Abstract
Background and aims: Management of infections caused by Pseudomonas aeruginosa is becoming difficult due to the rapid emergence of multi-antibiotic resistant strains. Antimicrobial photodynamic therapy (APDT) has a lot of potential as an alternative approach for inactivation of antibiotic resistant bacteria. In this study we report results of our investigations on the effect of poly-L-lysine conjugate of chlorine p6 (pl-cp6) mediated APDT on the healing of P.aeruginosa infected wounds and the role of Nuclear Factor kappa B (NF-kB) induced inflammatory response in this process.
Materials and method: Excisional wounds created in Swiss albino mice were infected with ∼107 colony forming units of P.aeruginosa. Mice with wounds were divided into three groups: 1) Uninfected, 2) Infected, untreated control (no light, no pl-cp6), 3) Infected, APDT. After 24 h of infection (day 1 post wounding), the wounds were subjected to APDT [pl-cp6 applied topically and exposed to red light (660 ± 25 nm) fluence of ∼ 60 J/cm2]. Subsequent to APDT, on day 2 and 5 post wounding (p.w), measurements were made on biochemical parameters of inflammation [toll like receptor-4 (TLR-4), NF-kB, Inteleukin (IL)-[1α, IL-β, and IL-2)] and cell proliferation [(fibroblast growth factor-2 (FGF-2), alkaline phosphatase (ALP)].
Results: In comparison with untreated control, while expression of TLR-4, NF-kB (p105 and p50), and proinflammatory interleukins (IL-1α, IL-1β,IL-2) were reduced in the infected wounds subjected to APDT, the levels of FGF-2 and ALP increased, on day 5 p.w.
Conclusion: The measurements made on the inflammatory markers and cell proliferation markers suggest that APDT reduces inflammation caused by P.aeruginosa and promotes cell proliferation in wounds.
Keywords: Antimicrobial photodynamic therapy, wound healing, Pseudomonas aeruginosa, lipopolysaccharide, toll like receptor-4, nuclear factor kappa B, fibroblast growth factor, alkaline phosphatase
1. Introduction
Pseudomonas aeruginosa is an opportunistic pathogen responsible for acute as well as chronic wound infections in immunocompromised hosts. While acute infections often spread rapidly and cause sepsis leading to high mortality rates, chronic infections in wounds lead to delay in healing 1,2). Management of infections caused by P. aeruginosa is difficult due to the rapid emergence of multi-antibiotic resistant strains. Further, the toxins and cell components of these bacteria such as lipopolysaccharide (LPS) can induce overproduction of local proinflammatory mediators, casue cell death 3–9). Proteases produced by these bacteria also degrade host matrix proteins, thereby impairing host tissue integrity 10–12). Although conventional treatments reduce bacterial load, they are unable to act on the virulent factors released by these bacteria 13, 14). Therefore, management of infections caused by these bacteria needs approaches which will not only kill bacteria but also inactivate the virulent factors of bacteria.
Antimicrobial photodynamic therapy (APDT) has been suggested as an alternative treatment approach for treating localized wound infection 15–19). APDT involves killing of target cells via formation of reactive oxygen species produced by interaction of photosensitizing compound with light of appropriate wavelength. As the mechanism of action of microbial killing is nonspecific and multiple sites are affected, it is less likely that bacteria develop resistance to APDT. Additionally, the biological activities of virulent factors produced by Gram-negative bacteria have been shown to be reduced by photodynamic action in cell free systems 20).
Recent studies carried out by us have shown that PDT also reduces inflammation in wounds infected with P. aeruginosa by down regulating proinflammatory cytokines Tumor Necrosis Factor-α, and Interleukin (IL)-6 21). However, these observations are in contrast to the activated immune response observed in tumors in response to PDT, which involves activation of the ubiquitous transcriptional activator, Nuclear Factor kappa-B 22). The NF-kB family is composed of five mammalian members: RelA/p65, RelB, c-Rel, NF-kB1 p105/p50, and NF-kB2 p100/p52. Active form of NF-kB subunit p50 is produced by ubiquitin-dependent proteolytic process of the COOH-terminal domains of NFkB1 p105. Activation of NF-kB in response to microbial pathogens, oxidative stress results in nuclear translocation of the dimmers, primarily, the p65/p50 dimer (classical pathway), which then bind to regulate expression of genes involved in inflammation, apoptosis, cell proliferation and angiogenesis 23, 24). However it is not clear how NF-kB will influence the APDT induced healing in bacteria infected wounds. To understand this, we investigated the expression of NF-kB p105/p50 and some of targets of NF-kB (IL-1α, IL-1β, IL-2), in P.aeruginosa infected wounds subjected to APDT mediated by poly-L-lysin conjugate of chlorine p6 (p1-cp6). We have also studied the effect of APDT on level of Toll like receptor-4 (TLR-4), as binding of lipopolysaccharide from Gram-negative bacteria to TLR-4 leads to NF-kB activation, which in turn also contributes to higher inflammation and tissue degradation in bacteria infected tissue 25, 26).
