Abstract
Aim: To review the applications of low level laser therapy on various soft and hard oral tissues.
A variety of therapeutic effects of Low Level Laser Therapy have been reported on a broad range of disorders. It has been found amenably practical in dental applications including soft as well as hard tissues of the oral cavity. LLLT has been found to be efficient in acceleration of wound healing, enhanced remodelling and bone repair, regeneration of neural cells following injury, pain attenuation, endorphin release stimulation and modulation of immune system. The aforementioned biological processes induced by Low level lasers have been effectively applied in treating various pathological conditions in the oral cavity. With is article, we attempt to review the possible application of Low Laser Therapy in the field of dentistry.
Keywords: Low Level Laser Therapy (LLLT), Biostimulation, Photobiomodulation
Introduction
With the advent of various advances in the field of laser dentistry there has been a growing interest in the effects of low level lasers on tissues. The effect of low level lasers on tissues was first demonstrated by Mester et al 1) in 1967. Since then it has been broadly termed as Low Level Laser Therapy (LLLT) or photobiomodulation. North American Association of Laser Therapy defines LLLT as “non-thermal laser light application using photons (light energy) from the visible and infrared spectrum for tissue healing and pain reduction”. 2)
Although several forms of light may be used for photobiomodulation, 3), 4) lasers are being increasingly used for this purpose due to better therapeutic effects of the latter.
The mechanism of action of LLLT was first proposed by Karu et al in 1981. 5) It was postulated that laser irradiation leads to production of singlet oxygen which in turn promotes RNA and DNA synthesis. In 1988, it was suggested that laser energy might cause photoexcitation of cytochrome c complex leading to alteration in redox state. 6) It has also been hypothesized that laser irradiation acts on mitochondria and reverses the inhibition caused by nitric oxide (NO) leading to activation of the respiratory chain. 7)
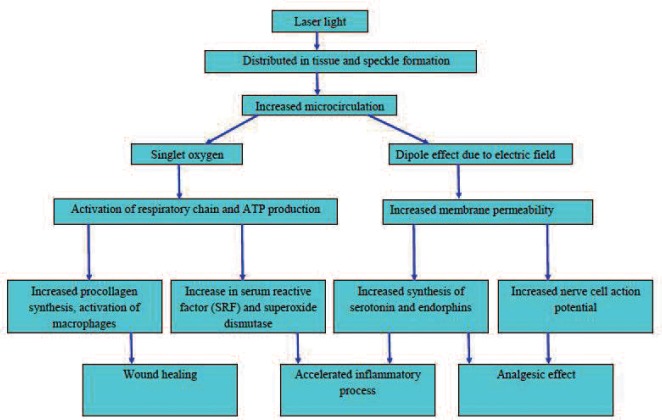
Although the exact mechanism is still not understood, it is proposed that several factors interact to produce the therapeutic effect of LLLT as summarized below 8–10) (Figure 1)
Figure 1.
Mechanism of action of low level laser therapy
As laser light gets diffusely distributed in the tissue it leads to speckle formation. These laser speckles create temperature and pressure gradients across cell membranes which increase the rate of diffusion across the membranes. Speckle formation occurs when coherent light such as laser is reflected or transmitted from an optically rough surface leading to secondary waves which intersect with each other. 11) The photons in each speckle are highly polarized which creates areas of partially polarized light. This increases the absorption of photons in cytochrome c oxidase molecules which stimulates the production of singlet oxygen. The singlet oxygen species activates the respiratory chain which enhances production of ATP. This also triggers the immunological chain reaction which stimulates mast cell and macrophages and also an increased procollagen synthesis is seen which promotes wound healing. The temperature and pressure gradients increase the permeability of cell membranes which affects ion transfer and results in increase in serum reactive factor (SRF) and superoxide dismutase levels which accelerate inflammatory process and increased synthesis of serotonin and endorphins which produce analgesic effect. There is membrane repolarisation & stimulation of mechanoreceptors along with decreased bradychinine production which further adds to analgesic effect. There is relaxation of smooth muscle associated with endothelium which causes vasodilation. This vasodilation causes increased oxygen supply to the area and results in greater inflow of immune cells into the tissue. Thus LLLT affects wound healing, inflammatory process as well as pain.
