Abstract
Many stroke survivors with severe impairment can grasp only with a power grip. Yet, little knowledge is available on altered power grip after stroke, other than reduced power grip strength. This study characterized stroke survivors’ static power grip during 100% and 50% maximum grip. Each phalanx force’s angular deviation from the normal direction and its contribution to total normal force was compared for 11 stroke survivors and 11 age-matched controls. Muscle activities and skin coefficient of friction (COF) were additionally compared for another 20 stroke and 13 age-matched control subjects. The main finding was that stroke survivors gripped with a 34% greater phalanx force angular deviation of 19±2° compared to controls of 14±1° (p<.05). Stroke survivors’ phalanx force angular deviation was closer to the 23° threshold of slippage between the phalanx and grip surface, which may explain increased likelihood of object dropping in stroke survivors. In addition, this altered phalanx force direction decreases normal grip force by tilting the force vector, indicating a partial role of phalanx force angular deviation in reduced grip strength post stroke. Greater phalanx force angular deviation may biomechanically result from more severe underactivation of stroke survivors’ first dorsal interosseous (FDI) and extensor digitorum communis (EDC) muscles compared to their flexor digitorum superficialis (FDS) or somatosensory deficit. While stroke survivors’ maximum power grip strength was approximately half of the controls’, the distribution of their remaining strength over the fingers and phalanges did not differ, indicating evenly distributed grip force reduction over the entire hand.
Keywords: Stroke, Power Grip, Hand, Grip Effort, Muscle Activation
1 Introduction
Currently more than 7 million stroke survivors reside in the United States of America (Roger et al. 2012). Many of these stroke survivors suffer from impaired motor function in their hands and arms (Parker et al. 1986; Gray et al. 1990; Nakayama et al. 1994). Loss of hand function leads to dependency on others to complete both simple and complex daily living activities. Many studies examined how pinch grip control is altered after stroke (Hermsdorfer et al. 2003; Nowak et al. 2003; McDonnell et al. 2006). However, many stroke survivors suffering from severe impairment can grasp only with a power grip, and cannot perform a pinch grip due to impaired finger individuation (Gowland et al. 1995; Lang and Schieber 2004b). Yet, currently little knowledge is available on altered power grip after stroke, other than a reduced power grip strength (Boissy et al. 1999).
Power grip characteristics, such as phalanx force direction and force distribution over the hand, may differ post stroke. Biomechanics studies have shown that not only the action of the long finger flexor muscles but also the action of the extensor muscles and intrinsic hand muscles are important for controlling the force direction and distribution (Li et al. 2000; Valero-Cuevas et al. 2000). Altered neurological activation of the muscles controlling the hand has previously been observed for stroke survivors leading to altered muscle activation patterns with under-activated intrinsic and extensor muscles and hyperactive long flexor muscles (Kamper and Rymer 2001; Kamper et al. 2003; Lang and Schieber 2004b; Cruz et al. 2005). These altered muscle activation patterns may disrupt the delicate balance among multiple hand muscles necessary for force directional control or natural force distribution during power grip (Li et al. 2001; Kutch and Valero-Cuevas 2011), leading to reduced phalanx force control. Alternatively, changes in skin frictional properties, if there are any after stroke potentially due to reduced limb use or altered autonomic nervous system function (Harms et al. 2011), could affect the slipperiness of the finger skin against the grip surface and modify grip force control as it did for aging adults (Cole 1991). Because altered grip post stroke could be biomechanically explained by an altered activation pattern of the muscles controlling the fingers or could be explained by altered physiological skin properties affecting the skin friction, an additional study was conducted to determine if altered muscle activation patterns or skin coefficient of friction accompanied stroke survivors’ power grip.
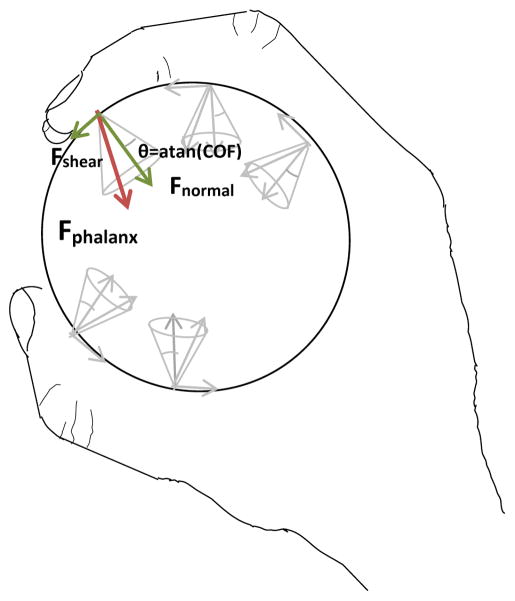
These stroke related changes could affect power grip characteristics such as phalanx force direction and force distribution over the hand, which can lead to the decreased object stability and object dropping that is frequently observed in persons with impaired hand function (Pazzaglia et al. 2010). Stable grip requires that phalanx force not deviate from the direction normal to a gripped object’s surface by more than an angle defined as the ‘cone of friction’ (Figure 1), which is calculated as the arctangent of the coefficient of friction (COF) between finger skin and the object’s surface (MacKenzie and Iberall 1994). Phalanx force direction outside the cone of friction leads to finger slippage, which has been observed in stroke survivors during pinch grip (Seo et al. 2010). In addition, deviation from the typical grip force distribution of the highest force concentration on the distal phalanx directed toward the palm (Amis 1987; Kong and Lowe 2005; Lee et al. 2009) could result in reduced grip force (Seo et al. 2007), object rotation out of the hand (MacKenzie and Iberall 1994; Kinoshita et al. 1997; Latash et al. 2002), and discomfort (Gurram et al. 1993).
Figure 1.
Stable grip without slippage requires that each phalanx’s force not deviate from the direction normal to the object surface more than the cone of friction angle (θ), determined as arctangent of the coefficient of friction (COF) between the hand and grasped object (MacKenzie and Iberall 1994). Phalanx force deviations outside the cone of friction can lead to hand-object slippage.
Despite these important functional implications for grip stability and strength, knowledge is sparse on the extent of altered phalanx force direction and altered force distribution across the fingers and phalanges during power grip post stroke. This knowledge gap is perhaps due to a lack of proper equipment. A recent development of an instrumented cylinder that has the capacity to measure not only normal force but also shear force from each phalanx and finger independently (Enders and Seo 2011) enables quantitative characterization of phalanx force direction and distribution during power grip post stroke. This new information on post-stroke power grip characteristics can provide greater insight into stroke survivors’ grip abnormality, especially the extent that altered phalanx force direction and distribution account for reduced whole-hand grip stability and strength.
The goal of this study was to characterize the altered power grip for people with stroke as compared with age-matched neurologically-intact (control) persons. The first experiment investigated the extent to which stroke survivors’ phalanx forces deviated from the normal direction and the distribution of normal forces across the phalanges and fingers compared to controls during static power grip at 100% and 50% maximum perceived effort. In addition, the ability to approximate 50% of the maximum power grip force was examined to gauge potential role of somatosensation in altered power grip post stroke. Upon observing greater phalanx force angular deviation post stroke, the second experiment was performed to examine potential mechanisms for altered phalanx force direction by comparing hand muscle activity between stroke survivors and healthy controls. In addition, the COF between the finger skin and grip surface was measured to determine any decrease in skin slipperiness that might allow greater phalanx force angular deviation after stroke.
