Abstract
This chapter focuses on neurodevelopmental diseases that are tightly linked to abnormal function of the striatum and connected structures. We begin with an overview of three representative diseases in which striatal dysfunction plays a key role—Tourette syndrome and obsessive-compulsive disorder, Rett's syndrome, and primary dystonia. These diseases highlight distinct etiologies that disrupt striatal integrity and function during development, and showcase the varied clinical manifestations of striatal dysfunction. We then review striatal organization and function, including evidence for striatal roles in online motor control/action selection, reinforcement learning, habit formation, and action sequencing. A key barrier to progress has been the relative lack of animal models of these diseases, though recently there has been considerable progress. We review these efforts, including their relative merits providing insight into disease pathogenesis, disease symptomatology, and basal ganglia function.
1. INTRODUCTION
The basal ganglia (BG) are a group of subcortical brain structures conserved across vertebrate species (Grillner, Robertson, & Stephenson-Jones, 2013).These interconnected structures contribute to the control of movement performance and several learning-related motor functions, including habit, reinforcement, and motor sequence learning. The BG are able to accomplish these functions as participants in recurrent neural loops, through which information flows to the BG from distinct cortical areas, is outputted to thalamus, and subsequently relayed back to cortex (cortico-BG-thalamocortical loops). The striatum (composed of the caudate and putamen in humans) is the principal “input” nucleus of the BG, receiving massive excitatory projections from the cortex. Myriad neuronal connections within and between BG structures process this cortical input—perhaps performing a “gating” or “selection” function—and subsequently project this information to the thalamus, which then projects back to more restricted areas of the neocortex. Evidence for the involvement of the striatum and related BG structures in varied behavioral processes and developmental disorders is reviewed.
The BG are affected by a range of debilitating diseases that can manifest throughout an individuals lifespan. For example, Tourette’s syndrome (TS) occurs primarily in school-aged children, while Parkinson disease (PD) primarily afflicts the aged. Treatment of these diseases has been challenging, in part because the function of these circuits remains poorly understood. Indeed, despite extensive research and clinical observation, a cohesive theory describing the functional roles of the striatum and connected BG circuits remains elusive.
Considerable focus has centered on diseases resulting from dysfunction or damage to the striatum. The diverse symptomatology of these diseases has strongly influenced hypotheses regarding striatal function. Broadly speaking, these hypotheses can be divided into “performance” and “learning” roles (Beeler et al., 2012; Leventhal et al., 2014). The short-duration response to levodopa is a striking example supporting a role for striatum in online motor performance. Evidence from diseases featuring pathological repetitive behaviors and thoughts such as TS and obsessive-compulsive disorder (OCD) has been critical to highlighting the importance of striatal function in learning and habit expression. A rapidly expanding literature supports a role for striatum in implicit learning processes, with implications for normal adaptive behavior, habit formation, and addiction.
This chapter focuses on neurodevelopmental diseases that are tightly linked to abnormal function of the striatum and connected structures. We begin with an overview of three representative diseases in which striatal dysfunction plays a key role—TS/OCD, Rett’s syndrome (RTT), and primary dystonia. These diseases highlight distinct etiologies that disrupt striatal integrity and function during development, and show-case the varied clinical manifestations of striatal dysfunction. We then review striatal organization and function, including evidence for striatal roles in online motor control/action selection, reinforcement learning, habit formation, and action sequencing. A key barrier to progress has been the relative lack of animal models of these diseases, though there recently has been considerable progress. We review these efforts, including their relative merits providing insight into disease pathogenesis, disease symptomatology, and BG function.
2. DISEASES AND LINKS TO STRIATAL DYSFUNCTION
2.1. Tic disorders, Tourette syndrome, and obsessive-compulsive disorder
Although transient tic disorders, TS and OCD are distinguished clinically, the common comorbidity of tics and obsessive-compulsive behaviors in TS suggest that these disorders exist on a spectrum. In support of this view, imaging studies demonstrate similar abnormalities between these disorders, and implicate abnormal BG function as a pathological feature of many of their associated symptoms (Cavanna & Seri, 2013; Worbe et al., 2010). As such, we will consider the disorders individually and as a unit.
2.1.1 Tics and Tourette syndrome: Clinical features
Tics are sudden, repetitive, involuntary movements, or vocalizations that have no intended purpose and interfere with ongoing behavior (Bronfeld & Bar-Gad, 2013). They range from mild movements or utterances involving few muscle groups (e.g., eye blinking or grimacing) to more complex, coordinated sequential activation of multiple muscle groups (Cohen, Leckman, & Bloch, 2013). To distinguish tics from other types of brief, abnormal movements, several features are helpful. Tics are often associated with premonitory feelings, ranging from a psychic urge to physical sensations like itching or even pain (Cavanna & Rickards, 2013). Tics are suppressible, at least transiently. When suppressed, the pressure to perform tics can become overwhelming, so that they are almost always eventually expressed. Some have termed tics an “unvoluntary” movement disorder to emphasize the inevitability of tic expression despite the temporary ability to suppress them (Cohen et al., 2013), though “semivoluntary” may be more accurate. Finally, tics are stereotyped—that is, patients tend to express a limited range of tic-like movements at any given time.
Tic disorders are classified into transient versus chronic tic disorders. Transient tic disorders persist for less than 1 year and represent the mildest and most common form of tic disorder (Cohen et al., 2013). The repetitive behaviors in transient tic disorder may not be consistent and often change in severity over time. In contrast, chronic tic disorders involve a more stereotyped motor or vocal tic behavior that persists for more than 1 year. TS, the most severe form of tic disorder, is characterized by multiple motor and at least one vocal tic which begin before 18 years of age, increase in severity over time, and persist for at least 1 year (Kurlan, 2010). TS, first described by Gilles de la Tourette in 1885, affects approximately 1% of the population, but is three to four times more common in boys (Kurlan, 2010; Robertson, Eapen, & Cavanna, 2009). TS often begins between 3 and 9 years of age, with the worst symptoms during the early teens. Symptoms often improve in the late teens and into adulthood (Bloch et al., 2006). Caudate nucleus volumes in childhood predict later tic severity, consistent with both BG dysfunction in TS and abnormal trajectory of brain development (Bloch, Leckman, Zhu, & Peterson, 2005). Despite this typical pattern, tic disorders remain a lifelong disabling condition for many. Pedigrees and twin studies suggest that TS has a strong hereditary component, but specific genetic risk factors remain elusive (Deng, Gao, & Jankovic, 2012; Scharf et al., 2013).
