Abstract
Fetal brain injury is often related to prenatal inflammation; however, there is a lack of effective therapy. Recently, molecular hydrogen (H2), a specific antioxidant to hydroxyl radical and peroxynitrite, has been reported to have anti-inflammatory properties. The aim of this study was to investigate whether maternal H2 administration could protect the fetal brain against inflammation. Pregnant C3H/HeN mice received an intraperitoneal injection of lipopolysaccharide (LPS) on gestational day 15.5 and were provided with H2 water for 24 h prior to LPS injection. Pup brain samples were collected on gestational day 16.5, and the levels of apoptosis and oxidative damage were evaluated using immunohistochemistry. Interleukin-6 (IL-6) levels were examined using real-time PCR. The levels of apoptosis and oxidative damage, as well as the levels of IL-6 mRNA, increased significantly when the mother was injected with LPS than that in the control group. However, these levels were significantly reduced when H2 was administered prior to the LPS-injection. Our results suggest that LPS-induced apoptosis, oxidative damage and inflammation in the fetal brain were ameliorated by maternal H2 administration. Antenatal H2 administration might protect the premature brain against maternal inflammation.
Keywords: molecular hydrogen, premature infant, anti-oxidant, anti-inflammation, brain injury
Introduction
Worldwide, the number of preterm birth is increasing. Currently, up to 11.1% of all infants are born prematurely.(1) Although the survival rates of these infants continuous to improve, the frequency of morbidities including brain injury remains high.(2) Perinatal brain injury can result in neonatal mortality or a long-term neurologic disability,(3) and the total number of children with lifelong health problems rises in developed countries.(2) Therefore, it is imperative to develop new strategies that can aid in reducing the number of premature births and perinatal brain injury. The poor prognosis has been reported to be related with prenatal exposure to inflammation.(4,5) Accumulating evidence suggests that elevation of interleukin-6 (IL-6) in the amniotic fluid is a marker of chorioamnionitis and indicates a poor prognosis for neonates.(6–9) Intrauterine infection or inflammation also causes preterm birth(10) and prematurity is a causative factor of neonatal brain injury.(11) These data suggest that prenatal administration of anti-inflammatory agents could be an effective therapeutic approach.(3)
Molecular hydrogen (H2) is a specific scavenger of hydroxyl radicals and peroxynitrite, and has been used as an innovated therapy in adult brain injury.(12–14) Several studies have reported the therapeutic effects of H2 in oxidative stress-related diseases, including Parkinson’s disease, and Diabetes Mellitus.(15) Several clinical trials with H2 have been initiated and so far, no side effects have been reported.(14) Recently, we found that maternal administration of H2 affects fetal cerebral damage in an ischemia-reperfusion model.(16) We demonstrated that maternal administration of H2 increased H2 concentrations in the fetal brain, which resulted in the reduction of oxidative damage and apoptosis in the hippocampus.(16)
From these findings, we hypothesized that maternal administration of H2 might also be effective at reducing fetal brain damage caused by intrauterine inflammation. Thus, we investigated the effects of maternal H2 administration on the fetal mouse brain by evaluating apoptosis, oxidative stress, and the level of IL-6.
Materials and Methods
Animals and treatments
The experimental procedures in this study were approved by the Animal experiment Committee of the Nagoya University Graduate School of Medicine (approval number: 25096). C3H/HeN mouse were purchased from Charles River Laboratories (Yokohama, Japan) and housed in plastic cages. All mice were maintained on a 12 h light/12 h dark lighting schedule (lights on at 9:00 am, off at 9:00 pm), and subjected to a standard chow diet (CE-2) and water ad libitum. As previously reported, virgin female mice were mated with fertile males of the same strain.(17) The timing of pregnancy was determined by visual inspection of the vaginal plug, which was defined as day 0.5 of pregnancy.(17) Normal term labor occurs on day 19.5 of gestation (G19.5), under our animal facility conditions. Approximately 50%-saturated hydrogen water (H2 water) was a kind gift from Blue Mercury, Inc. (Tokyo, Japan). The H2 water was exchanged every 24 h to maintain the concentration above 0.4 mM, as previously reported.(16) The mice were assigned randomly to three groups: a control group that was intraperitoneally (i.p.) injected with vehicle (0.5 ml of sterile PBS) on G15.5, a lipopolysaccharide (LPS) group receiving 5 µg of LPS (Escherichia coli LPS, serotype 055:B5; Sigma-Aldrich Japan, Tokyo, Japan) in 0.5 ml of sterile PBS by i.p. on G15.5, and an H2 water + LPS group, that in addition to the LPS i.p. injection on G15.5, received H2 water, starting from one day prior to LPS i.p. (on G14.5), until the end of the experiment (day 16.5 of pregnancy).