2 Materials and Methods
2.1 Subjects
Eleven chronic stroke survivors (mean age ± standard deviation (SD) = 64 ± 11 years) and 11 age matched neurologically intact control subjects (65 ± 10 years) participated in Experiment 1. Twenty chronic stroke survivors (mean age ± SD = 59 ± 11 years) and 13 age- matched control subjects (57 ± 8 years) participated in Experiment 2. Roughly half of the participants were females for each Experiment. Six of the subjects returned from Experiment 1 to participate in Experiment 2. All stroke survivors had time since stroke greater than 6 months. The mean motor impairment for the stroke survivors in both Experiment 1 and 2 was Stage 5 ± 2 out of the maximum score of 7 on the Chedoke-McMaster Stroke Assessment Hand Section (Gowland et al. 1995). For the hand and wrist subdivision of the Fugl-Meyer Assessment (Fugl-Meyer et al. 1975), the mean motor impairment was 20 ± 6 out of the maximum score of 24 for the stroke survivors in Experiment 1, and 19 ±5 in Experiment 2. All subjects signed a consent form and followed a protocol approved by the Institutional Review Board.
2.2 Procedure and Analysis
Experiment 1 quantified phalanx force direction and distribution during 100% and 50% maximum power grip for stroke survivors as compared to controls. Subjects sat in a chair with the elbow flexed at approximately 90° and the forearm horizontally rested on an arm rest. Subjects performed power grip at 100% and 50% of their maximum perceived effort on a custom-made grip dynamometer (Enders and Seo 2011) for at least five seconds, while individual phalanges’ normal force and shear force in the proximal-distal direction were recorded at 1000 Hz for one finger at a time. For each phalanx, normal and shear forces were measured using two sets of four strain gauges in a Wheatstone bridge configuration instrumented on a beam inside the custom-made grip dynamometer (Enders and Seo 2011). Two grip efforts were examined to facilitate comparison with previous literature using maximum grip (Radhakrishnan and Nagaravindra 1993) and to include submaximal grip for its relevance to daily activities. No visual feedback was provided to the subjects during gripping. Subjects were instructed to grip in a consistent manner regardless of the finger being measured. Subjects’ distal, middle, and proximal phalanges of the finger were aligned with the three measuring pads on the grip dynamometer during power grip. If subjects were unable to correctly align the fingers themselves, the experimenter assisted the subject by aligning their finger with the three pads. Stroke subjects’ paretic hand and control subjects’ non-dominant hand were used because this hand often acts as the “stabilizing hand” to hold objects while opening containers or performing finer manipulation with the non-paretic or dominant hand (Sainburg 2005; Wang and Sainburg 2007). In Experiment 1, for four of the eleven stroke survivors, the paretic hand was considered the non-dominant hand prior to the stroke event. The entire surface of the grip dynamometer was covered with a paper surface. Measurements of phalanx normal and shear forces for all five fingers at the two effort levels were repeated three times each to obtain averages.
For Experiment 1 data analysis, phalanx force direction and normal grip force distribution were determined during a two-second static grip period with the highest grip force within each trial. The phalanx force angular deviation for each phalanx of each finger was quantified as the absolute arctangent of the ratio of mean shear force to mean normal force of that phalanx during the static grip period. A deviation of 0° indicates that phalanx force was in the normal direction, perpendicular to the grip surface with no shear force. Phalanx force deviation of either distal or proximal direction was noted separately. For the normal grip force distribution across the phalanges and fingers, the percentage contribution for each of the 14 individual phalanges of the hand to the total normal force was calculated during the same static grip period. The accuracy of approximating 50% of the maximum grip was examined using the ratio of the sum of each phalanx’s resultant forces during the 50% maximum grip to that during the 100% maximum grip. Resultant force was used in this analysis since subjects may use feedback from both the normal and shear forces to approximate 50% of their maximum grip force.
After finding phalanx force deviation significantly increased for stroke survivors, a second experiment was carried out to determine if altered deviation was the result of altered neurological control of the finger muscles or due to a potential change in skin friction. Experiment 2 was performed in the same setting, except that electromyography (EMG) from hand muscles was additionally recorded. EMG data from two extrinsic hand muscles, the extensor digitorum communis (EDC) and flexor digitorum superficialis (FDS), and one intrinsic muscle, the first dorsal interosseous (FDI), were recorded at 1000 Hz (Bortec Biomedical Ltd., Calgary, AZ). The EDC and FDS muscles were investigated to sample the extrinsic muscles, and the FDI was investigated to sample the intrinsic muscles, similar to previous studies (Kamper et al. 2003), to determine if altered phalanx direction was due to specific muscle group-specific underactivation post stroke. Muscle activity during Experiment 2 was measured from stroke survivors’ paretic limb and the controls’ non-dominant hand. For Experiment 2, for nine of the twenty stroke survivors, the paretic hand was considered the non-dominant hand prior to the stroke event.
Skin was cleaned with alcohol swabs to reduce impedance before the bipolar surface electrodes were placed on the muscle bellies according to the literature (Basmajian 1989). The maximum voluntary contraction (MVC) EMG level was also recorded twice for each muscle by performing maximum voluntary contractions against resistance. For the EMG analysis, the root mean square (RMS) EMG with a 20-ms moving window was calculated for the two second static grip period with the highest grip force. These mean RMS values were further normalized by the RMS MVC for each muscle (%MVC). To examine altered muscle activation patterns post stroke, the relative FDI and EDC muscle activities in relation to the FDS muscle activity (calculated as the ratio of FDI to FDS EMG and that of EDC to FDS EMG in %MVC) were compared between stroke survivors and controls.
To compare the skin slipperiness between stroke survivors and healthy controls, the COF between the subjects’ finger skin and the paper surface was measured during a series of finger drag tests. The subject’s index finger tip was placed on a force transducer covered in paper and the experimenter applied 2N of normal force down on the finger tip, guided by a visual feedback display. Then force was increased in the shear direction until the finger slipped. The shear to normal force ratio at the point of the slip then determined the COF for that subject’s finger skin and the paper surface. COF was measured twice for each subject.
2.4 Statistical Analysis
For Experiment 1 results, two separate mixed-design Analysis of Variance tests (ANOVAs) were used to examine if the phalanx force angular deviation and normal force distribution varied significantly for the subject group, effort level, phalanx, finger, and interactions between the subject group and the effort level, the subject group and the phalanx, and the subject group and the finger. Once a significant subject group effect was found on the phalanx force angular deviation, Pearson correlation was performed to appreciate the relationship between the motor impairment levels (both in the Chedoke-McMaster Stroke Assessment Hand Section and the hand and wrist subdivision of the Fugl-Meyer Assessment) and the mean phalanx force deviation of stroke survivors. For the ability to estimate 50% of maximum grip force, one-sample t-tests determined if the ratio of grip force during 50% perceived grip to that during maximum grip was significantly different from 50% for each subject group.