2.1.2 Tics and Tourette syndrome: Evidence for striatal dysfunction
There is strong evidence that tic disorders have a basis in BG dysfunction. Secondary tics may arise from BG disease, including Huntington disease (Jankovic & Ashizawa, 1995), BG strokes (Kwak & Jankovic, 2002), and Wilson disease (Lorincz, 2012). The most effective treatment for tics is dopamine-blocking (or depleting) drugs, implicating nigrostriatal dopamine in their pathogenesis (Cavanna & Seri, 2013). Indeed, a syndrome of tardive tics after neuroleptic exposure has been reported (Bharucha & Sethi, 1995). Molecular imaging studies report abnormalities in the striatal dopaminergic system in patients with tics though results are not consistent (Albin et al., 2003, 2009; Denys et al., 2013; Singer et al., 2002; Wolf et al., 1996). Deep brain stimulation of thalamic nuclei tightly linked with the BG, or BG nuclei themselves, variably improves tics (Ackermans, Kuhn, Neuner, Temel, & Visser-Vandewalle, 2013; Priori et al., 2013; Vandewalle, van der Linden, Groenewegen, & Caemaert, 1999).
Consistent with these studies, several quantitative volumetric imaging studies have revealed differences in striatal size between patients with TS and controls (Felling & Singer, 2011; Hyde et al., 1995; Peterson et al., 2003; Roessner et al., 2011). Postmortem studies describe specific deficits in striatal and pallidal GABAergic and cholinergic neurons, but these studies are based on a small number of postmortem examinations (Kalanithi et al., 2005; Kataoka et al., 2010).
2.1.3 Obsessive-compulsive disorder: Clinical features
Up to 60% of patients with TS have comorbid OCD (Cavanna & Rickards, 2013). OCD is characterized by the presence of intrusive, unwanted thoughts (obsessions), and the performance of ritualized behaviors that are intended to neutralize the negative emotions resulting from obsessions (compulsions) (Sarvet, 2013). While these repeated behaviors do not provide pleasure or reward, they provide relief from obsessions. These distressing uncontrolled rituals vary in severity, leading to distress and diminished quality of life. OCD often begins during childhood, with a mean age at onset of ~20 years (Narayanaswamy et al., 2012), though a median onset age at onset of 10 has been reported (Sarvet, 2013). OCD is believed to affect 1–3% of the population, both in children and adults (Sarvet, 2013). Like TS, OCD has a strong genetic component, though specific genes have yet to be identified (Pauls, 2008).
2.1.4 Obsessive-compulsive disorder: Evidence for striatal dysfunction
As for tic disorders, dysfunction of corticostriatal circuits has been implicated in OCD. Structural abnormalities in frontal cortex and/or the BG have been observed in patients with secondary OCD (Berthier, Kulisevsky, Gironell, & Heras, 1996). Functional imaging has revealed enhanced connectivity between limbic cortical areas and the ventral striatum in patients with OCD compared to controls (Harrison et al., 2009), and decreased dopamine D2/3 receptor availability (Denys et al., 2013). DBS has also been attempted for OCD, targeting primarily ventral striatum or the internal capsule, with variable results (Blomstedt, Sjoberg, Hansson, Bodlund, & Hariz, 2013). DBS of the subthalamic nucleus for PD has been reported to both induce compulsive behaviors (Broen, Duits, Visser-Vandewalle, Temel, & Winogrodzka, 2011) and alleviate them (Fontaine et al., 2004). Finally, animals with OCD-like behavior (e.g., hyperdopaminergic mice, discussed in Section 4) have abnormalities of striatal function (Berridge, Aldridge, Houchard, & Zhuang, 2005; Welch et al., 2007).
While many similarities exist between tic disorders and OCD, including their frequent comorbidity, they exhibit distinct clinical pharmacology. Treatments for the two conditions differ. While dopamine-blocking (or depleting) agents are clearly effective for TS, they are at best adjunct therapy for OCD (Sarvet, 2013). Conversely, antidepressants, especially selective serotonin-reuptake inhibitors, are the pharmacologic treatment of choice for OCD (Sarvet, 2013), but are not effective for TS.
2.2. Rett syndrome
2.2.1 Rett syndrome—Clinical features
RTT is a childhood-onset neurodevelopmental disorder caused by mutations in the MECP2 gene encoding the X-linked methyl-CpG-binding protein 2 (MeCP2) protein (Amir et al., 1999). RTT is a leading cause of intellectual disability in girls (Jellinger, 2003). Subjects with RTT develop normally for 6–18 months, followed by loss of cognitive function and speech, regression of fine and gross motor skills, social withdrawal, and development of stereotypic hand movements (Hagberg, Hanefeld, Percy, & Skjeldal, 2002). Autism, ataxia, and seizures (Dolce, Ben-Zeev, Naidu, & Kossoff, 2013) are also characteristic following developmental regression, with Parkinsonism and dystonia occurring in older patients (FitzGerald, Jankovic, Glaze, Schultz, & Percy, 1990). While RTT occurs almost exclusively in females, in boys, MECP2 mutations can cause neonatal lethality (Villard et al., 2000) or, on rare occasion, an RTT-like phenotype (Armstrong, Pineda, Aibar, Gean, & Monros, 2001; Dayer et al., 2007; Masuyama et al., 2005), likely due to somatic mosaicism (Armstrong et al., 2001).
2.2.2 Rett syndrome: Evidence for striatal dysfunction
On purely clinical grounds, the highly stereotyped hand movements that occur in RTT are reminiscent of tic/compulsive-like behavior, and similar to the stereotypies that result from striatal dysfunction in experimental animals (Berridge et al., 2005; Welch et al., 2007). The occurrence of dystonia and Parkinsonism in RTT also point to BG involvement (FitzGerald, Jankovic, Glaze, et al., 1990; FitzGerald, Jankovic, & Percy, 1990).
