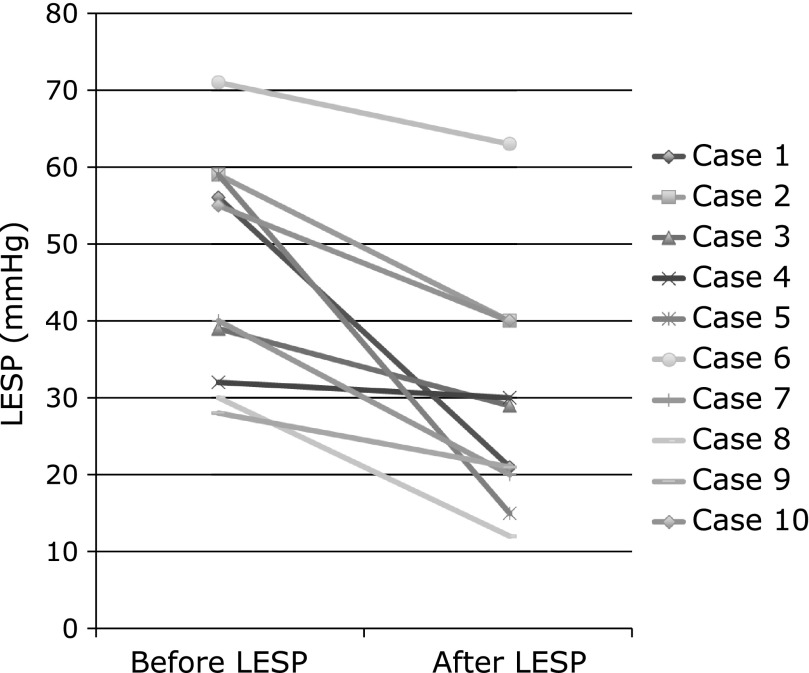
Before botulinum toxin injection, esophageal manometry showed each swallow for the contractile abnormality of peristalsis and markedly elevated residual LESP. After botulinum toxin injection, esophageal manometry showed improvement of residual LESP, and abnormal peristaltic waves had disappeared. Mean LESP was reduced from 46.9 (28–71) mmHg before treatment to 29.1 (12–63) mmHg after treatment (95% CI, 8.515–27.095, p = 0.002) (Fig. 2).
Fig. 2.
The upper panel shows mean LESP in before treatment of botulinum toxin injection, and LESP in one week after treatment. The mean LESP in before and after treatment was 46.9 mmHg (28–71) and 29.1 mmHg (12–63), respectively. (95% CI, 8.515–27.095, p = 0.002)
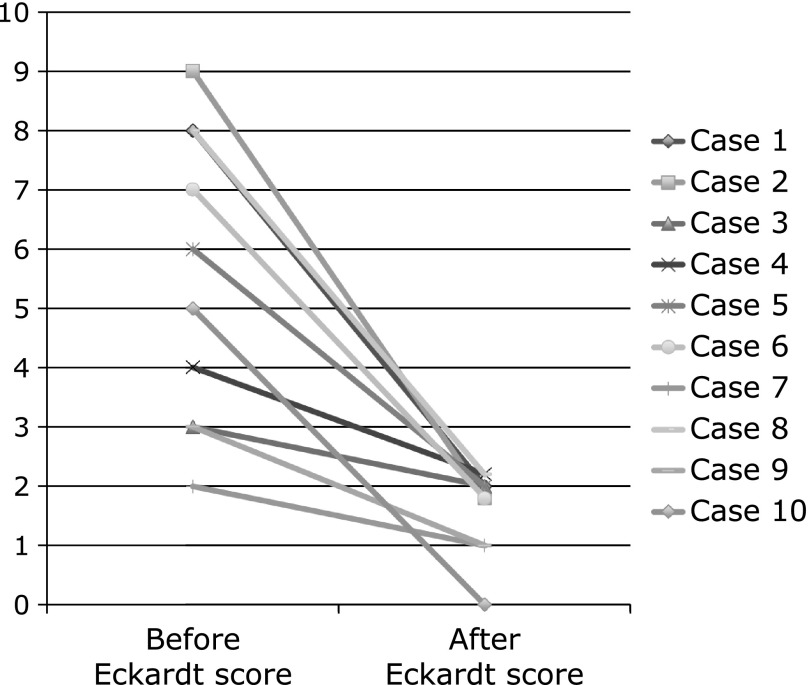
Mean Eckardt score was reduced from 5.5 (2–9) before treatment to 1.6 (0–2) after treatment [95% confidence interval (CI), 2.302–5.498, p<0.001] (Fig. 3).