Twenty-four hours after LPS or PBS i.p. (on G16.5), the brain tissue from the fetuses were collected (n = 10 for each group).
Measurement of IL-6 in amniotic fluid
The amniotic fluid from all sacs in a mother (6 mothers for each group) were aspirated by syringe and assembled on G16.5. IL-6 levels were was measured by ELISA (Quantikine® ELISA, M6000B; R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The experiments were performed in duplicate.
Quantitative real time polymerase chain reaction (qRT-PCR)
The fetal brains collected on G16.5 were grinded using a Power Masher (Nippi, Tokyo, Japan) and total RNA was isolated using the RNeasy Mini Kit (Qiagen Inc., Tokyo, Japan) following the manufacturer’s directions. Complementary DNAs were prepared with a first strand cDNA synthesis kit (Rever Tra Ace-α; Toyobo Co., Ltd, Osaka, Japan), according to the manufacturer’s directions using a MyCyclerTM thermal cycler (BIORAD, Hercules, CA). Subsequently, qRT-PCR was performed using the Thermal Cycler Dice (Takara Bio Inc., Tokyo, Japan) and SYBR II Premix Ex Taq (Takara Bio Inc.) according to the manufacturer’s directions. Data were normalized based on the expression of the housekeeping gene beta-actin. The primer sequences (NIPPON EGT CO., LTD., Toyama, Japan) were as follows: mouse β-actin forward: 5'-CGTGGGCCGCCCTAGGCACCA-3' and reverse: 5'-ACACGCAGCTCATTGTA-3'; mouse IL-6 forward: 5'-ACAACCACGGCCTTCCCTAC-3' and reverse: 5'-TCCACGATTTCCCAGAGAACA-3'.
Immunohistochemistry
Immunohistochemistry was performed as previously reported.(18) Briefly, formalin-fixed, paraffin-embedded tissue sections were cut at a thickness of 4 µm. For heat-induced epitope retrieval, deparaffinized sections placed in 1 mM citrate buffer (pH 6.0) were heated for 20 min each at 90°C and 750 W using an H2500 microwave oven. Staining was performed using Vector® M.O.MTM Immunodectection Kit Basic (Vector Laboratories, Inc., Burlingame, CA) to detect specifically to localize mouse primary antibodies on mouse tissues, and VECTASTAIN Elite ABC Reagent (Vector Laboratories, Inc.) for chromogenic detection, based on the manufacturer’s instructions. Anti-mouse monoclonal antibody against 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-OHdG) (Nikken Seil Co., Ltd., Shizuoka, Japan) was used at a 1:50 dilution for a primary antibody. Finally, the slides were counterstained with Meyer’s hematoxylin. For the quantification of 8-OHdG immunostaining, the intensity was evaluated as intensity score (IS), previously reported.(19) Briefly, two examiners determined IS in three segments per slide in a double blinded manner, based on a 4-point system: 0, 1, 2, and 3 (for none, light, medium, or dark staining, respectively). Apoptotic cells of fetal brain were identified using the In Situ Cell Death Detection Kit, Fluorescein (Roche Applied Science, Mannheim, Germany), according to the manufacturer’s instructions. The number of TUNEL positive cells were determined at ×400 magnifications in three segments per slide by two examiners independently.