Beyond mere pattern recognition, however, many lines of evidence indicate that striatal dysfunction is a key feature in the pathogenesis of RTT. Imaging studies of RTT subjects demonstrate decreased cerebral volumes (Carter et al., 2008; Jellinger, 2003; Murakami, Courchesne, Haas, Press, & Yeung-Courchesne, 1992), with reductions in caudate nucleus, cortical, and midbrain volumes disproportionately reduced compared to other structures (Casanova et al., 1991; Dunn et al., 2002; Reiss et al., 1993; Subramaniam, Naidu, & Reiss, 1997). PET imaging studies demonstrate specific changes in BG transmitter function. Several lines of evidence demonstrate significant dopaminergic deficits in RTT, including reduced fluorodopa uptake (Dunn et al., 2002), decreased dopamine transporter (DAT) levels (Wenk, 1995; Wong et al., 1998), and lower density of D2 receptors (Harris et al., 1986) in caudate nucleus and putamen. Other studies demonstrate increased striatal D2 receptor binding, believed to reflect receptor upregulation in response to dopaminergic deficits (Chiron et al., 1993). These differences are likely due to the difference in the ages of the patients that were examined (Dunn & MacLeod, 2001), and demonstrate a possible age-dependent change with early D2 receptor increases followed by later reductions (Cordes et al., 1994). In contrast to specific DAT and D2 receptor changes, caudate nucleus D1 receptors and dopamine reuptake sites in cortical regions remain unchanged (Wenk, 1995). These results suggest a degree of specificity in affected dopaminergic systems. There is also evidence for striatal cholinergic dysfunction in RTT (Wenk & Mobley, 1996). For example, striatal vesicular acetylcholine transporter densities are reduced in women with RTT, and their clinical abilities are correlated with these levels (Brasic et al., 2012).
Postmortem studies of humans with RTT reveal abnormalities of the striatum and its connections. Consistent with functional imaging, choline acetyl-transferase (ChAT) activity is decreased specifically in the hippocampus, caudate nucleus, and thalamus (Wenk et al., 1993). The nigrostriatal dopaminergic system is affected, with decreased pigmentation of nigral neurons and decreased immunoreactivity for tyrosine hydroxylase (TH) (the rate-limiting enzyme in dopamine synthesis) (Jellinger, Armstrong, Zoghbi, & Percy, 1988; Kitt & Wilcox, 1995). Other catecholamines and their metabolites are similarly decreased in the substantia nigra of RTT subjects (Lekman et al., 1989). AMPA and NMDA receptor density are decreased and GABA receptor density is increased, but no changes are observed in mGluR receptor density in the BG (Blue, Naidu, & Johnston, 1999). While it is impossible to disentangle definitively changes that reflect the underlying disease process from compensatory or medication effects, these data support strongly a primary role BG dysfunction in the behavioral features of RTT.
2.3. Primary dystonia
2.3.1 Primary dystonia—Clinical features
Dystonia is a movement disorder characterized by abnormal, sustained, or intermittent, usually twisting postures maintained by agonist/antagonist co-contraction (Albanese et al., 2013). Dystonia is classified as “primary” if it occurs in isolation without neuropathological changes. In contrast, “secondary” dystonic movements occur consequent to CNS damage (e.g., from stroke, trauma, or neurodegeneration) and are typically accompanied by additional neurological signs and symptoms. Among the primary dystonias, there is a bimodal age at onset with peaks during childhood (school age to teenage years) and the fifth to sixth decade of life (Bressman et al., 2000). Age at symptom onset is strongly associated with etiology and pattern of involvement. Childhood-onset dystonia typically begins in an arm or leg, spreads to other body parts and generally reflects an inherited mutation. In contrast, adult-onset dystonia is almost always sporadic and remains confined to the cranio-cervical region or, less commonly, the upper extremities (e.g., writer’s cramp) (Albanese et al., 2013). The pattern of involvement and time at symptom onset have been most extensively studied for DYT1 dystonia, the most common inherited form of primary dystonia. The DYT1 mutation (described in detail in Section 6) is only ~30% penetrant. Interestingly, manifesting subjects develop symptoms almost uniformly during the school-age years. Those that remain symptom free into their early twenties typically remain unaffected for life, strongly implying the presence of a “critical period” of vulnerability during CNS development as a key feature of disease pathogenesis.
2.3.2 Dystonia: Evidence for striatal dysfunction
A combination of clinical, neuroimaging, and basic scientific observations support a central role for striatal dysfunction in the pathophysiology of primary dystonia. Damage to the striatum and associated structures (e.g., pallidum, thalamus) are a common cause of secondary dystonia (Marsden, Obeso, Zarranz, & Lang, 1985; Mehanna & Jankovic, 2013). Metabolic diseases that disrupt striatal function (e.g., Wilson’s disease, pantothenate kinase-associated neurodegeneration) also cause secondary dystonia (Gordon, 2002; Lorincz, 2012).
There are strong links between dopaminergic dysfunction and dystonia. Dystonia is commonly an early manifestation of PD, or a complication of its treatment with levodopa. Among drugs that induce dystonia, dopamine-receptor blockers are the most prominent. An interesting age-dependent feature of these medications is that acute dystonic reactions are much more common in young patients, while tardive syndromes tend to emerge in older patients (Dayalu & Chou, 2008). Furthermore, patients with dopa-responsive dystonia are, as the name implies, exquisitely responsive to levodopa. These patients also have an age-dependent phenotype, with young onset associated with dystonia and older onset associated with Parkinsonism (Trender-Gerhard et al., 2009). Together with the clinical features of DYT1 dystonia reviewed above, this relationship between age and symptom onset further strengthens the link between abnormal striatal development and dystonia. Commonly used treatments for dystonia include anticholinergic and GABAergic agents, which strongly influence striatal circuitry (though are admittedly not specific to striatal function). Pallidal or subthalamic DBS is highly effective for dystonia (Vidailhet, Jutras, Grabli, & Roze, 2013), further implicating these circuits in disease pathophysiology.