With the above background, this review is an attempt to summarize various possible applications of LLLT on soft and hard dental tissue. (Table 1)
Table 1. Soft and Hard Tissue applications of Low Level Laser Therapy (LLLT).
| Soft Tissue Applications | Hard Tissue Applications |
|---|---|
| Herpetic Lesions | Temporomandibular Disorders |
| Aphthous/ Traumatic Ulcers | Orthodontic Treatment |
| Post Oncology Mucositis | Dentinal Hypersensitivity |
| Post Extraction Socket/ Post trauma Sites | Bone Remodelling |
| Trigeminal Nueralgia | Erosion |
| Periodontal Pocket Disinfection/Periodontitis | Stimulatory effect on root development |
| Edema | Implants |
| Sinusitis | |
| Gag Reflex/ Nausea | |
| Postsurgical Pain |
Herpetic Lesions (Herpes Labialis or post Herpetic neuralgia)
Herpes Simplex is caused by the human herpes virus types 1 and 2 that generally present a primary lesion, with periods of latency and a tendency to relapse. Symptoms may include painful vescicles or erosions involving gingival and labial/oral mucosa, fever and mucosis of mouth accompanied with painful lymph nodes. 12) Helium-Neon Lasers or diode lasers with low energy levels have been proved to be effective in producing analgesia and reduced inflammation in areas of vesicles. It has been proposed that laser radiation can proliferate the development of blood circulation within the regenerative tissue, which together with biochemical agents, secreted into inflamed tissue, can support the interpretation of the beneficial effects of laser radiation in Herpes Simplex Labialis therapy. Jovanovic L et al (1998) reported significant reduction in pain immediately after first session of He-Ne laser for five minutes. Crust formation had taken place after second session and all the signs and symptoms had disappeared at the fifth day. The patients were subjected to LLLT at 1mW power settings, wavelength (638 nm), spot size ∼ (2–3 cm2), Power density (14–32 W/cm2) for five minutes respectively, for about five days. 13)
Recurrent Aphthous ulcers or Ulcers of traumatic origin
LLLT for the treatment of aphthous or traumatic ulcers has been recommended for its analgesic effect and the shortened healing time. This may be attributed to the disruption of the Na-K pump in the cell membrane and serotonin release. 3) Diode laser (940 nm) could be considered as an effective and safe treatment modality in treating oral ulcers. Dhillon JK & Colleagues (2012) have reported excellent healing and immediate pain relief in aphthous ulcers when treated with Diode laser at 0.1 mW power settings, dissipating 3.5 J energy per cm2 for 3 minutes. 14)
Mucositis
Mucositis is reported to be inevitable sequelae of radiation or chemotherapy regimes. It has been documented that LLLT has been used effectively in such cases and has been able to reduce the incidence of inflammation and pain. Mainly He-Ne laser or red or infra red lasers have been proved fruitful in providing immediate symptomatic relief to such patients. 15), 16) Bensadoun RJ, Nair RG conducted a literature review and meta analysis on role of LLLT for preventing and treating mucositis. They recommended that red or infrared LLLT with diode output between 10–100 mW, dose of 2–3 J/cm2 for prophylaxis and 4 J/cm2 should be used for therapeutic effect. They also recommended that LLLT should be applied on single spot rather than using scanning motion. This should be repeated daily or every other day or a minimum of three times per week until resolution. 17)
Trigeminal Neuralgia
Low Level Laser Therapy has produced promising results in treatment of nerve injuries. 18) Studies have reported increased nerve function and improvement in myelin production with LLLT. (It has been shown to be effective in promoting axonal growth of injured nerves in animal models.) 19)
The direct application of this technique to dentistry has yielded positive results in promoting the regeneration of inferior dental nerve (IDN) tissue damaged during surgical procedures. 20) The proposed LLT protocols involve daily irradiation for prolonged periods, e.g., 10 days at 4.5 J per day. 21) Leonard F et al (2008) has reported two cases diagnosed with trigeminal neuralgia, who had tried all possible modalities such as NSAIDS and Steroids and could not be relieved from pain. GaAlAs Diode laser system (wavelength808 nm, power 200 mW and 6 J energy and a total of 20 applications) was applied to their affected facial areas once daily consecutively for five days with a two day interval. Symptomatic relief was reported immediately after fifth session and minimal discomfort after ninth session. 22)
Periodontal Pocket disinfection/Periodontitis
