Abstract
Human and nonhuman primate communication differs in various ways. In particular, humans base communicative efforts on mutual knowledge and conventions shared between interlocutors. In this study, we experimentally tested whether bonobos (Pan paniscus), a close relative to humans, are able to take into account the familiarity, i.e. the shared interaction history, when communicating with a human partner. In five experimental conditions we found that subjects took the recipients’ attentional state and their own communicative effectiveness into account by adjusting signal production accordingly. More importantly, in case of communicative failure, subjects repeated previously successful signals more often with a familiar than unfamiliar recipient, with whom they had no previous interactions, and elaborated by switching to new signals more with the unfamiliar than the familiar one, similar to what has previously been found in two year-old children. We discuss these findings in relation to the human capacity to establish common ground between interlocutors, a crucial aspect of human cooperative communication.
Human communication is punctuated by misunderstandings. Recipients can resolve them by requesting clarifications from signallers, who then must discern why the recipient has failed to comprehend them. Problems with perception or unfamiliarity with a linguistic convention are common causes, which are detected by assessments of the recipient’s mental states. In child development, the transition to understanding others as mental agents is a gradual process that derives, at least partly, from understanding intentions1. This is particularly important in linguistic discourse in which there is a continued need to take the listener’s perspective into account, which often differs from the signaller’s own. In case of failure, listeners will signal miscomprehension, an ability already observed in two-years olds2,3,4,5. For example, misunderstood infants are more likely to elaborate linguistic requests to an unfamiliar than a familiar adult, indicating that they possess some understanding of their shared interaction history6,7. In humans, in other words, shared experiences between a signaller and a recipient determine what inferences can be made about mutual knowledge, a foundation necessary to communicate cooperatively8.
Human cognitive evolution is often investigated with comparative research on non-human primates and particularly great apes, which continues to contribute to a clearer understanding of how hominids have diverged from their primate ancestors and what features continue to be shared with modern apes. Particularly impressive are studies with ‘enculturated’ great apes that have acquired artificial linguistic systems to communicate with their human caretakers9,10. Results of these studies have regularly been interpreted in terms of language-relevant capacities (e.g.11,12,13), including the claim that bonobos are capable of taking into account shared knowledge when interacting with familiar humans14,15. A general conclusion from this literature is that key cognitive abilities necessary for language were already present in the common ancestor of modern humans, chimpanzees and bonobos.
Although impressive, one weakness of this body of research has always been the difficulty to find comparable capacities in the natural communication of wild apes (e.g.16,17,18). In recent years, however, there have been concerted efforts to systematically investigate whether great apes and other animals share core abilities required for language, such as intentionality, reference, mental concepts, vocal imitation or signal combinations19,20,21,22,23,24. For great apes, there is now little doubt that individuals can address others intentionally, usually to alter a recipient’s behaviour in a goal-directed way25,26,27,28,29 or to convey information referentially30,31. Primates also seem to understand the communicative function of some of their signals, in that they require the presence and attention of a suitable recipient, as documented by a large number of studies (e.g.25,26,32,33,34,35,36,37,38,39). Here, a standard experimental paradigm has been to analyse signal production when requesting food from a human experimenter whose attentional state, comprehension or intention varied. A key finding has been that both monkeys and apes are more likely to use visual signals when the experimenter is oriented towards them, and audible signals when the experimenter is facing away (e.g., apes: 33,39,40; monkeys:41,42,43,44,45). Another finding has been that chimpanzees and orang-utans adjust their signalling behaviour according to the degree of comprehension manifest in a partner. When confronted with a response that suggested only partial understanding, apes persisted and continued to use the signals that they used before. When fully misunderstood, they elaborated their communication efforts by producing new signals46,47,48,49. The main conclusion from these studies is that great ape communication is intentional and that they act as if taking into account basic mental states of their recipients, such as attention and comprehension. What remains unknown, however, is whether any of their natural communication efforts are also based on taking into account familiarity, i.e. what others know based on shared interactions history.
We addressed this point with a study on bonobos living under semi-natural conditions using a combination of well-established experimental protocols developed for apes (e.g.38,46,50) and human infants7. Specifically, we tested whether subjects adjusted signal production to request food from a human partner depending on (a) the recipient’s attention (‘Attentive’ vs. ‘Inattentive’), (b) the success in communicating a goal (‘Fully successful’, ‘Partially successful’, or ‘Unsuccessful’) and (c) the familiarity of a human recipient (‘Familiar’ vs. ‘Unfamiliar’). While the first capacity (‘attention’) appears to be generally available to great apes and other social mammals34,51, the second one (‘communicative success’) has only been tested in orang-utans and chimpanzees46,47,48, while the third one (‘familiarity’) has only been tested in human children interacting with adults7,52.
Predictions
Recipient attention (a)
If bonobos are able to take others’ attentional state into account, they should adjust their signalling behaviour in accordance with the visual attention of the recipient. This ability has been demonstrated for other great apes in similar experimental protocols and for all great ape species during natural communication25,26,28. If subjects have an understanding of the communicative function of their signals, then they should indifferently use silent or audible signals provided the recipient is visually attentive. If the recipient is not visually attentive, however, subjects should decrease the use of silent gestures and increase, or at least maintain, the rate of audible signals relative to the attentive condition
Communicative success (b)
If bonobos are able to take their own signalling success into account, they should stop signalling when successful, repeat signals when partially successful (‘Repetition’) and switch to new signals when not successful (‘Elaboration’), as previously shown for chimpanzees and orang-utans46,47,48,49.