Behavioral functions typically attributed to striatal circuitry are disrupted in patients with dystonia, as well as carriers of dystonia mutations. A prominent theory of dystonia pathophysiology suggests that the action selection functions of the BG are deficient in dystonia, leading to the simultaneous activation of competing motor programs (Mink, 2003). A note of caution is in order, however, as the involvement of the BG in dystonia (and other movement disorders) is often cited as support for the action selection hypothesis. Motor sequence learning, which is more convincingly dependent on striatal function (see Section 3), is impaired in patients with DYT1 dystonia. On the other hand, similar impairments were found in nonmanifesting carriers of the DYT1 mutation, and impairments were not found in manifesting or nonmanifesting DYT6 mutation carriers (Carbon et al., 2011). It is therefore not clear if impairments in motor sequencing are sensitive or specific for primary dystonia.
Imaging studies strongly implicate BG circuits in the pathophysiology of dystonia. Though generally considered a disorder of abnormal function of motor circuits (as opposed to structure), consistent changes in the volume of motor-related areas (motor cortex, BG, and cerebellum) have been found in both focal and generalized primary dystonias (Delmaire et al., 2007; Draganski, Thun-Hohenstein, Bogdahn, Winkler, & May, 2003; Egger et al., 2007; Garraux et al., 2004). Some of these changes are specific to patients manifesting dystonia, while others also occur in mutation carriers. Other subtle structural changes have been found using tractography techniques. Patients with cervical dystonia have abnormalities of BG-pontine tracts (Blood et al., 2012). Reduced cerebellothalamic connectivity has been found in both manifesting and nonmanifesting DYT1 mutation carriers, but reduced thalamocortical connectivity was present only in nonmanifesting carriers (Argyelan et al., 2009).
There are very few neuropathological studies in primary dystonia patients (Paudel, Hardy, Revesz, Holton, & Houlden, 2012), making it difficult to know what, if any, histopathological abnormalities are responsible for the neuroimaging findings. Most neuropathological studies have found no pathological changes (Furukawa, Hornykiewicz, Fahn, & Kish, 2000; Gibb, Lees, & Marsden, 1988; Walker, Brin, Sandu, Good, & Shashidharan, 2002; Zweig et al., 1988). In those that did find changes, it is not clear if the findings were incidental (Holton et al., 2008). For example, two patients with Meige syndrome (Kulisevsky, Marti, Ferrer, & Tolosa, 1988; Mark et al., 1994) had Lewy Body pathology in the brainstem. These patients were ages 69 and 72, however, ages at which Lewy Body pathology is relatively common in people without clinical evidence of Parkinsonism. Another study found tau and ubiquitin-positive inclusions in the brainstems of patients with DYT1 dystonia (McNaught et al., 2004). While one of these patients was 33, the other three were ages 78–83, again raising the possibility that these were incidental findings. Overall, neuropathological studies in primary dystonia patients are simply too limited to draw any conclusions.
Functional imaging of subjects with dystonia demonstrates clear abnormalities of BG function. BG metabolic activity is altered in dystonia patients, with basal ganglia fMRI signals remaining elevated after finger tapping compared to controls (Blood et al., 2004). Some of the most consistent findings are in functional imaging of the dopaminergic system, though the specific abnormalities vary with the type of dystonia. For example, striatal D2-like receptor binding is decreased in patients with focal dystonia (Perlmutter et al., 1997) or nonmanifesting DYT1 mutations (Asanuma et al., 2005), but increased in patients with dopa-responsive dystonia (Rinne et al., 2004). A recent study suggests that at least some of these changes may be in the density of striatal D3 receptors (a member of the D2 receptor family) specifically (Karimi et al., 2011). D1 receptor density, at least in primary focal dystonia, however, seems to be unchanged from controls (Karimi et al., 2013).
3. STRIATAL ORGANIZATION AND FUNCTION
3.1. Summary of basic features of striatal organization
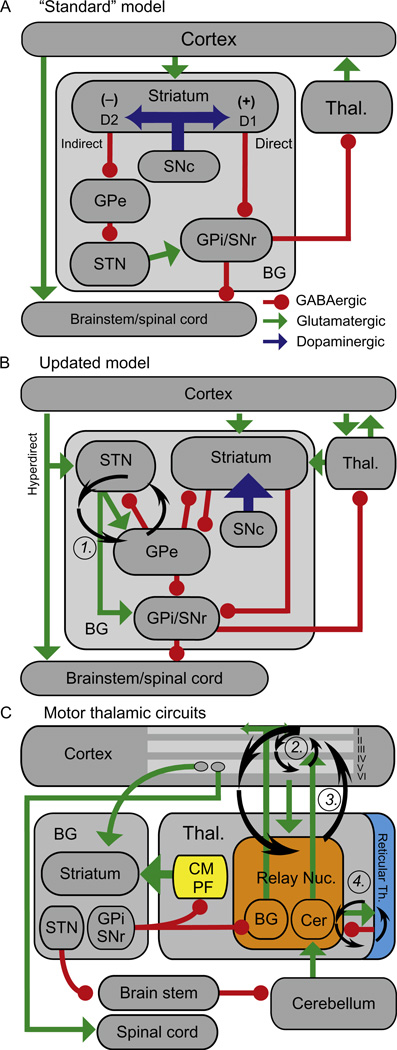
The striatum (composed of the caudate nucleus and putamen) is a major input nucleus for the BG, a group of subcortical gray matter structures that form recurrent loops with the thalamus and cortex (Fig. 3.1). It receives glutamatergic excitatory input from the whole neocortical mantle (Mathai & Smith, 2011), related structures such as the hippocampal formation and the amygdala (McGeorge & Faull, 1989), as well as thalamic intralaminar (centromedian and parafascicular nuclei—CM/Pf) and BG-recipient relay nuclei (Sadikot & Rymar, 2009). Some GABAergic striatal inputs come from within the BG, including from the globus pallidus, pars externa (GPe; Mallet et al., 2012). As detailed below, many of these afferents project preferentially to specific subpopulations of striatal neurons. The striatum sends a massive GABAergic efferent projection to GPe and the BG output nuclei (substantia nigra, pars reticulata (SNr) and globus pallidus, pars interna (GPi)).