Fig. 3.
The upper panel shows mean Eckardt score in before treatment of botulinum toxin injection, and Eckardt score in one week after treatment. The mean Eckardt score in before and after treatment was 5.5 (2–9) and 1.6 (0–2), respectively. (95% CI, 2.302–5.498, p<0.001)
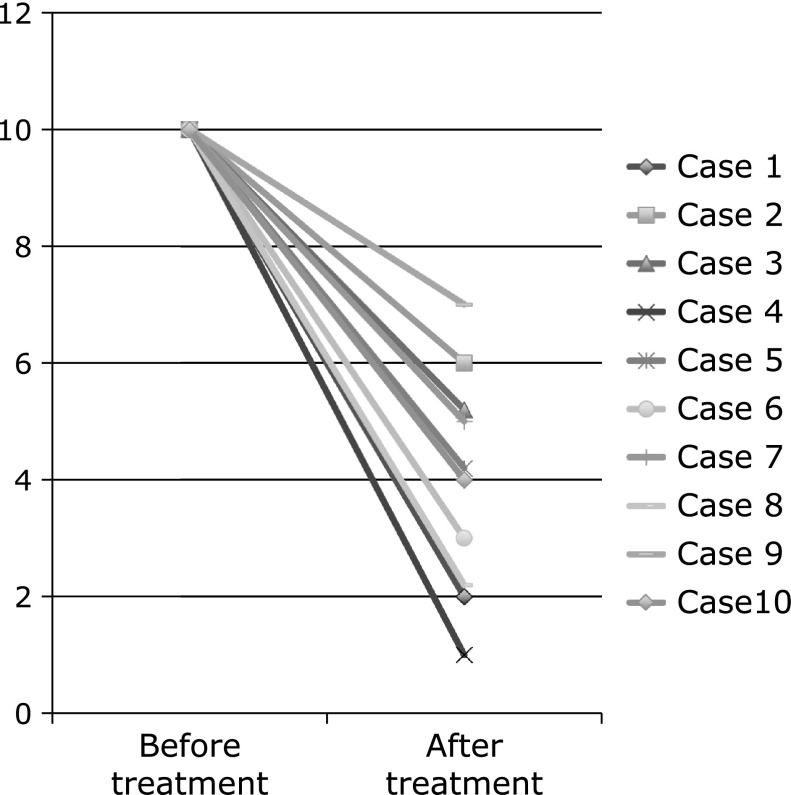
Mean score of subjective symptoms was reduced from 10 before treatment to 3.9 1 week after treatment (95% CI, 4.830–7.370, p<0.001) (Fig. 4).
Fig. 4.
The upper panel shows mean score of subjective symptoms in before treatment of botulinum toxin injection, and mean score of subjective symptoms in one week after treatment. The mean score of subjective symptoms in before and after treatment was 10 and 3.9 (1–7), respectively. (95% CI, 4.830–7.370, p<0.001)
During follow-up periods, five cases relapsed at 3–24 months after treatment (Table 2). The mean score of subjective symptoms was 5.4 (4–7) in five recurrent cases and 2.4 (1–4) in nonrecurrent cases. The scores for recurrent cases were significantly higher than those of the nonrecurrent cases (95% CI, 1.337–4.663, p<0.001). Three cases relapsed at 3, 6 and 24 months after treatment (Cases 3, 5 and 9) and received additional botulinum toxin therapy at their request.