For Experiment 2, a mixed-design ANOVA was used to determine if muscle activation, EMG (%MVC), significantly varied for subject group, effort level, muscle, and the interaction between group and effort and the interaction between group and muscle. To further investigate how muscle activation pattern is altered post stroke, another mixed-design ANOVA was used to examine if the relative FDI and EDC EMG (normalized to FDS EMG) varied significantly for the subject group, effort level, muscle, and interactions between the subject group and the effort level and between the subject group and the muscle. For the COF data, a two-sample t-test determined if the stroke survivors’ COF differed from healthy controls. To ensure normality, a square root transformation was applied to the phalanx force deviation, normal force distribution, and COF data and a log transformation was applied to the muscle activation pattern data to result in non-significant skewness (Tabachnick and Fidell 2007), and these transformed data were used for the ANOVAs and t-tests. A significance level of p-value<.05 was considered significant for all statistical analyses.
3. Results
The overall static power grip force profiles for stroke survivors and controls obtained from Experiment 1 are shown in Figure 2. The maximum total normal force for stroke survivors was 43% reduced compared to the control (154 N vs. 270 N). This extent of grip force reduction is comparable to the previous study (Boissy et al. 1999). The new finding of this study is that phalanx force angular deviation is substantially greater for the stroke survivors. Altered phalanx force direction post stroke was associated with an altered muscle activation pattern with more reduced FDI and EDC muscle activities compared to the FDS activity and larger force estimation error after stroke, but without any skin friction change. This increased phalanx force deviation was significantly correlated with lower hand motor function in stroke survivors. The distribution of the remaining force across the phalanges and fingers was unaltered in stroke survivors. Detailed results are described below.
Figure 2.
Mean phalanx force angular deviation, shown as the spread of the fan, was significantly greater for stroke survivors compared with healthy controls. Mean grip forces were substantially reduced for stroke survivors compared with healthy controls, as seen by the shorter fan height for stroke survivors.
3.1 Increased phalanx force angular deviation for stroke survivors
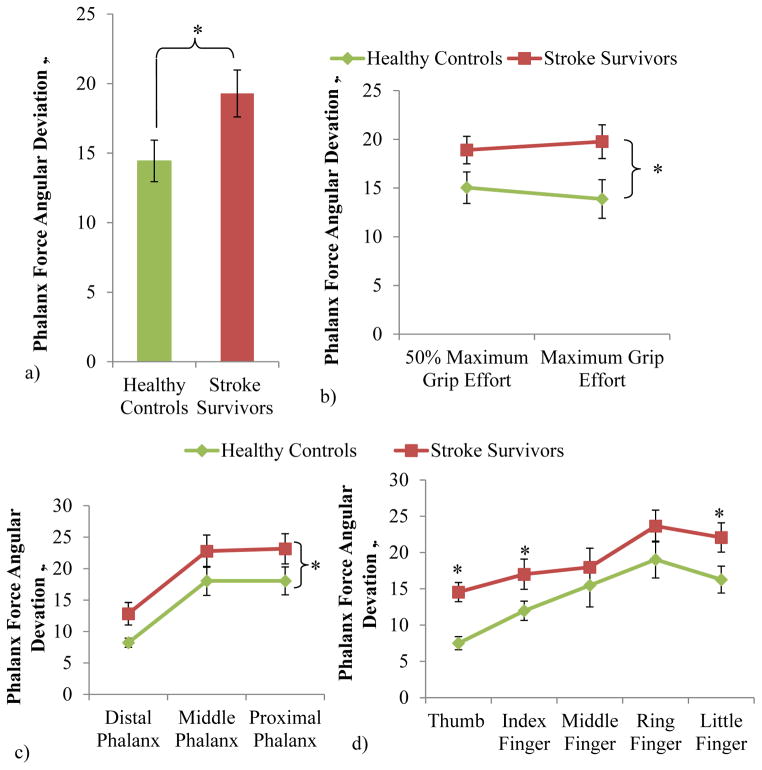
Stroke survivors gripped with 34% greater phalanx force deviation compared with controls on average (Figure 3a, ANOVA subject group main effect with p=.03). Phalanx force deviation was significantly dependent upon the subject group, phalanx, finger, and interaction between the subject group and the finger (p<.05). Stroke survivors’ phalanx force deviation was significantly greater than controls’ for both grip efforts and all phalanges (Figure 3b–c, ANOVA subject group main effect with p<.01 and non-significant interactions between subject group and effort and between subject group and phalanges with p>.05). The stroke survivors’ phalanx force deviation was significantly higher for the thumb, index, and little fingers, compared with the controls (Figure 3b, ANOVA subject group and finger interaction with p<.01, and post-hoc significance found for the three fingers with p<.05). The frequency of phalanx force being distally directed was 56% for the stroke subjects, which is comparable to 47% for the controls.
Figure 3.
Phalanx force angular deviation was significantly greater for stroke survivors compared with controls (ANOVA subject group main effect with p<.05) (effort levels, fingers, phalanges, and subjects pooled) (a), for both 50% and maximum grip effort (b), for all three phalanges (c), and especially for the thumb, index, and little fingers (ANOVA, subject group and finger interaction with p<.05, posthoc significance marked with stars) (d). Non-transformed mean ± SE data is shown in the figure.
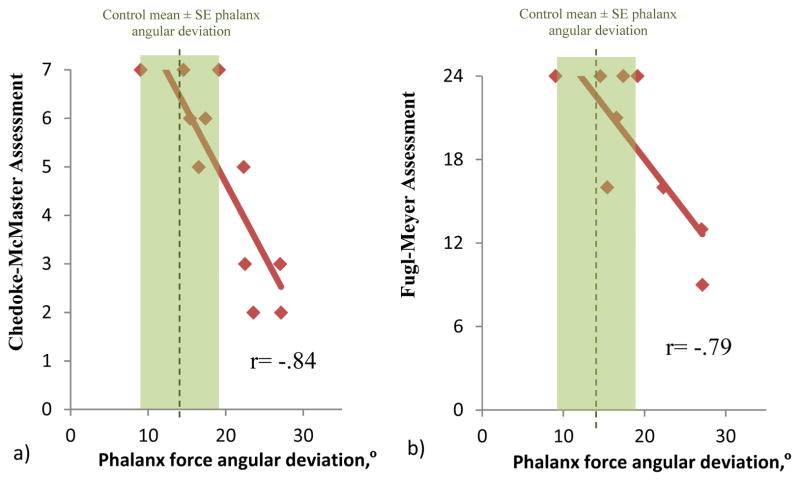
Increased phalanx force deviation was significantly and negatively correlated with motor impairment scores of the Chedoke-McMaster Assessment Hand Section (Figure 4a, Pearson Correlation, r = −.84 with p<.05). Stroke survivors’ increased phalanx force deviation was also significantly and negatively correlated with a lower motor function score on the hand and wrist subdivision of the Fugl-Meyer Assessment (Figure 4b, Pearson Correlation, r = −.79 with p<.05).
Figure 4.