Figure 3.1.
Cortical-BG-thalamic models. (A) The “standard” model. Green arrows, excitatory (glutamatergic) projections; Red circles, inhibitory (GABAergic) projections; Blue arrows, dopaminergic projections. (+) and (−) indicate excitatory and inhibitory effects of dopamine on MSNs, respectively. Abbreviations are defined in the text. (B) Updated model. Not all connections described in the text are illustrated for clarity. (C) Model emphasizing thalamic circuits. Roman numerals indicate cortical layers. Ovals in layer V indicate distinct populations of IT and PT neurons. Note the distinct projection patterns of the cerebellar and BG-recipient thalamus. In the thalamus, “BG” and “Cer” indicate the BG and cerebellar-recipient regions, respectively. Circular arrows in (B) and (C) indicate candidate oscillation generators. Figure and caption reprinted with modification from Ellens and Leventhal (2013), copyright 2013, with permission from IOS Press.
The organization of corticostriate projections is complex. Lesion studies and, more recently, functional imaging (Choi, Yeo, & Buckner, 2012; Kemp & Powell, 1970) demonstrate a topographic organization, suggesting that different striatal regions are functionally specialized depending on their cortical inputs. For example, dorsolateral striatum in rodents (primarily dorsolateral putamen in primates) receives afferents from primary sensorimotor cortex. Similarly, dorsomedial striatum in rodents (primarily caudate nucleus in primates) receives afferents from cortical association areas, and ventral striatum (nucleus accumbens) receives limbic afferents (Parent & Hazrati, 1995). This functional specialization is at least partially maintained throughout the BG and its thalamic projections (Alexander, DeLong, & Strick, 1986). Cortico-BG-thalamocortical loops tend to project back to their cortical site of origin, but also project to at least one other cortical area (Joel & Weiner, 1994). Thus, motor, associative, and limbic loops are not fully segregated. Sensitive tract-tracing studies also indicate that cortical regions project to the striatum in overlapping parasagittally arranged zones of considerable rostro-caudal extent, suggesting some interdigitation of corticostriate projections (Selemon & Goldman-Rakic, 1985). Other work suggests some convergence of corticostriate projections from cortical regions that are functionally linked by corticocortical connections (Yeterian & Van Hoesen, 1978).
The striatum is composed primarily of GABAergic medium spiny neurons (MSNs) and several interneuron types (Table 3.1). These cells exist in two interdigitated compartments known as “patch” (or striosomes) and “matrix” (Crittenden & Graybiel, 2011). MSNs represent 90–95% of neurons depending on the species, with lower percentages in primates (Graveland & DiFiglia, 1985; Tepper, Tecuapetla, Koos, & Ibanez-Sandoval, 2010). MSNs are characterized by the presence of multiple dendritic spines (small mushroom-shaped protuberances) that are the site of most excitatory synapses. They fire sparsely, requiring coordinated excitatory synaptic input to initiate spiking (Kreitzer, 2009). MSNs are divided into two groups of roughly equal proportions based on projection patterns (Gerfen & Surmeier, 2011). The monosynaptic connection from a subset of MSNs (dMSNs) to GPi/SNr is termed the “direct” pathway, but also sends collaterals to GPe. “Indirect” pathway MSNs (iMSNs), on the other hand, synapse only in GPe. dMSNs express D1 dopamine receptors with relatively low affinity for dopamine, while iMSNs express higher affinity D2 dopamine receptors (Gerfen & Surmeier, 2011). Thus, phasic increases or decreases in striatal dopamine concentration likely have distinct effects on dMSNs and iMSNs.
Table 3.1.
Striatal cell types
| Cell type | Transmitter | Nomenclature | Afferent connections |
Efferent connections |
Specific markers |
Major electrophysiological properties |
|---|---|---|---|---|---|---|
| Projection neurons | GABA | MSN— Direct pathway | Cortex, thalamus, interneurons, GPe | GPi, SNr, collaterals to GPe | DARPP-32, D1R | Fire sparsely. Require coordinated excitatory synaptic input to initiate spike |
| MSN— Indirect pathway | Cortex, thalamus, interneurons, GPe | GPe | DARPP-32, D2R, A2A receptors | Fire sparsely. Require coordinated excitatory synaptic input to initiate spike | ||
| Interneurons | GABA | FSI | Cortex, CM/Pf thalamus, GPe, interneurons | Proximal MSN dendrites/soma | Parvalbumin | Strong monosynaptic inhibition of MSN in vitro |
| PLTS | MSN distal dendrite | NPY, SST, nNOS | Small IPSP on postsynaptic neuron | |||
| CR+ | CR | |||||
| TH+ | TH | GABA-dependent IPSP on postsynaptic neuron | ||||
| Cholinergic | TAN | CM/pf thalamus, cortex, interneurons | MSN, interneurons, volume transmission | ChAT, VAChT, TrkA | Spontaneously fire at low frequencies (< 10 Hz). Pause in response to salient behavioral events |
The remaining neurons in the striatum are aspiny interneurons, including medium-sized GABAergic and large cholinergic neurons (Kreitzer, 2009). GABAergic interneurons can be further classified into at least four subtypes based on distinctive electrophysiological properties and expression of different neurochemical markers (Tepper, 2010; Tepper et al., 2010). Fast-spiking interneurons (FSIs) express the calcium-binding protein parvalbumin and represent approximately 1% of the neurons in the striatum (Luk & Sadikot, 2001) in a lateral to medial gradient (higher density laterally). This suggests a role in sensorimotor functions. These cells receive afferents from cortex (Ramanathan, Hanley, Deniau, & Bolam, 2002), the thalamic CM nucleus (Sidibe & Smith, 1999), other interneurons (Chang & Kita, 1992), and GPe (Berke, 2011; Bevan, Booth, Eaton, & Bolam, 1998; Kreitzer, 2009). One FSI may innervate hundreds of MSNs (Koos & Tepper, 1999). Single FSI discharges are capable of inhibiting action potential generation in MSNs (or at least delaying their onset) due to potent, proximal synapses (Bennett & Bolam, 1994; Kubota & Kawaguchi, 2000). Despite in vitro evidence of strong monosynaptic FSI–MSN inhibition, however, in vivo studies have not consistently found a sharp depression in MSN firing immediately after FSI spikes (Adler, Katabi, Finkes, Prut, & Bergman, 2013; Gage, Stoetzner, Wiltschko, & Berke, 2010; Lansink, Goltstein, Lankelma, & Pennartz, 2010). FSIs are linked with each other via dendritic gap junctions (Fukuda, 2009; Kita, Kosaka, & Heizmann, 1990), creating a syncytium hypothesized to synchronize FSI firing on a very fine timescale. More recent studies suggest that this is true only when FSIs receive coincident cortical input (Hjorth, Blackwell,&Kotaleski, 2009), and only in specific frequency ranges (Russo, Nieus, Maggi, & Taverna, 2013). In vivo experiments seem to bear this out, with coordinated FSI activity only during specific, temporally precise behavioral events (Adler et al., 2013; Berke, 2008; Gage et al., 2010). Nonetheless, the small increase in FSI coordination mediated via gap junctions may be critical for coordinating striatal cell assemblies and synchronizing corticostriatal oscillations.