Recipient familiarity (c)
If bonobos have some understanding of the fact that they only have a shared interaction history with a familiar recipient, then they should adjust their communicative behaviour accordingly. In the case of a communication failure, subjects should show persistence by repeating previously successful signals (i.e. signals that led to obtaining desirable food in past interactions) when communicating with a familiar recipient. In contrast, when failing to communicate with an unfamiliar recipient (with no shared history of interaction), subjects should elaborate by favouring alternative signals to increase their chances of being understood.
Materials and Methods
Ethics statement
The experiment was performed in accordance with the ethical ASAB/ABS Guidelines for the Use of Animals in Research and was conducted in compliance with animal care regulations and applicable national laws (research permit: MIN.RS/SG/004/2009). We received ethical approval from the scientific coordinator and scientific committee of ‘Les Amis des Bonobos’ for this study.
Study group
We collected data from 10 individuals in three social groups housed at the ‘Lola Ya Bonobo’ sanctuary, DRC, in January 2013. All groups live in large forested enclosures of 8, 10 and 15 ha, respectively, composed of patches of primary rainforest, lakes, swamps, streams, and open grassy areas. During the day individuals can move freely, forage for wild fruits, leaves, and herbaceous vegetation in the forested parts of their enclosures, in addition to three feedings provided by caregivers. The daily feeding routine is to distribute fruits in the morning, a mixture of soya milk (supplemented with milk, maize, honey and nutriments) around midday, and vegetables in the afternoon, approximately 6 kg of fruits and vegetables to each individual, complemented with daily supplemental feeds of seasonal fruits and nuts. Water is freely available from lakes, ponds and streams within their enclosures, with fresh water (with added salt and sugar) provided several times a week. At night, all individuals are kept in dormitories of approximately 75 m2, divided in several separable rooms and connected to the outside enclosures by a tunnel. During the study period, group 1 consisted of 25 individuals, group 2 of 16 individuals and group 3 of 11 individuals. All groups included adult, subadult, juvenile and infant males and females.
Experimental protocol
Twelve subjects were initially selected for this study, either because they produced idiosyncratic signals to request food (n = 4; Supplementary Table S1), probably developed through repetitive interactions with a familiar keeper, or because they could be easily tested with regards to their social position in the group. We had to exclude two individuals due to lack of motivation to interact with the human partners or because of a transient lack of interest in the foods provisioned. Consequently, the final analyses were carried out on the performance of 10 individuals (Supplementary Table S2).
All individuals habitually requested food from the keepers with gestures, vocalisations or combinations of both (Supplementary Tables S1 and S3). We first collected a database of signals produced by the subjects to request food from their keepers during daily feedings. For each individual we then selected a random sample of 10 video clips containing food requests from the subjects during these daily feedings with their main keepers, i.e. 100 clips total, from a sample of 192 clips collected during a two-week period (n = 10; mean ± SD = 17.45 ± 5.32).
We then classified and ranked the signals by frequency of use. Signals that accounted for at least 80% of a subject’s signalling production were considered as the ‘default’ signals to request food from its keeper, which was often a hand-reach gesture, a general begging gesture of great apes53,54 and humans (e.g. Supplementary Table S1). We also found that 4 of 10 individuals additionally produced idiosyncratic signals as their personal ‘default’ signals (e.g., hand-clap/rasp grunt or hand-reach/raspberry, Supplementary Table S1 & Videos S1–4).
All experimental trials took place during regular feeding times. To study signalling behaviour, we used banana (desirable) and corn (undesirable) as food incentives for all subjects. The familiar recipients were the main keepers of each group (i.e. three different recipients), who the subjects had been interacting with on a daily basis for years. The unfamiliar recipient was a person recruited from a village nearby the sanctuary whom the subjects had never met before. Each trial began with the human partner approaching the enclosure fence, looking at the subject and calling its name for him/her to approach. When the subject was present and attentive at the fence, the partner showed him or her a banana and, as soon as the subject produced the first request signal, the partner initiated one of five experimental conditions: (1) ‘Attentive’: Recipient facing subject, delivery of banana after 60 s; (2) ‘Inattentive’: Recipient turning his back to subject, delivery of banana after 60 s. In both conditions the banana was always equally visible to the subject (displayed in front or back of recipient); (3) ‘Fully successful’: Immediate delivery of whole banana, (4) ‘Partially successful’: Immediate delivery of half banana; (5) ‘Unsuccessful’: Immediate delivery of corn (hidden in food box during banana presentation) (Fig. 1). Each individual completed a total of 10 trials (one trial per condition with each recipient), one condition per day with each recipient (i.e., two conditions per day). Conditions were counter-balanced across subjects and recipients (Supplementary Table S4).
Figure 1. Schematic representation of the experimental protocol with five experimental conditions and two recipients.
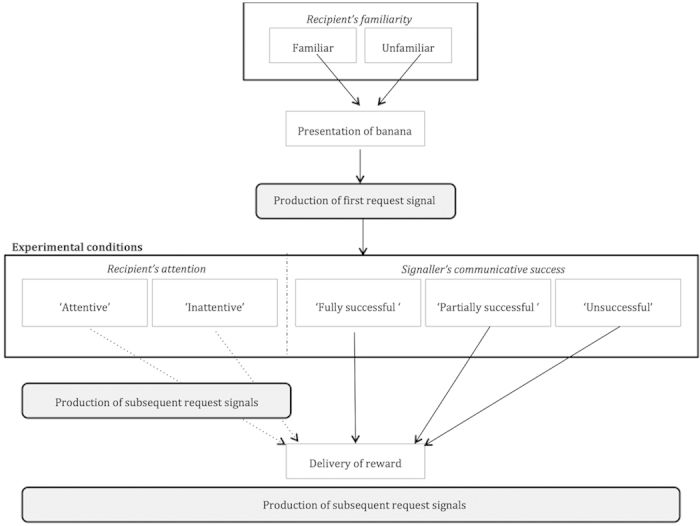
White boxes represent recipients’ actions and grey boxes represent signallers’ actions. Plain arrows represent an immediate transition to the next box, dotted arrows represent a 60 s delay before the delivery of the reward (a banana in the ‘Attentive’, ‘Inattentive’ and ‘Fully successful’ conditions, half a banana in the ‘Partially successful’ condition, and a corn in the ‘Unsuccessful’ condition). Italic captions represent the variables that are being tested: adjustment of signal production to (a) recipient’s attention (‘Attentive’ (1) and ‘Inattentive’ (2)), (b) communicative success (‘Fully successful’ (3), ‘Partially successful’ (4), ‘Unsuccessful’ (5)) and (c) familiarity of recipient (‘Familiar’, ‘Unfamiliar’).
