Abstract
Endoscopy using magnification narrow band imaging (mNBI) allows detailed assessment of mucosal surface and vascular pattern. This may help in better identification and prediction of the nature of the lesion. The role of this technology in duodenum is still evolving. Studies have shown that mNBI has high accuracy in predicting villous atrophy in the duodenum. Limited data suggests that this technique can provide additional information on duodenal polyps, nodules and ampullary tumour which can help guide their management. In this paper we describe the technique for duodenal assessment using NBI and review the existing literature evaluating its role in diagnosis of various duodenal pathologies.
Keywords: Narrow band imaging, Duodenum, Villous atrophy, Correlation, Polyp
Core tip: Narrow band imaging endoscopy with magnification (mNBI) enables detailed assessment of duodenal villous morphology. This advantage over white light endoscopy has potential clinical benefits. There is good evidence to show that villous morphology on mNBI correlates well with histopathology. Hence villous atrophy can be diagnosed with good accuracy during mNBI and targeted biopsy can be obtained from abnormal appearing areas. Preliminary data suggest that this technology may also aid in assessment of neoplastic lesions in duodenum.
INTRODUCTION
Endoscopic examination of mucosal surface of the gut is an integral part of evaluation of patients presenting with gastrointestinal symptoms. The quest for obtaining additional information from direct visualisation of mucosa has led to several advances in imaging techniques. These include narrow band imaging (NBI, Olympus), optical coherence tomography, Fujinon intelligent chromo endoscopy (FICE, Fujinon) system, I-Scan (Pentax) and confocal laser endomicroscopy[1,2]. NBI, as the name suggests, uses a narrow wavelength of light in the blue and green region instead of the entire visible spectrum which gives a dark appearance to blood vessels[3]. This in combination with magnification endoscopy enables characterisation of microsurface and microvascularity of mucosa and identifies abnormalities in different part of digestive tract[4]. Magnification NBI (mNBI) has been shown to play a useful role in evaluation of duodenal villus abnormalities seen in diseases associated with malabsorption as well as in assessment of polyps and tumours of the duodenum[5-9]. A search of published literature reveals that among the more than one thousand publications relating to NBI, less than thirty have focussed on duodenum. However, the number of publications on the role of NBI in duodenum has risen in recent years as its value in assessment of duodenal disorders is recognised. In this paper we have reviewed the available literature on use of NBI in evaluating the duodenum.
ASSESSMENT OF DUODENUM USING MAGNIFICATION NBI
In our experience we have found the following scheme of examination adequate for comprehensive assessment of duodenum[5]. The examination should begin with an initial assessment of duodenal mucosa with conventional white light endoscopy. Any debris on the mucosal surface should be cleared. In most situations examination is performed upto second part of duodenum. The characteristics of duodenal mucosal folds including atrophy, scalloping and nodularity should be assessed and presence of any surface lesion like polyp, nodule or tumour determined. Duodenal ampulla should also be examined although a forward viewing endoscope makes this slightly difficult.
Next the endoscope should be switched to magnification NBI mode. mNBI examination is undertaken in two steps. In the first step, the morphology of duodenal villi in second part of duodenum is evaluated. The magnification and contrast offered by mNBI enables clear visualisation of duodenal villi. Bile appears pink and blood appears black on NBI. Due to use of narrow band of light, the images on NBI are not very bright but as the duodenal lumen is narrower than stomach, this is less of a problem. This limitation is also being overcome by newer endoscope processors. Some centers have reported the use of water instillation in lumen to improve the visualisation of villi and we have also found it useful in our experience[10]. This technique can be used in selected situations where the assessment is otherwise difficult. Several studies have reported excellent performance of mNBI in assessing villous morphology[5,7,11,12]. In normal subjects the villi have greater length than breadth which gives them a leaf or finger like appearance (Figure 1)[5,7]. Atrophy of villi alters this ratio and makes them appear shortened or convoluted or stubbed or even absent in patients with total villous atrophy (Figure 1)[5,7]. The next step involves assessment of any protruding mucosal lesion like polyp, nodule and tumour. The microsurface and microvascular pattern with special attention to irregularity should be determined. There are only few reports on mNBI characteristics of various duodenal mucosal lesions which makes confident correlation of surface/vessel pattern with histology of lesion difficult but presence of irregularity of pattern generally signifies a high grade lesion[6,13].
Figure 1.