2. Materials and methods
2.1. Bacteria strain
Pseudomonas aeruginosa PAO1 (MTCC 3541, IMTECH, Chandigarh, India) used in this study was cultured in tryptone soya broth (TSB, Himedia, Mumbai, India) routinely. For experiments, a colony was inoculated into TSB and was grown for 18 h at 37°C using a shaker incubator. For wound infections studies, we used exponentially growing bacteria which was obtained by growing overnight culture in fresh TSB medium to an optical density (OD) of 0.4 at 600 nm. This corresponded to ∼107 Colony Forming Unit (CFU)/ml.
2.2. Antibodies
Primary mouse anti-NFkBp50 (sc-1190), anti-TLR-4 (sc-12511), anti-FGF-2 (sc-1884) were procured from Santa Cruz Biotechnology Inc (Santa Cruz, CA,USA) and Anti-GAPDH-HRP conjugate was procured from Cell Signaling Technology, USA.
2.3. Ethics statement
All the experimental procedures involving animals were approved by the Institutional Animal Ethics Committee, in accordance with the guidelines of the Committee for Purpose of Care and Supervision of Experimental Animals, Department of Environment and Forests, Government of India. The animals were housed individually in cages with free access to food, water and maintained on a 12 h light/dark cycle at 22°C (± 2°C). All the animal manipulations involving wounds were carried out in anesthetized conditions and animals were kept on warm cotton pads for recovery from anesthesia. Animals were euthanized by cervical dislocation. All efforts have been made to minimize the animal suffering and the number of animals sacrificed.
2.4. P. aeruginosa infection and photodynamic treatment of wounds
Swiss albino female mice (12-week-old) have been used for experiments. In mice anesthetized by intraperitoneal injection of Ketamine (80 mg/kg) and Xylazine (10 mg/kg) cocktail, the dorsal skin was shaved with help of an electric razor, treated with a depilatory cream, and then cleaned with povidone-iodine solution followed by 70 % alcohol wipe. Excisional wounds of ∼1.2 × 1 cm (L X W) were created on the back of mice by excising the skin down to panniculus carnosus, using sterile surgical scissors and forceps. Mice with wounds were divided into following groups: (1) Uninfected (2) Untreated infected (no light, no pl-cp6) and (3) Infected PDT group (pl-cp6 and light). For establishing infection, immediately after wound creation (day 0), 20 µl of bacterial suspension containing ∼ 107 CFU bacteria was applied onto each wound. For carrying out APDT, on day 1 p.w., 200 µM pl-cp6, prepared using the protocol as described in our earlier publication, was applied onto the infected wounds in dark 21). After 30 minutes of pl-cp6 application, wounds were exposed to red light (660 ± 25 nm) using a commercial light source LC-122A (LumaCare, Citek, USA) at a power density of ∼100 mW/cm2, for 10 minutes to achieve light fluence of ∼ 60 J/cm2, as described in 21)
2.5. Assessment of bacterial load
On day 1,2,3 and 5 p.w., bacteria load of photodynamically treated and untreated infected wounds was assessed by performing colony forming units assay in tissue swabs 21, 27).
2.6. Western blotting and densitometry
On day 2 and 5 p.w., wounds containing the scab and the granulation tissue were excised, snap frozen in liquid nitrogen, weighed and transferred to −80°C. The wound tissue homogenates were prepared according to the protocol described in 18, 21).Briefly, the tissue were first pulverized in liquid nitrogen with help of mortar/ pestle.Approximately 100 mg of tissue powder was suspended in 500 µl of lysis buffer (pH 7.4) containing 20 mM Tris Hcl buffer, 150 mM Nacl, 1 mM EDTA and a protease inhibitors cocktail[ ( INHIB1-1 KT Sigma Aldrich,USA)], kept on ice for 30 minutes followed by sonication (20 kHZ, 10 s, for 4 times) and centrifugation (5000 rpm, 10 minutes). Protein content in the supernatant was determined using the Bicinchoninic acid assay 28). Equal amount (∼50 µg) of protein from each group, was separated by SDS-PAGE and electro blotted to nitrocellulose membrane. Along with the samples, pre-stained colored molecular weight markers (Chemichrome™ Western Control, Sigma- Aldrich) were also separated and electro blotted to ensure optimal sample transfer. The western blots were developed according to the protocol described in an enhanced chemiluminescence western blotting detection kit (Amersham, GE Healthcare, USA). Chemiluminescence imaging of the western blots was carried out using a chemiluminescence imaging system (Chemi-HR-16, Syngene, UK). Ten serial images of each immunoblot were captured at 30 s exposure intervals using GENESNAP software (Syngene, UK). Densitometric band analysis of the blots was carried out using the GENETOOL software (Syngene, UK). All experiments were repeated thrice. Glyceral dehyde-3- phosphate dehydrogenase (GAPDH) was used as a loading control. Blot intensities of NF-kB, TLR-4 and FGF-2 of each goup were normalized with the GAPDH blot intensity of the corresponding group. To quantify effect of infection and APDT, the normalized intensity of each protein bands from these two groups, were divided with that of uninfected wounds.