Statistical analysis
Statistical analyses were performed using the SPSS software package ver. 22 (SPSS Inc., Chicago, IL). The distribution and variance were examined using the Shapiro-Wilk test. Student’s t test and The Mann-Whitney test were performed to assess variables with normal and non-normal distributions, respectively. Differences between groups were considered significant at a p value of <0.05.
Results
H2 water reduced LPS-induced intra-amniotic inflammation
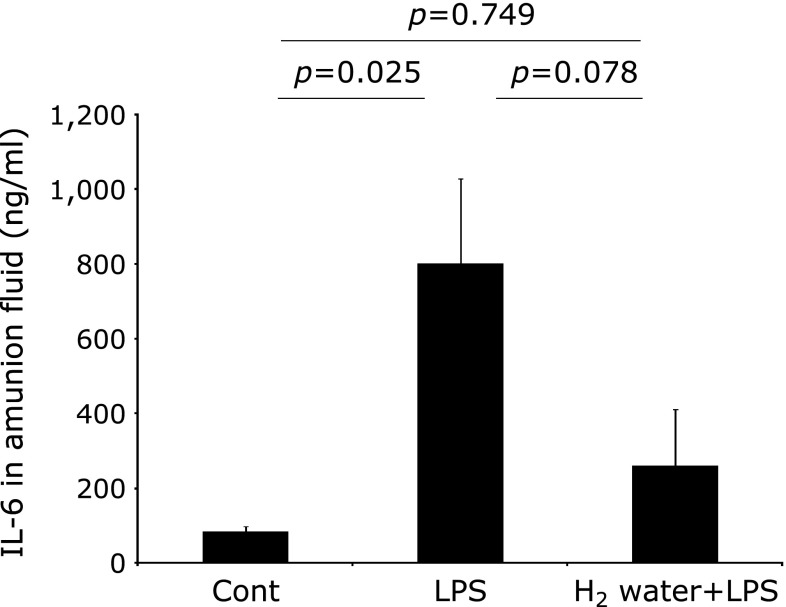
The concentration of IL-6 in the amniotic fluid of the LPS group showed an approximately 10-fold increase compared to the control group (Fig. 1, p = 0.025). This induction was partially decreased in the H2 water + LPS group compared to the LPS group, but this difference was found not to be statistically significant (Fig. 1, p = 0.078).
Fig. 1.
IL-6 proteins in the amniotic fluid. The results are expressed as the means ± SEM (n = 6). IL-6 in the amitotic fluid showed a tendency to decrease in the H2 water + LPS group compared with that observed in the LPS group. The p values were calculated according to the Student’s t test (Cont vs LPS) and the Mann–Whitney