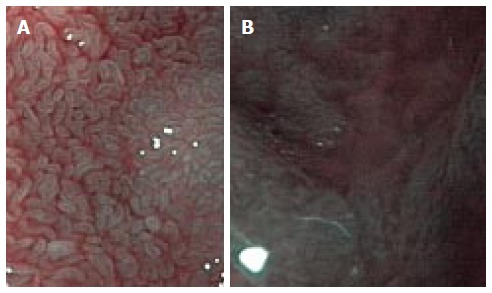
Appearance of normal duodenal villi on magnification narrow band imaging (A) and severe villous atrophy on magnification narrow band imaging (B).
NBI IN ASSESSMENT OF DUODENAL VILLOUS MORPHOLOGY
Emerging data from several studies have shown that mNBI has a very good correlation with histology in assessment of duodenal villous atrophy[5,7,10,11]. This has few potential benefits: (1) in situations where duodenal biopsy is taken only to evaluate for villous atrophy, normal villi on mNBI may preclude biopsy and save procedure cost and time. The same is not the case with white light endoscopy where mucosal fold abnormalities like atrophy, scalloping and nodularity have a poor sensitivity when compared to histology[14]. Therefore all patients undergoing white light endoscopy for suspected celiac disease will require duodenal mucosal biopsy irrespective of endoscopic appearance; (2) in subjects with patchy villous atrophy, mNBI may help target the biopsy to regions with atrophy and improve the diagnostic yield[15]; (3) ease of the procedure which does not require spray of dye to improve contrast; and (4) ease of identification of villous morphology as evidenced by most studies reporting very good interobserver agreement[5,10,11].
While celiac disease is the dominant cause of villous atrophy in developed nations, other diseases like tropical sprue associated with villous atrophy and malabsorption are seen in tropical countries. It is therefore not surprising that about half of the studies on the role of NBI in duodenal villous assessment are from India[5,11,16]. We have recently published a prospective study from our center in India on a hundred patients with suspected malabsorption and evaluated the ability of mNBI to assess duodenal villi[5]. Celiac disease was present in seven patients with villous atrophy while conditions like tropical sprue, infections and Crohn’s disease were present in eight patients and the cause of atrophy was unknown in one. Overall, villous atrophy was present in 16 patients and mNBI had a sensitivity of 87.5% and specificity of 95.2% in detecting this by one of the two examiners. The sensitivity and specificity were 81.3% and 92.9% respectively for the second assessor with high interobserver agreement (kappa 0.87). We therefore found mNBI examination of duodenum to be a promising modality in predicting villous atrophy. Subsequently another study from India on 105 subjects with suspected malabsorption (villous atrophy in 58 on histology) showed that mNBI had a sensitivity of 95% and specificity of 90.2% (interobserver kappa 0.89)[11] in predicting villous atrophy. A third study from north India, published only in abstract form also assessed correlation of mNBI with histology for detection of duodenal villous atrophy in 80 patients and reported a sensitivity of 87.03% and specificity of 84.61%[16].
A couple of studies have been published from Italy comparing standard white light endoscopy and mNBI in patients with celiac disease. De Luca et al[12] prospectively studied 44 patients and found that mNBI was able to identify villous atrophy in all 17 patients with confirmed celiac disease (100% sensitivity) while standard white light endoscopy showed abnormalities in only 7 of them (41% sensitivity). All cases of partial villous atrophy were identified on mNBI while standard endoscopy was normal in all of them. The mean additional time required for NBI examination was four and half minutes. Another study from Italy on pediatric patients with suspected celiac disease investigated the use of NBI with water immersion technique and obtained single NBI guided biopsy instead of conventional multiple biopsies[10]. NBI guided single biopsy had a sensitivity of 87.5% in diagnosing celiac disease, suggesting that this technique has the potential to reduce the need for multiple biopsies. An earlier study from Australia assessing villous atrophy using mNBI showed mNBI to have a sensitivity of 93.3% and specificity of 97.8% in patients with suspected celiac disease[7].
We performed a meta-analysis on diagnostic accuracy of mNBI to detect duodenal villous atrophy with histology as a reference standard. The above six studies were screened for inclusion[5,7,10-12,16]. The study by Sinha et al[16] was excluded as only abstract was published and more data was required for meta-analysis[16]. The study by Singh et al[7], included 10 videos from 3 patients with celiac disease which implied multiple assessments for same patient. This significantly differed from other study designs and hence was not appropriate for pooling of data with other studies. Valitutti and colleagues studied pediatric patients only and for this reason their data was also not included. Finally we included three studies in the meta analysis[5,11,12]. The analysis was performed using the software Meta-DiSc[17]. The pooled sensitivity (Figure 2) was 0.96 (95%CI: 0.89-0.99) and the pooled specificity (Figure 3) was 0.94 (95%CI: 0.89-0.97). These impressive data further strengthen the evidence in favour of mNBI in assessing duodenal villous atrophy.