The other GABAergic interneurons are less well-characterized, especially calretinin-positive interneurons, which are quite sparse in rodents (Gittis, Nelson, Thwin, Palop, & Kreitzer, 2010; Tepper, 2010). Another class of interneurons expresses neuropeptide Y, somatostatin, and nitric oxide synthase (hereafter referred to as NPY neurons). These cells synapse sparsely on more distal dendrites of MSNs, and generate small IPSPs. They are characterized physiologically by persistent low-threshold spikes (Kawaguchi, Wilson, Augood, & Emson, 1995; Tepper, 2010), leading to their other descriptor—PLTS neurons. Whether their effects are mediated primarily via GABAergic transmission or release of neuromodulatory peptides remains unknown (Tepper, 2010). Finally, there is a class of TH (the rate-limiting enzyme in dopamine and noradrenaline synthesis) positive interneurons that evoke GABA-dependent IPSPs in postsynaptic cells. The functional significance of the non-FSI GABAergic interneurons remains unclear, as they have been difficult to identify in awake, behaving subjects.
Cholinergic interneurons probably correspond to tonically active neurons (TANs) recorded in vivo, which fire spontaneously at low frequencies (<10 Hz) (Schulz & Reynolds, 2013). They receive excitatory inputs predominantly from CM/Pf (Ding, Guzman, Peterson, Goldberg, & Surmeier, 2010; Matsumoto, Minamimoto, Graybiel, & Kimura, 2001), minor excitatory input from the cortex (Thomas, Smith, Levey, & Hersch, 2000), and inhibitory input from GABAergic interneurons and possibly MSNs (Goldberg & Reynolds, 2011; Sullivan, Chen, & Morikawa, 2008). Striatal cholinergic signaling may be primarily via “volume neurotransmission” since there are relatively few typical cholinergic synapses within the striatum (Pisani, Bernardi, Ding, & Surmeier, 2007). Striatal cholinergic neurons directly influence GABAergic interneuron and MSN activity, and interact with dopamine to regulate synaptic plasticity (Gerfen & Surmeier, 2011). Their axons arborize densely and diffusely in the striatal matrix, with sparse crossings into the patch compartment (Graybiel, Baughman, & Eckenstein, 1986; Kawaguchi, 1992). Unlike most other neurons, TAN firing is correlated in awake, behaving animals, and may transmit coordinated signals to large striatal regions. An example is the “TAN pause” (Aosaki et al., 1994; Morris, Arkadir, Nevet, Vaadia, & Bergman, 2004), a transient, coordinated decrease in TAN firing in response to salient behavioral events dependent on afferents from the CM/Pf nucleus (Ding et al., 2010; Matsumoto et al., 2001). This pause is generally preceded and immediately followed by an increase in TAN firing, in a burst-pause-burst pattern. GABAergic interneurons are activated by nicotinic stimulation, and there is reason to believe that NPY neurons, in particular, may mediate the TAN pause by a feedback mechanism (Sullivan et al., 2008). Striatal output is transiently suppressed by the coordinated activity of TANs in vitro (English et al., 2012), and the TAN pause may provide a temporal window for corticostriatal plasticity in coordination with dopamine transients (Cragg, 2006). Interestingly, the TAN pause is lost after striatal dopamine depletion (Schulz & Reynolds, 2013).
Midbrain dopaminergic projections to striatum are especially strong, and likely play a major role in the pathophysiology of multiple disorders including Parkinsonism, tardive drug reactions, tic disorders, dystonia, and schizophrenia. The substantia nigra, pars compacta (SNc) innervates dorsal (motor and associative) regions of striatum, while the ventral tegmental area (VTA) innervates the ventral nucleus accumbens. These two cell populations have distinct physiologic features, but even this simple dichotomy may not capture their full diversity (Morikawa & Paladini, 2011). Indeed, there is a lateral (SNc) to medial (VTA) gradient of physiologic properties and anatomic connectivity. More medial cells project to ventral striatum, which project back to more lateral midbrain dopaminergic neurons, which project to more dorsal striatal regions. In this way, an “ascending spiral” of midbrain-striatal circuits is created, providing a mechanism by which distinct BG circuits can interact with each other (Belin & Everitt, 2008; Haber, Fudge, & McFarland, 2000). Midbrain dopaminergic projections feature a large number of terminals diverging from a relatively small number of neurons; each dopaminergic nigral neuron innervates many striatal neurons. The relative lack of specificity of nigrostriatal projections suggests that this pathway performs a diffuse “biasing” function for striatal neurons, though nigral dopamine neuron activity may not be as uniform as previously assumed (Matsumoto & Hikosaka, 2009). Dopaminergic terminals tend to synapse on the necks of MSN spines, with cortico- and thalamostriatal terminals on spine heads. These “triadic” synapse complexes allow tight regulation of the contributions of specific cortical and thalamic afferents to MSN firing.