Table 2.
Outcomes of treatment of botulinum toxin injection
| Recurrence | After score of symptoms (before 10) | Time to recurrence (months) | Duration of follow-up (months) | Side effects | |
|---|---|---|---|---|---|
| Case 1 | (–) | 2 | 0 | 72 | (–) |
| Case 2 | (+) | 6 | 6 | 18 | (–) |
| Case 3 | (+) | 5 | 24 | 60 | (–) |
| Case 4 | (–) | 1 | 0 | 48 | (–) |
| Case 5 | (+) | 4 | 6 | 48 | (–) |
| Case 6 | (–) | 3 | 0 | 36 | (–) |
| Case 7 | (+) | 5 | 3 | 18 | (–) |
| Case 8 | (–) | 2 | 0 | 24 | (–) |
| Case 9 | (+) | 7 | 3 | 18 | (–) |
| Case 10 | (–) | 4 | 0 | 18 | (–) |
Treatment was effective until the end of observation. However, one case received PD therapy 6 months after initial treatment (Case 2), and another received POEM 3 months after initial treatment (Case 7) because of recurrence. The therapeutic effect in eight cases was conservatively sustained until December 2014, with mean follow-up of 36 months. Side effects of botulinum toxin injection were not experienced up to December 2014 in all cases.
Discussion
In this study, we confirmed three important clinical observations. First, local injection of botulinum toxin was effective for Japanese as well as western achalasia patients. Second, botulinum toxin injection was safe compared with several other therapies for esophageal achalasia. Third, a high score for subjective symptoms after treatment became an important indicator of recurrence.
Therapeutic utility of botulinum toxin was first published in the 1980s.(12) Commercially available botulinum toxin is composed of botulinum toxin component (botulinum neurotoxin and complexing proteins) and excipients. Botulinum neurotoxin consists of a heavy amino acid chain connected to a lighter amino acid chain by means of a single disulfide bridge. After injection into the target tissue, the botulinum toxin binds to glycoprotein structures on the cholinergic nerve terminals, and the light chain, a protease, is internalized and cleaves the soluble N-ethylmaleimide-sensitive fusion attachment protein receptor proteins, thereby disabling vesicles from anchoring to the membrane and releasing acetylcholine. This blockage of the cholinergic synapse forces the neurons to form new synapses; a process known as sprouting.(13,14) There are eight different, antigenically distinct serotypes of Clostridium botulinum.(15) The most commonly used and studied serotype is botulinum toxin A, and achalasia studies have used it exclusively. However, botulinum toxin B is also commercially available.(16)
In the United States, botulinum toxin injection was most frequently used as the first-choice endoscopic therapy in 41% of cases, followed by balloon dilation in 21%, Savary dilation in 20%, Maloney dilation in 10%, Rigiflex in 4%, and other modalities in 4%.(6) Botulinum toxin injection has become the primary choice for initial endoscopic therapy.
A previous study has reported that 1 month after botulinum toxin injection, 31 of 37 patients (83.7%) showed significant improvement.(17) The treatment outcome of our study was similar. Botulinum toxin injection for esophageal achalasia is also useful in Japanese patients, as reported in western countries.
However, some studies have reported that most patients who are treated with botulinum toxin injection experience relapse, usually 6–12 months after the first treatment, and often require repeated treatment.(18) A meta-analysis of nine studies indicated that the percentage of patients with symptomatic improvement after one session of botulinum toxin injection was 78.7% at 1 month, 70% at 3 months, 53.3% at 6 months, and 40.6% at >12 months follow-up. However, at least a second botulinum toxin injection was required in 46.6% of patients.(19) Its efficacy decreases with repeat injections, possibly as a result of antibody formation. Therefore, botulinum toxin injection is considered to be effective in the short term, but has a risk of relapse. Our study was a small pilot study and the therapeutic effect was conservatively sustained in eight cases and good progress was achieved. The mean follow-up was 2 years, but we need to extend the surveillance periods and increase the number of patients.