Figure 2.
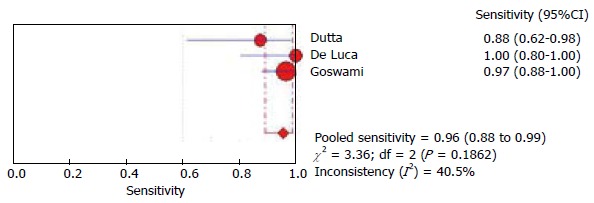
Pooled sensitivity of narrow band imaging in detecting duodenal villous atrophy.
Figure 3.
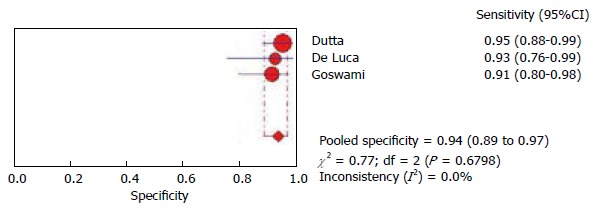
Pooled specificity of narrow narrow band imaging in detecting duodenal villous atrophy.
Atrophy of villi may be patchy in some patients with celiac disease and this may be missed on random mucosal biopsy. By identifying atrophic villi based on morphology, mNBI can overcome this limitation and help obtain targeted biopsies. Few case series and reports have demonstrated the capability of mNBI to detect patchy villous atrophy[15,18,19]. This perhaps is one area where mNBI can play an important role in avoiding false negative biopsies but more data is required. It is clear from the above studies that mNBI can play a useful role in aiding the diagnostic evaluation of celiac disease and other malabsorption syndromes.
NBI IN ASSESSING PROTRUDING DUODENAL MUCOSAL LESIONS
Unlike gastric, colonic and esophageal lesions, mNBI characteristics of duodenal mucosal lesions like polyps and tumours have been less well studied. Most of the available studies are from Japan and except for a few, these are in the form of case reports[6,13,20,21]. In contrast to assessment of villous morphology where surface characteristic is the focus of attention, both surface and vascular characteristics are important in assessing neoplastic lesions. Abnormal angiogenesis is a feature of neoplasia and alteration in vascular pattern is a reflection of this.
Kikuchi et al[6] retrospectively analysed the surface/vascular pattern of duodenal non-ampullary tumours on mNBI and identified characteristics suggestive of high grade dysplasia and invasive tumour. The surface patterns were classified as monotype or mixed type when they had single or multiple surface patterns respectively. Vascular patterns were classified as network, intrastructural vessel, unclassified or absent. They found that presence of mixed type surface pattern was suggestive of high grade dysplasia or invasive lesion in all 23 cases. In the remaining 23 lesions which had monotype surface, presence of unclassified vascular pattern and intrastructural vessels were associated with advanced lesions. Other vascular patterns were also seen with advanced lesions. The study therefore suggested that a mixed surface pattern was strongly suggestive of advanced lesion but confident diagnosis of low grade dysplastic or non-neoplastic lesion based on surface/vessel characteristics was not accurate. Another study assessed 65 duodenal sites in 36 subjects which were normal or had polyps[22]. Duodenal polyp with dysplasia was seen at 24 sites and mNBI had sensitivity of 83% and specificity of 78% in detecting dysplasia. They also examined the mucosa using probe based confocal endomicroscopy which was found to be better than mNBI. These data suggest that mNBI may help the endoscopist avoid biopsy (which makes subsequent resection difficult) and proceed directly to EMR in suspected dysplastic lesions.
A couple of case reports have described the appearance of follicular lymphoma in duodenum on mNBI. Chowdhury et al[20] from Morioka, Japan reported coiled, elongated microvascular pattern in two patients with follicular lymphoma and similar findings was observed by Iwamuro et al[23] in a 57-year-old patient from Niihama, Japan[20,23]. Inoue et al[24] reported whitish areas in enlarged villi in a patient with primary follicular lymphoma of duodenum. Elongated microvessels with white spots on surface was observed in a case of duodenal lymphangioma[21]. Another case report described saucer shaped lesions and multiple swollen villi like “moth eggs” on mNBI in a case of interdigitating dendritic cell sarcoma in duodenum[25]. While mNBI showed interesting abnormalities in the above reported lesions, its impact on management of patient is uncertain as biopsy may still be required.