All trials were recorded on video by EG using a Panasonic full HD digital camcorder (HC-X909) equipped with a directional microphone (Sennheiser MKE 400). EG stood about a 1.5 m from the recipient in order to film both his and the subject’s behaviours. At the beginning of each trial, EG announced the experimental condition. In the ‘Attentive’ and ‘Inattentive’ conditions, a trial began as soon as the subject produced a request signal and ended after a period of 60 s. The subjects’ behaviour was then recorded for another 30 s after the delivery of the food.
All signalling behaviours were recorded during the 60 s pre-delivery period and 30 s post-delivery in the ‘Attentive’ and ‘Inattentive’ conditions and during the 30 s post-delivery period in the ‘Fully successful’, ‘Partially successful’ and ‘Unsuccessful’ conditions. The subjects’ patterns of signalling across the two periods was then classified as (1) ‘Repetition’: number of repetitions of the first request signal in the bout (almost always the ‘default’ signal), (2) ‘Elaboration’: number of new signals in the bout (different signals from the first one). If a signal, other than the first one, was repeated in the bout it was not counted as a ‘Repetition’.
Differences in signalling patterns across conditions and recipient were analysed by assessing all signals produced during the 60 s of the ‘Attentive’ (1) and ‘Inattentive’ (2) conditions (subjects never persisted in signalling following delivery of the food in these two conditions) and the 30 s post-delivery in the ‘Partially successful’ (4) and ‘Unsuccessful’ (5) conditions (subjects never persisted in signalling after delivery of the food in the ‘Fully successful’ (3) condition).
All trials were coded from video by EG, using Filemaker Pro. A second rater, naïve to the hypotheses, but familiar with bonobo communicative signals, recoded 20% of the videos to assess inter-observer reliability. Inter-observer reliability was excellent for both gesture types (K = 0.93) and vocalisations types (K = 0.83).
Statistical analyses
To investigate whether subjects were able to adjust the signal modality to the attentional state of the recipient (a), we used non-parametric Wilcoxon paired-samples tests to compare production frequencies of silent and audible signals across the ‘Attentive’ and ‘Inattentive’ experimental conditions, pooled across ‘Familiar’ and ‘Unfamiliar’ recipients. We opted for this analysis strategy because we were not primarily interested in differences between recipients, but in the effect of the recipients’ attentional state on signal production.
In a second analysis, we used generalized linear mixed models55 with a binomial error and logit link function to test for differences in signalling patterns (i.e. ‘Repetition’ and ‘Elaboration’) across (b) experimental conditions (‘Attentive’, ‘Inattentive’, ‘Partially successful’ and ‘Unsuccessful’) and (c) recipient familiarity (‘Familiar’ and ‘Unfamiliar’). In two separate models, we modelled the proportion of signals that constituted ‘Repetition’ and ‘Elaboration’, respectively, from a database consisting of all signals produced in a given bout. Predictor variables in both models were experimental condition and recipient familiarity. Subject ID was fitted as random factor. Variance inflation factors were all <1.1, indicating that collinearity was not a problem. We assessed model stability by calculating Cook’s distance56, which indicated one influential individual in the ‘Repetition’ model. Reanalysing the data excluding this individual did not change our conclusions derived from the model and we present results with this subject included. We used likelihood ratio tests (LRT57) to assess significance of full models versus null models (containing only intercept and random effect) as well as for our categorical predictor variables.
The overall analysis strategy is summarised in Table 1. Models were fitted in R 3.1.158 with the function glmer in the lme4 package59. Variance inflation factors were calculated with the vif function in the car package60, Wilcoxon tests with exact p-values from the exactRankTests package61, and Cook’s distances with the influence. ME package56.
Table 1. Summary of statistical tests.
| Aim | Signal variables | Predictor variable | Test |
|---|---|---|---|
| a | Silent and Audible | Familiarity | Wilcoxon |
| b | Repetition and Elaboration | Condition | GLMM |
| c | Repetition and Elaboration | Familiarity | GLMM |
Results
Recipient attention (a)
As predicted, we found that subjects adjusted signal modality to the attentional state of their recipient by using fewer silent signals in the ‘Inattentive’ compared to the ‘Attentive’ condition (V = 1.5, p = 0.0059, N = 10 with 1 tie; median ‘Inattentive’ = 3.00, inter-quartile range: 2.00–3.75; median ‘Attentive’ = 10.00, inter-quartile range: 7.75–13.50, Fig. 2). For audible signals, we found no difference according to the attentional state of the recipient (V = 7, p = 0.2969, N = 10 with 3 ties; median ‘Inattentive’ = 3.00, inter-quartile range: 1.00–11.00; median ‘Attentive’ = 3.00, inter-quartile range: 0.50–4.0, Fig. 2), although the observed pattern went in the expected direction, with only two subjects presenting lower frequencies of audible signals in the ‘Inattentive’ condition.