Stroke survivors’ increased phalanx force deviation was significantly correlated with lower motor function scores of the Chedoke-McMaster Assessment Hand Section (Pearson Correlation, r = −.84 with p<.05) (a) and the hand and wrist subdivision of the Fugl-Meyer Assessment (Pearson Correlation, r = −.79 with p<.05) (b).
3.2 Similar grip force distribution
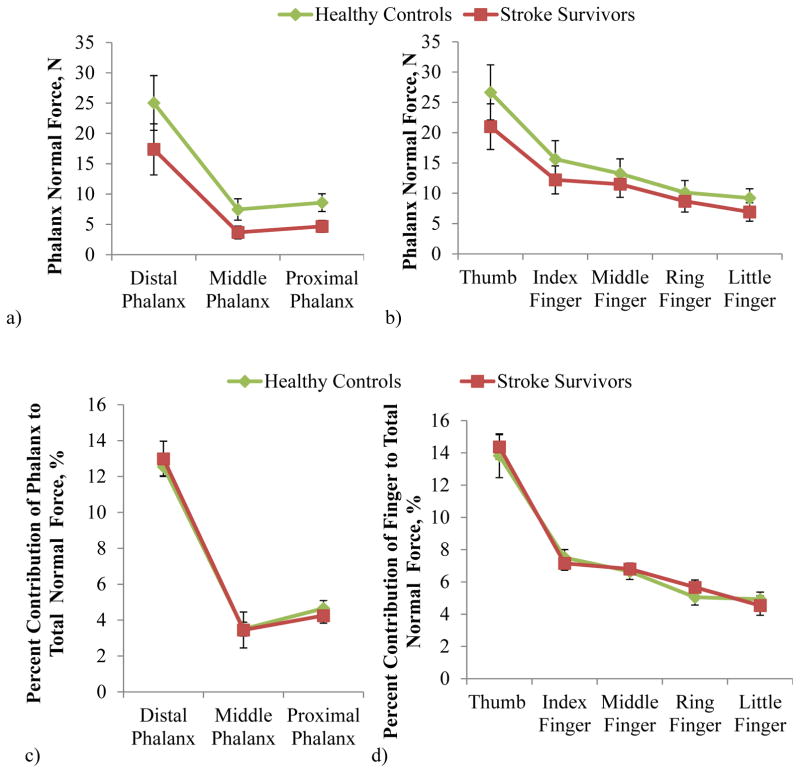
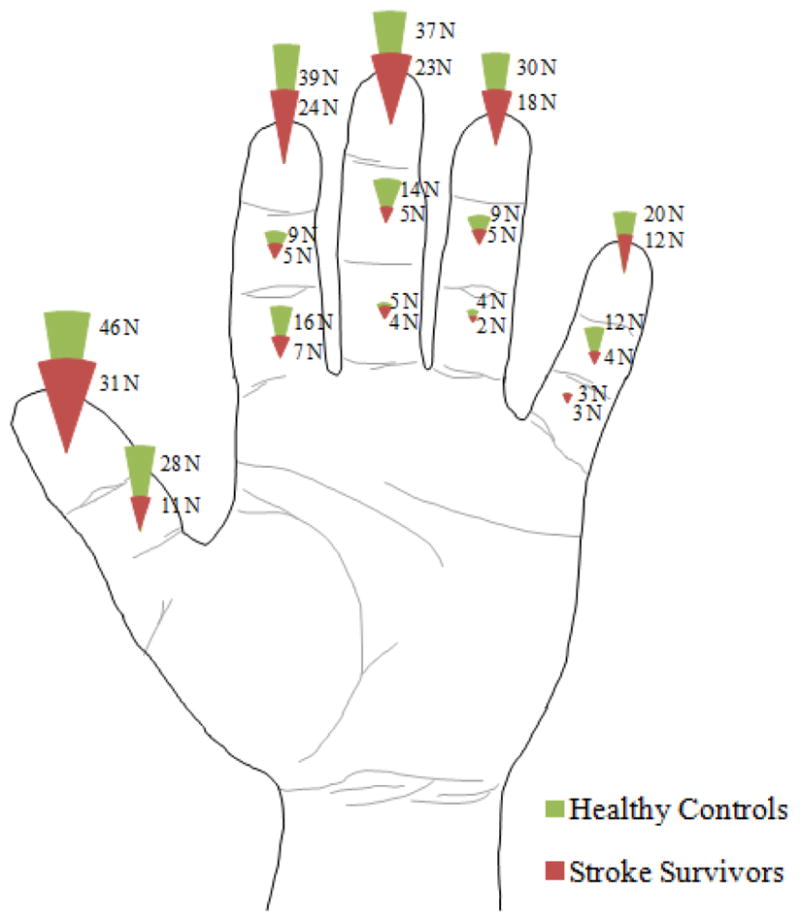
The distribution of normal force across the fingers and phalanges was similar between stroke and controls: They both gripped with the largest normal force produced by the distal phalanges (Figure 5a,c) and the thumb (Figure 5b,d), consistently with the previous study (Radhakrishnan and Nagaravindra 1993). The percent contribution of the phalanx normal force to the total normal force was significantly dependent upon the phalanx and the finger (ANOVA with p<.05), but not significantly dependent upon any other factor or interaction with the subject group (ANOVA with p>.05). Similar observations were made when resultant force magnitudes (instead of normal force) were examined for distribution across the fingers and phalanges, with no significant difference between the two subject groups.
Figure 5.
The distribution of phalanx normal force across the phalanges (a and c) and fingers (b and d) for stroke and control subjects. Percent contribution (c and d) of the individual phalanges to total normal force was not significantly dependent upon the interaction of subject group and phalanx or the interaction of subject group and finger (ANOVA with p>.05) (d). Non-transformed mean ± SE data is shown in the figure.
3.3 Overestimation of 50% grip for stroke survivors
During the 50% effort grip, the control subjects gripped with 46% ± 3% (mean ± SE) of their maximum grip force, which was not significantly different from 50% (t-test with p>.05). On the other hand, the stroke survivors gripped with, on average, 68% ± 11% of their maximum grip force, which was significantly different from 50% (t-test with p<.05).
3.4 Altered muscle activity pattern post stroke
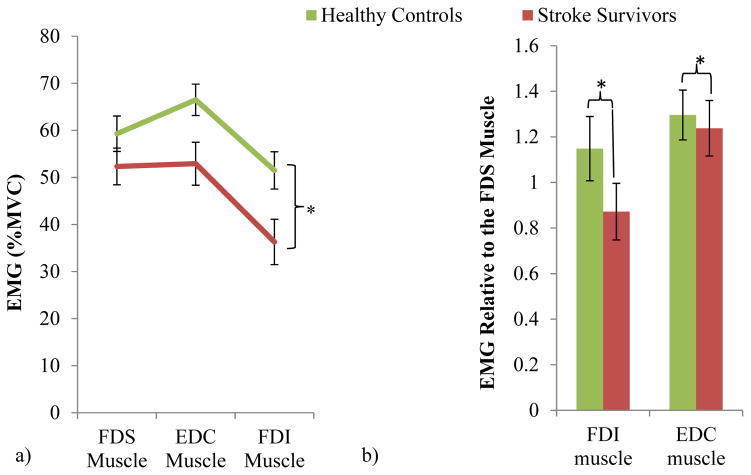
Each muscle’s activity (in %MVC) is shown for the stroke and control groups in Figure 6a. Muscle activity was significantly dependent upon subject group, effort level, muscle, and the interaction between group and muscle (ANOVA with p<.05). While the overall muscle activity was lower for stroke survivors compared with control (ANOVA subject group main effect p<.05), the reduction in muscle activity was more pronounced for the EDC and FDI muscles compared to the FDS muscle (ANOVA subject group and muscle interaction p<.05).”