NBI TO SCREEN FOR DUODENAL POLYPS IN POLYPOSIS SYNDROMES
Duodenum is an important site of polyps in familial adenomatous polyposis[26]. Surveillance endoscopy is recommended as duodenal tumours are the second most common cause of mortality in FAP after colonic tumours[27]. A study from Netherlands on 37 patients with FAP who underwent surveillance endoscopy using mNBI found NBI detected more adenomas than high resolution endoscopy and increased the Spigelman stage in 2 patients[28]. Another group used NBI for surveillance of polyps in patients with carriers of PTEN mutation seen in Cowden’s syndrome[29]. Nine out of ten patients were found to have duodenal polyps but the role mNBI in this high diagnostic yield was not very clear.
NBI FOR DUODENAL AMPULLARY LESIONS
Ampullary adenomas may harbour malignant foci which may be missed on random biopsy due to sampling error. A study on 14 patients with bulky ampulla investigated the correlation between findings on mNBI and histology[13]. The surface pattern was divided into three types (type I - oval villi; type II - pinecone/leaf shaped villi; type III - irregular or nonstructured villi). Presence of tortuous, dilated and network vessels were considered abnormal. Inflammatory and hyperplastic lesions had type I surface pattern while adenomas and adenocarcinomas had type II and/or III surface pattern. Adenomas did not have abnormal vessels. A case report also showed the absence of vascular abnormality to be associated with no foci of malignancy on histology[30]. This is another potential area where NBI may play a role in obtaining targeted biopsies and help decide management strategy. Apart from assessing lesion characteristics, mNBI may also help in determining the lesion margin in patients undergoing duodenal papillectomy. Itoi et al[31] found mNBI to be better than indigo carmine chromoendoscopy for this indication. These preliminary observations suggest that mNBI may be useful in assessment and management of ampullary lesions. Larger studies are however required before recommending this technique for routine use.
CONCLUSION
Magnification endoscopy with NBI (mNBI) seems to have a potential role in evaluating duodenal mucosal morphology. Table 1 summarises the merits, demerits and potential application of this technology in clinical practice. While there is robust evidence for its role in assessing villous morphology, more data is required before recommending it for routine use in assessment of duodenal neoplastic lesions. There is also a need for uniform terminology and classification in describing villous morphology and protruding lesions which can enable comparison of published literature and facilitate training. The recent increase in interest on NBI in duodenum is encouraging because it can lead to new diagnostic possibilities which may impact therapy.
Table 1.
Overview of current status of magnification narrow band imaging technology in duodenum
| Merits | Real time assessment of duodenal villous morphology with excellent accuracy Enables targeted biopsy of abnormal appearing area |
| Demerits | Added procedure time and cost |
| Potential applications | Assessment of duodenal villous atrophy in malabsorption syndromes Data on role in assessing duodenal neoplastic lesion is still preliminary |
Footnotes
Conflict-of-interest statement: None.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 27, 2015
First decision: July 29, 2015
Article in press: September 30, 2015
P- Reviewer: Kumagai Y, Tischendorf JJW S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
References
- 1.Coda S, Thillainayagam AV. State of the art in advanced endoscopic imaging for the detection and evaluation of dysplasia and early cancer of the gastrointestinal tract. Clin Exp Gastroenterol. 2014;7:133–150. doi: 10.2147/CEG.S58157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ianiro G, Gasbarrini A, Cammarota G. Endoscopic tools for the diagnosis and evaluation of celiac disease. World J Gastroenterol. 2013;19:8562–8570. doi: 10.3748/wjg.v19.i46.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larghi A, Lecca PG, Costamagna G. High-resolution narrow band imaging endoscopy. Gut. 2008;57:976–986. doi: 10.1136/gut.2007.127845. [DOI] [PubMed] [Google Scholar]
