Abstract
Vision begins in photoreceptor outer segments with light captured by opsins in continually synthesized disc membranes. The process by which rod photoreceptor discs are formed has been controversial. In this issue, Ding et al. (2015. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201508093) show conclusively that rod discs are formed by plasma membrane evagination.
The vertebrate retina contains two types of photoreceptors, rod cells and cone cells, whose outer segments initiate phototransduction under night and daytime conditions, respectively. The outer segments of these cells lack ER, Golgi, and mitochondria and are filled with hundreds to a few thousand flattened membrane organelles, called photoreceptor discs, which are loaded with the molecular machinery of phototransduction. The structural organization of outer segments differs between rods and cones. Although cone outer segments contain “open” discs that are infoldings of the plasma membrane, rod outer segments possess “closed” discs that are completely separated from the plasma membrane.
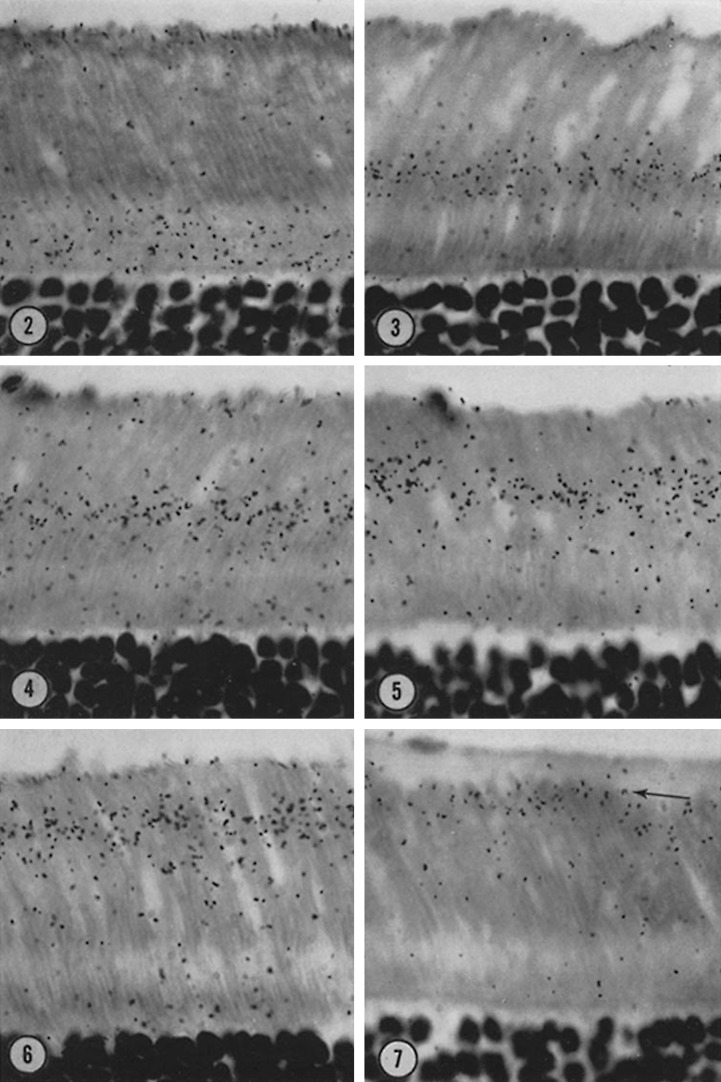
In 1967, in a paper that has been cited nearly 800 times, Richard Young reported the seminal finding that rod and cone outer segments are continually renewed (Young, 1967). Young’s classic experiment was elegantly simple: he injected [3H]methionine into a rat, mouse, and frog and performed autoradiograms of the excised retina on various days after the injection. He observed that the radiolabeled band moved along the outer segment as time after injection increased and ultimately disappeared at the apex of the cell (Fig. 1, republished from Young, 1967). (As Young was at the University of California, Los Angeles, this result was given the memorable moniker of “the UCLA marching band.”) Young’s seminal insight that outer segments are continually rebuilt posed a problem that has challenged photoreceptor cell biologists ever since: How are rod disc membranes initially formed? In this issue, Ding et al. present a compelling resolution to this question. Specifically, their work differentiates between currently competing models to determine whether rod discs are formed by evagination of plasma membrane at the base of the outer segment or by fusion of intracellular vesicles transported to the outer segment.
Figure 1.
Photoreceptor outer segments are continually renewed. Rats were injected with [3H]methionine, and radioautographs of photoreceptor cells were performed on various days after the injection. As time after injection increases (images 2–7), the radiolabel components are displaced from the inner segment along the outer segment toward the apex of the cell, revealing that the outer segment is continually renewed (figure republished from Young, 1967).
The classic hypothesis of disc morphogenesis is that they are formed by evagination of basal outer segment plasma membrane (Steinberg et al., 1980). This hypothesis is based largely on evidence that one surface of the most basal discs of rods is open to the extracellular space, as shown by EM (Carter-Dawson and LaVail, 1979; Steinberg et al., 1980), with lipophilic dye fluorescence (Laties et al., 1976), and by analysis of membrane capacitance (Rüppel and Hagins, 1973). In addition, rods and cones might be expected to share a common machinery of disc formation. Because most cone discs are well established by EM, lipophilic dye imaging, and electrophysiology to be continuous with the plasma membrane, nascent rod discs would seem likely to also be part of the plasma membrane. Thus, according to the classic hypothesis, new discs in both photoreceptor types are formed from outgrowths (evaginations) of the plasma membrane at the outer segment base. In both photoreceptor types, discs would begin life with one face exposed to the extracellular space, but at some point after formation, rod discs would pinch off from the outer segment plasma membrane to become self-contained and fully separated from the plasma membrane, whereas cones discs remain open. On the contrary, the vesicle fusion hypothesis postulates that nascent discs are born completely internalized in rods. Photoreceptor outer segments are now understood to be the plus end of a modified primary cilium (Bloodgood, 2009) and are joined to their inner segments by a narrow ciliary tube called the connecting cilium. This realization, combined with evidence of vesicles in the connecting cilium seen in electron micrographs, has been taken to support the model that vesicles are actively transported through the connecting cilium and generate nascent discs by membrane fusion at the base of the outer segment (Chuang et al., 2007, 2015).