Figure 2. Differences in signal modality according to recipient attentional state.
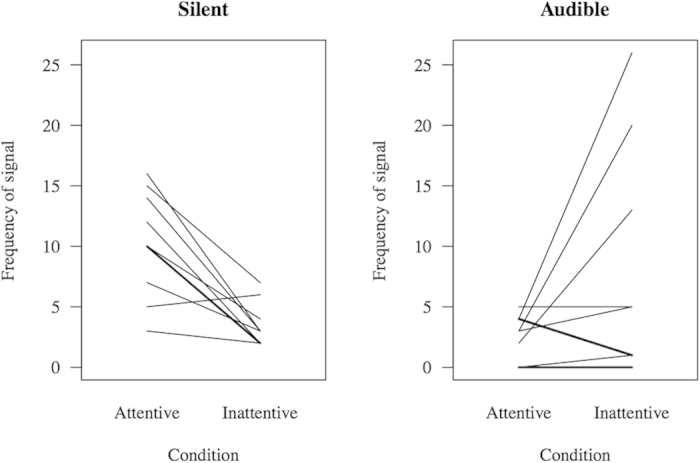
Each line represents one subject, except thick lines, which indicate that two subjects produced the same number of signals in both conditions. Numbers were pooled across familiar and unfamiliar recipients for each subject.
Although it was not the focus of this analysis, the result of the models (see below) indicated that the subjects used fewer repetitions with an inattentive recipient (estimate = −1.64, SE = 0.46, z = −3.59, p = 0.0003) than with an attentive one (see Fig. 3). However, we found no difference in elaboration with regard to the attentional state of the recipient (estimate = 0.22, SE = 0.31, z = 0.70, p = 0.4867).
Figure 3. Results of the GLMMs testing differences in signal repetition and elaboration according to experimental condition.
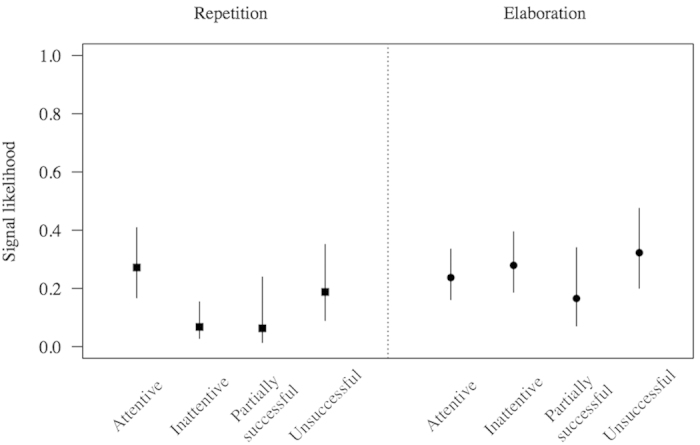
Shown are model estimates with associated standard errors.
Communicative success (b)
In the ‘Fully successful’ condition, no subject made any further attempts to communicate with the recipient after the delivery of the banana. In the ‘Partially successful’ condition, all 10 subjects immediately ate half the banana and only 3 out of 10 continued to signal afterwards (two only did so with the unfamiliar recipient). In the ‘Unsuccessful’ condition, half the subjects ate the corn but only after subsequent attempts to obtain the banana had failed.
Both full models differed from their respective null models (Repetition: χ2 = 32.7, df = 4, p = 0.0000, Elaboration: χ2 = 8.3, df = 4, p = 0.0817, Tables 2 and 3). We found that bonobos tended to persist more by repeating the first request signal (estimate = 1.24, SE = 0.84, z = 1.48, p = 0.1394, Fig. 3) and to elaborate more by producing new signals (estimate = 0.88, SE = 0.55, z = 1.58, p = 0.1134, Fig. 3) in the ‘Unsuccessful’ compared to the ‘Partially successful’ condition. Although statistically not significant, the directions of these results were consistent with our predictions.
Table 2. Results of the GLMM testing differences in Repetition of signals.
| estimate | se | z | p | |
|---|---|---|---|---|
| Intercept | −0.41 | 0.33 | −1.25 | 0.2110 |
| Experimental conditiona | ||||
| Inattentive | −1.64 | 0.46 | −3.59 | 0.0003 |
| Partially successful | −1.72 | 0.78 | −2.20 | 0.0278 |
| Unsuccessful | −0.48 | 0.42 | −1.14 | 0.2546 |
| Recipient familiarityb | ||||
| Unfamilar | −1.15 | 0.34 | −3.35 | 0.0008 |
Note that reference levels for both predictor variables (‘Attentive’ for experimental condition and ‘Familiar’ for recipient familiarity) are included in the intercept.
aLRT: χ2 = 18.5, df = 3, 0.0003.
bLRT: χ2 = 12.0, df = 1, p = 0.0005.
Table 3. Results of the GLMM testing differences in Elaboration of signals.
| estimate | se | z | p | |
|---|---|---|---|---|
| Intercept | −1.46 | 0.28 | −5.20 | 0.0000 |
| Experimental conditiona | ||||
| Inattentive | 0.22 | 0.31 | 0.70 | 0.4867 |
| Partially successful | −0.45 | 0.51 | −0.88 | 0.3806 |
| Unsuccessful | 0.43 | 0.37 | 1.15 | 0.2509 |
| Recipient familiarityb | ||||
| Unfamilar | 0.59 | 0.27 | 2.18 | 0.0290 |
Note that reference levels for both predictor variables (‘Attentive’ for experimental condition and ‘Familiar’ for recipient familiarity) are included in the intercept.
aLRT: χ2 = 3.2, df = 3, 0.3634.
bLRT: χ2 = 4.9, df = 1, p = 0.0273.