Diode lasers have been used extensively in the periodontal therapy for removal of diseased pocket lining epithelium and disinfection of periodontal pockets at low level energy. Optic fibre delivery systems, with 200–320 µm fibre diameters, enable extremely easy access into the periodontal pocket. 23) The anti-inflammatory effect of LLLT slows or stops the deterioration of periodontal tissues and reduces the swelling to facilitate the hygiene in conjunction with other scaling, root planning, curettage, or surgical treatment. Some studies demonstrated that diode laser is effective in bacterial elimination, resulting in a better healing. Moritz et al. (1997) showed a significant reduction of bacteria, as A. actenomycetemcomitans, with a parallel improvement of periodontal parameters. 24) Caruso et al. (2008) compared the effectiveness of a diode laser (980 nm wavelength) with a power output of 2.5 W in a pulse mode (30 Hz) and a tip (400 µm) angulated at 20° with each pocket lased for 30 s twice, with a 60 s interval. Findings indicated a slightly better periodontal healing, in terms of clinical parameters at 4, 8 and 12 weeks. 25)
Post surgical pain
Analgesic effects of soft tissue lasers at lower energy levels have been evidently seen in literature. The effect has been explained in terms of interference with the mediation of the pain impulses and/or the stimulation of endorphin production. 26), 27) It also inhibits nociceptive signals arising from peripheral nerves. 28) Less post operative pain and swelling may be encountered if the surgical site is irradiated with low level powers. Post extraction irradiation with about 2 J/cm2, in a non contact mode for about one minute may reduce any complications. 29) A single irradiance episode of LLLT with Ga-Al-As laser (wavelength 830-nm and energy 0.9–2.7 J/cm2) is most effective in apical periodontitis following root canal treatment and post extraction pain. 30)
Edema
Edema, caused either by pathologies or by dental interventions, may be found commonly occurring in dental patients. LLLT decreases the permeability of the lymph vessels and can also stimulate lymph vessel collaterals. Edema may be controlled by augmenting phagocytosis and increase in number and size of lymph vessels. 31) Giuliani A et al (2004) confirmed that LLLT was effective in reducing edema and hyperalgesia in acute and chronic inflammation if administered at the points usually selected for acupuncture in rats. 32) Aras MH, Güngörmüş M (2009) reported that immediately after surgery, intraoral application of LLLT at 4 J/cm2 and at a distance of 1 cm from the target tissue and extraorally to masseter muscle leads to reduction in postoperative edema and trismus. 33)
Sinusitis
Literature reports that LLLT can effectively reduce pain and inflammation in cases of acute exacerbations of sinusitis. In a study involving 65 children aged 6 to 15 years with sinusitis, LLLT was instituted and was found that LLLT improved microcirculation, reduced oedema, and reduced the frequency of relapses of sinusitis. 34) Naghdi S et al (2013) reported in a pilot study on effects of LLLT on chronic rhinosinusitis that Ga-Al-As laser (wavelength 830 nm, power output 30 mW and energy dose of 1 J, 3 times a week) in continuous-wave mode applied for 4 weeks improves symptoms. 35)
Nausea/ Gag Reflex
Acupuncture may be practiced with the help of LLLT. It has been proposed that a useful and risk free point on wrist known as the meridian P 6, if irradiated with laser wavelength 880 nm, an energy of about 3–5 Joules/cm2 for a period of 1 min held in contact and perpendicular to the tissue prove to be effective in reducing gag reflex or nausea in patients. However, acupuncture should be performed only by trained individuals. 36), 37)
TMD
Non dental pain in the orofacial region is mainly associated with the TMD (temporomandibular disorder). A characteristic feature is the pain in the masticatory muscles associated with restricted mandibular movement. 38) Various treatment modalities are available amongst which LLLT has gained interest as it is conservative in nature and the analgesic, regenerative, and non inflammatory effects are in the target tissue. Several mechanisms have been involved in pain reduction and therapeutic effects of low-level lasers, including promoting the release of endogenous opioids, enhancing cell respiration and tissue healing, increasing vasodilatation, increasing pain threshold by affecting the cellular membrane potential, and decreasing inflammation, possibly due to the reduction of prostaglandin E2 and suppression of cyclooxygenase 2 levels. 39), 40) Controversial results have been reported in literature regarding the pain reduction and mouth opening. Kulekcioglu S and colleagues conducted a study to compare the effect of reduction of pain in both study and placebo group where study group received LLLT (frequency: 1000 Hz, duration: 180 seconds, dosage: 3 J/cm2) at the four most tender points selected during examination. 41) please check the result- comparing test and placebo group.