Ding et al. (2015) addressed these competing hypotheses with two distinct approaches. First, they treated sections of retinas of mice perfused with a membrane-staining mixture of tannic acid and uranyl acetate and performed EM. Because tannic acid penetrates intact membranes poorly, this treatment distinguishes between membranes exposed to the extracellular space and intracellular membrane structures. The researchers found that, like the plasma membrane, a small number of basal rod discs were intensely stained by tannic acid, whereas the staining of fully internalized discs was weak, confirming that newly formed rod discs are open to the extracellular space. Consistently and strikingly, EM analysis also revealed a single basal disc face (approximately five to seven discs north of the most basal disc) that is contiguous with the plasma membrane. Second, Ding et al. (2015) performed EM with an immunogold-tagged antibody raised against an intracellular epitope of peripherin, a protein that plays an essential role in disc stacking (Arikawa et al., 1992; Goldberg, 2006). Quantification of gold particle counts showed that the peripherin antibody closely associated intracellularly with the edges of fully internalized discs but was negligibly associated with the surface of nascent discs identified as facing the extracellular space, suggesting that peripherin redistributes along the rod disc edge upon its separation from the plasma membrane and enclosure into the outer segment. Finally, Ding et al. (2015) performed experiments using the fixation techniques reported by other investigators and demonstrated that artifacts of tissue fixation were responsible for the erroneous interpretation that basal discs are fully internalized and for the evidence supporting the vesicular fusion hypothesis.
Other tools, such as superresolution microscopy of living rods stained with lipophilic dyes or fluorescent antibodies raised against epitopes on the extracellular face of the rod plasma membrane, could further test aspects of the evagination model of disc formation. Nonetheless, the work of Ding et al. (2015) unequivocally shows that basal rod discs are open to the extracellular space and provides a new system and conceptual framework for the investigation of the fundamental biological mechanism of plasma membrane evagination. As outer segment discs exhibit a specialized composition of lipids and phototransduction proteins, further work will also focus on how disc lipids and proteins are transported from the inner segment to the basal outer segment. The current hypotheses about such transport include (a) vesicular transport through the connecting cilium followed by fusion with the outer segment plasma membrane; (b) directed transport through the connecting cilium membrane after vesicle fusion at the base of the connecting cilium in the inner segment; and (c) exocytotic release from the inner segment followed by endocytotic capture in the outer segment. As the molecular details of disc formation and specialization become clearer, Richard Young’s “UCLA marching band” (Young, 1967) will continue to have a broad conceptual impact on the cell biology of photoreceptor development and cilia.
Acknowledgments
The author declares no competing financial interests.
References
- Arikawa K., Molday L.L., Molday R.S., and Williams D.S.. 1992. Localization of peripherin/rds in the disk membranes of cone and rod photoreceptors: relationship to disk membrane morphogenesis and retinal degeneration. J. Cell Biol. 116:659–667. 10.1083/jcb.116.3.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood R.A. 2009. From central to rudimentary to primary: the history of an underappreciated organelle whose time has come. The primary cilium. Methods Cell Biol. 94:3–52. 10.1016/S0091-679X(08)94001-2 [DOI] [PubMed] [Google Scholar]
- Carter-Dawson L.D., and LaVail M.M.. 1979. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J. Comp. Neurol. 188:245–262. 10.1002/cne.901880204 [DOI] [PubMed] [Google Scholar]
- Chuang J.Z., Zhao Y., and Sung C.H.. 2007. SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell. 130:535–547. 10.1016/j.cell.2007.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang J.Z., Hsu Y.C., and Sung C.H.. 2015. Ultrastructural visualization of trans-ciliary rhodopsin cargoes in mammalian rods. Cilia. 4:4 10.1186/s13630-015-0013-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J.-D., Salinas R.Y., and Arshavsky V.Y.. 2015. Discs of mammalian rod photoreceptors form through the membrane evagination mechanism. J. Cell Biol. 10.1083/jcb.201508093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A.F. 2006. Role of peripherin/rds in vertebrate photoreceptor architecture and inherited retinal degenerations. Int. Rev. Cytol. 253:131–175. 10.1016/S0074-7696(06)53004-9 [DOI] [PubMed] [Google Scholar]
- Laties A.M., Bok D., and Liebman P.. 1976. Procion yellow: A marker dye for outer segment disc patency and for rod renewal. Exp. Eye Res. 23:139–148. 10.1016/0014-4835(76)90197-4 [DOI] [PubMed] [Google Scholar]
- Rüppel H., and Hagins W.A.H.. 1973. Spatial origin of the fast photovoltage in retinal rods. In Biochemistry and Physiology of Visual Pigments. Langer H., editor. Springer-Verlag New York Inc., New York: 257–261. 10.1007/978-3-642-85769-0_30 [DOI] [Google Scholar]
- Steinberg R.H., Fisher S.K., and Anderson D.H.. 1980. Disc morphogenesis in vertebrate photoreceptors. J. Comp. Neurol. 190:501–518. 10.1002/cne.901900307 [DOI] [PubMed] [Google Scholar]
- Young R.W. 1967. The renewal of photoreceptor cell outer segments. J. Cell Biol. 33:61–72. 10.1083/jcb.33.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]