Abstract
Excess consumption of trans-fatty acid could increase the risk of non-alcoholic steatohepatitis (NASH); however, treatment targeting trans-fatty acid-induced NASH has not been examined. Here we focused on the influence of trans-fatty acid intake on endoplasmic reticulum (ER) stress in hepatocytes, so we investigated the effect of the chemical chaperone 4-phenylbutyric acid (PBA), on trans-fatty acid-caused steatohepatitis using diabetic KK-Ay mice. Elaidic acid (EA, trans-fatty acid) alone did not cause definitive liver injury. In contrast, EA plus low-dose fructose induced extensive apoptosis in hepatocytes with severe fat accumulation. EA plus fructose significantly increased ER stress markers such as glucose-regulated protein 78 (GRP78), eukaryotic initiation factor 2α (eIF2α) and phosphorylated c-jun N-terminal kinase (JNK), while PBA significantly reduced this response. In vitro, EA promoted expression of GRP78 and phosphorylation of eIF2α in primary-cultured hepatocytes. EA also increased hepatocellular susceptibility to low-dose tert-butyl hydroperoxide. Treatment with PBA significantly reduced these responses. In conclusion, EA potentiates susceptibly to non-hazardous dose of fructose, and increases ER and oxidative stress. PBA improved steatohepatitis induced by EA plus fructose through amelioration of ER stress. Therefore, ER stress-targeted therapy using a chemical chaperone is a promising novel strategy for trans-fatty acid-induced steatohepatitis.
Keywords: non-alcoholic steatohepatitis, trans-fatty acid, endoplasmic reticulum stress, fructose, 4-phenylbutyric acid
Introduction
Non-alcoholic steatohepatitis (NASH) is defined as hepatic steatosis with inflammation and hepatocyte injury in the form of ballooning degeneration in the absence of other know causes of secondary hepatic fat accumulation such as significant alcohol consumption, use of steatogenic medication or hereditary disorders.(1) NASH eventually leads to liver cirrhosis and hepatocellular carcinoma, however, there is no established pharmacotherapy to prevent disease progression.(2,3)
Currently, the prevalence of NASH is increasing in Japan, and lifestyle westernization characterized by increased intake of trans-fatty acids and fructose may contribute to this risk.(4–6) Trans-fatty acids are unsaturated fatty acids with at least one double bond in the trans configuration, and hydrogenated oils such as margarine and shortening made from vegetable oils contain 1–2% trans-fatty acid.(7) Intake of trans-fatty acids is known as a potent risk factor for coronary heart disease.(7,8) Recently, some animal-based studies have suggested that excess consumption of trans-fatty acid increases the risk of NASH.(9,10)
Endoplasmic reticulum (ER) stress is a cellular stress caused by the accumulation of unfolded proteins in the ER, and the response to this stress is called the unfolded protein response (UPR). This response includes the induction of several molecular chaperones to restore cellular homeostasis; however, if the protein load continues to exceed the folding capacity of the ER, cells tend to undergo apoptosis.(11) Several recent studies have suggested that ER stress is involved in NASH pathogenesis.(12,13) Sodium 4-phenylbutyric acid (PBA) is a salt of an aromatic fatty acid and one of several reagents known as chemical chaperones based on their ability to reduce protein misfolding. PBA has been used for treatment of urea cycle disorders, sickle cell disease, and thalassemia.(14,15) It has also been shown that PBA protects against liver ischemia-reperfusion injury by inhibiting ER stress in animal models,(16) however, the effect of PBA on trans-fatty acid intake-related steatohepatitis has not been demonstrated.
KK-Ay mice are a strain derived from crossing the diabetic KK mouse with the lethal yellow (Ay) mouse, which carries a mutation of the agouti gene on chromosome 2.(17) KK-Ay mice exhibit phenotypes including obesity, dyslipidemia and insulin resistance and develop mild steatohepatitis spontaneously; thus this animal is potentially useful as a model of metabolic syndrome-related steatohepatitis. Indeed, we have reported that KK-Ay mice exhibit increased susceptibility to methionine- and choline-deficiency diet-induced steatohepatitis and acetaminophen-induced liver injury.(17,18) We also reported that KK-Ay mice exhibit proportional and functional alterations in hepatic NKT cells and disability in liver regeneration after partial hepatectomy.(19,20)
We therefore examined the impact of trans-fatty acid intake on hepatic steatosis and injury in KK-Ay mice focusing on ER stress, and evaluated the effect of treatment with PBA on pathogenesis. Additionally, we confirmed the direct effect of PBA on primary cultured hepatocytes exposed to trans-fatty acid.