Recipient familiarity (c)
As predicted, bonobos used fewer repetitions with the unfamiliar recipient compared to the familiar ones (estimate = −1.15, SE = 0.34, z = −3.36, p = 0.0008, Fig. 4), but at the same time elaborated more by using more new signals with the unfamiliar than the familiar ones (estimate = 0.59, SE = 0.27, z = 2.18, p = 0.0290, Fig. 4).
Figure 4. Results of the GLMMs testing differences in signal repetition and elaboration according to recipient familiarity.
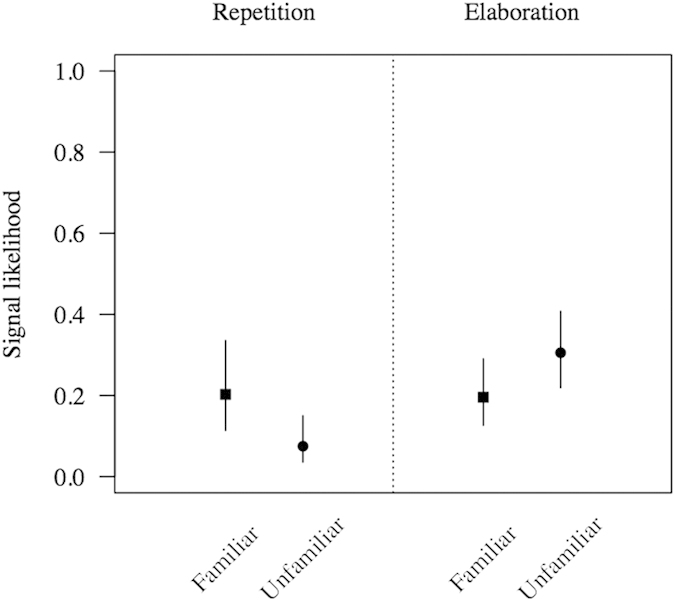
Shown are model estimates with associated standard errors.
Discussion
In this study, we found that bonobos communicated with a range of signals to obtain desirable food from human recipients in goal-directed and strategic ways. With our first analysis, we were able to replicate findings of earlier studies (e.g.33,34,35,37,38,39,50) by showing that bonobos take into account the attentional states of their recipients during communication. With our second analysis, we were able to replicate findings in orang-utans and chimpanzees46,47,48,49, demonstrating that bonobos can adjust and refine communication signals depending on the degree of success of previous communication efforts. If successful in obtaining the banana, subjects never continued to signal, but if unsuccessful they tended to elaborate their communicative efforts by switching to new signals, suggesting an apparent attempt to rectify an unsuccessful communication event and misunderstanding. With our third analysis, we showed that, similarly to human infants interacting with adults7,52 and language-trained bonobos interacting with their caretakers14,15, subjects adapted their signal production according to whether or not they knew the recipient. In particular, they used more repetitions with a familiar recipient but elaborated more, by using new signals with an unfamiliar one.
Our results are consistent with the hypothesis that bonobos take into account recipient familiarity, and possibly consider the knowledge they share with recipients that has been acquired jointly during their common history of interactions. Presumably, subjects have learnt, over the course of previous interactions, which signals are likely to persuade the familiar human recipient to deliver a food reward and they seemingly quickly understand that these signals are not necessarily equally effective when interacting with an unfamiliar recipient with whom they have no shared history. Whether this is the result of mere operant conditioning or reasoning cannot be determined here. Operant conditioning theory predicts that a subject learns to associate a previously neutral behaviour (e.g. producing an idiosyncratic gesture) to human recipients with a positive outcome (food). One version of this type of learning has been referred to as ‘conditional discrimination’, i.e. a discrimination between two stimuli in which the reinforcement for responding to one stimulus (food) depends on the presence of another stimulus (human partner)62. In our opinion, these results do not support such an interpretation because in conditional discrimination subjects should transfer the initially trained association to any human recipient, regardless of familiarity. The alternative is that signalling behaviour would have had to be learned by conditioning with each recipient separately. However, in our study the unfamiliar recipient was unknown to the subjects and yet we found that signalling behaviour was influenced by this variable in ways that suggest that recipients were aware of the differences. Subjects reacted to the unfamiliar human by changing signals more often than to a familiar one from the first exposure and without training, indicating that request signals are not just blindly acquired and delivered, but deployed strategically by taking the targeted recipient into account.
We find it thus more likely to assume that great apes can partially discriminate between private and shared knowledge when communicating with a partner, something that has recently been suggested for chimpanzee alarm calls30. In our current study, this ability has revealed itself without any specific training but while using idiosyncratic signals and other usual food request signals that are part of the bonobos’ natural signalling repertoire.
Familiarity judgements are an important component for the mental capacity to form a mutual ‘common ground’, a key capacity in human social interactions6. To this end, humans routinely take the knowledge shared with their social partners into account, both in linguistic conversations63,64 as well as during joint attention and joint actions65. ‘Common ground’ is a hallmark of human cooperative behaviour and communication and, according to some accounts, an exclusive capacity as it is based on the ability to attribute mental states to others64,66,67. For example, if two interlocutors have previously shared an experience relating to an object, they will both know that they can make relevant inferences about this object even in its absence, but only with this partner8,68, an ability that appears in human infants from 12 months of age65.
