Abstract
With the newly developed dynamic jaws technology, radiation dose for the cranio-caudal edges of a target can be lowered in the treatment with tomotherapy. We compared dynamic-jaw- and fixed-jaw-mode plans for lung cancer. In 35 patients, four plans using the 2.5-cm dynamic-, 2.5-cm fixed-, 5.0-cm dynamic-, and 5.0-cm fixed-jaw modes were generated. For 10 patients with upper lobe stage I lung cancer, the helical tomotherapy mode was used. Fifty-six Gy in 8 fractions was prescribed as a minimum coverage dose for 95% of the target (D95%). For 25 patients with locally advanced lung cancer, plans using four static ports (TomoDirect® mode) were made. Sixty Gy in 30 daily fractions for the primary tumor and swollen lymph nodes and 51 Gy in 30 fractions for prophylactic lymph node areas were prescribed as median doses. The mean conformity index of the planning target volume were similar among the four plans. The mean V5 Gy of the lung for 2.5-cm dynamic-, 2.5-cm fixed-, 5.0-cm dynamic-, and 5.0-cm fixed-jaw mode plans were 18.5%, 21.8%, 20.1%, and 29.4%, respectively (p < 0.0001), for patients with stage I lung cancer, and 37.3%, 38.7%, 40.4%, and 44.0%, respectively (p < 0.0001), for patients with locally advanced lung cancer. The mean V5 Gy of the whole body was 1,826, 2,143, 1,983, and 2,939 ml, respectively (p < 0.0001), for patients with stage I lung cancer and 4,849, 5,197, 5,220, and 6,154 ml, respectively (p < 0.0001), for patients with locally advanced lung cancer. Treatment time was reduced by 21-39% in 5.0-cm dynamic-jaw plans compared to 2.5-cm plans. Regarding dose distribution, 2.5-cm dynamic-jaw plans were the best, and 5.0-cm dynamic-jaw plans were comparable to 2.5-cm fixed-jaw plans with shorter treatment times. The dynamic-jaw mode should be used instead of the conventional fixed-jaw mode in tomotherapy for lung cancer.
Keywords: Dynamic jaws, Lung cancer, Static port, Tomotherapy
Introduction
The TomoTherapy® (Accuray Inc., Sunnyvale, CA, USA) is a radiation delivery system that combines dynamic intensity-modulated radiation therapy (IMRT) and an on-board imaging system (1, 2). The role of tomotherapy has now been established for the treatment of various targets (3–14). With the conventional tomotherapy delivery mode, the cranio-caudal “penumbra”, i.e., dose scattering at the cranio-caudal edges of a target, has been an issue that should be improved. In the treatment of lung cancer with IMRT, low dose irradiation to the lung has been reported to be a risk factor for radiation pneumonitis (3, 4). To reduce the lung volume receiving low dose radiation, we reported the usefulness of tomotherapy using static ports (TomoDirect® mode) for the treatment of locally advanced lung cancer (5). However, scattered doses were not negligible, especially in the 5.0-cm fixed-jaw mode. Recently, a newly developed dynamic jaws technology (TomoEDGE®) has been introduced in our institution first in Japan. With this technology, radiation doses for the cranio-caudal edges of the target can be lowered by using narrower jaws around the edges (6–8). The purpose of this study is to evaluate the characteristics of dynamic-jaw-mode plans compared to conventional fixed-jaw-mode plans for lung cancer.
Methods
In 35 patients, four plans using the 2.5-cm dynamic-, 2.5-cm fixed-, 5.0-cm dynamic-, and 5.0-cm fixed-jaw modes were made and ompared. For 10 patients with upper lobe stage I lung cancer, helical tomotherapy plans were generated to obtain better conformity than with the TomoDirect mode. For 25 patients with locally advanced lung cancer, treatment plans using four ports with the TomoDirect mode were made to reduce the lung volume receiving low dose irradiation. The location of the primary tumor in the 25 patients was the upper lobe in 10, middle lobe in 2, lower lobe in 5, and mediastinum in 8. This is a planning comparison study, and actually the patients were treated with linac-based stereotactic radiotherapy for stage I lung cancer (15, 16) or conventional radiotherapy for locally advanced lung cancer.
CT Simulation and Planning
The 10 patients with stage I lung cancer were immobilized in a supine position with a vacuum bag system (BodyFIX; Medical Intelligence, Schwabmünchen, Germany) alongside the whole body. Computed tomography (CT) scans were performed with a slice thickness of 3.2-mm using a 4-row multi-detector CT (Mx8000; Philips Medical Systems, Best, The Netherlands) in a supine position under normal breathing, and with breath holding during the expiratory and inspiratory phases as described in detail previously (15). For the 25 patients with locally advanced lung cancer, actually treated with conventional radiotherapy, CT scans were performed under normal breathing without immobilization devices. CT images were reconstructed with a 2.5-mm thickness. Contouring of target volumes and normal structures was performed on the Pinnacle3 version 9 treatment planning system (Philips Medical System, Eindhoven, The Netherlands). The contours created in the treatment planning system were exported to the TomoTherapy treatment planning system (Tomo HD version 2.0), where all plans were generated. For stage I lung cancer, the clinical target volume (CTV) was defined as the visible gross tumor volume (GTV). The CTV on CT during the 3 phases were superimposed to represent the internal target volume. We defined the planning target volume (PTV) margin for the internal target volume as 5-mm in all directions. As a minimum coverage dose for 95% of the PTV (D95%), 56 Gy in 8 fractions was prescribed. We contoured the ispirateral lung and total lung excluding the PTV. As dose constraints, 1) D95% > 95% of the prescribed dose, 2) V90% of the PTV ≥ 95%, 3) mean lung dose (MLD) < 18 Gy, V20 Gy < 20% of the total lung, and 4) spinal cord maximum dose < 25 Gy were satisfied. V90% was defined as the percentage of the PTV receiving at least 90% of the prescribed dose. The Vx Gy value represents the percentage or absolute volume (V) receiving the specified dose (x) in Gy, e.g., V10 Gy is the percentage volume receiving 10 Gy.