Figure 6.
Mean ± SE EMG was significantly reduced for all muscles of the stroke survivors compared with healthy controls (a). Relative to the FDS EMG, mean ± SE FDI and EDC EMG were significantly reduced for stroke survivors compared with controls (significant subject group and finger muscle interaction with p<.05, significant difference in relative FDI and EDC EMG between stroke and control with Tukey post-hoc p<.05) (b), showing an altered muscle activity pattern with a particularly underactivated intrinsic FDI muscle and the extrinsic EDC muscle for stroke survivors compared with controls. Non-transformed data is shown in the figure.
This greater reduction in the FDI and EDC muscle activity than the FDS muscle for stroke survivors is apparent when the relative muscle activity is examined (Figure 6b). Compared to healthy controls, stroke survivors’ FDI and EDC activities relative to FDS muscle were significantly lower (ANOVA subject group main effect with p<.05 as well as Tukey posthoc p<.05 for stroke vs. control for both muscles). The under-activation was greater for the FDI than EDC muscle (ANOVA, muscle main effect and subject group and muscle interaction with p<.05). The interaction between group and effort was also found to be significant (p<.05) while the effort main effect was not (p>.05).
3.5 Similar skin COF
There was no significant difference in COF between the finger skin and the paper grip surface between the two subject groups (t-test with p>.05). The mean COF of the paper surface with the finger skin was 0.43 ± .03 (mean ± SE), similar to previous findings for healthy adults ranging from 0.3 (Buchholz et al. 1988) to 0.5 (Gee et al. 2005). This COF value means that the threshold for slippage (the maximum phalanx force deviation allowed before slippage, calculated as arctangent of the COF) for the grip surface used in this study was 23° for both stroke survivors and controls.
4. Discussion
4.1 Altered power grip force profile for stroke
Post-stroke power grip was characterized by 34% greater phalanx force angular deviation accompanied by reduced FDI and EDC muscle activities relative to FDS and inaccurate force estimation, compared to age-matched controls. The skin friction did not differ, indicating that this change in phalanx force direction is not mediated by skin friction change. This increased phalanx force deviation was significantly associated with lower motor function scores. Consistent with the previous study (Boissy et al. 1999), stroke survivors produced reduced maximum grip force compared with the age-matched controls. However, the distribution of the remaining grip force across the fingers and phalanges of the hand was similar to the controls.
4.2 Potential mechanism for altered power grip force profile following stroke – altered muscle activation pattern
One of the possible explanations for this greater phalanx force angular deviation during power grip after stroke is disruption in the coordinated force outputs across individual muscles for the hand. Both the FDI and EDC muscles were under-activated for stroke survivors, with greater underactivation observed for the FDI muscle. Grip requires coordination among all muscles for the hand to produce grip force toward the object, and disruption in the balance among individual muscles’ force outputs can directly alter the direction of phalanx forces (Valero-Cuevas 2000; Johanson et al. 2001). For instance, weakening of any single muscle can limit force production in a specific direction (Kutch and Valero-Cuevas 2011). Specifically, the intrinsic muscles, including the FDI muscle, are important for directional force control (Long et al. 1970; Valero-Cuevas et al. 2000; Milner and Dhaliwal 2002). Weakening of the FDI and other intrinsic muscles has been shown to increase digit force angular deviation from the normal direction based on a biomechanical model (Valero-Cuevas et al. 2000), and weakening of the intrinsic muscle group has been speculated to contribute to altered digit force deviation in older adults (Cole 2006). Therefore, the increased phalanx force angular deviation may be attributable to the observed altered muscle activation pattern with greater underactivation of the FDI and the EDC muscles, and potentially other intrinsic and extensor muscles, relative to the long finger flexor muscles after stroke. This specific pattern of altered muscle activation after stroke was observed in the past (Seo et al. 2010) and is thought to be mediated by a disinhibited reticulospinal tract resulting in the hyperexcited long finger flexor muscle (Zaaimi et al. 2012) relative to other muscles. In addition, the distal intrinsic muscles may require more corticospinal drive than more proximal muscles (Palmer and Ashby 1992; Turton and Lemon 1999), leaving them more vulnerable to reduced neural activation post-stroke. Furthermore, intrinsic muscles may suffer from disproportionately greater underactivation due to changes occurring within the muscles: intrinsic muscles are composed predominantly of Type II muscle fibers (Hwang et al. 2013), which have been shown to be particularly prone to atrophy post stroke (Dattola et al. 1993; Hu et al. 2007).
Although underactivation of the FDI and EDC muscles may have contributed to increased phalanx force deviation, the normal force distribution over the phalanges and fingers remained unchanged after stroke. This finding is consistent with the literature that describes the role of the intrinsic muscles as controlling the direction of finger force (Milner and Dhaliwal 2002) and the role of finger extensor muscles as stabilizing the finger joints (Chao et al. 1976), while the large long finger flexor muscles play the major role in force production in the normal direction (Li et al. 2000). As such, the trend with the major force concentrated on the distal phalanges powered by the extrinsic flexor muscles did not change after stroke in this study.
4.3 Potential mechanism for altered power grip force profile following stroke – posture
Another explanation for altered phalanx force direction for stroke survivors is a difference in finger posture with respect to the gripping surface compared to healthy controls. Although subjects’ finger alignment to the three measuring pads was controlled when the subjects’ hand was placed on the dynamometer initially for each trial, it is possible that the position and orientation of the phalanges shifted during power grip exertion. For instance, stroke subjects’ altered muscle activation could have caused a curling or rotation of the finger during the grip, affecting the direction of the force vector and causing an increase in the proximal-distal shear force. Although finger postures were not recorded in the present study to substantiate this possibility, the results of the present study show that the force vectors applied to the object differed for stroke survivors compared to healthy controls, which has functional implications for grip stability during daily activities involving power grip as discussed in the next section.
4.4 Potential mechanism for altered power grip force profile following stroke – somatosensory deficit
An alternative explanation for the greater phalanx force angular deviation during power grip after stroke is impaired somatosensation post stroke (Di Fabio and Badke 1991; Carey 1995; Turton and Butler 2001; Hermsdorfer et al. 2003; Niessen et al. 2008). Somatosensory feedback is critical in the control of digit force magnitudes and trajectories during gripping (Nowak et al. 2001; Zatsiorsky and Latash 2004). The diminished somatosensation often found for stroke survivors (Carey 1995; Turton and Butler 2001) has been shown to contribute to stroke survivors’ excessive force fluctuation (Blennerhassett et al. 2007), inappropriate grip force regulation (Blennerhassett et al. 2007), and improper safety margins (Hermsdorfer et al. 2003). The stroke survivors tested in this study exhibited impaired somatosensation, as seen by the overshooting of grip force estimation at 50% of maximum effort. The impaired somatosensation could have hindered stroke survivors from correcting their phalanx force direction reaching toward the threshold of slippage, resulting in the increased phalanx force deviation observed in this study.