Although our results appear to be consistent with such an interpretation, simpler mechanisms may have been at work. For example, it could be argued that differences in familiarity generate differences in emotional states, which may affect communication behaviour. However, our study is unable to describe in more detail the cognitive abilities deployed by the bonobos to discriminate between familiar and unfamiliar recipients. Whatever the underlying mechanism, the fact remains that differences in partner familiarity caused differences in signalling behaviour that was structured in a way that suggested subjects took their partners’ knowledge into account. In future research it would be necessary to let subjects interact with a range of familiar and unfamiliar recipients in a matched design (i.e. some recipients being familiar to some subjects but unfamiliar to others). In our case, this would have required 20 human experimenters, something that unfortunately was impractical within the constraints of the sanctuary setting. Instead we opted for a previously established protocol with human children7, which enabled us to use the main bonobo keeper for a given enclosure as the ‘Familiar’ recipient and one unfamiliar human partner as the ‘Unfamiliar’ recipient for all individuals, allowing comparisons to the human infant study7. Further research is needed to investigate whether experimentally created shared knowledge between a subject and a human partner (e.g. training of novel arbitrary signals with different social partners) has any measurable behavioural consequences and indications of shared knowledge and understanding.
One limitation of the present and similar types of studies is that the experimental design is based on testing interactions with human partners. Obviously, this is very different from natural situations in which individuals are communicating with each other and as part of differentiated relationships. It could therefore be argued that our experimental design is based on a situation that rarely occurs in the wild, i.e. an individual communicating to an unfamiliar partner to obtain food. One interesting exception is the arrival of an immigrant in an established group, a situation that naturally occurs in wild chimpanzees and bonobos. Do immigrants progressively adapt their communication signals on the basis of shared history with the different group members, as recently suggested for the food calls of captive chimpanzees69 Documenting the communication behaviour of newly arriving individuals throughout the immigration phase would generate relevant data to study the communicative interactions between individuals of different familiarity. Here, our prediction is that individuals, who are unsuccessful in communicating their goal, should show more persistence (more repetitions) when interacting with already familiar individuals compared to still unfamiliar individuals, to whom they should show more signs of elaboration.
Conclusion
Parts of the human psychological architecture are shared with the great apes, in particular the ability to flexibly communicate by taking into account the visual attentive state of the recipient and one’s own communicative success. Our study further suggests that bonobos also possess an understanding of the shared history of interaction. What remains unknown, however, is the exact nature of the underlying mechanism responsible for the distinction between familiar and unfamiliar recipients and to what degree mental state attribution is involved.
Additional Information
How to cite this article: Genty, E. et al. Bonobos modify communication signals according to recipient familiarity. Sci. Rep. 5, 16442; doi: 10.1038/srep16442 (2015).
Supplementary Material
Acknowledgments
We are grateful to Stany Mokando Nzeli, Jean-Claude Nzumbi-Belinda, Henriette Lubondo and Fils Okwe for assisting with the experiment, to Gladez Shorland for second coding the data, to Albert Ros for comments on a previous version of the manuscript, to Zanna Clay for correcting the English, to Claudine André and Brian Hare for permission to work at ‘Lola Ya Bonobo’, to Pierrot Mbonzo, Dominique Morel, Valéry Dhanani, Raphaël Belais for their administrative support, and to the Ministry of Research and the Ministry of Environment in the Democratic Republic of Congo for giving us permission to carry out this research. This project has received funding from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no 283871.
Footnotes
Author Contributions E.G. and K.Z. designed the experiment, E.G. conducted the experiment, E.G. and C.N. analysed the data, all authors wrote the manuscript.
References
- Tomasello M. & Rakoczy H. What makes human cognition unique? From individual to shared to collective intentionality. Mind Lang. 18, 121–147 (2003). [Google Scholar]
- Gallagher T. M. Revision behaviors in the speech of normal children developing language. J. Speech Lang. Hear. Res. 20, 303–318 (1977). [DOI] [PubMed] [Google Scholar]
- Gallagher T. M. & Darnton B. A. Conversational aspects of the speech of language-disordered children: Revision behaviors. J. Speech Lang. Hear. Res. 21, 118–135 (1978). [DOI] [PubMed] [Google Scholar]
- Gallagher T. M. Contingent query sequences within adult–child discourse. J. Child Lang. 8, 51–62 (1981). [PubMed] [Google Scholar]
- Wilcox M. J. & Webster E. J. Early discourse behavior: An analysis of children’s responses to listener feedback. Child Dev. 51, 1120–1125 (1980). [Google Scholar]
- Clark H. H. & Brennan S. E. in Perspectives on socially shared cognition (eds. Resnick L. M., Levine J. M. & Teasley S.) 127–149 (APA Books, 1991). [Google Scholar]