For the 25 patients with locally advanced lung cancer, contrast-enhanced CT images were acquired and fused to the planning CT images to delineate the target, but unenhanced CT images were used for dose calculation to keep calculation accuracy (17). The visible primary tumor, swollen lymph nodes, and prophylactic lymph node area were contoured as the CTV1, CTV2, and CTV3, respectively. For the PTV1, the CTV1 was expanded by 5-mm for mediastinal primary tumors, 10-mm in all directions for upper-lobe and 1 of 5 lower-lobe primary tumors that invaded the chest wall. For four patients with a lower-lobe primary tumor, we defined the margin as 15-mm in the cranio-caudal direction. The CTV2 and CTV3 were expanded by 5-mm in all directions for the PTV2 and PTV3, respectively. Using the simultaneous integrated boost technique, 60 Gy in 2-Gy daily fractions to the PTV1 and PTV2, and 51 Gy in 1.7-Gy fractions to the PTV3 were prescribed as median doses (18). We contoured the ispirateral lung and total lung excluding the PTV1. Dose constraints were: 1) D95% > 90% of the prescribed dose; 2) total lung: MLD > 17 Gy, V10 Gy < 40%, and V20 Gy < 30%; 3) spinal cord: maximum dose < 50 Gy; and 4) esophagus: maximum dose < 66 Gy. Inverse planning procedure of optimization using the TomoTherapy planning station was described in detail previously (5). The same pitch (0.287 or 0.215 for helical tomotherapy and 0.251 or 0.500 for TomoDirect) and modulation factor (2.0) were used in each patient. When the dose constrains could not be fulfilled, the dose of the cord and the esophagus took priority. A fine calculation grid (1.95 × 1.95-mm) was used for the final calculation process.
To compare 2.5-cm dynamic-, 2.5-cm fixed-, 5.0-cm dynamic-, and 5.0-cm fixed-jaw-mode plans, the conformity index, uniformity index, dose distribution in organs at risk, and beam-on time were evaluated in the TomoTherapy planning system. The conformity index and uniformity index were calculated according to the following formulae (5, 19).
where VPTV = PTV (ml), TVPV = lesion volume (ml) covered by the prescribed isodose, VTV = prescribed isodose volume (ml), and D5% = minimum dose delivered to the 5% of the PTV. The lower conformity index indicates the higher conformity, and the lower uniformity index indicates the better homogeneity. An ideal conformity index and uniformity index are both 1.
Statistical Analysis
The conformity index, uniformity index, dose distribution in organs at risk, integral dose, monitor unit, and beam-on time were compared. Paired t-test was used to analyze the difference within the same-size jaws as a priori comparisons. We used parametric analysis of variance for the dependent samples among the whole groups. These were performed when the normality of the distribution and homogeneity of the variance in the analyzed groups were confirmed by Kolmogorov-Sminov test and Bartlett’s test. When these criteria were not met, Wilcoxon signed-rank test as a priori comparisons and Friedman analysis of variance for the whole groups were performed. Statistical analyses were carried out with the statistical software package ‘R’ (20). All plannings and evaluations were performed by one radiation oncologist (Y. M.).
Results
Stage I Lung Cancer
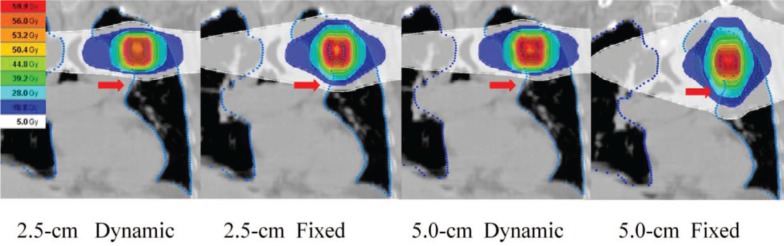
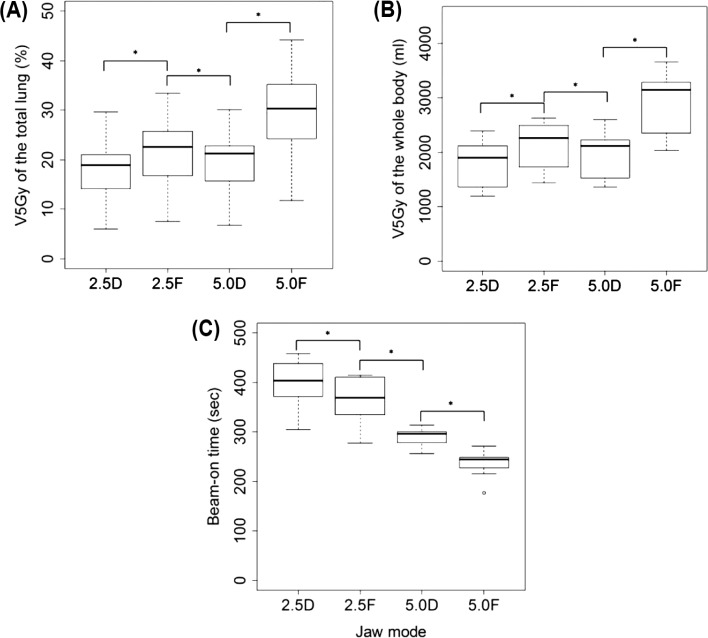
Figure 1 shows representative dose distributions for the four plans in a patient with stage I lung cancer. The treatment parameters, dose-volume parameters, beam-on times, monitor units, and gantry periods of the four plans for 10 patients are summarized in Tables I and II. The conformity index of the PTV and the maximum dose of the esophagus were similar among the four plans. The uniformity index was slightly better in the dynamic-jaw-mode than fixed-jaw-mode plans, but these differences appeared to be clinically of no significance (Table I). The differences in the maximum spinal cord dose were small between the dynamic-jaw and fixed-jaw plans. All dose-volume parameters for the whole body (V5-30 Gy) and lung (V5-50 Gy and MLD) were better in the 2.5-cm dynamic-jaw plans than in the 2.5-cm fixed-jaw plans; this was also observed between the 5.0-cm dynamic-jaw and fixed-jaw plans, except V40 Gy for the ipsilateral lung and V50 Gy for the total and ipsilateral lungs (Tables I and II; Figure 2A and 2B). The V10 Gy of the contralateral lung were all <1% (data not shown). The mean of the V5 Gy of the total lung, ispilateral lung, and the whole body in 5.0-cm dynamic-jaw plans were lower than in 2.5-cm fixed-jaw plans (Figure 2A and 2B). The beam-on time was longer by 32-54 seconds in dynamic-jaw plans probably due to slower gantry rotation and higher monitor units. The 5.0-cm dynamic-jaw plans reduced the beam-on time by 21-27% compared to the 2.5-cm jaw plans (Figure 2C).