Orthodontic movement
The force application required for the movement of tooth in orthodontics lead to pain. Decreasing the duration of orthodontic treatment could decrease the incidence of side-effects, leading to greater patient comfort and satisfaction. 42) Considering the long term effect of orthodontic tooth movement on root resorption and periodontal concerns, use of lasers has gained importance. There is some documentation for the use of LLLT to reduce the pain experienced during tooth movements and accelerate tooth movement. 43) Low dosage seems to accelerate the speed of movement, whereas higher dosage appears to slow movement. In the latter case, this could possibly be used for stabilization of a finished orthodontic therapy. The effects of laser application in biostimulation of bone depend on the irradiation dose. 44) Various studies have provided the evidence towards amelioration of pain and expediting tooth movement. Long H et al suggest that low-level laser therapy would be effective in accelerating orthodontic tooth movement at the fluence of 5 J/cm2 within 2 and 3months. 45) It also leads to osteoclastic activity on pressure side and osteoblastic activity on tension side. 37)
Dentinal hypersensitivity
Dentin hypersensitivity has been one of the most distressing symptoms to the patients for ages. 46) Elimination of pain and discomfort due to dental hypersensitivity has always been a great concern. Analgesic effect has been the need regardless of the origin of the hypersensitivity. Low-level laser effect on dentinal hypersensitivity relies mainly upon inducing changes in the neural transmission networks within the dental pulp (depressed nerve transmission) rather than alterations in the exposed dentine surface as observed in other treatment modalities. 47) Tuner and Hode concluded that laser effects on endorphin release could be the reason for the immediate pain relief in patients, but biostimulative effects happens gradually in probably in few days. 48) Besides the immediate analgesic effect, the laser therapy used with correct parameters, may stimulate the normal physiological cellular functions. Stimulation of odontoblasts, production of reparatory irregular dentin and obliteration of dentinal tubules provoked by laser are reasons for the prolonged suppression of pain in dentinal hypersensitivity. 48) Orhan Kaan and co-authors conducted a short term clinical trial with the irradiance of 4 J/cm2 per treatment site which revealed reduction in dentinal hypersensitivity in 7 days 49). Pesevska S and colleagues compared low level laser and fluoride therapy and observed complete resolution of pain achieved in 86.67% of the laser-treated group, compared to 26.67% of the control group with topical fluoride treatment. 50)
Bone remodelling
Considerable work in arena of bone remodelling, including the use of cell cultures, animal models and clinical studies, has been conduced to evaluate the cell bio stimulation effects of LLLT. 51) It is equivocal whether low-level laser bio modulation of bone formation is a consequence of stimulation of mesenchymal cells or direct stimulation of osteoblasts. 52) It is possible that bio stimulation is a repercussion of an increased release of fibroblast growth factor, which is found in bone tissue. It acts on differentiated cells, increasing both cell proliferation and secretion of components of the matrix. 52) Khadra et al. investigated the effect of LLLT on the attachment, proliferation, differentiation and production of transforming growth factor-β1 from human mandibular bone exposed to gallium-aluminum-arsenium (GaAlAs) laser (1.5 J/cm2 or 3 J/cm2). 53) The results of this study indicated that LLLT can modulate the activity of cells and tissues surrounding implant material. 37) Bone fractures exhibited speedy formation of bone tissue, with a tighter mesh of trabeculae. Some authors have pointed out that LLLT can accelerate bone formation by increasing osteoblastic activity, vascularization, and organization of collagen fibres. 54–57) Depending on the phase of bone repair, LLLT can accelerate resorption or formation activities. 58) Literature has shown that LLLT helps in formation of new bone around implant. The mechanism of action is ambiguous. Further research is required to explain it completely.
Erosion
There has been an increase in prevalence of erosion due to amplified consumption of acidic food and gastroesophageal reflux. Dental erosion involves a multifactorial pathogenesis where chemical, biological, and behavioural factors are related to the outcome, which is progressive and irreversible demineralization of the outer layer of the dental tissues. Several studies have demonstrated that high-power laser irradiation (Nd: YAG, argon, CO2, and Er: YAG) may reduce the progression of the demineralization process. However undesirable thermal alterations have been associated with high power laser. In the search for alternatives to protect dental tissues from constant exposure to high acidity, researchers have shown that the use of the diode lasers at wavelengths in the visible and near-infrared regions may lead to an increase in the resistance of teeth against demineralization. 59) Vlacic J and colleagues reported that after application of 1.23% neutral sodium fluoride gel when lased with 488, 514.5, 532, 633, 670, 830 or 1064 nm wavelength (energy density 15J/cm(-2); spot size 5mm), then exposed to an erosive challenge (1.0M HCI for five minutes). They concluded laser energy to increase the resistance of human enamel and dentine to acid dissolution. 60) In future it can be promised that LLLT may be an alternative approach as their application can possibly induce modification of the organic matrix content of enamel, which may then lead to an increased resistance against demineralization.