4.5 Functional implications of stroke survivors’ altered phalanx force deviation
As previously discussed, the phalanx skin slips against the gripped object surface when phalanx force deviation reaches the cone of friction, calculated as the arctangent of the COF between the finger and the grasped object (MacKenzie and Iberall 1994) (Figure 1). Stroke survivors produced an average 19° ± 2° phalanx force deviation, closer to the slip threshold of 23° for the grip surface used in the present study, compared with controls who kept their phalanx force deviation low at 14° ± 1°. The COF between the finger skin and the grip surface was not significantly different between stroke survivors and controls, indicating that both groups had the same slip threshold and the increased phalanx force deviation was not afforded by increased skin COF post stroke.
This phalanx force deviation near the slip threshold post stroke represents grip instability and likelihood of object dropping. Indeed, a previous study found that excessive digit force deviation for stroke survivors was accompanied by finger slippage of at least 1 cm in 55% of all pinch grips (Seo et al. 2010). The increased phalanx force deviation near the slip threshold also implies potential hand slippage while stroke survivors try to hold onto a support pole in a bus or in the shower, which could lead to falling and serious injury. Given the tight relationship between the phalanx force deviation and grip stability, it is not surprising that stroke survivors with greater phalanx force deviation were found to have lower motor function scores indicating difficulty in hand grip function
In addition to greater finger-object slippage, greater phalanx force deviation can lead to reduced phalanx normal force. Increasing phalanx force deviation can decrease the phalanx normal force by tilting the force vector such that the force in the normal direction decreases and force in the shear direction increases. Specifically, in the present study, the phalanx force deviation reduced stroke survivors’ potential normal force by approximately 14% (calculated by taking the average difference between phalanx resultant and normal force).
4.6 Clinical implications of stroke survivors’ altered phalanx force deviation
Clinically, this research finding may encourage a new therapy to improve stroke survivors’ gripping and motor functional scores via a force direction training rehabilitation paradigm using biofeedback (Seo et al. 2011). Furthermore, since an altered muscle activation pattern with EDC and FDI underactivation could have contributed to increasing the phalanx force deviation, a muscle specific strength training program such as resistance training (Laidlaw et al. 1999; Barry and Carson 2004) or neuromuscular electrical stimulation could improve activation of the specific underactivated muscles (Chae et al. 1998) and reduce the phalanx force deviation thereby potentially improving stroke survivors’ grip. In addition, design modification for commonly used household items could assist in accommodating stroke survivors’ altered force direction (Slota et al. 2014).
Furthermore, applying more grip force than is required by the task, such as the grip force overshoot observed in this study during the 50% maximum grip task, can lead to an earlier onset of muscle fatigue and decrease one’s ability to perform daily activities (Nowak et al. 2003). This could be especially true for the tasks where a high grip effort is required, such as cooking, holding onto a bar while riding the bus or train, or pushing and pulling a cart in a store.
Increased phalanx force deviation could also partially explain reduced grip strength in the aging, in addition to the age-associated muscle atrophy(Kallman et al. 1990), changes in muscle fiber type composition (Klitgaard et al. 1990; Lexell 1995), and alterations in the neural activation of the muscles(Akima et al. 2001; Barry and Carson 2004). In general, stroke survivors and ageing adults’ reduced grip strength is especially important to consider since there is evidence that grip strength is an indicator for life expectancy (Cooper et al.).
4.7 Limitation
One of the limitations of this study is that only the shear force in the proximal-distal direction was recorded, due to space constraints for the strain gauges inside the custom built grip dynamometer. Force in the medial-lateral direction is expected to be minimal because grip is performed primarily in the finger flexion direction and abduction/adduction strength is reduced post stroke (Lang and Schieber 2004a), and because the grip dynamometer was supported against gravity in this study. Nonetheless, if stroke survivors gripped with greater medial-lateral shear force than healthy individuals, the extent of tilt in stroke survivors’ force vector reported in this study may be an underestimation and the true deviation in force direction could actually be greater than reported in this study
5. Conclusions
The present study demonstrated that stroke survivors perform power grip with greater phalanx force deviation compared to age-matched controls, although the slip threshold between the skin and grip surface was not significantly different between stroke survivors and controls. Distribution of phalanx grip normal force was similar for stroke survivors and healthy controls. Altered muscle activation patterns with reduced activation of the FDI and EDC muscles compared with the FDS muscle may account for increased phalanx force deviation. Impaired somatosensation following stroke may also account for the increased phalanx force deviation as well as the grip force overshoot. Increased phalanx force deviation could reduce the grip strength and increase the likelihood of finger-object slippage, thus leading to reduced grip stability and an increased rate of object dropping or loss of grip. In addition, stroke survivors’ grip force overshoot may indicate that they develop muscle fatigue earlier in tasks requiring submaximal force. Decreased phalanx force control and grip force overshoot may limit stroke survivors in completing everyday tasks and progressing in rehabilitation, leading to long-term negative effects on hand function post stroke. The knowledge obtained in this research could be applied to developing more sophisticated rehabilitation therapies or assistive devices that correct altered phalanx force deviations and assist in approximating target grip force levels.
Acknowledgments
This study was supported by the American Heart Association Midwest Affiliate Predoctoral Fellowship 12PRE9320004, Grant-In-Aid award from the American Society of Biomechanics, University of Wisconsin-Milwaukee Research Growth Initiative, Mary E. Switzer Distinguished Fellowship from the National Institute of Disability and Rehabilitation Research, grant number H133F110005. However, those contents do not necessarily represent the policy of the Department of Education, and you should not assume endorsement by the Federal Government. Also, this project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 8UL1TR000055. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors thank Dr. Thomas Armstrong and Mr. Charles Woolley for their aid in developing the custom-made grip dynamometer. The authors also thank Mr. Jeffrey King for his editorial service.
Footnotes
7. Conflict of Interest Statement
The authors listed in this manuscript participated in the study design, collection, and preparation of the manuscript. The information conveyed in this article has not been submitted for publication elsewhere.