2.7. Cytokine ELISA
The levels of inflammatory cytokines IL-1α, IL-1β, and IL-2 in wound tissues of different groups excised on day 5 p.w. were measured using a mouse inflammatory cytokines and chemokines multi analyte™ ELISA array kit (SA Biosciences Corp, USA). In the tissue supernatant prepared (section 2.6), the assay was carried out according to the protocol described in the kit and the colored product formed was quantified by reading absorbance at 450 nm and reference at 570 nm using a mircoplate reader. The results were expressed as absorbance values (optical density) per mg protein 21, 29).
2.8. Alkaline phosphatase assay
Alkaline phosphatase (ALP) activity in homogenates of wound tissue excised on day 2 and 5 p.w. was measured using p-nitrophenyl phosphate (p-NPP) as a substrate, according to the method described in 30). Briefly, 50 µL of the tissue supernatant was incubated with 50 µL of 0.5M N-methyl-D-glucamine buffer (pH 10.5) containing 0.5 mM magnesium acetate, 110 mM NaCl, and 0.2 % Triton X-100, for 30 minutes at 37°C. Twenty mM p-NPP; (Himedia, Mumbai, India) was added and the reaction was incubated at 37°C for 30 minutes. The amount of p-NPP liberated was measured at 405 nm in a mircoplate reader. The level of ALP is expressed as value of optical density (OD) per mg protein.
2.9. Statistical analysis
Data were presented as mean ± SD. For assessment of treatment effects, comparisons between the means of different groups were carried out by one-way analysis of variance (ANOVA). A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Effect of PDT on TLR-4, NF-kB levels
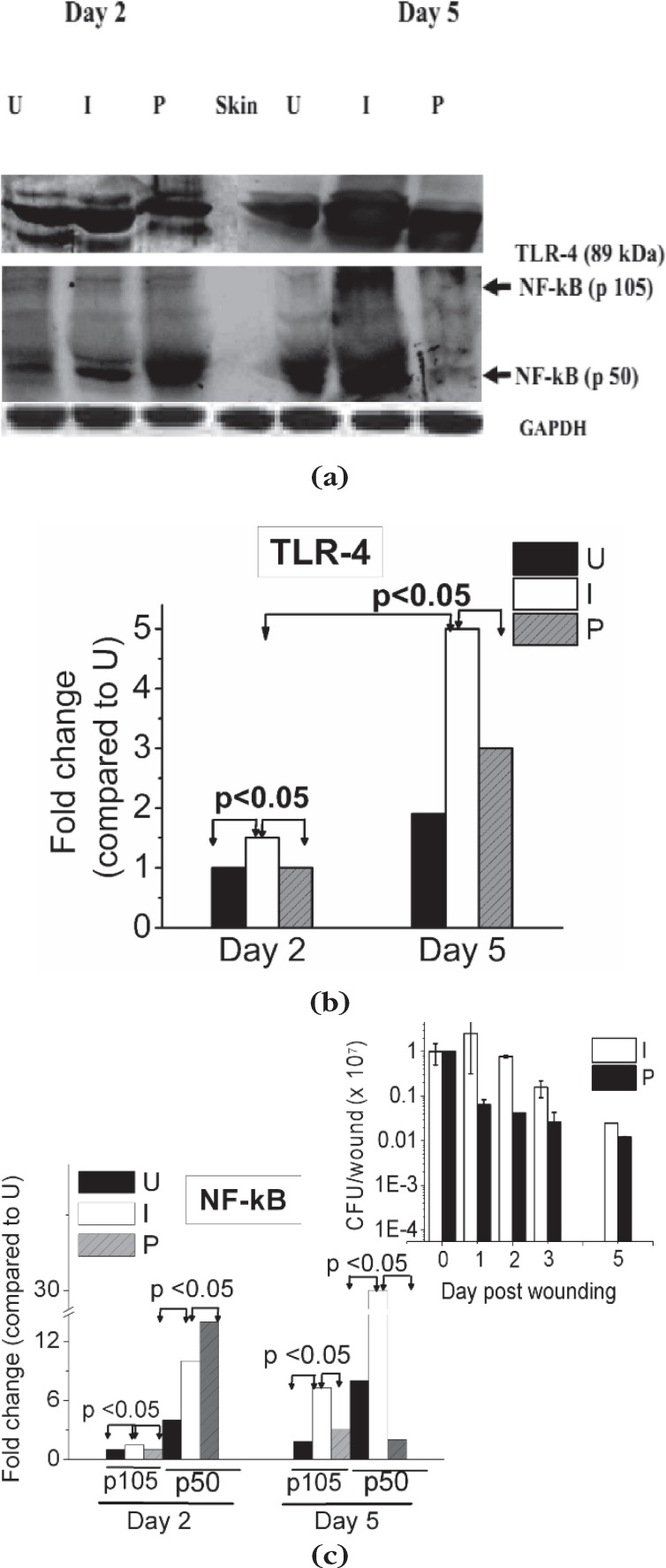
In Fig. 1 (a–c) we show the expression of TLR-4, NF-kB p50 and NF-kB p105, in uninfected, infected and photodynamically treated infected wounds on day 2 and 5 p.w. As can be observed from the figure, on day 2 p.w, compared to uninfected wounds, levels of NFkB p105, p50 and TLR-4 were higher in the untreated infected wounds. As expected the level of the precursor