Stimulatory effect on root development
Dental trauma and caries can cause necrosis of pulp leading to arrest in root development. The primary goal is to maintain the vitality of the tooth for the root development. 61) Studies have demonstrated that LLLT used on exposed pulp reduces the inflammatory process. 62) LLLT also accelerates tissue repair by formation of a fibrous matrix and dentin bridge and increases the production of collagenous and non-collagenous proteins from the extracellular matrix (ECM). These proteins are able to perform an important role in mineralized tissue formation, and are also able to act in differentiation, migration, and proliferation during the various stages of dentinogenesis. A marked acceleration in root development in rats was observed in a study conducted by Toomarian L et al. This can be supported by the findings that GaAlAs laser irradiation increases alkaline phosphatase (ALP) activity and certain molecular expression of dental pulp cells. ALP is an early differentiation marker for both osteoblasts and odontoblasts. This enzyme plays a vital role in calcified tissue formation, probably by regulating phosphate transport. 63)
Implants
Osseointegration is the key point in determining the success of an implant. Researchers have been inquest of a technique for bringing the implant into function and reduce the osseointegration time, by altering the bone-implant contact surface area. Multiple sessions of application of low-level lasers (LLL) in peri-implant tissues stimulates local blood circulation, bone-implant contact surface area, accelerates bone maturation in peri-implant area resulting in higher quantity of viable osteocytes and higher metabolic bone activity. Guzzardella GA and workers observed higher boneimplant contact values and hardness nearby implant compared to control. 64) Khadra et al concluded use of LLLT at the range of doses between 1.5 and 3 J/cm2 may modulate the activity of cells interacting with an implant, thereby enhancing tissue healing and ultimate implant success.53)
Contraindications and Safety measures
Generally, low level laser therapy, also known as therapeutic lasers have not been found to report any side effects or have caused harm to patients being operated. These lasers usually belong to Class III or Class IV (Based on potential to cause damage). The risk of eye injury must be considered, especially for high-output lasers in the invisible range. Diode laser light is generally divergent; however, if the light is collimated, the risk of eye injury increases significantly. Protective goggles, specific for the wavelength, must be used for the patient and the dental professional. The laser beam should not be seen under magnification aids such as microscope or surgical loupes. Non inflammable products must be used in the operatory.
Although there are no contraindications to the application of LLLT, however, patients with malignancies and coagulation disorders may be handled with utmost care and caution.
Conclusion
LLLT can prove to be an effective treatment modality for various oral maladies provided that the clinician takes proper training and adopts necessary safety measures. Future research might result in several more potential applications of LLLT in dentistry.
References
- 1: Mester E, Szende B, Tota JG. (1967): Effect of laser on hair growth of mice. Kiserl Orvostud, 19:628-631 [Google Scholar]
- 2:NAALT 2003 Post conference standards report. Available online at http://www.naalt.org/post-conferencehtm Last accessed on 1st July 2013.
- 3: Karu TI. (1989): Photobiology of low-power laser effects. Health Physics, 56:691-704 [DOI] [PubMed] [Google Scholar]
- 4: Laakso EL, Richardson CR, Cramond T. (1993): Factors affecting low level laser therapy. Australian Journal of Physiotherapy,39:95-99 [DOI] [PubMed] [Google Scholar]
- 5: Karu TJ, Kalendo GS, Letokhov VS. (1981): Control of RNA synthesis rate in tumor cells HeLa by action of low-intensity visible light of copper laser. Nuovo Cimento,32:55-59 [Google Scholar]