Dopamine modulates striatal output on multiple timescales via pre- and postsynaptic mechanisms. Presynaptic D2 receptors on multiple cell types regulate dopamine, acetylcholine, glutamate, and GABA release (Gerfen & Surmeier, 2011). Postsynaptic D1 receptor stimulation acutely enhances MSN excitability, while D2 receptor activation has opposite effects (Gerfen & Surmeier, 2011). The differential expression of D1 and D2 receptors by MSNs (D1-dMSN; D2-iMSN) results in contrasting effects of acute changes in dopamine levels on the direct and indirect BG pathways. Striatal interneurons also express multiple dopamine-receptor subtypes with complex responses to dopaminergic stimulation. In addition to direct effects on striatal neuronal activity, dopamine influences synaptic plasticity at cortico- and thalamostriatal synapses of dMSNs and iMSNs in distinct ways (Gerfen & Surmeier, 2011; Shen, Flajolet, Greengard, & Surmeier, 2008).
According to the prevailing “rate” model of BG physiology (Albin, Young, & Penney, 1989), striatal dopamine increases dMSN firing, decreases iMSN firing, and, ultimately, suppresses BG output to release thalamocortical circuits from tonic inhibition. In Parkinsonism, dMSN activity decreases and iMSN activity increases, resulting in excessive BG inhibition of thalamocortical activity. Strong experimental support for this model comes from selective activation of dMSNs or iMSNs using optogenetic techniques, which dramatically alters locomotor activity and BG output in a manner consistent with the rate model (Freeze, Kravitz, Hammack, Berke, & Kreitzer, 2013; Kravitz et al., 2010). Though the rate model successfully predicts many clinical and physiological phenomena, it is also clearly incomplete. For one thing, there are multiple types of hyperkinetic movement disorders (e.g., dystonia, chorea), yet the rate model generically predicts increased movement from excessive direct, or insufficient indirect, pathway activity. More telling, the rate model predicts that lesions of BG output should provoke abnormal movements, but such lesions are used to treat chorea and dystonia. Finally, direct recordings from BG-thalamocortical circuits in PD and other movement disorders show only partial agreement with rate model predictions, instead emphasizing correlations between neuronal activity patterns and behavioral output (Cleary et al., 2013; Ellens & Leventhal, 2013; Rubin, McIntyre, Turner, & Wichmann, 2012; Tang et al., 2009).
3.2. Overview of striatal function
3.2.1 Online motor control/action selection
Based largely on observations of patients with PD and Huntington disease, it has been long assumed (at least among neurologists) that the primary function of the striatum is to regulate moment-to-moment motor function (Albin et al., 1989; DeLong, 1990). The “short-duration response” of PD patients to levodopa, in which motor function improves on approximately the same timescale that levodopa enters the brain (Chan, Nutt, & Holford, 2004), provides strong support for this hypothesis. There is also experimental evidence that the striatum regulates online motor performance: motor output is acutely altered by intrastriatal drug infusions (Gittis et al., 2011; Leventhal et al., 2014; Worbe et al., 2009), electrical stimulation (Watanabe & Munoz, 2010), or optogenetic stimulation (Kravitz et al., 2010).
It has been suggested that the BG, in general, and striatum specifically, participate in action selection and initiation (Mink, 2003; Ratcliff & Frank, 2012; Redgrave, Vautrelle, & Reynolds, 2011). In part, this hypothesis grows out of the observation that decorticate rats can properly sequence stereotyped movement patterns and associate specific cues with preferred behaviors (Berridge & Whishaw, 1992; Whishaw & Kolb, 1984). It is often stated that the BG, which are highly conserved from invertebrates through primates, provide blanket suppression of brainstem motor programs (Grillner et al., 2013) given the high tonic firing of BG output neurons. Though details of these models vary, the basic idea is that the striatum uses convergent cortical and thalamic input regarding the current state of the animal to determine the next optimal motor program, which is released for execution by focused inhibition of BG output via the direct pathway. Meanwhile, the indirect pathway suppresses all alternative options so that the motor system is accessed by only one “action” at a time (Cui et al., 2013). Several computational models of the striatum (Humphries, Wood, & Gurney, 2009) and full BG circuits (Lo & Wang, 2006; Ratcliff & Frank, 2012) demonstrate that striatal circuitry could implement action selection. These models make implicit assumptions that neuronal assemblies turned off or on by model circuits represent individual “action plans.” Further, they assume that the primary function of GABAergic BG output is to suppress neuronal activity in target structures. Recent studies, however, suggest that BG output plays a subtler role in manipulating the fine timing of spikes generated by target neurons (Goldberg, Farries, & Fee, 2013; Goldberg & Fee, 2012; Rubin et al., 2012).
Physiologic studies of the BG are broadly consistent with the action selection hypothesis. For example, many BG output (GPi/SNr) neurons abruptly decrease their firing rates just prior to movement initiation (Gulley, Kuwajima, Mayhill, & Rebec, 1999; Schmidt, Leventhal, Mallet, Chen, & Berke, 2013; but see Mink & Thach, 1991a). Indeed, firing rate changes in this subset of neurons strongly predicts movement onset (Freeze et al., 2013). Many BG output neurons increase their firing rates at movement onset, however, and both dMSNs and iMSNs fire at movement onset (Cui et al., 2013). These observations have been interpreted as suppression of competing motor programs by the indirect pathway.