test (LPS vs H2 water + LPS and Cont vs H2 water + LPS, respectively. Cont = PBS i.p. control group, LPS = LPS i.p. injected group, H2 water + LPS = maternal H2 water intake prior to LPS i.p. injection.
H2 water alleviated cerebral irrigation
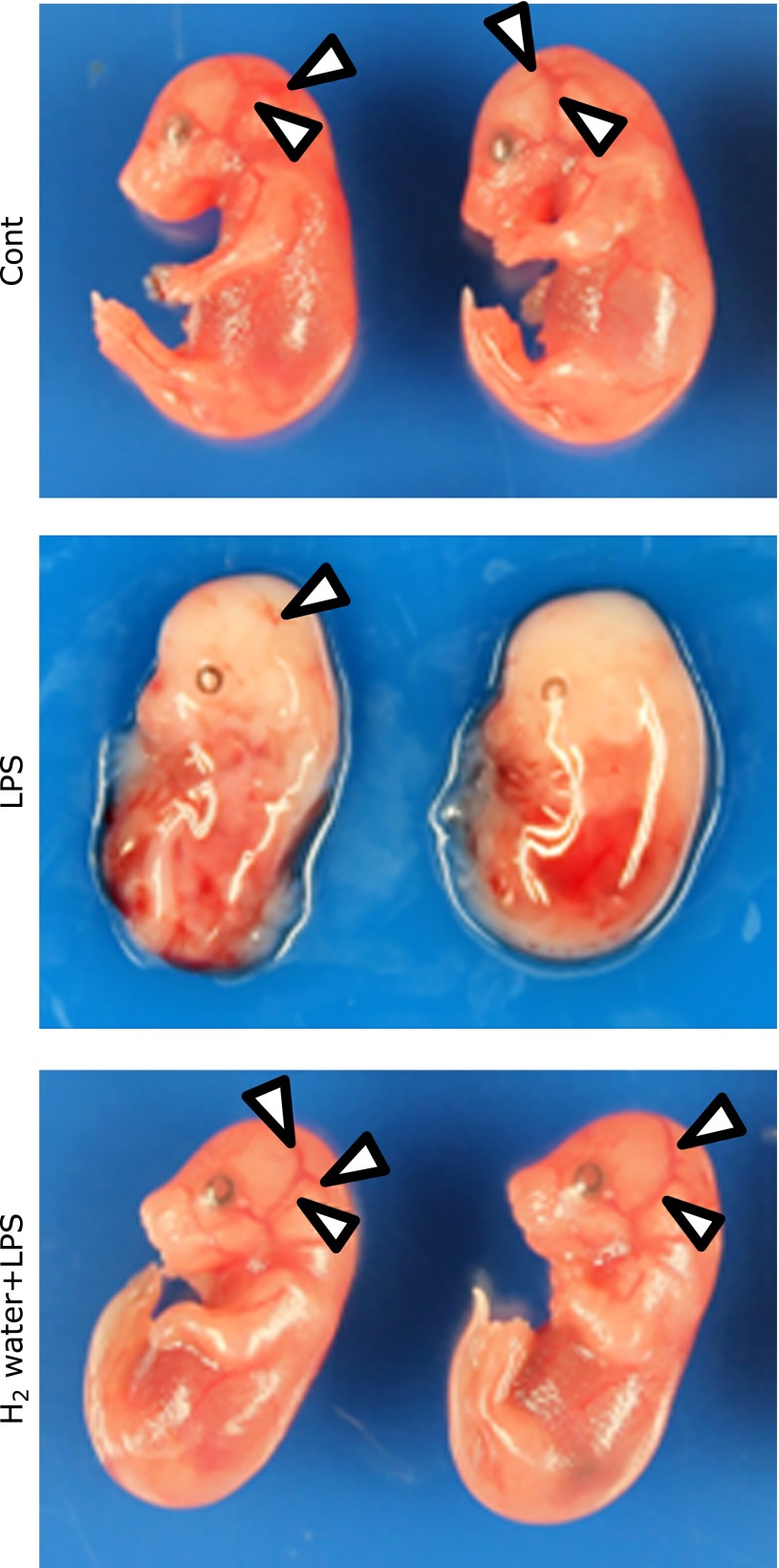
Representative images of the macroscopic findings of fetuses from three groups 24 h after PBS or LPS i.p. injection are shown in Fig. 1. The reduction of cerebral irrigation with partial melting limbs was observed in 45.8% of the fetuses in the LPS group (Fig. 2, middle panel). However, these read-outs were improved in the H2 water + LPS group (Fig. 2, bottom panel), and the population of fetuses with those findings in the H2 water + LPS group was reduced to only 26.1%.
Fig. 2.
The effect of H2 and LPS on fetuses. Representative photographs of fetuses from gestational day 16.5, 24 h after the injection of LPS. Arrows indicate decrease in cerebral irrigation in the fetuses of the LPS group. Cerebral irrigation was improved in the H2 water + LPS group. Cont = PBS i.p. control group, LPS = LPS i.p. injected group, H2 water + LPS = maternal H2 water intake prior to LPS i.p. injection.