- 6: Karu TI. (1988): Molecular mechanism of the therapeutic effect of low-intensity laser radiation. Lasers Life Science, 2(1): 53-74. [Google Scholar]
- 7: Karu TI. (2000): Mechanisms of low-power laser light action on cellular level. Proc. SPIE 4159, Effects of Low-Power Light on Biological Systems V, 1 (November 3, 2); 10.1117/12.405918; http://dx.doi.org/10.1117/12.405918. [DOI] [Google Scholar]
- 8: Karu TI. (2003): Low-Power Laser Therapy. In Biomedical Photonics Handbook. Ed Tuan Vo-Dinh. CRC Press. ISBN: 978-0-8493-1116-1117. [Google Scholar]
- 9: Hansen HJ, Thorøe U. (1990): Low power laser biostimulation of chronic oro-facial pain. A double-blind placebo controlled cross-over study in 40 patients. Pain, 43(2):169-179 [DOI] [PubMed] [Google Scholar]
- 10: Walker J. (1983): Relief from chronic pain by low power laser irradiation. Neuroscience Letters, 30;43(2–3):339-344 [DOI] [PubMed] [Google Scholar]
- 11: Dainty JC, Laser Speckle and Related Phenomena, 1984, Sprinter Verlag, ISBN 0-387-13169-8. [Google Scholar]
- 12: Simunovic Z. (2012): Herpes virus infection low level laser therapy (LLLT)- photobiostimulation applied as monotherapy in treatment of human pathogen Herpes virus. Laser medicine,1:1-12 [Google Scholar]
- 13: Jovanovi L, Mirkovi B, Živkovi B. (1998): Soft Laser in the therapy of Herpes simplex labialis. Medicine and Biology,5(1):61-63 [Google Scholar]
- 14: Dhillon JK, Kalra G, Mathur VP. (2012): Laser biostimulation of oral ulcers in children. International Journal of Laser Dentistry,2(2):59-62 [Google Scholar]
- 15: Wong SF, Wilder-Smith P. (2002): Pilot study on laser effects on oral mucositis in patients receiving chemotherapy. Cancer Journal,8:247-254 [DOI] [PubMed] [Google Scholar]
- 16: Marie-Thérèsea G, Jean K. (2005): Low-level laser for prevention and therapy of oral mucositis induced by chemotherapy or radiotherapy. Current Opinion in Oncology,17(3):236-240 [DOI] [PubMed] [Google Scholar]
- 17: Bensadoun RJ, Nair RG. (2012): Low-level laser therapy in the prevention and treatment of cancer therapy-induced mucositis: 2012 state of the art based on literature review and meta-analysis. Current Opinion in Oncology. 24(4):363-370 [DOI] [PubMed] [Google Scholar]
- 18: Mohammed IF, Al-Mustawfi N, Kaka LN. (2007): Promotion of regenerative processes in injured peripheral nerve induced by low-level laser therapy. Photomedicine Laser Surgery,25(2):107-111 [DOI] [PubMed] [Google Scholar]
- 19: Gigo-Benato D, Russo TL, Tanaka EH, Assis L, Salvini TF, Parizotto NA. (2010): Effects of 660 and 780 nm low-level laser therapy on neuromuscular recovery after crush injury in rat sciatic nerve. Lasers Surgery and Medicine,42(9):673-682 [DOI] [PubMed] [Google Scholar]
- 20: Rochkind S, Drory V, Alon M, et al. (2007): Laser phototherapy (780 nm), a new modality in treatment of long-term incomplete peripheral nerve injury: a randomized double-blind placebo-controlled study. Photomedicine and Laser Surgery; 25:436-442. [DOI] [PubMed] [Google Scholar]
- 21: Mester AF, Snow JB, Shaman P. (1991): Photochemical effects of laser irradiation on neuritic outgrowth of olfactory neuroepithelial explants. Otolaryngology Head Neck Surgery,105:449-456 [DOI] [PubMed] [Google Scholar]
- 22: Leonard F, Vernon DC, Hasbun RJ. (2008): Low Level Laser Therapy for Trigeminal Neuralgia. Practical Pain Management, 56-63. [Google Scholar]
- 23: Pirnat S. (2007): Versatility of an 810 nm Diode Laser in Dentistry: An Overview. Journal of Laser and Health Academy,4:1-9 [Google Scholar]
- 24: Moritz A, Gutknecht N, Doertbudak O, Goharkhay K, Schoop U, Schauer P, Sperr W. (1997): Bacterial reduction in periodontal pockets through irradiation with a diode laser: a pilot study. Journal of Clinical Laser Medicine Surgery, 15:33-37 [DOI] [PubMed] [Google Scholar]
- 25: Caruso U, Nastri L, Piccolomini R, d'Ercole S, Mazza C, Guida L. (2008): Use of diode laser 980 nm as adjunctive therapy in the treatment of chronic periodontitis. A randomized controlled clinical trial. New Microbiology,31(4):513-518 [PubMed] [Google Scholar]
- 26: Ohshiro T, Fujino T. (1993): Laser applications in plastic and reconstructive surgery. Keio Journal of Medicine, 42:191-195 [DOI] [PubMed] [Google Scholar]