Contributor Information
Leah R. Enders, Department of Industrial and Manufacturing Engineering, University of Wisconsin-Milwaukee, Milwaukee WI 53211, US
Na Jin Seo, Email: seon@musc.edu, Department of Health Professions, Department of Health Sciences and Research, Medical University of South Carolina, 151B Rutledge Ave., Charleston, SC 29425, USA
References
- Akima H, Kubo K, Imai M, Kanehisa H, Suzuki Y, Gunji A, et al. Inactivity and muscle: effect of resistance training during bed rest on muscle size in the lower limb. Acta Physiol Scand. 2001;172:269–278. doi: 10.1046/j.1365-201x.2001.00869.x. [DOI] [PubMed] [Google Scholar]
- Amis AA. Variation of Finger Forces in Maximal Isometric Grasp Tests on a Range of Cylinder Diameters. Journal of Biomedical Engineering. 1987;9:313–320. doi: 10.1016/0141-5425(87)90079-3. [DOI] [PubMed] [Google Scholar]
- Barry BK, Carson RG. The consequences of resistance training for movement control in older adults. J Gerontol A Biol Sci Med Sci. 2004;59:730–754. doi: 10.1093/gerona/59.7.m730. [DOI] [PubMed] [Google Scholar]
- Basmajian J. Biofeedback Principles and Practice for Clinicians. Williams and Wilkins; Baltimore, MD: 1989. [Google Scholar]
- Blennerhassett JM, Matyas TA, Carey LM. Impaired discrimination of surface friction contributes to pinch grip deficit after stroke. Neurorehabil Neural Repair. 2007;21:263–272. doi: 10.1177/1545968306295560. [DOI] [PubMed] [Google Scholar]
- Boissy P, Bourbonnais D, Carlotti MM, DGBAA Maximal grip force in chronic stroke subject and its relationship to global upper extremity function. Clinical Rehabilitation. 1999;13:354–362. doi: 10.1191/026921599676433080. [DOI] [PubMed] [Google Scholar]
- Buchholz B, Frederick LJ, Armstrong TJ. An investigation of human palmar skin friction and the effects of materials, pinch force and moisture. Ergonomics. 1988;31:317–325. doi: 10.1080/00140138808966676. [DOI] [PubMed] [Google Scholar]
- Carey LM. Somatosensory loss after stroke. Critical Reviews in Physical and Rehabilitation Medicine. 1995;7:51–91. [Google Scholar]
- Chao EY, Opgrande JD, Axmear FE. Three-dimensional force analysis of finger joints in selected isometric hand functions. Journal of Biomechanics. 1976;9:387–396. doi: 10.1016/0021-9290(76)90116-0. [DOI] [PubMed] [Google Scholar]
- Cole KJ. Grasp force control in older adults. J Mot Behav. 1991;23:251–258. doi: 10.1080/00222895.1991.9942036. [DOI] [PubMed] [Google Scholar]
- Cole KJ. Age-related directional bias of fingertip force. Experimental Brain Research. 2006;175:285–291. doi: 10.1007/s00221-006-0553-0. [DOI] [PubMed] [Google Scholar]
- Cooper R, Strand BH, Hardy R, Patel KV, Kuh D. Physical capability in mid-life and survival over 13 years of follow-up: British birth cohort study. BMJ. 348:g2219. doi: 10.1136/bmj.g2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz EG, Waldinger HC, Kamper DG. Kinetic and kinematic workspaces of the index finger following stroke. Brain. 2005;128:1112–1121. doi: 10.1093/brain/awh432. [DOI] [PubMed] [Google Scholar]
- Dattola R, Girlanda P, Vita G, Santoro M, Roberto ML, Toscano A, et al. Muscle rearrangement in patients with hemiparesis after stroke: an electrophysiological and morphological study. Eur Neurol. 1993;33:109–114. doi: 10.1159/000116915. [DOI] [PubMed] [Google Scholar]
- Di Fabio RP, Badke MB. Stance duration under sensory conflict conditions in patients with hemiplegia. Arch Phys Med Rehabil. 1991;72:292–295. [PubMed] [Google Scholar]
- Enders LR, Seo NJ. Phalanx force magnitude and trajectory deviation increased during power grip with an increased coefficient of friction at the hand-object interface. Journal of Biomechanics. 2011;44:1447–1453. doi: 10.1016/j.jbiomech.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Gee MG, Tomlins P, Calver A, Darling RH, Rides M. A new friction measurement system for the frictional component of touch. Wear. 2005;259:1437–1442. [Google Scholar]
- Gowland C, VanHullenaar S, Torresin W, Moreland J, Vanspall B, Barrecca S, et al. Chedoke-McMaster Stroke Assessment: Development, Validation and Administration Manual. Hamilton, Canada: Chedoke-McMaster Hospitals and McMaster University; 1995. [Google Scholar]
- Gray CS, French JM, Bates D, Cartlidge NE, James OF, Venables G. Motor recovery following acute stroke. Age and Ageing. 1990;19:179–184. doi: 10.1093/ageing/19.3.179. [DOI] [PubMed] [Google Scholar]
- Gurram R, Gouw GJ, Rakheja S. Grip Pressure Distribution Under Static and Dynamic Loading Experimental Mechanics. 1993;33:169–173. [Google Scholar]
- Harms H, Reimnitz P, Bohner G, Werich T, Klingebiel R, Meisel C, et al. Influence of stroke localization on autonomic activation, immunodepression, and post-stroke infection. Cerebrovasc Dis. 2011;32:552–560. doi: 10.1159/000331922. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J, Hagl E, Nowak DA, Marquardt C. Grip force control during object manipulation in cerebral stroke. Clinical Neurophysiology. 2003;114:915–929. doi: 10.1016/s1388-2457(03)00042-7. [DOI] [PubMed] [Google Scholar]
- Hu XL, Tong KY, Li L. The mechanomyography of persons after stroke during isometric voluntary contractions. Journal of Electromyography and Kinesiology. 2007;17:473–483. doi: 10.1016/j.jelekin.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Hwang K, Huan F, Kim DJ. Muscle fibre types of the lumbrical, interossei, flexor, and extensor muscles moving the index finger. Joural of Plastic Surgery and Hand Surgery. 2013;47:268–272. doi: 10.3109/2000656X.2012.755988. [DOI] [PubMed] [Google Scholar]
- Johanson ME, Valero-Cuevas FJ, Hentz VR. Activation patterns of the thumb muscles during stable and unstable pinch tasks. Journal of Hand Surgery American Volume. 2001;26:698–705. doi: 10.1053/jhsu.2001.26188. [DOI] [PubMed] [Google Scholar]
- Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol. 1990;45:M82–88. doi: 10.1093/geronj/45.3.m82. [DOI] [PubMed] [Google Scholar]
- Kamper DG, Harvey RL, Suresh S, Rymer WZ. Relative contributions of neural mechanisms versus muscle mechanics in promoting finger extension deficits following stroke. Muscle and Nerve. 2003;28:309–318. doi: 10.1002/mus.10443. [DOI] [PubMed] [Google Scholar]
- Kamper DG, Rymer WZ. Impairment of voluntary control of finger motion following stroke: role of inappropriate muscle coactivation. Muscle and Nerve. 2001;24:673–681. doi: 10.1002/mus.1054. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Backstrom L, Flanagan JR, Johansson RS. Tangential torque effects on the control of grip forces when holding objects with a precision grip. J Neurophysiol. 1997;78:1619–1630. doi: 10.1152/jn.1997.78.3.1619. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G, Saltin B. Ageing alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiol Scand. 1990;140:55–62. doi: 10.1111/j.1748-1716.1990.tb08975.x. [DOI] [PubMed] [Google Scholar]
- Kong Y-K, Lowe BD. Optimal cylindrical handle diameter for grip force tasks. International Journal of Industrial Ergonomics. 2005;35:495–507. [Google Scholar]