Material and Methods
Materials
Oleic acid (OA), elaidic acid (EA), fructose, PBA and tert-butyl hydroperoxide (tert-BuOOH), were purchased from Sigma Aldrich (St. Louis, MO). AIN-93G diet was purchased from CLEA Japan (Tokyo, Japan). Anti-4-hydroxy-2-nonenal (4-HNE) antibody was purchased from the Japan Institute for the Control of Aging, Nikken SEIL Co., Ltd. (Shizuoka, Japan). Secondary biotinylated antimouse IgG, anti-c-Jun N-terminal kinase (JNK) antibody, anti-eukaryotic initiation factor 2α (eIF2α) antibody, anti-phosphorylated eIF2α antibody, anti-glucose-regulated protein 78 (GRP78) antibody, anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody, and anti-horseradish peroxidase-conjugated IgG antibody were purchased from Cell Signaling Technology Inc. (Danvers, MA). Anti-M30 CytoDeath monoclonal antibody was purchased from Roche Diagnostics (Basel, Switzerland). Protease inhibitors cocktail (Complete Mini) was purchased from Roche Diagnostics. Other reagents were purchased from Sigma Aldrich unless otherwise specified.
Animals and experimental design
The experimental protocols were approved by the Committee of Laboratory Animals according to institutional guidelines. KK-Ay and C57Bl/6 mice were purchased from CLEA Japan. Mice were housed in air-conditioned, specific pathogen-free animal quarters with lighting from 08:00 to 20:00 and were given unrestricted access to a standard laboratory chow and water throughout this study.
After acclimation, 8 week-old male KK-Ay mice were fed AIN-93G diet containing 4% (w/w) dextran as control, 2% (w/w) OA (C18:1 cis-fatty acid) or 2% (w/w) EA (C18:1 trans-fatty acid) for 4 weeks. Some of the mice in each diet group were also given 4% (w/v) fructose in their drinking water during the same period, and some mice in the EA diet/fructose water group were treated with PBA (120 mg/kg/day) via intraperitoneal infusion. After 4 weeks, mice were sacrificed by exsanguination from the inferior vena cava, and liver and serum samples were obtained.
Serum transaminase levels
The levels of serum alanine aminotransferase (ALT) activity were measured colorimetrically and determined with a Fuji DRI-CHEM system (Fuji, Japan).
Histological analysis and Immunohistochemistry
For histological evaluations, liver tissues were fixed in 10% buffered formalin and embedded in paraffin, and Hematoxylin-Eosin (H-E) staining was performed. Hepatic lipid accumulation was measured morphometrically.
The expression and localization of tissue 4-HNE in the liver was detected by immunohistochemical staining as previously described elsewhere.(18) Briefly, deparaffinized tissue sections were incubated with a monoclonal anti-4-HNE antibody and secondary biotinylated antimouse IgG, and the specific binding was visualized with the abidin-biotin complex solution followed by incubation with a 3,3-diaminobenzidine tetrahydrochloride solution using Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA). Specimens for histology and immunohistochemistry were observed under an optical microscope (PH-2; Olympus, Tokyo, Japan) equipped with a digital microscope camera (VB6000; Keyence, Osaka, Japan).
The M30 cytodeath assay, which stains a caspase cleavage product of cytokeratin (ccCK) 18, was performed following the manufacturer's instructions. Briefly, deparaffinized tissue sections were incubated with a monoclonal anti-M30 antibody and secondary biotinylated antimouse IgG, and specific binding was visualized as above.
Primary cultured hepatocytes
Hepatocytes were isolated from overnight-fasted male C57Bl/6 mice at 8 to 12 weeks of age via collagenase perfusion through the inferior vena cava, as previously described.(18) Hepatocytes were resuspended in Waymouth’s medium MB752/1 containing 2 mM L-glutamine, 10% fetal calf serum, 100 nM insulin, 100 nM dexamethasone, 100 units/ml penicillin, and 100 µg/ml streptomycin. Cell viability was greater than 90%, as determined via trypan blue exclusion. Hepatocytes were plated in 24-well microtiter collagen-coated plates (1.5 × 105 cells per well) or 60 mm collagen-coated dishes (4.5 × 105 cells per dish, Cosmo Bio, Tokyo, Japan). Hepatocytes were incubated for 2 h in humidified 5% CO2, 95% air at 37°C for attachment of hepatocytes to plates. Hepatocytes were then incubated with 1 mM EA or OA for 6 h, allowing lipid droplets to accumulate within the hepatocytes. Subsequently, PBA dissolved in demineralized water, pH 7.4, was added to a final concentration of 3 mM and the cells were incubated overnight.
Fluorometric assay of cell viability
After overnight attachment to 24-well plates, hepatocytes were washed once and the medium was changed with Krebs-Ringer-HEPES buffer containing 115 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM KH2PO4, 1.2 mM MgSO4, 25 mM HEPES (pH 7.4), and 30 µM propidium iodide (PI). Next, 20 µM tert-BuOOH was added to exert oxidative stress on the hepatocytes as previously described.(18) Fluorescence was measured using a multiwall fluorescence plate reader (Fluoroskan Ascent, Thermo Fisher Scientific, Waltham, MA). Cell death assessed by PI fluorometry correlates closely with trypan blue exclusion and enzyme release as an indicator of oncotic necrosis.