NF-kB p105 was lower than the p50 level in wounds. With increase in post infection time (day 5 p.w), NF-kB (p105/p50) and TLR-4 levels increased further by ∼4 fold and ∼2 fold, respectively in these wounds, although the bacteria number decreased (Fig. 1 c, inset). In the infected wounds subjected to PDT fluence ∼60 J/cm2, as compared to their untreated controls, the levels of TLR-4, reduced significantly, on day 2 and day 5 p.w. In contrast, the level of NF-κB p50 in the photodynamically treated wounds, compared to the untreated wounds, increased on day 2 p.w. However, with increase in post wounding time (day 5 p.w.), a substantial decrease in NF-κB p50 was observed and the levels were equal to that of uninfected wounds although the bacteria number in both treated and untreated wounds were similar at this time point.
Fig. 1.
(a) Immunoblot for expression profile of TLR-4, NF-kB p105 and p50, and GAPDH measured in wound tissues day 2 and day 5 p.w. (b),(c) Mean values of densitometric quantification of TLR-4, NF-kB, respectively. (c, inset) Bacterial load of wounds. (U): Uninfected, (I): Untreated infected and (P): Infected wounds treated with pl-cp6 and exposed to red light (660 ± 25 nm) fluence of ∼60 J/cm2, as described in section 2.4.
3.2. Effect of APDT on inflammatory cytokine levels
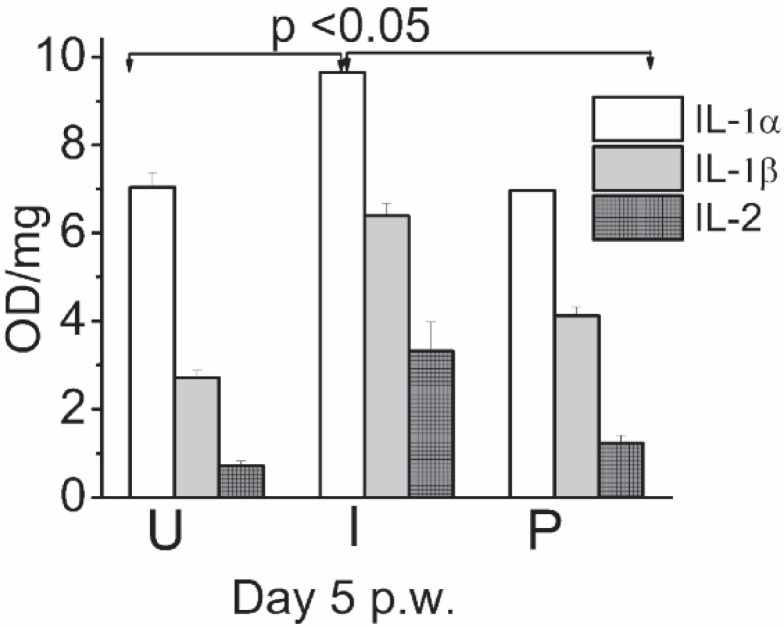
Levels of IL-1α, IL-1β, and IL-2 were measured on day 5 p.w. in the wound tissue homogenates. As compared to the uninfected group, in infected group, all the cytokines measured were elevated significantly (Fig. 2). In contrast, wounds of photodynamically treated groups showed comparatively low levels of these cytokines as against untreated infected wounds.
Fig. 2.
Effect of PDT on expression of proinflammatory cytokines measured in wound tissue homogenates day 5 p.w.by ELISA. (U): Uninfected, (I): Untreated infected and (P): Infected wounds treated with pl-cp6 and then exposed to red light (660 ± 25 nm) (660 nm fluence of ∼60 J/cm2, as described in section 2.4.