- Tomasello M., Farrar M. J. & Dines J. Children’s speech revisions for a familiar and an unfamiliar adult. J. Speech Lang. Hear. Res. 27, 359–363 (1984). [DOI] [PubMed] [Google Scholar]
- Tomasello M. Origins of Human communication. (MIT Press, Cambridge, Massachusetts, 2008). [Google Scholar]
- Savage-Rumbaugh E. S. et al. Language comprehension in ape and child. Monogr. Soc. Res. Child Dev. 58, 1–252 (1993). [PubMed] [Google Scholar]
- Sevcik R. A. & Savage-Rumbaugh E. S. Language comprehension and use by great apes. Lang. Commun. 14, 37–58 (1994). [Google Scholar]
- Greenfield P. M. & Savage-Rumbaugh E. S. in ‘Language’ and intelligence in monkeys and apes: Comparative developmental perspectives (eds. Parker S. T. & Gibson K. R.) 540–578 (New York, NY, US: Cambridge University Press, 1990). [Google Scholar]
- Greenfield P. M. & Savage-Rumbaugh E. S. in Biological and behavioral determinants of language development (eds. Krasnegor N. A., Rumbaugh D. M., Schiefelbusch R. L. & Studdert-Kennedy M.) 235–258 (Hillsdale, NJ, England: Lawrence Erlbaum Associates, 1991). [Google Scholar]
- Greenfield P. M. & Savage-Rumbaugh E. S. Comparing communicative competence in child and chimp: the pragmatics of repetition. J. Child Lang. 20, 1–26 (1993). [DOI] [PubMed] [Google Scholar]
- Pedersen J. & Fields W. M. Aspects of repetition in bonobo–human conversation: creating cohesion in a conversation between species. Integr. Psychol. Behav. Sci. 43, 22–41 (2009). [DOI] [PubMed] [Google Scholar]
- Savage-Rumbaugh S., Fields W. M. & Taglialatela J. Ape consciousness–human consciousness: A perspective informed by language and culture. Am. Zool. 40, 910–921 (2000). [Google Scholar]
- Boesch C. & Boesch-Achermann H. The chimpanzees of the Taï forest: Behavioural ecology and evolution. (Oxford University Press Oxford, 2000). [Google Scholar]
- Goodall J. The chimpanzees of Gombe: Patterns of behavior. (Belknap Press of Harvard University Press, 1986). [Google Scholar]
- Reynolds V. The Chimpanzees of the Budongo Forest: Ecology, behaviour, and conservation. (Oxford University Press, 2005). [Google Scholar]
- Arnold K. & Zuberbühler K. Call combinations in monkeys: Compositional or idiomatic expressions? Brain Lang. 120, 303–309 (2012). [DOI] [PubMed] [Google Scholar]
- Moura L. N. et al. Gestural communication in a new world parrot. Behav. Processes 105, 46–48 (2014). [DOI] [PubMed] [Google Scholar]
- Pepperberg I. M. The Alex studies: cognitive and communicative abilities of grey parrots. (Cambridge, MA: Harvard University Press, 1999). [Google Scholar]
- Pika S. & Bugnyar T. The use of referential gestures in ravens (Corvus corax) in the wild. Nat. Commun. 2, 560, 10.1038/ncomms1567 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage-Rumbaugh E. S., Wilkerson B. J. & Bakeman R. in Progress in ape research (ed. Bourne G. H.) 97–116 (Academic Press, 1977). [Google Scholar]
- Vail A. L., Manica A. & Bshary R. Referential gestures in fish collaborative hunting. Nat. Commun. 4, 1765, 10.1038/ncomms2781 (2013). [DOI] [PubMed] [Google Scholar]
- Call J. & Tomasello M. The gestural communication of apes and monkeys. (Taylor & Francis Group/Lawrence Erlbaum Associates, 2007). [Google Scholar]
- Genty E., Breuer T., Hobaiter C. & Byrne R. W. Gestural communication of the gorilla (Gorilla gorilla): repertoire, intentionality and possible origins. Anim. Cogn. 12, 527–546 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty E., Clay Z., Hobaiter C. & Zuberbühler K. Multi-modal use of a socially directed call in bonobos. PloS One 9, e84738, 10.1371/journal.pone.0084738 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobaiter C. & Byrne R. W. The gestural repertoire of the wild chimpanzee. Anim. Cogn. 14, 745–767 (2011). [DOI] [PubMed] [Google Scholar]
- Schel A. M., Machanda Z., Townsend S. W., Zuberbühler K. & Slocombe K. E. Chimpanzee food calls are directed at specific individuals. Anim. Behav. 86, 955–965 (2013). [Google Scholar]
- Crockford C., Wittig R. M., Mundry R. & Zuberbühler K. Wild chimpanzees inform ignorant group members of danger. Curr. Biol. 22, 142–146 (2012). [DOI] [PubMed] [Google Scholar]
- Genty E. & Zuberbühler K. Spatial Reference in a bonobo gesture. Curr. Biol. 24, 1601–1605, 10.1016/j.cub.2014.05.065 (2014). [DOI] [PubMed] [Google Scholar]
- Call J. & Tomasello M. Production and comprehension of referential pointing by orangutans (Pongo pygmaeus). J. Comp. Psychol. 108, 307 (1994). [DOI] [PubMed] [Google Scholar]
- Hostetter A. B., Cantero M. & Hopkins W. D. Differential use of vocal and gestural communication by chimpanzees (Pan troglodytes) in response to the attentional status of a human (Homo sapiens). J. Comp. Psychol. 115, 337 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetter A. B., Russell J. L., Freeman H. & Hopkins W. D. Now you see me, now you don’t: evidence that chimpanzees understand the role of the eyes in attention. Anim. Cogn. 10, 55–62 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski J., Call J. & Tomasello M. Body orientation and face orientation: two factors controlling apes? begging behavior from humans. Anim. Cogn. 7, 216–223 (2004). [DOI] [PubMed] [Google Scholar]
- Leavens D. A., Hopkins W. D. & Thomas R. K. Referential Communication by Chimpanzees (Pan troglodytes). J. Comp. Psychol. 118, 48–57 (2004). [DOI] [PubMed] [Google Scholar]
- Pika S., Liebal K. & Tomasello M. Gestural communication in subadult bonobos (Pan paniscus): Repertoire and use. Am. J. Primatol. 65, 39–61 (2005). [DOI] [PubMed] [Google Scholar]