Western blot analysis
Protein extracts were obtained by homogenizing frozen tissues in a buffer containing 50 mM Tris, pH 8.0, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1% Triton X-100, and protease inhibitors (Complete Mini) followed by centrifugation at 17,400 g for 15 min, and the protein concentration was determined using a Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA). Five micrograms of protein was electrophoresed in 12.5% sodium dodecyl sulfate (SDS)-polyacrylamide gels and electrophoretically transferred onto polyvinylidene fluoride membranes. After blocking with 5% nonfat dry milk in Tris-buffered saline, membranes were incubated with primary antibody against JNK (1:500), phosphorylated JNK (1:500), eIF2α (anti-rabbit polyclonal, 1:500), phosphorylated eIF2α (anti-rabbit polyclonal, 1:500) GRP78 (anti-rabbit monoclonal, 1:1,000) or GAPDH (1:1,000) followed by a secondary horseradish peroxidase-conjugated anti-rabbit IgG antibody. Subsequently, specific bands were visualized using the ECL plime detection kit and detected using a LAS3000 (Fuji Film, Japan).
Statistical analysis
Morphometric and densitometric analyses were performed using Scion Image (ver. Beta 4.0.2, Scion Corp., Fredrick, MD). Data were expressed as means ± SEM. Statistical differences between means were determined using one way analysis of variance (ANOVA) or Kruskal-Wallis ANOVA on ranks followed by an all pairwise multiple comparison procedure (Student-Newman-Keuls Method) as appropriate. P<0.05 was selected before the study to reflect significance.
Results
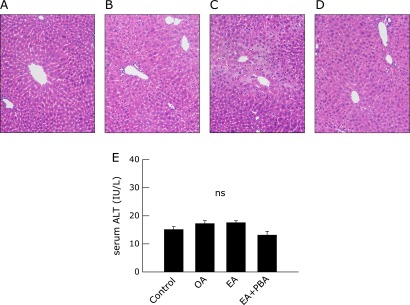
EA potentiates the toxicity of low-dose fructose. First, to evaluate the toxicity of trans-fatty acid alone on liver tissue in vivo, OA or EA-supplemented diets were given to KK-Ay mice for 4 weeks. EA-supplemented diets alone caused minimal hepatic micro vesicular steatosis, but not definitive liver injury. OA-supplemented diets induced no pathological changes. Serological measurements indicated no significant elevation in serum ALT levels in either group (Fig. 1).
Fig. 1.
The effect of trans-fatty acid intake alone on liver histology and serum ALT levels. KK-Ay mice fed an oleic acid- or elaidic acid-containing diet for 4 weeks. Each mouse was treated by daily intraperitoneal injection of saline or 4-phenyl butyric acid (PBA, 120 mg/kg BW). Representative photomicrographs (n = 4) of the liver from mice of control (A), oleic acid (B) or elaidic acid (C) containing diet and elaidic acid containing diet with treatment with PBA (D) are shown (H-E staining, original magnification: ×100). Serum ALT levels were measured by the colorimetric method, and average values are plotted (E).
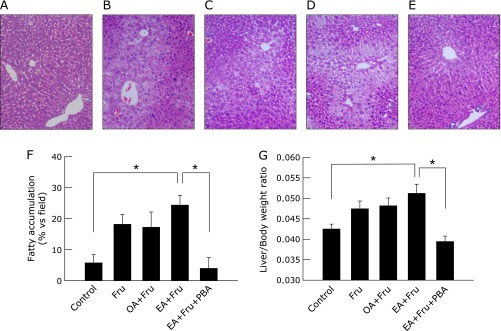
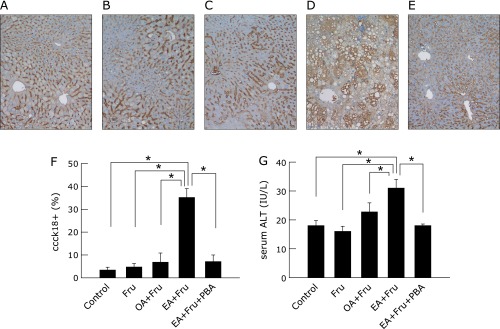
Next, fructose was added to the drinking water at a concentration of 4% in conjunction with the OA-/EA-supplemented diets to evaluate the effect of OA/EA on susceptibility of liver to secondary stress. It has been well documented in prior animal models that high-fructose intake causes liver injury with steatosis,(21,22) so fructose solution at a non-hazardous dose of 4% was given to mice. The intake of low dose fructose tended to increase fatty accumulation, and fructose in conjunction with EA additively increased it, reaching a significant difference compared with control (Fig. 2). Hepatocytes undergoing apoptosis were detected by immunohistological staining for ccCK18, and death of hepatocytes increased as quantitatively evaluated by serum ALT levels. Whereas 4% fructose alone did not affect ccCK18 positive cells or serum ALT elevation, the combination of 4% fructose plus EA markedly increased the percentage of ccCK18-positive cells in liver to 35.2 ± 3.8%, an increase of over 7-fold compared with fructose alone (p<0.05). In contrast, 4% fructose in conjunction with OA did not increase ccCK18-positive cells. The treatment with PBA significantly decreased fructose plus EA-induced ccCK 18-positive cells to 7.2 ± 2.9% (p<0.05). Serum ALT levels were also significantly elevated in EA plus fructose-fed mice, and this elevation was significantly reduced by PBA (Fig. 3, p<0.05).