H2 water relieved LPS-induced inflammation, oxidative stress and apoptosis in fetal brains
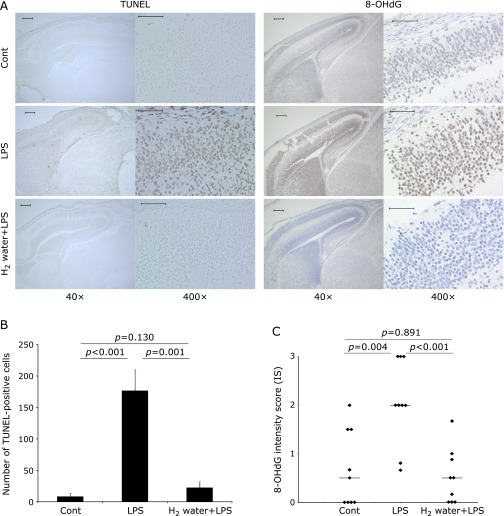
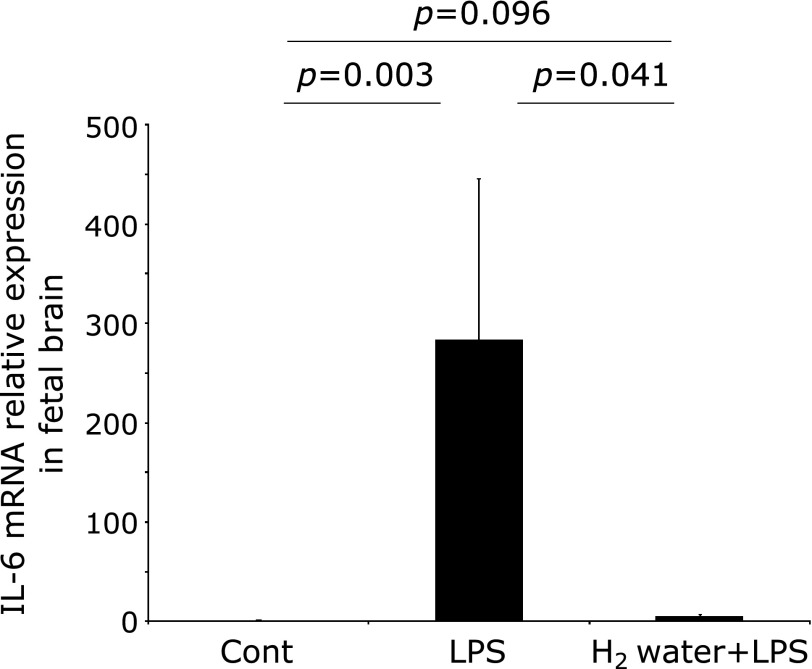
To evaluate the effect of H2 on the fetal brain damage induced by maternal administration of LPS, the number of apoptotic cells was measured in fetal brain specimens of the three groups. The number of TUNEL-positive cells in the LPS group was significantly increased, in comparison to the control group (Fig. 3A and B, p<0.001), whereas the number of TUNEL-positive cells in the H2 water + LPS group was completely restored to the same level as that of the control group (Fig. 3A and B, p = 0.001). To investigate whether LPS induced oxidative stress in the fetal brain, anti-8-OHdG immunostaining was performed. The intensity score calculated in the LPS group was significantly increased beyond that in the Control group (Fig. 3A and C, p = 0.004), but that detected in the H2 water + LPS group was significantly reduced, back to the same level as that in the control group (Fig. 3A and C, p<0.001). The expression of IL-6 mRNA in whole fetal brains from the LPS group was significantly increased, compared with the levels found in the control group (Fig. 4, p = 0.003). Again, IL-6 mRNA levels were significantly reduced in the H2 water + LPS group (Fig. 4, p = 0.041), and there was no significant difference in IL-6 mRNA levels between the control group and the H2 water + LPS group. TNF-α and IL-1β expression were not detected in the control or the LPS group (data not shown).
Fig. 3.
(A) Representative data for TUNEL staining (left columns) and immunohistochemical staining for 8-OHdG (right columns) of fetal brain tissue. Number of TUNEL positive cells and intensity score of 8-OHdG were significantly increased in the LPS group compared with the control group, but these induction was completely attenuated in the H2 water + LPS group. The original magnification is 40× (scale bar = 200 µm) in the left side and 400× (scale bar = 50 µm) in the images on the right side, respectively. The arrows indicate cells positive for TUNEL, an apoptotic marker, or 8-OHdG, a marker for oxidative damage on DNA. (B) The TUNEL positive cells were quantified and presented as the mean ± SEM. P values were calculated according to the Mann–Whitney test. (C) 8-OHdG staining was scored as intensity score (IS) and is presented as the mean ± SEM. The p values were calculated according to the Mann–Whitney test (Cont vs LPS and Cont vs H2 water + LPS) or the Student’s t test (LPS vs H2 water + LPS). Cont = PBS i.p. control group, LPS = LPS i.p. injected group, H2 water + LPS = maternal H2 water intake prior to LPS i.p. injection.