Figure 1:
Dose distribution of the four mode plans in a patient with stage I lung cancer. Cranio-caudal dose penumbra can be reduced using dynamic jaw mode (arrows).
Table I.
Treatment and dose-volume parameters, monitor units, gantry period, and beam-on times of the four helical tomotherapy plans for patients with stage I lung cancer.
| Mean ± standard deviation | |||||||
|---|---|---|---|---|---|---|---|
| 2.5-cm Dynamic | 2.5-cm Fixed | p* | 5.0-cm Dynamic | 5.0-cm Fixed | p** | p*** | |
| Patient number | 10 | ||||||
| Total PTV (ml) | 22.7 ± 10.3 | ||||||
| Pitch | 0.215, 0.287 | ||||||
| Modulation factor | 2.0 | ||||||
| Conformity index | 1.28 ± 0.12 | 1.30 ± 0.06 | 0.32‡ | 1.29 ± 0.05 | 0.16‡ | 0.39† | |
| Uniformity index | 1.09 ± 0.02 | 1.10 ± 0.02 | 0.02|| | 1.10 ± 0.02 | 1.11 ± 0.03 | 0.05|| | 0.0002§ |
| Monitor unit | 5525 ± 644 | 5066 ± 635 | 0.002‡ | 3988 ± 245 | 3219 ± 363 | 0.002‡ | <0.0001† |
| Gantry period (sec) | 38.1 ± 5.8 | 35.2 ± 5.1 | <0.0001|| | 41 ± 8.3 | 32.1 ± 3.9 | 0.002|| | <0.0001§ |
| Beam-on time (sec) | 397.9 ± 45.1 | 365.9 ± 44.6 | 0.002‡ | 290.3 ± 17.4 | 236.2 ± 25.4 | 0.002‡ | <0.0001† |
| Body | |||||||
| V5 Gy (ml) | 1825.6 ± 409.4 | 2143.4 ± 441.6 | <0.0001|| | 1983.4 ± 401.6 | 2938.9 ± 559.9 | <0.0001|| | <0.0001§ |
| V10 Gy (ml) | 806.1 ± 255.2 | 909.0 ± 271.8 | <0.0001|| | 881.5 ± 264.7 | 1196.4 ± 335.7 | <0.0001|| | <0.0001§ |
| V20 Gy (ml) | 240.3 ± 89.5 | 269.5 ± 91.3 | <0.0001|| | 265.6 ± 96.3 | 354.0 ± 113.2 | <0.0001|| | < 0.0001§ |
| V30 Gy (ml) | 115.4 ± 43.3 | 128.0 ± 43.4 | <0.0001|| | 127.9 ± 46.4 | 163.4 ± 51.9 | <0.0001|| | < 0.0001§ |
| Spinal cord maximum (Gy) | 10.3 ± 4.6 | 10.9 ± 4.8 | 0.003|| | 9.2 ± 4.6 | 8.9 ± 4.9 | 0.80|| | 0.02§ |
| Esophagus maximum (Gy) | 14.1 ± 4.1 | 14.3 ± 4.2 | 0.14|| | 14.0 ± 3.8 | 14.1 ± 4.1 | 0.68|| | 0.31§ |
Abbreviation: PTV: Planning target volume.
*p-value between 2.5-cm dynamic- and 2.5-cm fixed- jaw plans.
**p-value between 5.0-cm dynamic- and 5.0-cm fixed- jaw plans.
***p-value among the four plans.
†Calculated by Friedman analysis of variance.
‡Calculated by Wilcoxon signed-rank test.
§Calculated by parametric analysis of variance for depended samples.
||Calculated by paired t-test.
Table II.