Botulinum toxin injection into the LES is an established therapy for achalasia and has few side effects.(20,21) Reported side effects of botulinum toxin injection include mild chest pain and mild heartburn, which is easily controlled by antacid therapy.(5) Rare systemic side effects such as diffuse neuromuscular jitters and autonomic side effects such as dry mouth and eye accommodation difficulties have been reported, especially with high doses of botulinum toxin B. In the present study, there were no serious side effects. One study has shown that there was no significant difference in remission rates between PD and botulinum toxin at 4 weeks of intervention. However, PD appeared to be more effective after long-term follow-up of >6 months.(4) According to five studies that compared botulinum toxin injection with PD, the remission rate was 65.8% for PD compared with 36% for botulinum toxin injection, and the time to relapse after the first intervention was shorter for patients who received botulinum toxin compared with PD.(22) However, esophageal perforation is a serious complication of PD. In one study, perforation occurred at a rate of 3–5% (range 0–20%). Some perforations can be managed nonoperatively, but free perforation requires urgent laparotomy or thoracotomy.(23) POEM is a new endoscopic procedure to treat achalasia, and is a modification of Pasricha’s submucosal endoscopic esophageal myotomy.(8) In POEM, visible complete transmural openings into the mediastinum and peritoneal cavity are created in the majority of patients. Therefore, POEM potentially carries the risk of mediastinitis, peritonitis and damage to surrounding organs. The following complications were reported for POEM: perforation (4%), mucosal injury (4%), bleeding requiring intervention (1%), and mediastinal hematoma (1%).(24) Esophageal perforation is the most common major complication for LHM, with an incidence of 0–4.6%.(25) In another study, LHM had complications such as gastrointestinal perforation (1.5%), pneumothorax (1.9%), and pulmonary complications (1.3%).(26) The difference in safety between botulinum toxin injection and other invasive treatment options such as PD or LHM is arguably the reason why botulinum toxin is the most commonly used initial therapy for achalasia in the United States.(27) The outcome of botulinum toxin injection was comparable to the outcome of several other therapies for esophageal achalasia, while injection therapy was safer than other therapies.
In our study, the subjective symptoms score in five recurrent cases was significantly higher than in the nonrecurrent cases. However, there was no difference in Eckardt score and LESP between the recurrent and nonrecurrent cases. Patients with high subjective symptom scores are likely to experience recurrence within a short time. Intensive follow-up might be required in such cases.
The limitations of our study were its small number of participants and single center analysis. A large number of participants are necessary to validate our results. In addition, there is no universal gold standard technique for injecting botulinum toxin into the LES. Various researchers have used different techniques.(23,28) A previous study has reported that relapse of symptoms was evident in 19% of patients who received two injections of 100 U compared with 47% and 43% in the single 50 U and 200 U groups, respectively.(28) Further studies are required to validate botulinum toxin injection for achalasia and establish an adequate method of injection.
In conclusion, botulinum toxin injection for achalasia was safe and effective, with few complications in Japanese patients. Therefore, botulinum toxin could be one of the less invasive treatment modalities for esophageal achalasia in Japan.