Fig. 4.
IL-6 mRNA expression in the fetal brain was significantly increased in the LPS group, compared with that detected in the control group. This induction was completely decreased in the H2 water + LPS group. The data were normalized to the expression of beta-actin and are presented as the mean ± SEM. P values were calculated according to the Mann–Whitney test. Cont = PBS i.p. control group, LPS = LPS i.p. injected group, H2 water + LPS = maternal H2 water intake prior to LPS i.p. injection.
Discussion
In the present study, we have demonstrated that maternal administration of H2 has a suppressive effect on fetal brain injury caused by intrauterine inflammation. Maternal intraperitoneal injection of LPS in animals has previously been used to model maternal inflammation-induced perinatal brain injury.(20,21) Using this model, we found that the levels of IL-6 in the amniotic fluid were elevated, consistent with a previous report.(21) Clinical evidence has demonstrated that the elevated levels of IL-6 in the amniotic fluid are a marker of intrauterine infection,(22) and a predictor of preterm birth(22) and histological chorioamnionitis.(23) Elevated IL-6 levels are associated with adverse perinatal outcomes and fetal brain injury.(24,25) In this study, we observed a reduction in cerebral irrigation in the fetus, similar to another report.(17) Moreover, immunohistochemical analysis of the fetal brain revealed that the number of apoptotic cells was markedly increased, which was accompanied with an increased expression of 8-OHdG, a marker of DNA oxidative damage, and IL-6, an inflammation marker. Thus, LPS-induced intrauterine inflammation caused reduced blood flow, increased oxidative stress and inflammation in the fetal brain of the animals, eventually leading to fetal brain damage. It mimics the pathological state of the neonatal brain after injury caused by chorioamnionitis. All of these symptoms were attenuated by maternal H2 administration prior to LPS injection.
H2 was initially reported to act as an antioxidant, preventing brain injury by selectively targeting cytotoxic oxygen radicals.(12) H2 has a rapid rate of diffusion and effectively reaches the nucleus, where it can protect nuclear DNA from oxidative damage.(12) Several researchers also observed that H2 reduces oxidative DNA damage in brain tissues, indicating its potential as a neuroprotective potential.(26–28) In addition, H2 can penetrate the blood-brain barrier, an import advantage of using H2 over other neuroprotective drugs.(16) We have previously confirmed that maternal H2 administration increases the H2 concentrations in the fetal brains.(16,18) Thus, it could be postulated that H2 administered to the mother could pass through the blood-brain barrier and protect fetal brain cells against oxidative stress or inflammation. Indeed, it has been reported that H2 has an anti-inflammatory effect.(14) H2 reduced the mRNA expression of pro-inflammatory cytokines including IL-6 in an adult mouse model of LPS-induced neuroinflammation.(29) Other studies reported that H2 improves memory function via suppression of IL-6 expression in brain tissues in a rat model of Alzheimer’s disease.(30) Based on those results, it can be concluded that H2 suppresses oxidative DNA damage and the expression of IL-6 in the adult brain. Moreover, our results now show that these effects of H2 are also detected in the fetal brain after maternal H2 administration. The effects of H2 water administration were milder in the amniotic fluid than in the fetal brain. This might be due to the fact that maternal H2 water administration leads to an increase in H2 concentration in the fetal brain, but not in the amniotic fluid.(16) We speculate that the effects of H2 on the fetal brain tissue are mainly due to its direct action in the fetal brain tissue, but they may be partially explained by ant-inflammatory effect on the intrauterine inflammation itself.