Dose-volume parameters of the four helical tomotherapy plans for patients with stage I lung cancer.
| Mean ± standard deviation | |||||||
|---|---|---|---|---|---|---|---|
| 2.5-cm Dynamic | 2.5-cm Fixed | p* | 5.0-cm Dynamic | 5.0-cm Fixed | p** | p*** | |
| Total lung | |||||||
| V5 Gy (%) | 18.5 ± 6.8 | 21.8 ± 7.5 | <0.0001|| | 20.1 ± 7.1 | 29.4 ± 9.8 | 0.0001|| | <0.0001§ |
| V10 Gy (%) | 9.2 ± 2.8 | 10.8 ± 3.2 | <0.0001|| | 10.0 ± 2.9 | 14.5 ± 4.0 | <0.001|| | <0.0001§ |
| V20 Gy (%) | 4.0 ± 1.4 | 4.7 ± 1.6 | <0.0001|| | 4.4 ± 1.5 | 6.2 ± 1.9 | <0.0001|| | <0.0001§ |
| V30 Gy (%) | 1.9 ± 0.9 | 2.3 ± 0.8 | 0.009|| | 2.3 ± 0.8 | 3.0 ± 1.0 | <0.0001|| | <0.0001§ |
| V40 Gy (%) | 1.0 ± 0.4 | 1.2 ± 0.5 | <0.0001|| | 1.2 ± 0.5 | 1.5 ± 0.5 | <0.0001|| | <0.0001§ |
| V50 Gy (%) | 0.4 ± 0.2 | 0.5 ± 0.2 | 0.01|| | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.06|| | <0.0001§ |
| MLD (Gy) | 3.3 ± 0.9 | 3.9 ± 1.0 | <0.0001|| | 3.6 ± 0.9 | 5.1 ± 1.3 | <0.0001|| | <0.0001§ |
| Ispilateral lung | |||||||
| V5 Gy (%) | 25.3 ± 7.1 | 31.1 ± 8.4 | <0.0001|| | 27.0 ± 7.4 | 42.8 ± 10.6 | <0.0001|| | <0.0001§ |
| V10 Gy (%) | 18.0 ± 5.8 | 21.3 ± 6.5 | <0.0001|| | 19.5 ± 5.9 | 28.4 ± 7.7 | <0.0001|| | <0.0001§ |
| V20 Gy (%) | 8.1 ± 3.6 | 9.4 ± 4.0 | <0.0001|| | 8.9 ± 3.7 | 12.4 ± 4.5 | <0.0001|| | <0.0001§ |
| V30 Gy (%) | 4.0 ± 1.9 | 4.7 ± 2.1 | <0.0001|| | 4.6 ± 2.0 | 6.2 ± 2.3 | <0.0001|| | <0.0001§ |
| V40 Gy (%) | 2.1 ± 1.1 | 2.4 ± 1.1 | <0.0001|| | 2.4 ± 1.1 | 3.7 ± 2.2 | 0.09|| | 0.009§ |
| V50 Gy (%) | 0.9 ± 0.5 | 0.9 ± 0.5 | 0.03‡ | 1.0 ± 0.6 | 1.6 ± 1.6 | 0.06‡ | <0.0001† |
| Contralateral lung | |||||||
| V5 Gy (%) | 11.6 ± 7.1 | 12.5 ± 7.1 | 0.007|| | 13.1 ± 7.5 | 15.7 ± 9.2 | 0.004|| | <0.0001§ |
*p-value between 2.5-cm dynamic- and 2.5-cm fixed-jaw plans.
**p-value between 5.0-cm dynamic- and 5.0-cm fixed-jaw plans.
***p-value among the four plans.
†Calculated by Friedman analysis of variance.
‡Calculated by Wilcoxon signed-rank test.
§Calculated by parametric analysis of variance for depended samples.
||Calculated by paired t-test.
Figure 2:
V5 Gy of the total lung (A), V5 Gy of the whole body (B), and beam-on time (C) in each mode for patients with stage I lung cancer. The box includes the central 50% of data (25-75%), and the central 99% of data are contained within the error bars. The solid line within each box indicates the median of the data. V5 Gy refers to the percentage or absolute volume receiving 5 Gy, 2.5D refers to 2.5-cm dynamic-jaw mode, 2.5F refers to 2.5-cm fixed-jaw mode, 5.0D refers to 5.0-cm dynamic-jaw mode, and 5.0F refers to 5.0-cm fixed-jaw mode. *p < 0.01.
Locally Advanced Lung Cancer
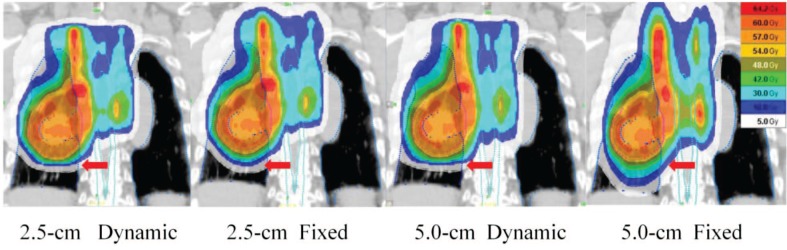
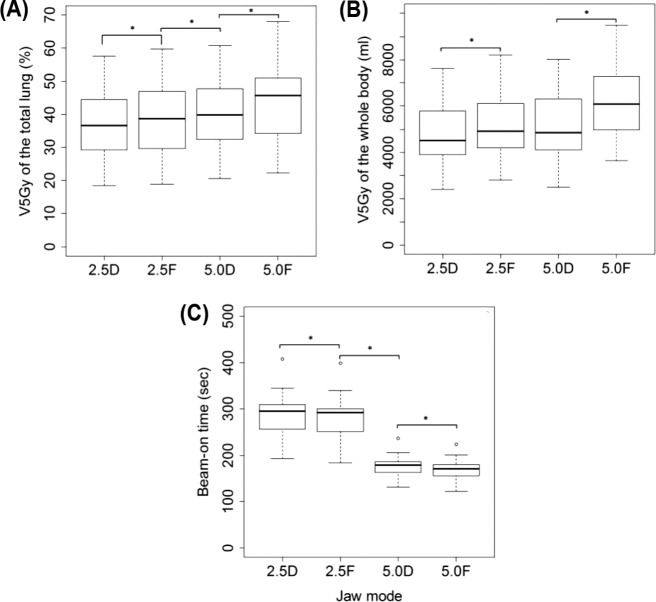
Figure 3 shows representative dose distributions for the four plans in a patient with locally advanced lung cancer. The treatment parameters, dose-volume parameters, beam-on times, and monitor units of the four plans for all 25 patients are summarized in Tables III and IV. The conformity index and uniformity index of the PTV and the maximum spinal cord dose were similar when all four plans were compared (Table III). The difference in the maximum esophagus dose did not seem to be of clinical significance. The V40 Gy for the heart was slightly lower in the dynamic-jaw than fixed-jaw plans. The V5-30 Gy for the whole body, MLD and V5-20 Gy for the total, ipsilateral, and contralateral lungs were consistently lower in the dynamic-jaw than fixed-jaw plans (Tables III and IV; Figure 4A and 4B). For V30-50 Gy, the superiority of either plan was not constant. The beam-on time was longer in dynamic-jaw plans probably due to higher monitor units, but the difference was relatively small (about 7 seconds longer in the dynamic-jaw plans). The 5.0-cm dynamic-jaw plans reduced the beam-on time by 38-39% compared to the 2.5-cm jaw plans (Figure 4C).