3.3. Effect of PDT on ALP and FGF-2 levels
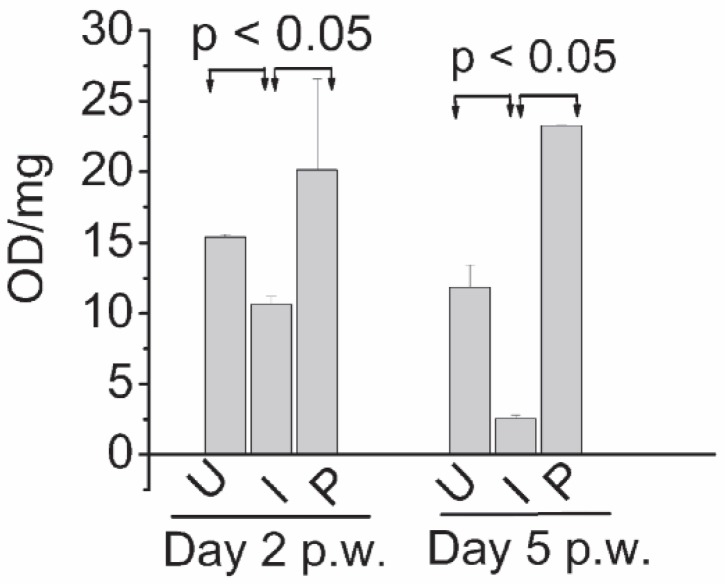
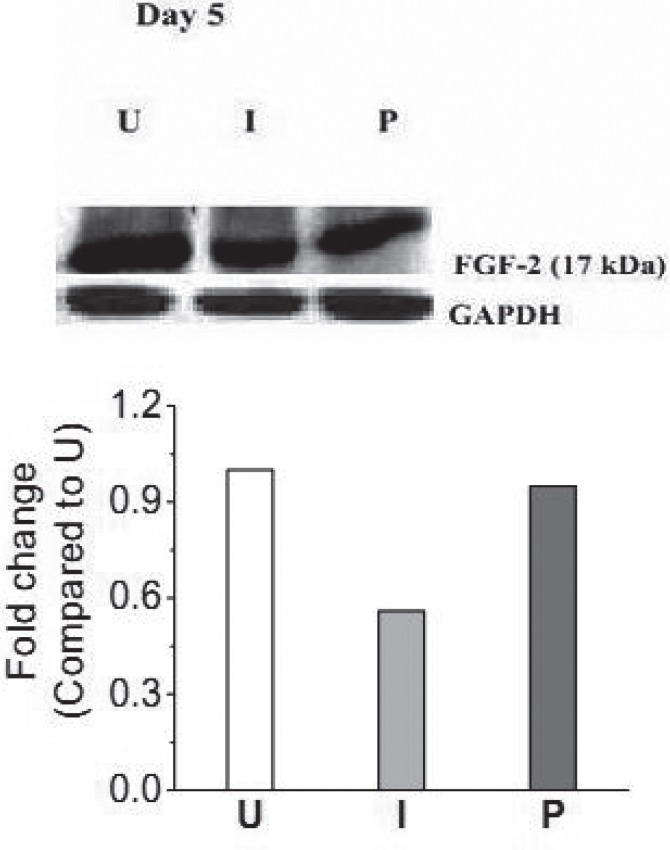
Fig. 3 shows ALP activity on day 2 and 5 in homogenates of uninfected, untreated infected and photodynamically treated infected wounds. Compared to uninfected wounds, in infected wounds, ALP activity was reduced by ∼1.5 and ∼4.5 fold on day 2 and 5 p.w respectively. In wounds subjected to PDT, with respect to the untreated groups, the levels of ALP were significantly higher, on both the time points. In Fig. 4, immunoblot of FGF-2 of uninfected, untreated infected and photodynamically treated infected wounds on day 5 p.w. is shown. The level of FGF-2 in photodynamically treated infected wounds was also observed to be higher (p< 0.05) than that of untreated infected wounds.
Fig. 3.
Time dependent alkaline phosphatase (ALP) activity in homogenates of wound excised on day 2 and 5 p.w. (U); uninfected wounds, (I); infected untreated wounds, (P); infected wounds treated with pl-cp6 and exposed to red light (660 ± 25 nm) fluence ∼ 60 J/cm2 (P), as described in section 2.4.
Fig. 4.
(a) Expression profile of FGF-2 and GAPDH measured in wound tissues on day 5 p.w. Mean values of densitometric quantification of each of the band of different groups with respect to uninfected wounds of day 5 p.w are shown below the blots. (U);Uninfected, (I); Untreated infected, and (P); Infected wounds treated with pl-cp6 and then exposed to red light (660 ± 25 nm) fluence of ∼60 J/cm2, as described in section 2.4.