- 27: Tsuchiya K, Kawatani M, Takeshige C, Matsumoto I. (1994). Laser irradiation abates neuronal responses to nociceptive stimulation of rat paw skin. Brain Research Bulletin.;34:369-374 [DOI] [PubMed] [Google Scholar]
- 28: Walsh LJ. (1997). The current status of low level laser therapy in dentistry. Part 1. Soft tissue applications. Australian Dental Journal, 42:4. [DOI] [PubMed] [Google Scholar]
- 29: Takeda Y. (1988). Irradiation effect of low-energy laser on alveolar bone after tooth extraction. International Journal of Oral Maxillofacial Surgery,17:388-391 [DOI] [PubMed] [Google Scholar]
- 30: Kawakami T, Ibaraki Y, Haraguchi K, Odachi H, Kawamura H, Kubota M, Miyata T, Watanabe T, Iioka A, Nittono M. (1989): The effectiveness of GaAlAs semiconductor laser treatment to decrease pain after irradiation. Higashi Nippon Shigaku Zasshi,8:57-62 [PubMed] [Google Scholar]
- 31: Aimbire F, Albertini R, Pacheco M.T.T., et al. (2006): Low-Level Laser Therapy Induces Dose-Dependent Reduction of TNFα Levels in Acute Inflammation. Photomedicine and Laser Surgery,24(1):33-37 [DOI] [PubMed] [Google Scholar]
- 32: Giuliani A, Fernandez M, Farinelli M, et al. (2004): Very low level laser therapy attenuates edema and pain in experimental models. International Journal of Tissue Reaction, 26(1–2):29-37 [PubMed] [Google Scholar]
- 33: Aras MH, Güngörmü M. (2009): The effect of low-level laser therapy on trismus and facial swelling following surgical extraction of a lower third molar. Photomedicine and Laser Surgery,27(1):21-24 [DOI] [PubMed] [Google Scholar]
- 34: Kruchinina I, Feniksova LV, Rybalkin SV, Pekli FF. (1991): Therapeutic effect of helium-neon laser on microcirculation of nasal mucosa in children with acute and chronic maxillary sinusitis as measured by conjunctival biomicroscopy. Vestnik otorinolaringoloji, 3:26-30 [PubMed] [Google Scholar]
- 35: Naghdi S, Ansari NN, Fathali M, Bartley J, Varedi M, Honarpishe R. (2013): A pilot study into the effect of low-level laser therapy in patients with chronic rhinosinusitis. Physiotherapy Theory and Practice Informa health care, 29(8):596-603 [DOI] [PubMed] [Google Scholar]
- 36: Kotlow L. (2009): Photobiomodulating Lasers and Children's Dental Care. Journal of Laser Dentistry,17(3):125-130 [Google Scholar]
- 37: Sun G, Tuner J. (2004): Low-level laser therapy in dentistry. Dental Clinics of North America,48:1061-1076 [DOI] [PubMed] [Google Scholar]
- 38: Ahrari F, Madani AS, Ghafouri ZS, Tuner J. (2013): The efficacy of low-level laser therapy for the treatment of myogenous temporomandibular joint disorder. Lasers Medicine Science, 29(2):551-557 [DOI] [PubMed] [Google Scholar]
- 39: Sayeed N. (2013): Evaluation of low-level laser therapy in patients with acute and chronic temporomandibular disorders. Journal of Maxillofacial and Oral Surgery,28(1):57-64 [DOI] [PubMed] [Google Scholar]
- 40: Brito JALS. (2012):Management of Temporomandibular Disorders with Low Level Laser Therapy Lasers Medicine Science,28:57-64 [Google Scholar]
- 41: Sevinc Kulekcioglu1, Koncuy Sivrioglu1, Orhan Ozcan1, Mufit Parlak. (2003): Effectiveness of low-level laser therapy in temporomandibular disorder. Scandanavian Journal of Rheumatology,32:114-118. [DOI] [PubMed] [Google Scholar]
- 42: Genc G. (2013): Effect of low-level laser therapy (LLLT) on orthodontic tooth movement Lasers Medicine Science,28:41-47 [DOI] [PubMed] [Google Scholar]
- 43: Somayeh Heidari, Sepideh Torkan: Laser Applications in Orthodontics (2013) Journal of Lasers Medicine and Science, 4(4): 151-158. [PMC free article] [PubMed] [Google Scholar]
- 44: Youssef M, et al. (2008): The effect of low-level laser therapy during orthodontic movement: a preliminary study. Lasers Medicine and Science, 23:27-33 [DOI] [PubMed] [Google Scholar]
- 45: Long H, et al. (2015): The effectiveness of low-level laser therapy in accelerating orthodontic tooth movement: a meta-analysis. Lasers Medicine and Science,30:1161-1170 [DOI] [PubMed] [Google Scholar]