The present study has several limitations. Only IL-6 was found to be increased in the LPS group in this study, but other pro-inflammatory cytokines such as TNF-α and IL-1β, which are also be important in the pathogenesis of brain injury resulting from chorioamnionitis or fetal inflammatory response syndrome, were not detected.(4) It has previously been reported that these cytokines are increased in the fetal brain after intrauterine injection of LPS.(31,32) and the effect of H2 on the expression of these cytokines remains to be established and confirmed in the other strains. Furthermore, the H2 administration protocol should be revised for clinical research. The timing to start therapy is important for efficacy.(33) After obtaining promising results in this proof-of-principal study, we will now proceed with a further evaluation of H2 dosages suitable for clinical use.
In conclusion, maternal administration of H2 water prevents fetal brain damage caused by intrauterine inflammation. Prophylactic use of H2 might improve the neurological prognosis of premature neonates delivered from mothers suffering from intrauterine inflammation.
Acknowledgments
We acknowledge Mrs. Kaori Ushida (Department of Pathology, Nagoya University Graduate School of Medicine) for her valuable technical support. 50%-saturated hydrogen water were kind gifts from Blue Mercury, Inc. (Tokyo, Japan). This work was supported by JSPS KAKENHI, Grant Number 26462484.
Abbreviations
- 8-OHdG
8-hydroxy-2'-deoxyguanosine
- PBS
phosphate buffered saline
- LPS
lipopolysaccharide
Conflict of Interest
The authors have no conflicts of interest to declare in association with this study. The authors are responsible for the content and writing of the paper.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies) BMJ. 2012;345:e7976. doi: 10.1136/bmj.e7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salmeen KE, Jelin AC, Thiet MP. Perinatal neuroprotection. F1000Prime Rep. 2014;6:6. doi: 10.12703/P6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chau V, McFadden DE, Poskitt KJ, Miller SP. Chorioamnionitis in the pathogenesis of brain injury in preterm infants. Clin Perinatol. 2014;41:83–103. doi: 10.1016/j.clp.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Chau V, Poskitt KJ, McFadden DE, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol. 2009;66:155–164. doi: 10.1002/ana.21713. [DOI] [PubMed] [Google Scholar]
- 6.Girard S, Kadhim H, Roy M, et al. Role of perinatal inflammation in cerebral palsy. Pediatr Neurol. 2009;40:168–174. doi: 10.1016/j.pediatrneurol.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol. 2012;71:444–457. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- 8.Murthy V, Kennea NL. Antenatal infection/inflammation and fetal tissue injury. Best Pract Res Clin Obstet Gynaecol. 2007;21:479–489. doi: 10.1016/j.bpobgyn.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Normann E, Lacaze-Masmonteil T, Eaton F, Schwendimann L, Gressens P, Thébaud B. A novel mouse model of Ureaplasma-induced perinatal inflammation: effects on lung and brain injury. Pediatr Res. 2009;65:430–436. doi: 10.1203/PDR.0b013e31819984ce. [DOI] [PubMed] [Google Scholar]
- 10.Burdet J, Rubio AP, Salazar AI, Ribeiro ML, Ibarra C, Franchi AM. Inflammation, infection and preterm birth. Curr Pharm Des. 2014;20:4741–4748. doi: 10.2174/1381612820666140130202224. [DOI] [PubMed] [Google Scholar]
- 11.Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG. 2005;112 Suppl 1:16–18. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 12.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 13.Ohta S. Recent progress toward hydrogen medicine: potential of molecular hydrogen for preventive and therapeutic applications. Curr Pharm Des. 2011;17:2241–2252. doi: 10.2174/138161211797052664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohta S. Molecular hydrogen as a novel antioxidant: overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015;555:289–317. doi: 10.1016/bs.mie.2014.11.038. [DOI] [PubMed] [Google Scholar]