Fig. 2.
The effect of trans-fatty acid containing diet in combination with fructose on liver histology and liver/body ratio. Mice fed a diet with oleic acid or elaidic acid in combination with fructose in drinking water for 4 weeks. Mice fed elaidic acid with fructose drinking water were treated by daily intraperitoneal injection of 4-phenyl butyric acid (PBA, 120 mg/kg BW) or saline. Representative photomicrographs (n = 4) of the liver from control mice with tap water (A) or fructose drinking water alone (B), or supplemented with dietary oleic acid (C) or elaidic acid (D), or PBA-treated mice given fructose drinking water and an elaidic acid supplemented diet (E) are shown (H-E staining, original magnification: ×100). Area ratio of lipid deposit in each field was quantified morphometrically. Average percentages of lipid deposit from 4 different animals are plotted. More than 3 fields per animal were measured. Liver/body weight ratio is also plotted to evaluate hepatic steatosis (G) by ANOVA on ranks and Student-Neuman-Keuls post-hoc test.
Fig. 3.
The effect of 4-phenyl butyric acid on liver apoptotic cell death in mice exposed to fructose plus elaidic acid. Mice fed an oleic acid- or elaidic acid-supplemented diet in combination with 4% fructose drinking water for 4 weeks. Mice fed elaidic acid with fructose drinking water were treated by daily intraperitoneal injection of PBA (120 mg/kg BW) or saline. Representative photomicrographs (n = 4) of the liver from mice of control with tap water (A) or fructose water (B), oleic acid- (C) or elaidic acid- (D) supplemented diets with fructose water, and from PBA-treated mice fed elaidic acid plus fructose water (E) are shown (M30 cytodeath assay staining, original magnification: ×100). Numbers of caspase-cleaved cytokeratin 18 positive (ccCK18+) hepatocytes were counted. The average ratio of percentages of ccCK18+ cells from 4 different animals are plotted. More than 500 cells per animal were scored. (F) Serum ALT levels were measured by the colorimetric method, and average values are plotted (G).*p<0.05 by ANOVA on ranks and Student-Neuman-Keuls post-hoc test.
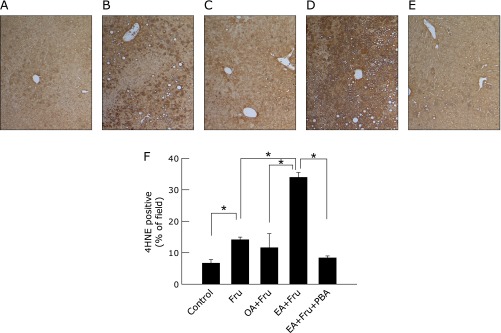
PBA reduces oxidative stress and ER stress caused by EA plus fructose
Immunohistological staining of 4-HNE revealed that intake of 4% fructose solution significantly increased the proportion of 4-HNE-positive staining cells by itself from 6.7 ± 1.1% to 14.1 ± 0.8% (Fig. 4, p<0.05), and induced a much higher proportion of positive cells when given together with EA, rising to 34.0 ± 1.5% (p<0.05 vs fructose alone). Treatment with PBA significantly reduced the increment attributable to EA plus fructose, lowering 4-HNE-positive cells to 8.4 ± 0.6%.
Fig. 4.
The effect of 4-phenyl butyric acid on oxidative stress in the livers of mice exposed to fructose plus elaidic acid. Mice were fed an oleic acid- or elaidic acid-supplemented diet in combination with fructose drinking water for 4 weeks. Mice fed elaidic acid with fructose drinking water were treated by daily intraperitoneal injection of PBA (120 mg/kg BW) or saline. Representative photomicrographs (n = 4) of the livers from mice given tap water (A) or fructose water (B), an oleic acid- (C) or elaidic acid- (D) supplemented diet with fructose water, or elaidic acid plus fructose water with PBA treatment (E) are shown (4-HNE immunohistochemical staining, original magnification: ×100). The staining area ratio was measured morphometrically. Average percentages of 4-HNE staining area from 4 different animals are plotted. More than 3 fields per animal were measured (F). *p<0.05 by ANOVA on ranks and Student-Newman-Keuls post-hoc test).