- Kutch JJ, Valero-Cuevas FJ. Muscle redundancy does not imply robustness to muscle dysfunction. Journal of Biomechanics. 2011;44:1264–1270. doi: 10.1016/j.jbiomech.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw DH, Kornatz KW, Keen DA, Suzuki S, Enoka RM. Strength training improves the steadiness of slow lengthening contractions performed by old adults. J Appl Physiol (1985) 1999;87:1786–1795. doi: 10.1152/jappl.1999.87.5.1786. [DOI] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Human finger independence: limitations due to passive mechanical coupling versus active neuromuscular control. Journal of Neurophysiology. 2004a;92:2802–2810. doi: 10.1152/jn.00480.2004. [DOI] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Reduced muscle selectivity during individuated finger movements in humans after damage to the motor cortex or corticospinal tract. J Neurophysiol. 2004b;91:1722–1733. doi: 10.1152/jn.00805.2003. [DOI] [PubMed] [Google Scholar]
- Latash ML, Scholz JP, Schoner G. Motor control strategies revealed in the structure of motor variability. Exerc Sport Sci Rev. 2002;30:26–31. doi: 10.1097/00003677-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Kong YK, Lowe BD, Song S. Handle grip span for optimising finger-specific force capability as a function of hand size. Ergonomics. 2009;52:601–608. doi: 10.1080/00140130802422481. [DOI] [PubMed] [Google Scholar]
- Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- Li ZM, Zatsiorsky VM, Latash ML. Contribution of the extrinsic and intrinsic hand muscles to the moments in finger joints. Clinical Biomechanics. 2000;15:203–211. doi: 10.1016/s0268-0033(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Li ZM, Zatsiorsky VM, Latash ML. The effect of finger extensor mechanism on the flexor force during isometric tasks. Journal of Biomechanics. 2001;34:1097–1102. doi: 10.1016/s0021-9290(01)00061-6. [DOI] [PubMed] [Google Scholar]
- Long C, Conrad PW, Hall EA, Furler SL. Intrinsic-extrinsic muscle control of the hand in power grip and precision handling. An electromyographic study. Journal of Bone and Joint Surgery. 1970;52-A:853–867. [PubMed] [Google Scholar]
- MacKenzie CL, Iberall T. The grasping hand. North Holland; 1994. [Google Scholar]
- McDonnell MN, Hillier SL, Ridding MC, Miles TS. Impairments in precision grip correlate with functional measures in adult hemiplegia. Clinical Neurophysiology. 2006;117:1474–1480. doi: 10.1016/j.clinph.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Milner TE, Dhaliwal SS. Activation of intrinsic and extrinsic finger muscles in relation to the fingertip force vector. Experimental Brain Research. 2002;146:197–204. doi: 10.1007/s00221-002-1177-7. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Archives of Physical Medicine and Rehabilitation. 1994;75:394–398. doi: 10.1016/0003-9993(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Niessen MH, Veeger DH, Koppe PA, Konijnenbelt MH, van Dieen J, Janssen TW. Proprioception of the shoulder after stroke. Arch Phys Med Rehabil. 2008;89:333–338. doi: 10.1016/j.apmr.2007.08.157. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J, Glasauer S, Philipp J, Meyer L, Mai N. The effects of digital anaesthesia on predictive grip force adjustments during vertical movements of a grasped object. Eur J Neurosci. 2001;14:756–762. doi: 10.1046/j.0953-816x.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J, Topka H. Deficits of predictive grip force control during object manipulation in acute stroke. Journal of Neurology. 2003;250:850–860. doi: 10.1007/s00415-003-1095-z. [DOI] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. Jounral of Physiology. 1992;448:397–412. doi: 10.1113/jphysiol.1992.sp019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker VM, Wade DT, Langton Hewer R. Loss of arm function after stroke: measurement, frequency, and recovery. International Rehabilitation Medicine. 1986;8:69–73. doi: 10.3109/03790798609166178. [DOI] [PubMed] [Google Scholar]
- Pazzaglia C, Caliandro P, Granata G, Tonali P, Padua L. “Dropping objects”: a potential index of severe carpal tunnel syndrome. Neurol Sci. 2010;31:437–439. doi: 10.1007/s10072-010-0242-4. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan S, Nagaravindra M. Analysis of hand forces in health and disease during maximum isometric grasping of cylinders. Biomechanics Medical and Biological Engineering & Computing. 1993:372–376. doi: 10.1007/BF02446690. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL. Handedness: differential specializations for control of trajectory and position. Exerc Sport Sci Rev. 2005;33:206–213. doi: 10.1097/00003677-200510000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo NJ, Armstrong TJ, Ashton-Miller JA, Chaffin DB. The effect of torque direction and cylindrical handle diameter on the coupling between the hand and a cylindrical handle. Journal of Biomechanics. 2007;40:3236–3243. doi: 10.1016/j.jbiomech.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Seo NJ, Fischer HW, Bogey RA, Rymer WZ, Kamper DG. Use of visual force feedback to improve digit force direction during pinch grip in persons with stroke: a pilot study. Archices of Physical Medicine and Rehabilitation. 2011;92:24–30. doi: 10.1016/j.apmr.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Seo NJ, Rymer WZ, Kamper DG. Altered Digit Force Direction during Pinch Grip Following Stroke. Experimental Brain Research. 2010;202:891–901. doi: 10.1007/s00221-010-2193-7. [DOI] [PubMed] [Google Scholar]
- Slota GP, Enders LR, Seo NJ. Improvement of hand function using different surfaces and identification of difficult movement post stroke in the Box and Block Test. Appl Ergon. 2014;45:833–838. doi: 10.1016/j.apergo.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. Ally and Bacon; New York: 2007. [Google Scholar]
- Turton A, Lemon RN. The contribution of fast corticospinal input to the voluntary activation of proximal muscles in normal subjects and in stroke patients. Experimental Brain Research. 1999;129:559–572. doi: 10.1007/s002210050926. [DOI] [PubMed] [Google Scholar]
- Turton AJ, Butler SR. Referred Sensations Following Stroke. Neurocase. 2001;7:397–405. doi: 10.1076/neur.7.5.397.16251. [DOI] [PubMed] [Google Scholar]
- Valero-Cuevas FJ. Predictive modulation of muscle coordination pattern magnitude scales fingertip force magnitude over the voluntary range. Journal of Neurophysiology. 2000;83:1469–1479. doi: 10.1152/jn.2000.83.3.1469. [DOI] [PubMed] [Google Scholar]
- Valero-Cuevas FJ, Towles JD, Hentz VR. Quantification of fingertip force reduction in the forefinger following simulated paralysis of extensor and intrinsic muscles. Journal of Biomechanics. 2000;33:1601–1609. doi: 10.1016/s0021-9290(00)00131-7. [DOI] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. The dominant and nondominant arms are specialized for stabilizing different features of task performance. Exp Brain Res. 2007;178:565–570. doi: 10.1007/s00221-007-0936-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. 2012;135:2277–2289. doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatsiorsky VM, Latash ML. Prehension Synergies. Excercise Sport Science Review. 2004;32 doi: 10.1097/00003677-200404000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]