Figure 3:
Dose distribution of the four mode plans in a patient with locally advanced lung cancer. Cranio-caudal dose penumbra can be reduced using dynamic jaw mode (arrows).
Table III.
Treatment and dose-volume parameters, monitor units, and beam-on times of the four TomoDirect plans for patients with locally advanced lung cancer.
| Mean ± standard deviation | |||||||
|---|---|---|---|---|---|---|---|
| 2.5-cm Dynamic | 2.5-cm Fixed | p* | 5.0-cm Dynamic | 5.0-cm Fixed | p** | p*** | |
| Patient number | 25 | ||||||
| Total PTV (ml) | 338.7 ± 179.0 | ||||||
| Pitch | 0.251 | 0.500 | |||||
| Modulation factor | 2.0 | ||||||
| Conformity index | 12.5 ± 13.0 | 13.3 ± 13.8 | 0.002‡ | 11.3 ± 10.4 | 13.3 ± 12.3 | 0.77‡ | 0.08† |
| Uniformity index | 1.09 ± 0.04 | 1.10 ± 0.05 | 0.003|| | 1.10 ± 0.05 | 1.11 ± 0.05 | 0.0001|| | 0.08§ |
| Monitor unit | 3509 ± 601 | 3417 ± 596 | <0.0001‡ | 1912 ± 294 | 1806 ± 292 | <0.0001‡ | <0.0001† |
| Beam-on time (sec) | 287.9 ± 42.1 | 281.3 ± 41.9 | <0.0001‡ | 175.7 ± 20.6 | 168.3 ± 20.4 | <0.0001‡ | <0.0001† |
| Body | |||||||
| V5 Gy (ml) | 4848.5 ± 1337.5 | 5197.4 ± 1402.4 | <0.0001|| | 5220.2 ± 1442.8 | 6153.9 ± 1599.0 | <0.0001|| | <0.0001§ |
| V10 Gy (ml) | 4287.4 ± 1175.3 | 4602.2 ± 1241.8 | <0.0001|| | 4628.7 ± 1282.5 | 5418.1 ± 1433.5 | <0.0001|| | <0.0001§ |
| V20 Gy (ml) | 3737.7 ± 1046.8 | 3929.5 ± 1089.1 | <0.0001|| | 3999.5 ± 1134.9 | 4500.7 ± 1247.8 | <0.0001|| | <0.0001§ |
| V30 Gy (ml) | 3282.8 ± 933.6 | 3405.4 ± 972.5 | <0.0001|| | 3488.7 ± 1000.2 | 3762.4 ± 1074.1 | <0.0001|| | <0.0001§ |
| Spinal cord maximum (Gy) | 43.3 ± 2.2 | 43.5 ± 2.4 | 0.11|| | 43.1 ± 2.2 | 43.1 ± 2.0 | 0.94|| | 0.09§ |
| Esophagus maximum (Gy) | 59.4 ± 2.2 | 59.0 ± 2.4 | 0.05|| | 58.2 ± 2.7 | 58.3 ± 2.8 | 0.27|| | <0.0001§ |
| Heart V40 Gy (%) | 7.9 ± 7.7 | 8.3 ± 7.7 | 0.01|| | 10.4 ± 9.6 | 11.2 ± 9.6 | 0.02|| | <0.0001§ |
Abbreviation: PTV: Planning target volume.
*p-value between 2.5-cm dynamic- and 2.5-cm fixed-jaw plans.
**p-value between 5.0-cm dynamic- and 5.0-cm fixed-jaw plans.
***p-value among the four plans.
†Calculated by Friedman analysis of variance.
‡Calculated by Wilcoxon signed-rank test.
§Calculated by parametric analysis of variance for depended samples.
||Calculated by paired t-test.
Table IV.