4. Discussion
The primary objective of this study was to understand the role of NF-kB in APDT induced healing of P. aeruginosa infected wounds of mice. The transcription factor NF-kB is a key regulator of the immune response and also controls cell survival and proliferation. It is activated by various stimuli, including bacterial components, inflammatory cytokines as well as oxidative stress 23,24). Therefore it is important to assess its activation status and its impact on the target genes involved in inflammatory response, to understand the response of APDT on wound healing.
The TLR-4 plays an important role in innate host defense especially in the infections caused by Gram negative bacteria. In fact, it has been shown that NF-kB is activated as soon as LPS of Gram negative bacteria binds to TLR-4 gets translocated to nucleus for transcription activity 25). Our results show that, compared to uninfected wounds, TLR-4 and NF-kB were upregulated in infected wounds on day 2 p.w. Levels of these markers increased further with increase in post infection time (day 5 p.w.) although bacterial load decreased. A further comparative analysis of the band intensities of the immunoblots show that the magnitude of upregulation was much higher for NF-kB than TLR-4. This suggests that besides LPS, other virulent factors such as pyocyanin, proteases, flagella released to the wound milieu are also involved in activation of NF-kB on day 2 and 5 p.w. These virulent factors might be released by the bacteria themselves or due to the lysis of bacteria by immune cells recruited to the wound site and delay resolution of tissue inflammation in individuals 31,32). As transcriptional activation of IL-1α, IL-1β, IL-2 are under control of NF-kB, in the infected wounds, compared to the uninfected counterparts, we also observed overexpression of these interleukins 24).
Results presented in this study show that TLR-4 level reduces significantly in infected wounds following PDT which could have been due to damage/ inactivation of the LPS. Previous studies have shown that ability of cell free LPS to stimulate proinflammatory cytokines from peripheral blood mononuclear cells was reduced when irradiated with light in presence of TBO 33). In addition, studies carried out by us previously and others have shown that proteases activity of P.aeruginosa culture supernatants reduces after photodynamic treatment 20, 21, 33). NF-kB level in photodynamically treated wounds compared to untreated wounds, was observed to be higher initially (day 2). This might have occurred due to oxidative stress induced by the treatment as well as accumulation of inflammatory cells 22,34,35). However, with increase in wound healing time point, loss of viability of P.aeruginosa, and inactivation of its virulent factors possibly led to significant down regulation of NF-kB and inflammatory cytokines.
It has been shown that virulent factors secreted by P.aeruginosa such as LPS, phospholipase, proteolytic enzymes and pyocyanin induce damage to fibroblast, endothelial cells in the wound milieu and delay the wound healing 4–6, 31). Also, a prolonged inflammatory phase induced by infection, causes increased levels of proteases which destroy components of the extracellular matrix and damage the growth factors 31). In our previous study, we also observed that P.aerugionsa infection leads to overexpression of MMPs in wounds 18). These reasons could have contributed for the decrease in levels of ALP and FGF-2, which are the markers of cell proliferation and wound healing, in the untreated infected wounds. In fact, ALP activity is maximum during cell migration, granulation tissue deposition, but its expression is reduced considerably in severe inflammation, tissue damage and chronic non healing wounds 36–39). At the same time, FGF-2 level is known to be lowered in wounds due to high inflammation and high proteases levels 40). Therefore, enhancement of both ALP and FGF-2 observed in the photodynamically treated infected wounds, compared to their untreated controls, suggests that the reduction in inflammation due to APDT accelerates cell proliferation and thereby healing of wounds.
5. Conclusion
Results of the current study suggest involvement of the TLR-4, NF-kB induced inflammation response in APDT induced enhanced healing. Further, the increased FGF-2 and ALP levels suggest APDT may contribute to increased cell proliferation and healing of wounds.
Acknowledgement
The authors would like to thank Dr Alok Dube for providing pl-cp6.