- 4.East JE, Tan EK, Bergman JJ, Saunders BP, Tekkis PP. Meta-analysis: narrow band imaging for lesion characterization in the colon, oesophagus, duodenal ampulla and lung. Aliment Pharmacol Ther. 2008;28:854–867. doi: 10.1111/j.1365-2036.2008.03802.x. [DOI] [PubMed] [Google Scholar]
- 5.Dutta AK, Sajith KG, Shah G, Pulimood AB, Simon EG, Joseph AJ, Chacko A. Duodenal villous morphology assessed using magnification narrow band imaging correlates well with histology in patients with suspected malabsorption syndrome. Dig Endosc. 2014;26:720–725. doi: 10.1111/den.12285. [DOI] [PubMed] [Google Scholar]
- 6.Kikuchi D, Hoteya S, Iizuka T, Kimura R, Kaise M. Diagnostic algorithm of magnifying endoscopy with narrow band imaging for superficial non-ampullary duodenal epithelial tumors. Dig Endosc. 2014;26 Suppl 2:16–22. doi: 10.1111/den.12282. [DOI] [PubMed] [Google Scholar]
- 7.Singh R, Nind G, Tucker G, Nguyen N, Holloway R, Bate J, Shetti M, George B, Tam W. Narrow-band imaging in the evaluation of villous morphology: a feasibility study assessing a simplified classification and observer agreement. Endoscopy. 2010;42:889–894. doi: 10.1055/s-0030-1255708. [DOI] [PubMed] [Google Scholar]
- 8.Dutta AK. Narrow band imaging endoscopy for real-time assessment of duodenal villi. Indian J Gastroenterol. 2014;33:408–409. doi: 10.1007/s12664-014-0486-7. [DOI] [PubMed] [Google Scholar]
- 9.Pittayanon R, Imraporn B, Rerknimitr R, Kullavanijaya P. Advances in diagnostic endoscopy for duodenal, including ampullary, adenoma. Dig Endosc. 2014;26 Suppl 2:10–15. doi: 10.1111/den.12244. [DOI] [PubMed] [Google Scholar]
- 10.Valitutti F, Oliva S, Iorfida D, Aloi M, Gatti S, Trovato CM, Montuori M, Tiberti A, Cucchiara S, Di Nardo G. Narrow band imaging combined with water immersion technique in the diagnosis of celiac disease. Dig Liver Dis. 2014;46:1099–1102. doi: 10.1016/j.dld.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 11.Goswami A, Dadhich S, Bhargava N. Use of narrow band imaging in assessing duodenal villous atrophy. Indian J Gastroenterol. 2014;33:440–444. doi: 10.1007/s12664-014-0489-4. [DOI] [PubMed] [Google Scholar]
- 12.De Luca L, Ricciardiello L, Rocchi MB, Fabi MT, Bianchi ML, de Leone A, Fiori S, Baroncini D. Narrow band imaging with magnification endoscopy for celiac disease: results from a prospective, single-center study. Diagn Ther Endosc. 2013;2013:580526. doi: 10.1155/2013/580526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uchiyama Y, Imazu H, Kakutani H, Hino S, Sumiyama K, Kuramochi A, Tsukinaga S, Matsunaga K, Nakayoshi T, Goda K, et al. New approach to diagnosing ampullary tumors by magnifying endoscopy combined with a narrow-band imaging system. J Gastroenterol. 2006;41:483–490. doi: 10.1007/s00535-006-1800-7. [DOI] [PubMed] [Google Scholar]
- 14.Bardella MT, Minoli G, Radaelli F, Quatrini M, Bianchi PA, Conte D. Reevaluation of duodenal endoscopic markers in the diagnosis of celiac disease. Gastrointest Endosc. 2000;51:714–716. doi: 10.1067/mge.2000.104653. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee R, Reddy DN. High-resolution narrow-band imaging can identify patchy atrophy in celiac disease: targeted biopsy can increase diagnostic yield. Gastrointest Endosc. 2009;69:984–985. doi: 10.1016/j.gie.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Sinha SK, Siddappa PK, Basha J, Vaiphei K, Prasad KK, Appasani S, Berry N, Ashat M, Singh K, Kochhar R. Can narrow band imaging predict duodenal histology in celiac disease? A prospective double blind pilot study. United Eur Gastroent. 2014;2:A10. [Google Scholar]
- 17.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh R, Mei SL, Jayanna M, Ruszkiewicz A. Education and imaging: Gastrointestinal: Patchy distribution of coeliac disease diagnosed with narrow band imaging and optical magnification. J Gastroenterol Hepatol. 2013;28:584. doi: 10.1111/jgh.12097. [DOI] [PubMed] [Google Scholar]