Dose-volume parameters of the four TomoDirect plans for patients with locally advanced lung cancer.
| Mean ± standard deviation | |||||||
|---|---|---|---|---|---|---|---|
| 2.5-cm Dynamic | 2.5-cm Fixed | p* | 5.0-cm Dynamic | 5.0-cm Fixed | p** | p*** | |
| Total lung | |||||||
| V5 Gy (%) | 37.3 ± 11.0 | 38.7 ± 11.4 | <0.0001|| | 40.4 ± 11.7 | 44.0 ± 12.4 | <0.0001|| | <0.0001§ |
| V10 Gy (%) | 31.1 ± 9.6 | 32.3 ± 10.0 | <0.0001|| | 34.4 ± 10.6 | 37.1 ± 11.2 | <0.0001|| | <0.0001§ |
| V20 Gy (%) | 25.0 ± 7.9 | 25.5 ± 8.4 | 0.007|| | 27.5 ± 8.9 | 29.2 ± 9.5 | <0.0001|| | <0.0001§ |
| V30 Gy (%) | 20.7 ± 6.6 | 21.0 ± 7.0 | 0.09|| | 22.7 ± 7.4 | 23.3 ± 7.7 | <0.0001|| | <0.0001§ |
| V40 Gy (%) | 17.2 ± 6.2 | 16.8 ± 5.8 | 0.40|| | 18.1 ± 6.1 | 18.1 ± 6.2 | 0.82|| | <0.0001§ |
| V50 Gy (%) | 12.7 ± 5.3 | 12.3 ± 4.2 | 0.43|| | 12.8 ± 4.6 | 12.5 ± 4.6 | 0.0006|| | 0.53§ |
| MLD (Gy) | 13.7 ± 4.0 | 14.1 ± 4.1 | <0.0001|| | 14.8 ± 4.4 | 15.6 ± 4.5 | <0.0001|| | <0.0001§ |
| Ispilateral lung | |||||||
| V5 Gy (%) | 53.5 ± 19.4 | 55.5 ± 20.0 | <0.0001|| | 56.8 ± 19.7 | 62.7 ± 20.7 | <0.0001|| | <0.0001§ |
| V10 Gy (%) | 46.3 ± 17.4 | 48.2 ± 18.1 | <0.0001|| | 50.0 ± 18.1 | 55.2 ± 19.6 | <0.0001|| | <0.0001§ |
| V20 Gy (%) | 38.4 ± 14.7 | 39.6 ± 15.1 | <0.0001|| | 42.4 ± 16.0 | 45.3 ± 17.1 | <0.0001|| | <0.0001§ |
| V30 Gy (%) | 32.6 ± 12.9 | 33.3 ± 13.1 | 0.0002|| | 35.9 ± 14.1 | 37.0 ± 14.4 | <0.0001|| | <0.0001§ |
| V40 Gy (%) | 26.9 ± 11.2 | 27.3 ± 11.3 | 0.03|| | 29.3 ± 12.2 | 28.8 ± 2.2 | 0.22|| | 0.009§ |
| V50 Gy (%) | 20.6 ± 9.1 | 21.0 ± 9.4 | 0.13|| | 21.7 ± 9.8 | 21.1 ± 9.3 | 0.003|| | 0.003§ |
| Contralateral lung | |||||||
| V5 Gy (%) | 23.4 ± 9.9 | 24.1 ± 10.3 | <0.0001|| | 27.0 ± 11.9 | 28.7 ± 13.0 | 0.0005|| | <0.0001§ |
| V10 Gy (%) | 18.1 ± 8.1 | 18.8 ± 8.4 | 0.0008|| | 21.1 ± 9.9 | 22.2 ± 10.9 | 0.002|| | <0.0001§ |
| V20 Gy (%) | 13.4 ± 6.8 | 13.6 ± 7.0 | 0.006|| | 15.3 ± 8.2 | 15.8 ± 8.7 | 0.007|| | <0.0001§ |
| V30 Gy (%) | 10.6 ± 6.0 | 10.6 ± 6.1 | 0.49|| | 11.7 ± 7.1 | 12.0 ± 7.3 | 0.02|| | 0.001§ |
| V40 Gy (%) | 8.2 ± 5.2 | 8.2 ± 5.2 | 0.41|| | 8.9 ± 5.9 | 9.0 ± 6.0 | 0.12|| | 0.03§ |
| V50 Gy (%) | 5.2 ± 3.9 | 5.2 ± 3.9 | 0.65|| | 5.6 ± 4.5 | 5.6 ± 4.4 | 0.66|| | 0.29§ |
*p-value between 2.5-cm dynamic- and 2.5-cm fixed-jaw plans.
**p-value between 5.0-cm dynamic- and 5.0-cm fixed-jaw plans.
***p-value among the four plans.
§Calculated by parametric analysis of variance for depended samples.
||Calculated by paired t-test.
Figure 4:
V5 Gy of the total lung (A), V5 Gy of the whole body (B), and beam-on time (C) in each mode for patients with locally advanced lung cancer. The box includes the central 50% of data (25-75%), and the central 99% of data are contained within the error bars. The solid line within each box indicates the median of the data. V5 Gy refers to the percentage or absolute volume receiving 5 Gy, 2.5D refers to 2.5-cm dynamic-jaw mode, and 2.5F refers to 2.5-cm fixed-jaw mode, 5.0D refers to 5.0-cm dynamic-jaw mode, and 5.0F refers to 5.0-cm fixed-jaw mode. $p < 0.01.
Discussion
It is well known that V20 Gy of the lung and the MLD are predictors of pulmonary complications (21–23). In addition, low dose irradiation to the lung as expressed by V5 Gy or V10 Gy has also been reported to be a risk factor for pulmonary toxicity in IMRT of lung cancer, especially when combined with chemotherapy (3, 4). This study suggested that low dose irradiation to the lung could be reduced by using the dynamic-jaw mode in tomotherapy for lung cancer.