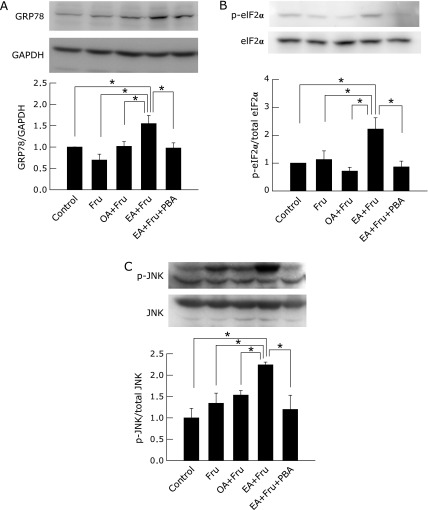
To evaluate ER stress in liver tissue, ER stress-related proteins were measured by western blotting. EA plus fructose significantly increased expression of GRP78 to one-and-a-half times that of control (p<0.05), phosphorylation of eIF2α to over twice that of other groups (p<0.05) and significantly induced phosphorylation of JNK (p<0.05). PBA significantly counteracted these changes caused by EA plus fructose (Fig. 5, p<0.05).
Fig. 5.
The effect of 4-phenyl butyric acid on ER stress markers in the liver from mice exposed to fructose plus elaidic acid. Mice fed an oleic acid- or elaidic acid-supplemented diet in combination with fructose water for 4 weeks. Mice fed elaidic acid with fructose water drinking were treated by daily intraperitoneal injection of PBA (120 mg/kg BW) or saline. Expression of GRP78, GAPDH, eIF2α, phosphorylated-eIF2α (p-eIF2α), JNK and phosphorylated-JNK (p-JNK) in hepatic tissue was detected by western blotting. (A) Representative photographs of 78 kDa bands (GRP78) and 37 kDa bands (GAPDH) are shown. The GRP78 to GAPDH ratio, as determined by the densitometrical values of specific bands is plotted in arbitrary units normalized to the control ratio. (B) Representative photographs of 38 kDa bands for p-eIF2α and eIF2α are shown. The p-eIF2α to eIF2α ratio, as determined by the densitometric values of their specific bands, is plotted in arbitrary units normalized to the control ratio. (C) Representative photographs of 46 kDa and 54 kDa bands for p-JNK and JNK are shown. The ratio of densitometric values of specific bands for p-JNK to JNK are plotted in arbitrary units normalized to the control value (n = 4, *p<0.05 by ANOVA on ranks and Student-Newman-Keuls post-hoc test).
PBA prevents EA-enhanced susceptibility to oxidative stress in primary-cultured hepatocytes
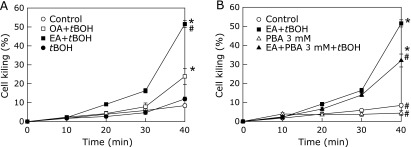
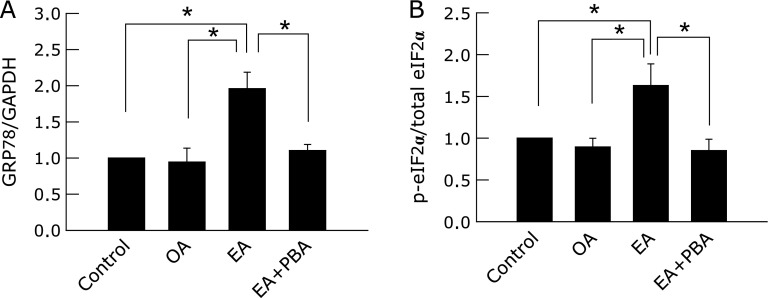
We next evaluated the direct effect of EA on ER stress using primary cultured hepatocytes. When control cells or cells preincubated with OA were exposed to 20 µM tert-BuOOH, significant cell killing did not occur until 40 min had passed. However, when cells preincubated with EA were exposed to tert-BuOOH, significant cell killing was evident by 30 min and rose to 51.5 ± 1.9% by 40 min of exposure to tert-BuOOH (p<0.05). Treatment with PBA ameliorated cell killing significantly, reducing it to 32.0% ± 3.4% after 40 min of tert-BuOOH exposure (Fig. 6, p<0.05). Western blotting showed that EA induced expression of GRP78 and phosphorylation of eIF2α, and treatment with PBA significantly reduced both effects (Fig. 7, p<0.05). In contrast, exposure to OA did not increase expression of GRP78 or phosphorylation of eIF2α.
Fig. 6.