- 15.Ohno K, Ito M, Ichihara M, Ito M. Molecular hydrogen as an emerging therapeutic medical gas for neurodegenerative and other diseases. Oxid Med Cell Longev. 2012;2012:353152. doi: 10.1155/2012/353152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mano Y, Kotani T, Ito M, et al. Maternal molecular hydrogen administration ameliorates rat fetal hippocampal damage caused by in utero ischemia-reperfusion. Free Radic Biol Med. 2014;69:324–330. doi: 10.1016/j.freeradbiomed.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 17.Domínguez Rubio AP, Sordelli MS, Salazar AI, et al. Melatonin prevents experimental preterm labor and increases offspring survival. J Pineal Res. 2014;56:154–162. doi: 10.1111/jpi.12108. [DOI] [PubMed] [Google Scholar]
- 18.Hattori Y, Kotani T, Tsuda H, et al. Maternal molecular hydrogen treatment attenuates lipopolysaccharide-induced rat fetal lung injury. Free Radic Res. 2015;49:1026–1037. doi: 10.3109/10715762.2015.1038257. [DOI] [PubMed] [Google Scholar]
- 19.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 20.Rose KM, Parmar MS, Cavanaugh JE. Dietary supplementation with resveratrol protects against striatal dopaminergic deficits produced by in utero LPS exposure. Brain Res. 2014;1573:37–43. doi: 10.1016/j.brainres.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 21.Awad N, Khatib N, Ginsberg Y, et al. N-acetyl-cysteine (NAC) attenuates LPS-induced maternal and amniotic fluid oxidative stress and inflammatory responses in the preterm gestation. Am J Obstet Gynecol. 2011;204:450.e415–420. doi: 10.1016/j.ajog.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 22.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–1400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kacerovsky M, Musilova I, Hornychova H, et al. Bedside assessment of amniotic fluid interleukin-6 in preterm prelabor rupture of membranes. Am J Obstet Gynecol. 2014;211:385.e1–9. doi: 10.1016/j.ajog.2014.03.069. [DOI] [PubMed] [Google Scholar]
- 24.Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210:125.e1–125.e15. doi: 10.1016/j.ajog.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 25.Rees S, Harding R, Walker D. The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int J Dev Neurosci. 2011;29:551–563. doi: 10.1016/j.ijdevneu.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashida K, Sano M, Kamimura N, et al. H2 gas improves functional outcome after cardiac arrest to an extent comparable to therapeutic hypothermia in a rat model. J Am Heart Assoc. 2012;1:e003459. doi: 10.1161/JAHA.112.003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagatani K, Takeuchi S, Kobayashi H, et al. The effect of hydrogen gas on a mouse bilateral common carotid artery occlusion. Acta Neurochir Suppl. 2013;118:61–63. doi: 10.1007/978-3-7091-1434-6_10. [DOI] [PubMed] [Google Scholar]
- 28.Shen L, Wang J, Liu K, et al. Hydrogen-rich saline is cerebroprotective in a rat model of deep hypothermic circulatory arrest. Neurochem Res. 2011;36:1501–1511. doi: 10.1007/s11064-011-0476-4. [DOI] [PubMed] [Google Scholar]
- 29.Spulber S, Edoff K, Hong L, Morisawa S, Shirahata S, Ceccatelli S. Molecular hydrogen reduces LPS-induced neuroinflammation and promotes recovery from sickness behaviour in mice. Plos One. 2012;7:e42078. doi: 10.1371/journal.pone.0042078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Wang C, Zhang JH, Cai JM, Cao YP, Sun XJ. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer’s disease by reduction of oxidative stress. Brain Res. 2010;1328:152–161. doi: 10.1016/j.brainres.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 31.Burd I, Bentz AI, Chai J, et al. Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. J Neurosci Res. 2010;88:1872–1881. doi: 10.1002/jnr.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang EY, Zhang J, Sullivan S, Newman R, Singh I. N-acetylcysteine attenuates the maternal and fetal proinflammatory response to intrauterine LPS injection in an animal model for preterm birth and brain injury. J Matern Fetal Neonatal Med. 2011;24:732–740. doi: 10.3109/14767058.2010.528089. [DOI] [PubMed] [Google Scholar]
- 33.Shichiri M. The role of lipid peroxidation in neurological disorders. J Clin Biochem Nutr. 2014;54:151–160. doi: 10.3164/jcbn.14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]