For the patients with stage I lung cancer, although the beam-on time was relatively long when the dynamic-jaw mode was used, dose-volume parameters of plans using the dynamic-jaw mode were better than those using the fixed-jaw mode. The 2.5-cm dynamic-jaw mode appeared to be the best among these four modes in terms of dose distribution. It is notable that those parameters including the integral dose in the 5.0-cm dynamic-jaw mode were superior to those in the 2.5-cm conventional fixed jaw mode while reducing the beam-on time by 21%. Thus, 5.0-cm dynamic-jaw mode would be suitable for patients who have difficulty in lying immobile during a longer treatment time.
For the patients with locally advanced lung cancer, dose-volume parameters of plans using the dynamic-jaw mode were generally better than those using the fixed-jaw mode, and the differences in beam-on time were small between the plans; therefore, the dynamic-jaw mode is generally superior to the conventional fixed-jaw mode for the treatment of locally advanced lung cancer. The 2.5-cm dynamic-jaw mode appeared to be the best among these four modes in terms of dose distribution. The 5.0-cm dynamic-jaw mode required shorter treatment time while maintaining dose distribution comparable to that obtained by the conventional 2.5-cm fixed-jaw mode. Thus, this mode might be alternatively used for patients for whom a shorter treatment time is desirable as well as for patients with stage I lung cancer.
In this planning comparison study, CT simulation data without immobilization devices was used for the patients with locally advanced lung cancer. However, immobilization devices should be used in clinical practice when using IMRT technique (24).
A few planning comparison studies using the dynamic-jaw and dynamic-couch modes have been reported for tumors other than lung cancer (6–8). This mode offers dynamic jaw alignment throughout the treatment and dynamic couch speed, but it is not available for clinical application yet. The previous studies only employed the TomoHelical mode. With these new options of tomotherapy, beam-on time could be reduced by 66% compared to the conventional 2.5-cm fixed-jaw mode (from 595 to 199 seconds) in the treatment of nasopharyngeal cancer without significant difference in dose distribution (6). The current study showed that the dynamic-jaw mode is useful when used with both TomoHelical and TomoDirect modes. In the present study, the 5.0-cm dynamic-jaw mode could reduce beam-on time by 21-39% in the treatment of lung cancer. Adding the dynamic couch mode may achieve a shorter treatment time, but the characteristics of dose distribution including those in the lung and the heart are unclear for the lung target volume. Further investigation is warranted.
Conclusion
The dynamic-jaw mode should be used instead of the conventional fixed-jaw mode in tomotherapy for lung cancer. The 2.5-cm dynamic-jaw mode would be the best in terms of dose distribution. The 5.0-cm dynamic-jaw mode might be an alternative to reduce beam-on time.
Acknowledgments
This study was carried out by an offered mechanical option of tomotherapy from Accuray, Japan without any financial support.
Footnotes
Conflict of Interest: All authors certify that this manuscript has not been published in whole or in part nor it is being considered for publication elsewhere. The authors have no conflicts of interest to declare.
References
- 1.Mackie TR. History of tomotherapy. Phys Med Biol 51, R427–453 (2006). DOI: 10.1088/0031-9155/51/13/R24 [DOI] [PubMed] [Google Scholar]
- 2.Mackie TR, Holmes T, Swerdloff S, Reckwerdt P, Deasy JO, Yang J, Paliwal B, Kinsella T. Tomotherapy: a new concept for the delivery of dynamic conformal radiotherapy. Mel Phys 20, 1709–1719 (1993). [DOI] [PubMed] [Google Scholar]
- 3.Song CH, Pyo H, Moon SH, Kim TH, Kim DW, Cho KH. Treatment-related pneumonitis and acute esophagitis in non-smallcell lung cancer patients treated with chemotherapy and helical tomotherapy. Int J Radiat Oncol Biol Phys 78, 651–658 (2010). DOI: 10.1016/j.ijrobp.2009.08.068 [DOI] [PubMed] [Google Scholar]
- 4.Kim Y, Hong SE, Kong M, Choi J. Predictive factors for radiation pneumonitis in lung cancer treated with helical tomotherapy. Cancer Res Treat 45, 295–302 (2013). DOI: 10.4143/crt.2013.45.4.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murai T, Shibamoto Y, Manabe Y, Murata R, Sugie C, Hayashi A, Ito H, Miyoshi Y. Intensity-modulated radiation therapy using static ports of tomotherapy (TomoDirect): comparison with the TomoHelical mode. Radiat Oncol 8, 68 (2013). DOI: 10.1186/1748-717X-8-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sterzing F, Uhl M, Hauswald H, Schubert K, Sroka-Perez G, Chen Y, Lu W, Mackie R, Debus J, Herfarth K, Oliveira G. Dynamic jaws and dynamic couch in helical tomotherapy. Int J Radiat Oncol Biol Phys 76, 1266–1273 (2010). DOI: 10.1016/j.ijrobp.2009.07.1686 [DOI] [PubMed] [Google Scholar]