The effect of tert-butyl hydroperoxide on necrotic cell death in primary-cultured hepatocytes exposed to elaidic acid. Isolated hepatocytes were incubated in Waymouth’s medium containing 10% FBS for 2 h followed by overnight exposure to 1 mM oleic acid or elaidic acid. Cells were then incubated in Krebs-Ringer-HEPES-buffer supplemented with 30 µM propidium iodide (PI) for 30 min and exposed to 20 µM tert-BuOOH (A, n = 4 *p<0.05 vs control and #p<0.05 vs oleic acid + tert-BuOOH by ANOVA on ranks and Student-Newman-Keuls post-hoc test). Some cells were treated with 3 mM 4-phenyl butyric acid (PBA) overnight after exposure to 1 mM elaidic acid for 6 h (B, n = 4 *p<0.05 vs control and #p<0.05 vs elaidic acid + tert-BuOOH by ANOVA on ranks and Student-Newman-Keuls post-hoc test).
Fig. 7.
The effect of elaidic acid on ER stress markers in primary-cultured hepatocytes. Isolated hepatocytes were incubated in Waymouth’s medium containing 10% FBS for 2 h followed by exposure to 1 mM oleic acid or elaidic acid for 6–8 h, and some cells were treated with 3 mM 4-phenyl butyric acid (PBA) overnight. Expression of eIF2α, phosphorylated-eIF2α (p-eIF2α), GRP78 and GAPDH in hepatocytes was detected by western blotting. (A) The ratio of densitometric values for the specific bands corresponding to p-eIF2α and eIF2α are plotted in arbitrary units normalized to the control. (B) The ratio of densitometrical values for the specific bands corresponding to GRP78 and GAPDH are plotted (n = 4, *p<0.05 by ANOVA on ranks and Student-Newman-Keuls post-hoc test).
Discussion
In the present study, EA-supplemented diet alone caused trivial steatosis but not detectable cell death (Fig. 1). EA-supplemented diet in conjunction with 4% fructose additively increased fatty accumulation (Fig. 2), whereas surprisingly, the combination diet induced massive hepatocyte apoptosis (Fig. 3). Immunohistological staining for 4-HNE revealed that the combination diet drastically increased oxidative stress (Fig. 4). Fructose alone at low concentration caused significant oxidative stress in hepatocytes but not apoptosis, so that the massive apoptosis observed with EA and fructose in combination must be attributed to enhanced susceptibility conferred by EA to the secondary oxidative stress induced by fructose. This effect was specific to the trans-fatty acid because the OA-supplemented diet with fructose did not cause hepatic apoptosis. This striking effect of trans-fatty acid was also shown in vitro. Preincubation of primary cultured hepatocytes with EA significantly increased susceptibility to a non-lethal dose of tert-BuOOH in comparison to preincubation with OA (Fig. 6). Increased susceptibility to secondary stress has been proposed as a key event in development of NASH.(23)
Additionally, we investigated the involvement of the UPR pathway. UPR responses occur in response to ER stress, and are therefore considered to be ER stress markers. In the unstressed state, GRP78 is bound to the ER lumenal domains of several stress sensors. Upon ER stress, GRP78 binds to unfolded proteins, causing it to dissociate from ER stress sensors and allow downstream activation of the UPR.(24,25) The UPR induces the expression of several genes that ameliorate the stressed state, thus activation of GRP78 triggers responses to alleviate ER stress.(26) EIF2α is located downstream of protein kinase RNA-like ER kinase, which is one of the ER lumenal domains, and decreases the ER protein load by attenuating general translation in order to prevent the generation of further ER stress.(27) JNK is known as stress-activated protein kinase, and is related to several signaling pathways including ER stress-related cell death.(28) JNK can phosphorylate and activate proapoptotic B-cell CLL/lymphoma 2 family proteins and inactivate anti-apoptotic proteins. Sustained ER stress induces apoptotic cell death via activation of JNK.(29,30) Puri et al.(31) have reported that the phosphorylations of eIF2α and JNK are observed in the biopsy specimens of NASH patients. Our findings demonstrated that EA plus fructose caused expression of GRP78 and activated downstream UPR pathways. Moreover, this combination induced the ER stress-related apoptosis via JNK activation. So we see strong points of commonality between the mechanism of steatohepatitis in our model and the ER stress pathway observed in human NASH. The treatment with PBA decreased the UPR and subsequent activation of JNK, reflecting a reduction in ER stress itself (Fig. 5).
Oxidative stress and ER stress are thought to contribute to various kinds of liver injury but the relationship between oxidative stress and ER stress remains controversial;(13,18,26,32,33) not only can oxidative stress be a factor in ER stress, but also ER stress can induce oxidative stress via protein misfolding.(34,35) In the present study, both oxidative stress and ER stress caused by EA plus fructose are reduced by PBA, which acts as a chemical chaperone (Fig. 4 and 5). These findings indicate that ER stress is located upstream of oxidative stress in the present model.