- 46: Davari Davari, Ataei E, Assarzadeh H. (2013): Dentin Hypersensitivity: Etiology, Diagnosis and Treatment; A Literature Review. Journal of Dentistry,14(3): 136-145 [PMC free article] [PubMed] [Google Scholar]
- 47: Hashim, et al. (2014): Effect of the clinical application of the diode laser (810 nm) in the treatment of dentine hypersensitivity. BMC Research Notes, 7:31-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48: Ardavan Etemadi, Mostafa Sadeghi, Mohamad Hossein Dadjou. (2011): The Effects of Low Level 660nm Laser Irradiation on Pain and Teeth Hypersensitivity after Periodontal Surgery. Journal of Lasers in Medical Sciences, 2(3):103-109. [Google Scholar]
- 49: Orhan K, Aksoy U, -Karabulut D, Kalender A. (2011): Low-level laser therapy of dentin hypersensitivity: a short-term clinical trial. Lasers Medicine Science,26:591-598. [DOI] [PubMed] [Google Scholar]
- 50: Pesevska S. (2010): Dentinal hypersensitivity following scaling and root planing: comparison of low-level laser and topical fluoride treatment. Lasers Medicine Science,25:647-650. [DOI] [PubMed] [Google Scholar]
- 51: Obradovi Radmila R., Kesa Ljiljana G., Peševska Svetlana. (2009): Influence of low-level laser therapy on biomaterial osseointegration: a mini-review. Lasers Medicine Science, 24:447-451. [DOI] [PubMed] [Google Scholar]
- 52: Pinheiro ALB, et al. (2003): Effect of Low Level Laser Therapy on the Repair of Bone Defects Grafted with Inorganic Bovine Bone. Brazilian Dental Journal, 14(3):177-181 [DOI] [PubMed] [Google Scholar]
- 53: Khadra M, Lyngstadaas SP, Haanæs HR, Mustafa K. (2005): Effect of laser therapy on attachment, proliferation and differentiation of human osteoblastlike cells cultured on titanium implant material. Biomaterials,26:3503-3509 [DOI] [PubMed] [Google Scholar]
- 54: Trelles MA, Mayayo E. (1987): Bone fracture consolidates faster with low-power laser. Lasers Surgery Medicine,7:36-45 [DOI] [PubMed] [Google Scholar]
- 55: Barushka O, Yaakobi T, Oron U. (1995): Effect of low energy laser (He-Ne) irradiation on the process of bone repair in the rat tibia. Bone, 16:47-55 [DOI] [PubMed] [Google Scholar]
- 56: Ozawa Y, Shimizu N, Kariya G, et al. (1998): Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone,22:347-354 [DOI] [PubMed] [Google Scholar]
- 57: Hamajima S, Hiratsuka K, Kiyama-Kishikawa M, et al. (2003): Effect of low-level laser irradiation on osteoglycin gene expression in osteoblasts. Lasers Medicine Science,18:78-82 [DOI] [PubMed] [Google Scholar]
- 58: Nicola RA, Jorgetti V, Rigau J, et al. (2003): Effect of low power GaAlAs laser (660 nm) on bone structure and cell activity: an experimental animal study. Lasers Medicine Science,18:89-94 [DOI] [PubMed] [Google Scholar]
- 59: Melo MA. (2011): The effect of diode laser irradiation on dentin as a preventive measure against dental erosion: an in vitro study. Lasers Medicine Science ,26:615-621 [DOI] [PubMed] [Google Scholar]
- 60: Vlacic J, Meyers IA, Walsh LJ. (2007): Laser-activated fluoride treatment of enamel as prevention against erosion. Australian Dental Journal,52(3):175-180 [DOI] [PubMed] [Google Scholar]
- 61: Witherspoon DE, Small JC, Harris GZ. (2006): Mineral trioxide aggregate pulpotomies: a case series outcomes assessment. Journal of American Dental Association,137(5):610-618 [DOI] [PubMed] [Google Scholar]
- 62: Utsunomiya T. (1998): A histopathological study of the effects of low-power laser irradiation on wound healing of exposed dental pulp tissues in dogs, with special reference to lectins and collagens. Journal of Endodontics,24:187-193 [DOI] [PubMed] [Google Scholar]
- 63: Toomarian L, Fekrazad R, Tadayon N, Ramezani Jamileh, Tunér Jan. (2012): Stimulatory effect of low-level laser therapy on root development of rat molars: a preliminary study. Lasers Medicine Science,27:537-542. [DOI] [PubMed] [Google Scholar]
- 64: Guzzardella GA, Toricelli P, Nicoli-Aldini N, et al. > (2003): Osseointegration of endosseous ceramic implants after postoperative low power laser stimulation: an in vivo comparative study. Clinical Oral Implants Research,14:226-232. [DOI] [PubMed] [Google Scholar]