References
- 1: Bjarnsholt T, Kirketerp-Møller K, Jensen PØ, Madsen KG, Phipps R, Krogfelt K, Høiby N, Givskov M. (2008): Why chronic wounds will not heal: a novel hypothesis. Wound Rep Regen, 16:2-10. [DOI] [PubMed] [Google Scholar]
- 2: Gjødsbøl K, Christensen JJ, Karlsmark T, Jørgensen B, Klein BM, Krogfelt KA. (2006): Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J, 3:225-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3: McCarty SM, Percival SL. (2013): Proteases and Delayed Wound Healing. Adv Wound Care (New Rochelle), 2:438-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4: Schwarzer C, Fu Z, Fischer H, Machen TE. (2008): Redox-independent activation of NF-kappaB by Pseudomonas aeruginosa pyocyanin in a cystic fibrosis airway epithelial cell line. J Biol Chem, 283:27144-27153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5: Wenshu Chai, Jia Zhang, Yan Duan, Dianzhu Pan, Wei Liu, Ying Li, Xue Yan, Baiyi Chen. (2014): Pseudomonas pyocyanin stimulates IL-8 expression through MAPK and NF- B pathways in differentiated U937 cells. BMC Microbiology, 14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6: Kida Y, Higashimoto Y, Inoue H, Shimizu T, Kuwano K. (2008): A novel secreted protease from Pseudomonas aeruginosa activates NF-kappa B through protease-activated receptors. Cell Microbiol,10:1491-1504. [DOI] [PubMed] [Google Scholar]
- 7: Takeuchi R, Matsumoto H, Akimoto Y, Fujii A. (2011): Reduction in lipopolysaccharide-induced apoptosis of fibroblasts obtained from a patient with gingival overgrowth during nifedipine-treatment. Arch Oral Biol,56:1073-1080. [DOI] [PubMed] [Google Scholar]
- 8: Soler C, Valdés R, Garcia-Manteiga J, Xaus J, Comalada M, Casado FJ, Modolell M, Nicholson B, MacLeod C, Felipe A, Celada A, Pastor-Anglada M. (2001): Lipopolysaccharide-induced apoptosis of macrophages determines the up-regulation of concentrative nucleoside transporters Cnt1 and Cnt2 through tumor necrosis factor-alpha-dependent and -independent mechanisms. J Biol Chem, 276:30043-30049. [DOI] [PubMed] [Google Scholar]
- 9: Hull C, McLean G, Wong F, Duriez PJ, Karsan A. (2002): Lipopolysaccharide signals an endothelial apoptosis pathway through TNF receptor-associated factor 6-mediated activation of c-Jun NH2-terminal kinase. J Immunol, 169:2611-2618 [DOI] [PubMed] [Google Scholar]
- 10: Heck LW, Morihara K, McRae WB, Miller EJ. (1986): Specific cleavage of human type III and IV collagens by Pseudomonas aeruginosa elastase. Infect Immun, 51:115-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11: Harrington DJ. (1996): Bacterial collagenases and collagen-degrading enzymes and their potential role in human disease. Infect Immun, 64: 1885-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12: Metzger Z, Nitzan D, Pitaru S, Brosh T, Teicher S. (2002): The effect of bacterial endotoxin on the early tensile strength of healing in surgical wounds. J Endod, 28: 30-33. [DOI] [PubMed] [Google Scholar]
- 13: Kadurugamuwa JL, Beveridge TJ. (1997): Natural release of virulence factors in membrane vesicles by Pseudomonas aeruginosa and the effect of aminoglycoside antibiotics on their release. J Antimicrob Chemother, 40: 615-621. [DOI] [PubMed] [Google Scholar]
- 14: Van Langevelde P, Kwappenberg KM, Groeneveld PH, Mattie H, van Dissel JT. (1998): Antibiotic-induced lipopolysaccharide release from Salmonella typhi: delay between killing by ceftazidime and imipenem and release of LPS. Antimicrob Agents Chemother, 42:739-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15: Maisch T. (2009): A new strategy to destroy antibiotic resistant microorganisms: antimicrobial photodynamic treatment. Mini Rev Med Chem, 9;974-983. [DOI] [PubMed] [Google Scholar]
- 16: Hashimoto MC, Prates RA, Kato IT, Nnnez SC, Courrol LC, Ribeiro MS. (2012): Antimicrobial photodynamic therapy on drug resistant Pseudomonas aeruginosa induced infection. An in vivo study. Photochem.Photobiol, 88:590-595. [DOI] [PubMed] [Google Scholar]
- 17: Vecchio D, Dai T, Huang L, Fantetti L, Roncucci G, Hamblin MR. (2013): Antimicrobial photodynamic therapy with RLP068 kills methicilin resistant Staphylococcus aureus and improves wound healing in a mouse model of infected skin abrasion. J Biophotonics, 6: 733-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18: Sahu K, Sharma M, Sharma P, Verma Y, Rao KD, Bansal H, Dube A, Gupta P.K. (2014): Effect of poly-L-lysine-chlorin p6-mediated antimicrobial photodynamic treatment on collagen restoration in bacteria-infected wounds. Photomed Laser Surg, 32:23-29. [DOI] [PubMed] [Google Scholar]