The hepatotoxicity of saturated fatty acids has been reported in many studies.(36–39) However, the effect of unsaturated trans-fatty acid on death of hepatocytes has not been clarified. Obara et al.(9) reported that trans-fatty acid at a very high concentration (18% of total calories) induced liver steatosis in mice. Our present study showed that EA at much lower concentration of 2% did not cause prominent hepatocyte death by itself; however, it enhanced hepatotoxicity at an otherwise non-hazardous dose of fructose. No previous reports refer to this interaction between a trans-fatty acid and fructose. Actually recent meta-analysis of healthy participants has reported that isocaloric exchange of fructose for other carbohydrates does not induce non-alcoholic fatty liver disease, which concluded that fructose itself was not toxic to liver.(40) Our present data suggests that the interaction of fructose with trans-fatty acid contributes to the development of NASH by increasing ER stress, even if intake of each factor is not excessive. Therefore, in the future, it will be necessary to evaluate each risk factor on the development of NASH as members of a pool of factors that may interact with each other.
In summary, exposure to fructose, even at low dose, may cause hepatotoxicity if trans-fatty acid is also present in the diet. We demonstrated that trans-fatty acid potentiates hepatocellular susceptibility to the stimulus, which causes ER stress-related liver steatosis and hepatocyte apoptosis, while treatment with PBA averted this response. This study suggests the possibility of a new treatment for NASH aimed at mitigating ER stress using a chemical chaperone.
Acknowledgments
The authors thank Professor Takashi Ueno Ph.D. for technical advice, Takako Ikegami and Tomomi Ikeda (Laboratory of Molecular and Biochemical Research, Research Support Center, Juntendo University Graduate School of Medicine, Tokyo, Japan) for technical assistance and R. F. Whittier for critical reading of the manuscript and thoughtful discussions.
This work was supported in part by Grant-in-Aid for Scientific Research (20790508 and 24590995 to K.K., 24590996 to K.I., 24390191 to S.W.) and High Technology Research Center Grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abbreviations
- ALT
alanine aminotransferase
- ANOVA
analysis of variance
- ccCK
caspase cleavage product of cytokeratin
- EA
elaidic acid
- eIF2α
eukaryotic initiation factor 2α
- ER
endoplasmic reticulum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GRP78
glucose-regulated protein 78
- H-E
Hematoxylin-Eosin
- 4-HNE
4-hydroxy-2-nonenal
- JNK
c-Jun N-terminal kinase
- NASH
non-alcoholic steatohepatitis
- OA
oleic acid
- PBA
4-phenyl butyric acid
- PI
propidium iodide
- tert-BuOOH
tert-butyl hydroperoxide
- UPR
unfolded protein response
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe S, Hashimoto E, Ikejima K, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. J Gastroenterol. 2015;50:364–377. doi: 10.1007/s00535-015-1050-7. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe S, Hashimoto E, Ikejima K, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatol Res. 2015;45:363–377. doi: 10.1111/hepr.12511. [DOI] [PubMed] [Google Scholar]
- 4.Simopoulos AP. Is insulin resistance influenced by dietary linoleic acid and trans fatty acids? Free Radic Biol Med. 1994;17:367–372. doi: 10.1016/0891-5849(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 5.Simopoulos AP. Dietary omega-3 fatty acid deficiency and high fructose intake in the development of metabolic syndrome brain, metabolic abnormalities, and non-alcoholic fatty liver disease. Nutrients. 2013;5:2901–2923. doi: 10.3390/nu5082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basaranoglu M, Basaranoglu G, Sabuncu T, Senturk H. Fructose as a key player in the development of fatty liver disease. World J Gastroenterol. 2013;19:1166–1172. doi: 10.3748/wjg.v19.i8.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oomen CM, Ocké MC, Feskens EJ, van Erp-Baart MA, Kok FJ, Kromhout D. Association between trans fatty acid intake and 10-year risk of coronary heart disease in the Zutphen Elderly Study: a prospective population-based study. Lancet. 2001;357:746–751. doi: 10.1016/s0140-6736(00)04166-0. [DOI] [PubMed] [Google Scholar]
- 8.Willett WC, Stampfer MJ, Manson JE, et al. Intake of trans fatty acids and risk of coronary heart disease among women. Lancet. 1993;341:581–585. doi: 10.1016/0140-6736(93)90350-p. [DOI] [PubMed] [Google Scholar]
- 9.Obara N, Fukushima K, Ueno Y, et al. Possible involvement and the mechanisms of excess trans-fatty acid consumption in severe NAFLD in mice. J Hepatol. 2010;53:326–334. doi: 10.1016/j.jhep.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 10.Machado RM, Stefano JT, Oliveira CP, et al. Intake of trans fatty acids causes nonalcoholic steatohepatitis and reduces adipose tissue fat content. J Nutr. 2010;140:1127–1132. doi: 10.3945/jn.109.117937. [DOI] [PubMed] [Google Scholar]