Acknowledgments
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Abbreviations
- BTX
botulinum toxin
- LES
lower esophageal sphincter
- LESP
lower esophageal sphincter pressure
- LHM
laparoscopic Heller myotomy
- PD
pneumatic dilation
- POEM
per-oral endoscopic myotomy
- VAS
visual analog scale
Authors’ Contributions
Guarantor of the article: Daisuke Yamaguchi, MD. Specific author contributions: Principal investigator, subject recruitment, subject evaluation, data collection and manuscript preparation: Daisuke Yamaguchi; manuscript preparation and statistical analysis: Nanae Tsuruoka, Yasuhisa Sakata, Ryo Shimoda, Kazuma Fujimoto, Ryuichi Iwakiri.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hoshino M, Omura N, Yano F, Tsuboi K, Kashiwagi H, Yanaga K. Immunohistochemical study of the muscularis externa of the esophagus in achalasia patients. Dis Esophagus. 2013;26:14–21. doi: 10.1111/j.1442-2050.2011.01318.x. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka Y, Iwakiri K, Kawami N, et al. Predictors of a better outcome of pneumatic dilatation in patients with primary achalasia. J Gastroenterol. 2010;45:153–158. doi: 10.1007/s00535-009-0145-4. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita H, Ashida K, Fukuchi T, et al. Predictive factors associated with the success of pneumatic dilatation in Japanese patients with primary achalasia: a study using high-resolution manometry. Digestion. 2013;87:23–28. doi: 10.1159/000343902. [DOI] [PubMed] [Google Scholar]
- 4.Ramzan Z, Nassri AB. The role of Botulinum toxin injection in the management of achalasia. Curr Opin Gastroenterol. 2013;29:468–473. doi: 10.1097/MOG.0b013e328362292a. [DOI] [PubMed] [Google Scholar]
- 5.Pasricha PJ, Ravich WJ, Hendrix TR, Sostre S, Jones B, Kalloo AN. Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med. 1995;332:774–778. doi: 10.1056/NEJM199503233321203. [DOI] [PubMed] [Google Scholar]
- 6.Enestvedt BK, Williams JL, Sonnenberg A. Epidemiology and practice patterns of achalasia in a large multi-centre database. Aliment Pharmacol Ther. 2011;33:1209–1214. doi: 10.1111/j.1365-2036.2011.04655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Renteln D, Inoue H, Minami H, et al. Peroral endoscopic myotomy for the treatment of achalasia: a prospective single center study. Am J Gastroenterol. 2012;107:411–417. doi: 10.1038/ajg.2011.388. [DOI] [PubMed] [Google Scholar]
- 8.Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265–271. doi: 10.1055/s-0029-1244080. [DOI] [PubMed] [Google Scholar]
- 9.Tominaga N, Iwakiri R, Tsuruoka N, et al. Two cases of esophageal achalasia, in which localized botulinum toxin injections were effective to improve symptoms. Nihon Shokakibyo Gakkai Zasshi. 2010;107:598–604. (in Japanese) [PubMed] [Google Scholar]
- 10.The Japan Esophageal Society . Descriptive Rules for Achalasia of the Esophagus (4th ed.) Tokyo: Kanehara-syuppan; 2012. (in Japanese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldenberg SP, Burrell M, Fette GG, Vos C, Traube M. Classic and vigorous achalasia: a comparison of manometric, radiographic, andclinical findings. Gastroenterology. 1991;101:743–748. doi: 10.1016/0016-5085(91)90534-r. [DOI] [PubMed] [Google Scholar]
- 12.Ascher B, Fanchon C, Kanoun-Copy L, Bouloc A, Benech F. A skincare containing retinol adenosine and hyaluronic acid optimises the benefits from a type A botulinum toxin injection. J Cosmet Laser Ther. 2012;14:234–238. doi: 10.3109/14764172.2012.712700. [DOI] [PubMed] [Google Scholar]
- 13.Lee SK. Multiple intradermal small bolus injection of botulinum toxin: the limit and the potentiality. J Cosmet Laser Ther. 2012;14:304–306. doi: 10.3109/14764172.2012.738914. [DOI] [PubMed] [Google Scholar]