- 19: Morley S, Griffiths J, Philips G, Moseley H, O'Grady C, Mellish K, Lankester CL, Faris B, Young RJ, Brown SB, Rhodes LE. (2013): Phase IIa randomized, placebo-controlled study of antimicrobial photodynamic therapy in bacterially colonized, chronic leg ulcers and diabetic foot ulcers: a new approach to antimicrobial therapy. Br J Dermatol, 168:617-624. [DOI] [PubMed] [Google Scholar]
- 20: Kömerik N, Wilson M, Poole S. (2000): The effect of photodynamic action on two virulence factors of gram-negative bacteria. Photochem Photobiol, 72:676-680 [DOI] [PubMed] [Google Scholar]
- 21: Sahu K, Sharma M, Bansal H, Dube A, Gupta PK. (2013): Topical photodynamic treatment with poly-L-lysine-chlorin p6 conjugate improves wound healing by reducing hyperinflammatory response in Pseudomonas aeruginosa infected wounds of mice. Lasers Med Sci, 28: 465-471. [DOI] [PubMed] [Google Scholar]
- 22: Matroule JY, Volanti C, Piette J. (2006) : NF-kappaB in photodynamic therapy: discrepancies of a master regulator. Photochem Photobiol, 82:1241-1246. [DOI] [PubMed] [Google Scholar]
- 23: Rahman MM, McFadden G. (2011): Modulation of NF- B signalling by microbial pathogens. Nat Rev Microbiol, 9:291-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24: Pahl HL. (1999): Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene, 8:6853-6866. [DOI] [PubMed] [Google Scholar]
- 25: Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors (2007). Trends Mol Med., 13:460-469. [DOI] [PubMed] [Google Scholar]
- 26: Covert MW, Leung TH, Gaston JE, Baltimore D. (2005): Achieving stability of lipopolysaccharideinduced NF-kappaB activation. Science, 309:1854-1857. [DOI] [PubMed] [Google Scholar]
- 27: Mc Ripley RJ, Whitney RR. (1976): Characterization and quantitation of experimental surgical-wound infections used to evaluate topical antibacterial agents. Antimicrob Agents Chemother, 10:38-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28: Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. (1985): Measurement of protein using bicinchoninic acid. Anal Biochem, 150: 76-85. [DOI] [PubMed] [Google Scholar]
- 29: Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM. (2010). Th17 and Th1 T-cell responses in giant cell arteritis. Circulation, 21:906-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30: Abe T, Abe Y, Aida Y, Hara Y, Maeda K. (2001): Extracellular matrix regulates induction of alkaline phosphatase expression by ascorbic acid in human fibroblasts. J Cell Physiol, 189:144-151. [DOI] [PubMed] [Google Scholar]
- 31: Walcott RD, Rhoads DD, Dowd SE. (2008): Biofilms and chronic wound inflammation. J Wound Care,17:333-341. [DOI] [PubMed] [Google Scholar]
- 32: Lu M, Varley AW, Munford RS. (2013): Persistently active microbial molecules prolong innate immune tolerance in vivo. PLoS Pathog, 9:e1003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33: Sharma M, Bansal H, Gupta PK. (2005): Virulence of Pseudomonas aeruginosa cells surviving photodynamic treatment with toluidine blue. Curr. Microbiol,50: 277-280. [DOI] [PubMed] [Google Scholar]
- 34: Huang Ying-Ying, Tanaka M, Vecchio D, Garcia-Diaz M, Chang J, Morimoto Y, Hamblin MR. (2013): Photodynamic therapy induces an immune response against a bacterial pathogen. Expert Rev Clin Immuno,8: 479-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35: Tanaka M, Mroz P, Dai T, Huang L, Morimoto Y, Kinoshita M, Yoshihara Y, Nemoto K, Shinomiya N, Seki S, Hamblin MR. (2012) : Photodynamic therapy can induce a protective innate immune response against murine bacterial arthritis via neutrophil accumulation. PLoS One, 7: e39823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36: Alpaslan G, Nakajima T, Takano Y. (1997): Extracellular alkaline phosphatase activity as a possible marker for wound healing: a preliminary report. Oral Mazillofac Surg; 55: 56-62. [DOI] [PubMed] [Google Scholar]
- 37: Malo MS, Biswas S, Abedrapo MA, Yeh L, Chen A, Hodin RA. (2006): The pro-inflammatory cytokines, IL-1beta and TNF-alpha, inhibit intestinal alkaline phosphatase gene expression. DNA Cell Biol, 25:684-695. [DOI] [PubMed] [Google Scholar]
- 38: Bol-Schoenmakers M, Fiechter D, Raaben W, Hassing I, Bleumink R, Kruijswijk D, Maijoor K, Tersteeg-Zijderveld M, Brands R, Pieters R. (2010): Intestinal alkaline phosphatase contributes to the reduction of severe intestinal epithelial damage. Eur J Pharmacol, 633:71-77. [DOI] [PubMed] [Google Scholar]
- 39: Krötzsch E, Salgado RM, Caba D, Lichtinger A, Padilla L, Di Silvio M. (2005): Alkaline Phosphatase Activity is Related to Acute Inflammation and Collagen Turnover During Acute and Chronic Wound Healing. Wound Repair and Regeneration, 13: A28-A48. [Google Scholar]
- 40: Werner S, Grose R. (2003): Regulation of wound healing by growth factors and cytokines. Physiol Rev, 83:835-870. [DOI] [PubMed] [Google Scholar]