- 11.Kapoor A, Sanyal AJ. Endoplasmic reticulum stress and the unfolded protein response. Clin Liver Dis. 2009;13:581–590. doi: 10.1016/j.cld.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XQ, Xu CF, Yu CH, Chen WX, Li YM. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:1768–1776. doi: 10.3748/wjg.v20.i7.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 14.Maestri NE, Hauser ER, Bartholomew D, Brusilow SW. Prospective treatment of urea cycle disorders. J Pediatr. 1991;119:923–928. doi: 10.1016/s0022-3476(05)83044-6. [DOI] [PubMed] [Google Scholar]
- 15.Brusilow SW. Phenylacetylglutamine may replace urea as a vehicle for waste nitrogen excretion. Pediatr Res. 1991;29:147–150. doi: 10.1203/00006450-199102000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Vilatoba M, Eckstein C, Bilbao G, et al. Sodium 4-phenylbutyrate protects against liver ischemia reperfusion injury by inhibition of endoplasmic reticulum-stress mediated apoptosis. Surgery. 2005;138:342–351. doi: 10.1016/j.surg.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Okumura K, Ikejima K, Kon K, et al. Exacerbation of dietary steatohepatitis and fibrosis in obese, diabetic KK-Ay mice. Hepatol Res. 2006;36:217–228. doi: 10.1016/j.hepres.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Kon K, Ikejima K, Okumura K, Arai K, Aoyama T, Watanabe S. Diabetic KK-Ay mice are highly susceptible to oxidative hepatocellular damage induced by acetaminophen. Am J Physiol Gastrointest Liver Physiol. 2010;299:G329–G337. doi: 10.1152/ajpgi.00361.2009. [DOI] [PubMed] [Google Scholar]
- 19.Yamagata H, Ikejima K, Takeda K, et al. Altered expression and function of hepatic natural killer T cells in obese and diabetic KK-Ay mice. Hepatol Res. 2013;43:276–288. doi: 10.1111/j.1872-034X.2012.01062.x. [DOI] [PubMed] [Google Scholar]
- 20.Aoyama T, Ikejima K, Kon K, Okumura K, Arai K, Watanabe S. Pioglitazone promotes survival and prevents hepatic regeneration failure after partial hepatectomy in obese and diabetic KK-Ay mice. Hepatology. 2009;49:1636–1644. doi: 10.1002/hep.22828. [DOI] [PubMed] [Google Scholar]
- 21.Kanuri G, Spruss A, Wagnerberger S, Bischoff SC, Bergheim I. Fructose-induced steatosis in mice: role of plasminogen activator inhibitor-1, microsomal triglyceride transfer protein and NKT cells. Lab Invest. 2011;91:885–895. doi: 10.1038/labinvest.2011.44. [DOI] [PubMed] [Google Scholar]
- 22.Spruss A, Kanuri G, Stahl C, Bischoff SC, Bergheim I. Metformin protects against the development of fructose-induced steatosis in mice: role of the intestinal barrier function. Lab Invest. 2012;92:1020–1032. doi: 10.1038/labinvest.2012.75. [DOI] [PubMed] [Google Scholar]
- 23.Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010;103:71–83. doi: 10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 25.Oikawa D, Kimata Y, Kohno K, Iwawaki T. Activation of mammalian IRE1alpha upon ER stress depends on dissociation of BiP rather than on direct interaction with unfolded proteins. Exp Cell Res. 2009;315:2496–2504. doi: 10.1016/j.yexcr.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brewer JW, Diehl JA. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci U S A. 2000;97:12625–12630. doi: 10.1073/pnas.220247197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 29.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puri P, Mirshahi F, Cheung O, et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–576. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 32.Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52:59–69. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Adachi T, Kaminaga T, Yasuda H, Kamiya T, Hara H. The involvement of endoplasmic reticulum stress in bile acid-induced hepatocellular injury. J Clin Biochem Nutr. 2014;54:129–135. doi: 10.3164/jcbn.13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Back SH, Scheuner D, Han J, et al. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10:13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malhotra JD, Miao H, Zhang K, et al. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei Y, Wang D, Pagliassotti MJ. Saturated fatty acid-mediated endoplasmic reticulum stress and apoptosis are augmented by trans-10, cis-12-conjugated linoleic acid in liver cells. Mol Cell Biochem. 2007;303:105–113. doi: 10.1007/s11010-007-9461-2. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008;47:1495–1503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao J, Dai DL, Yao L, et al. Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol Cell Biochem. 2012;364:115–129. doi: 10.1007/s11010-011-1211-9. [DOI] [PubMed] [Google Scholar]
- 39.Ristić-Medić D, Takić M, Vučić V, Kandić D, Kostić N, Glibetić M. Abnormalities in the serum phospholipids fatty acid profile in patients with alcoholic liver cirrhosis - a pilot study. J Clin Biochem Nutr. 2013;53:49–54. doi: 10.3164/jcbn.12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiu S, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr. 2014;68:416–423. doi: 10.1038/ejcn.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]