- 7.Krause S, Beck S, Schubert K, Lissner S, Hui S, Herfarth K, Debus J, Sterzing F. Accelerated large volume irradiation with dynamic jaw/dynamic couch helical tomotherapy. Radiat Oncol 7, 191 (2012). DOI: 10.1186/1748-717X-7-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krause S, Beck S, Schramm O, Schubert K, Hauswald H, Zabel-du Bois A, Herfarth K, Debus J, Sterzing F. Tomotherapy radiosurgery for arteriovenous malformations—current possibilities and future options with helical tomotherapy dynamic jaws? Technol Cancer Res Treat 12, 421–428 (2013). DOI: 10.7785/tcrt.2012.500335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiandra C, Filippi AR, Catuzzo P, Botticella A, Ciammella P, Franco P, Borca VC, Ragona R, Tofani S, Ricardi U. Different IMRT solutions vs. 3D-conformal radiotherapy in early stage Hodgkin’s lymphoma: dosimetric comparison and clinical considerations. Radiat Oncol 7, 186 (2012). DOI: 10.1186/1748-717X-7-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piotrowski T, Czajka E, Bak B, Kazmierska J, Skorska M, Ryczkowski A, Adamczyk M, Jodda A. Tomotherapy: implications on daily workload and scheduling patients based on three years’ institutional experience. Technol Cancer Res Ttreat 13, 233–242 (2014). DOI: 10.7785/tcrt.2012.500374 [DOI] [PubMed] [Google Scholar]
- 11.Aibe N, Yamazaki H, Nakamura S, Tsubokura T, Kobayashi K, Kodani N, Nishimura T, Okabe H, Yamada K. Outcome and toxicity of stereotactic body radiotherapy with helical tomotherapy for inoperable lung tumor: analysis of Grade 5 radiation pneumonitis. J Radiat Res 55, 575–582 (2014). DOI: 10.1093/jrr/rrt146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugie C, Shibamoto Y, Ayakawa S, Mimura M, Komai K, Ishii M, Miyamoto A, Oda K. Craniospinal irradiation using helical tomotherapy: evaluation of acute toxicity and dose distribution. Technol Cancer Res Treat 10, 187–195 (2011). DOI: 10.7785/tcrt.2012.500194 [DOI] [PubMed] [Google Scholar]
- 13.Franco P, Zeverino M, Migliaccio F, Sciacero P, Cante D, Casanova Borca V, Torielli P, Arrichiello C, Girelli G, Numico G, La Porta MR, Tofani S, Ricardi U. Intensity-modulated adjuvant whole breast radiation delivered with static angle tomotherapy (TomoDirect): a prospective case series. J Cancer Res Clin Oncol 139, 1927–1936 (2013). DOI: 10.1007/s00432-013-1515-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franco P, Zeverino M, Migliaccio F, Cante D, Sciacero P, Casanova Borca V, Torielli P, Arrichiello C, Girelli G, La Porta MR, Tofani S, Numico G, Ricardi U. Intensity-modulated and hypofractionated simultaneous integrated boost adjuvant breast radiation employing statics ports of tomotherapy (TomoDirect): a prospective phase II trial. J Cancer Res Clin Oncol 140, 167–177 (2014). DOI: 10.1007/s00432-013-1560-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baba F, Shibamoto Y, Tomita N, Ikeya-Hashizume C, Oda K, Ayakawa S, Ogino H, Sugie C. Stereotactic body radiotherapy for stage I lung cancer and small lung metastasis: evaluation of an immobilization system for suppression of respiratory tumor movement and preliminary results. Radiat Oncol 4, 15 (2009). DOI: 10.1186/1748-717X-4-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibamoto Y, Hashizume C, Baba F, Ayakawa S, Manabe Y, Nagai A, Miyakawa A, Murai T, Iwata H, Mori Y, Mimura M, Ishikura S. Stereotactic body radiotherapy using a radiobiology-based regimen for stage I nonsmall cell lung cancer: a multicenter study. Cancer 118, 2078–2084 (2012). DOI: 10.1002/cncr.26470 [DOI] [PubMed] [Google Scholar]
- 17.Shibamoto Y, Naruse A, Fukuma H, Ayakawa S, Sugie C, Tomita N. Influence of contrast materials on dose calculation in radiotherapy planning using computed tomography for tumors at various anatomical regions: a prospective study. Radiother Oncol 84, 52–55 (2007). DOI: 10.1016/j.radonc.2007.05.015 [DOI] [PubMed] [Google Scholar]
- 18.Hodapp N. The ICRU report 83: prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT). Strahlenther Onkol 188, 97–99 (2012). DOI: 10.1007/s00066-011-0015-x [DOI] [PubMed] [Google Scholar]
- 19.Paddick I.A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg 93(Suppl. 3), 219–222 (2000). DOI: 10.3171/jns.2000.93.supplement 3.0219 [DOI] [PubMed] [Google Scholar]
- 20.R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: (2010). http://www.R-project.org/ 16 March 2014, last accessed. [Google Scholar]
- 21.Graham MV, Purdy JA, Emami B, Harms W, Bosch W, Lockett MA, Perez CA. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 45, 323–329 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Tucker SL, Jin H, Wei X, Wang S, Martel MK, Komaki R, Liu HH, Mohan R, Chen Y, Cox JD, Liao Z. Impact of toxicity grade and scoring system on the relationship between mean lung dose and risk of radiation pneumonitis in a large cohort of patients with non-small cell lung cancer. Int J Radiat Oncol Biol Phys 77, 691–698 (2010). DOI: 10.1016/j.ijrobp.2009.05.055 [DOI] [PubMed] [Google Scholar]
- 23.Piotrowski T, Matecka-Nowak M, Milecki P. Prediction of radiation pneumonitis: dose-volume histogram analysis in 62 patients with non-small cell lung cancer after three-dimensional conformal radiotherapy. Neoplasma 52, 56–62 (2005). [PubMed] [Google Scholar]
- 24.Holmes T, Das R, Low D, Yin FF, Balter J, Palta J, Eifel P. American Society of Radiation Oncology recommendations for documenting intensity-modulated radiation therapy treatments. Int J Radiat Oncol Biol Phys 74, 1311–1318 (2009). DOI: 10.1016/j.ijrobp.2009.04.037 [DOI] [PubMed] [Google Scholar]