- 14.Shen Y, Vasandani P, Iyer J, et al. Virtual trainer for intra-detrusor injection of botulinum toxin to treat urinary incontinence. Stud Health Technol Inform. 2012;173:457–462. [PubMed] [Google Scholar]
- 15.Al-Halfawy A, Gomaa NE, Refaat A, Wissa M, Wahidi MM. Endobronchial injection of botulinum toxin for the reduction of bronchial hyperreactivity induced by methacholine inhalation in dogs. J Bronchology Interv Pulmonol. 2012;19:277–283. doi: 10.1097/LBR.0b013e318271179e. [DOI] [PubMed] [Google Scholar]
- 16.Kuo YC, Kuo HC. Botulinum toxin injection for lower urinary tract dysfunction. Int J Urol. 2013;20:40–55. doi: 10.1111/j.1442-2042.2012.03035.x. [DOI] [PubMed] [Google Scholar]
- 17.D'Onofrio V, Miletto P, Leandro G, Iaquinto G. Long-term follow-up of achalasia patients treated with botulinum toxin. Dig Liver Dis. 2002;34:105–110. doi: 10.1016/s1590-8658(02)80238-9. [DOI] [PubMed] [Google Scholar]
- 18.Pasricha PJ, Rai R, Ravich WJ, Hendrix TR, Kalloo AN. Botulinum toxin for achalasia: long-term outcome and predictors of response. Gastroenterology. 1996;110:1410–1415. doi: 10.1053/gast.1996.v110.pm8613045. [DOI] [PubMed] [Google Scholar]
- 19.Campos GM, Vittinghoff E, Rabl C, et al. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg. 2009;249:45–57. doi: 10.1097/SLA.0b013e31818e43ab. [DOI] [PubMed] [Google Scholar]
- 20.Çiftçi T, Akıncı D, Yurttutan N, Akhan O. US-guided botulinum toxin injection for excessive drooling in children. Diagn Interv Radiol. 2013;19:56–60. doi: 10.4261/1305-3825.DIR.5940-12.1. [DOI] [PubMed] [Google Scholar]
- 21.Skaf GS, Domloj NT, Salameh JA, Atiyeh B. Pseudoaneurysm of the superficial temporal artery: a complication of botulinum toxin injection. Aesthetic Plast Surg. 2012;36:982–985. doi: 10.1007/s00266-012-9881-6. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Li YM, Li L. Meta-analysis of randomized and controlled treatment trials for achalasia. Dig Dis Sci. 2009;54:2303–2311. doi: 10.1007/s10620-008-0637-8. [DOI] [PubMed] [Google Scholar]
- 23.Triadafilopoulos G, Boeckxstaens GE, Gullo R, et al. The Kagoshima consensus on esophageal achalasia. Dis Esophagus. 2012;25:337–348. doi: 10.1111/j.1442-2050.2011.01207.x. [DOI] [PubMed] [Google Scholar]
- 24.Von Renteln D, Fuchs KH, Fockens P, et al. Peroral endoscopic myotomy for the treatment of achalasia: an international prospective multicenter study. Gastroenterology. 2013;145:309–311. doi: 10.1053/j.gastro.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 25.Lynch KL, Pandolfino JE, Howden CW, Kahrilas PJ. Major complications of pneumatic dilation and Heller myotomy for achalasia: single-center experience and systematic review of the literature. Am J Gastroenterol. 2012;107:1817–1825. doi: 10.1038/ajg.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onimaru M, Inoue H, Ikeda H, et al. Peroral endoscopic myotomy is a viable option for failed surgical esophagocardiomyotomy instead of redo surgical Heller myotomy: a single center prospective study. J Am Coll Surg. 2013;217:598–605. doi: 10.1016/j.jamcollsurg.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 27.Kelly EA, Koszewski IJ, Jaradeh SS, Merati AL, Blumin JH, Bock JM. Botulinum toxin injection for the treatment of upper esophageal sphincter dysfunction. Ann Otol Rhinol Laryngol. 2013;122:100–108. doi: 10.1177/000348941312200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Annese V, Bassotti G, Coccia G, et al. A multicentre randomised study of intrasphincteric botulinum toxin in patients with oesophageal achalasia. GISMAD Achalasia Study Group. Gut. 2000;46:597–600. doi: 10.1136/gut.46.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]