- 19.Tchekmedyian AJ, Coronel E, Czul F. “Leopard skin sign”: the use of narrow-band imaging with magnification endoscopy in celiac disease. Rev Gastroenterol Peru. 2014;34:321–324. [PubMed] [Google Scholar]
- 20.Chowdhury M, Endo M, Chiba T, Kudara N, Oana S, Sato K, Akasaka R, Tomita K, Fujiwara S, Mizutani T, et al. Characterization of follicular lymphoma in the small intestine using double-balloon endoscopy. Gastroenterol Res Pract. 2009;2009:835258. doi: 10.1155/2009/835258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwamuro M, Kawai Y, Takata K, Okada H, Yamamoto K. Observation of lymphangioma of the duodenum by a magnifying endoscope with a narrow-band imaging system. Case Rep Gastroenterol. 2013;7:229–233. doi: 10.1159/000351831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shahid MW, Buchner A, Gomez V, Krishna M, Woodward TA, Raimondo M, Wallace MB. Diagnostic accuracy of probe-based confocal laser endomicroscopy and narrow band imaging in detection of dysplasia in duodenal polyps. J Clin Gastroenterol. 2012;46:382–389. doi: 10.1097/MCG.0b013e318247f375. [DOI] [PubMed] [Google Scholar]
- 23.Iwamuro M, Okuda M, Yumoto E, Suzuki S, Shirakawa A, Takata K, Yoshino T, Okada H, Yamamoto K. Magnifying endoscopy for intestinal follicular lymphoma is helpful for prompt diagnosis. Gut Liver. 2013;7:258–261. doi: 10.5009/gnl.2013.7.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inoue N, Isomoto H, Shikuwa S, Mizuta Y, Hayashi T, Kohno S. Magnifying endoscopic observation of primary follicular lymphoma of the duodenum by using the narrow-band imaging system. Gastrointest Endosc. 2009;69:158–159; discussion 159. doi: 10.1016/j.gie.2008.06.055. [DOI] [PubMed] [Google Scholar]
- 25.Nonaka K, Honda Y, Gushima R, Maki Y, Sakurai K, Iyama K, Sasaki Y. Narrow-band imaging of interdigitating dendritic cell sarcoma originating in the duodenum. Endoscopy. 2011;43 Suppl 2 UCTN:E113–E114. doi: 10.1055/s-0030-1256146. [DOI] [PubMed] [Google Scholar]
- 26.Brosens LA, van Hattem WA, Jansen M, de Leng WW, Giardiello FM, Offerhaus GJ. Gastrointestinal polyposis syndromes. Curr Mol Med. 2007;7:29–46. doi: 10.2174/156652407779940404. [DOI] [PubMed] [Google Scholar]
- 27.Brosens LA, Keller JJ, Offerhaus GJ, Goggins M, Giardiello FM. Prevention and management of duodenal polyps in familial adenomatous polyposis. Gut. 2005;54:1034–1043. doi: 10.1136/gut.2004.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Ceron M, van den Broek FJ, Mathus-Vliegen EM, Boparai KS, van Eeden S, Fockens P, Dekker E. The role of high-resolution endoscopy and narrow-band imaging in the evaluation of upper GI neoplasia in familial adenomatous polyposis. Gastrointest Endosc. 2013;77:542–550. doi: 10.1016/j.gie.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 29.Levi Z, Baris HN, Kedar I, Niv Y, Geller A, Gal E, Gingold R, Morgenstern S, Baruch Y, Leach BH, et al. Upper and Lower Gastrointestinal Findings in PTEN Mutation-Positive Cowden Syndrome Patients Participating in an Active Surveillance Program. Clin Transl Gastroenterol. 2011;2:e5. doi: 10.1038/ctg.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamao T, Isomoto H, Yamaguchi N, Irie J, Ito Y, Nakashima Y, Shikuwa S, Mizuta Y, Kohno S, Imamura S, et al. Magnified endoscopic observation using narrow-band imaging of periampullary adenoma in a patient with familial adenomatous polyposis. Med Sci Monit. 2009;15:CS169–CS173. [PubMed] [Google Scholar]
- 31.Itoi T, Tsuji S, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Ikeuchi N, Igarashi M, Gotoda T, et al. A novel approach emphasizing preoperative margin enhancement of tumor of the major duodenal papilla with narrow-band imaging in comparison to indigo carmine chromoendoscopy (with videos) Gastrointest Endosc. 2009;69:136–141. doi: 10.1016/j.gie.2008.07.036. [DOI] [PubMed] [Google Scholar]