- Poss S. R., Kuhar C., Stoinski T. S. & Hopkins W. D. Differential use of attentional and visual communicative signaling by orangutans (Pongo pygmaeus) and gorillas (Gorilla gorilla) in response to the attentional status of a human. Am. J. Primatol. 68, 978–992 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempelmann S., Kaminski J. & Liebal K. Focus on the essential: all great apes know when others are being attentive. Anim. Cogn. 14, 433–439 (2011). [DOI] [PubMed] [Google Scholar]
- Liebal K., Call J., Tomasello M. & Pika S. To move or not to move: how apes adjust to the attentional state of others. Interact. Stud. 5, 199–219 (2004). [Google Scholar]
- Bourjade M., Meguerditchian A., Maille A., Gaunet F. & Vauclair J. Olive baboons, Papio anubis, adjust their visual and auditory intentional gestures to the visual attention of others. Anim. Behav. 87, 121–128 (2014). [Google Scholar]
- Canteloup C., Bovet D. & Meunier H. Do Tonkean macaques (Macaca tonkeana) tailor their gestural and visual signals to fit the attentional states of a human partner? Anim. Cogn. 18, 451–461 (2014). [DOI] [PubMed] [Google Scholar]
- Hattori Y., Kuroshima H. & Fujita K. Tufted capuchin monkeys (Cebus apella) show understanding of human attentional states when requesting food held by a human. Anim. Cogn. 13, 87–92 (2009). [DOI] [PubMed] [Google Scholar]
- Maille A., Engelhart L., Bourjade M. & Blois-Heulin C. To beg, or not to beg? That is the question: mangabeys modify their production of requesting gestures in response to human’s attentional states. PLoS One 7, e41197, 10.1371/journal.pone.0041197 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick S.-J. & Anderson J. R. Use of human visual attention cues by olive baboons (Papio anubis) in a competitive task. J. Comp. Psychol. 117, 209 (2003). [DOI] [PubMed] [Google Scholar]
- Cartmill E. A. & Byrne R. W. Orangutans modify their gestural signaling according to their audience’s comprehension. Curr. Biol. 17, 1345–1348 (2007). [DOI] [PubMed] [Google Scholar]
- Leavens D. A., Russell J. L. & Hopkins W. D. Intentionality as measured in the persistence and elaboration of communication by chimpanzees (Pan troglodytes). Child Dev. 76, 291–306 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. I., Vick S.-J., Roberts S. G. B. & Menzel C. R. Chimpanzees modify intentional gestures to coordinate a search for hidden food. Nat. Commun. 5, 10.1038/ncomms4088 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russon A. & Andrews K. Orangutan pantomime: elaborating the message. Biol. Lett. 7, 627–630 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens D. A., Hostetter A. B., Wesley M. J. & Hopkins W. D. Tactical use of unimodal and bimodal communication by chimpanzees, Pan troglodytes. Anim. Behav. 67, 467–476 (2004). [Google Scholar]
- Gácsi M., Miklósi Á., Varga O., Topál J. & Csányi V. Are readers of our face readers of our minds? Dogs (Canis familiaris) show situation-dependent recognition of human’s attention. Anim. Cogn. 7, 144–153 (2004). [DOI] [PubMed] [Google Scholar]
- Liebal K., Behne T., Carpenter M. & Tomasello M. Infants use shared experience to interpret pointing gestures. Dev. Sci. 12, 264–271 (2009). [DOI] [PubMed] [Google Scholar]
- Kano T. The last ape: Pygmy chimpanzee behavior and ecology. (Stanford University Press, 1992). [Google Scholar]
- de Waal F. B. Bonobo: The forgotten ape. (Univ of California Press, 1997). [Google Scholar]
- Bolker B. M. et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 (2009). [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis R., Grotenhuis M. Te. & Pelzer B. Influence. ME: tools for detecting influential data in mixed effects models. R J. 4, 38–47 (2012). [Google Scholar]
- Quinn G. P. & Keough M. J. Experimental Design and Data Analysis for Biologists. (Cambridge University Press, 2002). [Google Scholar]
- R. Core Team. R: A Language and Environment for Statistical Computing. (2014). at < http://www.R-project.org/> Date of access: 10/12/2014.
- Bates D. M., Maechler M., Bolker B. M. & Walker S. C. lme4: linear mixed-effects models using Eigen and S4, version 1.1–7. (2014). at < http://CRAN.R-project.org/package=lme4> Date of access: 10/12/2014.
- Fox J. & Weisberg S. An R Companion to Applied Regression. (Sage, 2011). at < http://socserv.socsci.mcmaster.ca/jfox/Books/Companion>.
- Hothorn T. & Hornik K. exact Rank Tests: exact distributions for rank and permutation tests, version 0.8–27. (2013). at < http://CRAN.R-project.org/package=exactRankTests> Date of access: 07/03/2015.
- Robinson T. & Justice J. in Experimental Analysis of Behavior, Part 2 (eds. Iverson I. H. & Lattal K. A.) 6, 1–282 (Elsevier B.V., 1991). [Google Scholar]
- Grice P. Studies in the Way of Words. (Harvard University Press, 1991). [Google Scholar]
- Stalnaker R. Common ground. Linguist. Philos. 25, 701–721 (2002). [Google Scholar]
- Tomasello M., Carpenter M. & Liszkowski U. A new look at infant pointing. Child Dev. 78, 705–722 (2007). [DOI] [PubMed] [Google Scholar]
- Flombaum J. I. & Santos L. R. Rhesus monkeys attribute perceptions to others. Curr. Biol. 15, 447–452 (2005). [DOI] [PubMed] [Google Scholar]
- Tomasello M., Carpenter M., Call J., Behne T. & Moll H. Understanding and sharing intentions: The origins of cultural cognition. Behav. Brain Sci. 28, 675–691 (2005). [DOI] [PubMed] [Google Scholar]
- Moll H., Richter N., Carpenter M. & Tomasello M. Fourteen-month-olds know what ‘we’ have shared in a special way. Infancy 13, 90–101 (2008). [Google Scholar]
- Watson S. K. et al. Vocal learning in the functionally referential food grunts of chimpanzees. Curr. Biol. 25, 495–499, 10.1016/j.cub.2014.12.032 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