While the studies described above are consistent with the concept that action selection is a primary role for the striatum, they are not proof. During tasks in which decisions are indicated with orienting movements to the left or right (i.e., saccades in primates or nose pokes in rodents), intrastriatal manipulations strongly bias decisions. For example, caudate microstimulation suppresses contralateral saccades (Watanabe & Munoz, 2010), and inactivation of dorsolateral striatum biases rats to move toward the inactivated side (Leventhal et al., 2014). Further, unilateral blockade of dopamine receptors or dopamine depletion biases rats toward responses ipsilateral to the lesion (Brown & Robbins, 1989; Carli, Evenden, & Robbins, 1985; Leventhal et al., 2014). In some sense, this biasing is similar to the rapid rotation observed in hemidopamine lesioned rodents exposed to amphetamine or apomorphine (Ungerstedt & Arbuthnott, 1970). Thus, there is strong evidence that the striatum participates online in the decision to orient to the left or right. Since the striatum is a bilateral structure, however, decisions regarding head/neck movements cannot be determined by the output of one striatum alone. Further computations must take place in downstream structures, perhaps by comparing BG output from both hemispheres. The superior colliculus, which receives bilateral afferents from SNr, likely plays a key role in decisions regarding eye/head/neck movements (Holmes et al., 2012; Lo & Wang, 2006). Regardless of the underlying neural mechanisms, it is clear that for orienting type movements, the striatum participates in action selection processes.
A natural question is whether this function is generalizable to limb movements. Since striatal “decisions” must be conveyed to the rest of the brain via BG output nuclei, lesion/inactivation studies of sensorimotor regions of GPi are informative. Disruption of BG output slows limb movements and causes hypometria (target undershoot), but with relatively preserved reaction times (Horak & Anderson, 1984; Inase, Buford, & Anderson, 1996; Limousin et al., 1999; Mink & Thach, 1991b; Wenger, Musch, & Mink, 1999). Critically, previously acquired motor sequences are completed in the correct order (Desmurget & Turner, 2010; Turner & Desmurget, 2010), but patients with therapeutic pallidotomies have difficulty acquiring new sequences (Brown et al., 2003; Obeso et al., 2009). These results argue against a primary role for the striatum in online decision-making processes, at least for some types of movements.
3.2.2 Reinforcement learning
An impressive convergence of theoretical and experimental results accumulating in the past couple of decades suggest that nigrostriatal dopamine carries signals critical to reward-based striatal learning processes (for a concise review, see Glimcher, 2011). The basic concept is that nigrostriatal dopaminergic neurotransmission mediates reward prediction errors (RPEs) for reinforcement learning. In these models, an error signal indicates the difference between the expected and actual value of rewards. This error signal is used to refine predictions regarding the expected reward given a specific action performed in a specific context. In seminal experiments performed by Schultz and colleagues, it became apparent that phasic nigrostriatal dopaminergic neuron signaling exhibits the properties of an RPE signal (Schultz, Apicella, & Ljungberg, 1993). Phasic activity of these neurons is characterized by high firing rates with resulting marked elevation of striatal dopamine release. Schultz’s group studied SNc dopaminergic neuron spiking rates as monkeys learned associations between visual cues and rewards. These cells have a baseline tonic firing rate of about 5–10 Hz that is modulated by behavioral events. In untrained animals, unpredictable reward presentation was followed rapidly by a phasic increase in nigral activity. As animals learned that specific cues predict rewards, the burst of SN activity associated with the primary reward subsided. Instead, the phasic increase in dopamine neuron firing became associated with the (unpredictably timed) reward-predicting cue. Migration of the positive RPE signal from previously unexpected reward to the predicting cue is an explicit prediction of computational temporal difference models of reinforcement learning, which were formulated years prior to Schultz’s experiments.
Just as an unexpected reward results in a burst of nigral activity, omission of an expected reward results in transient depression nigral activity (Schultz, Dayan, & Montague, 1997), compatible with a negative RPE signal. More recent experiments investigating the fine timing of phasic dopamine release in rodent striatum using fast-scan cyclic voltammetry have confirmed rapid changes in striatal dopamine concentration that correlate well with the phasic changes in dopamine neuron firing rates observed by Schultz and others (Brown, McCutcheon, Cone, Ragozzino, & Roitman, 2011; Flagel et al., 2011; Kiyatkin & Gratton, 1994). It should be noted, however, that cholinergic activity intrinsic to the striatum also modulates dopamine release independently of ascending activity in midbrain dopaminergic neurons (Threlfell et al., 2012). The details of intrinsic striatal microcircuitry notwithstanding, selective activation of VTA dopaminergic neurons is still sufficient to induce conditioned place preference (Tsai et al., 2009). Activating dMSNs or iMSNs independently of dopamine signaling is sufficient to reinforce or diminish, respectively, instrumental responding (Kravitz, Tye, & Kreitzer, 2012), further supporting a role for the striatum in reinforcement learning.
Though many dopamine neuron responses are consistent with an RPE signal, this is not uniformly true (Matsumoto & Hikosaka, 2009; Schultz, 2013). Many putative midbrain dopamine neurons fire in response to aversive as well-rewarding events. Others respond to novel stimuli, even if not previously associated with rewarding outcomes (Schultz, 2013). This may in part be attributable to contamination of putative dopaminergic neuronal populations with recordings from GABAergic interneurons (Cohen, Haesler, Vong, Lowell, & Uchida, 2012). These experiments suggest, however, that midbrain dopaminergic neurons do not code a pure RPE signal.
It should be stressed that while an associative learning role for striatal dopamine is the predominant view of nigrostriatal dopaminergic signaling, this is not the only cogent interpretation of these data. Berridge and Robinson (1998) put forward an alternative explanation in which nigrostriatal dopaminergic signaling provides what they term incentive salience (Berridge, 2007), essentially a mapping of the motivational significance of rewards and stimuli, as opposed to learning per se. This interpretation leads to many of the same predictions as interpreting dopaminergic signaling as an example of temporal difference learning, but may account for “dopamine-free” associative learning observed under special circumstances (Cannon & Palmiter, 2003; Flagel et al., 2011; Hnasko, Sotak, & Palmiter, 2005). Thus, it seems that midbrain dopaminergic signaling is sufficient, but not necessary, for certain types of implicit learning.
Specific hypotheses have been advanced regarding the cellular mechanisms mediating the effects of striatal dopamine (Robinson, Sotak, During, & Palmiter, 2006). The so-called “3-factor rule” postulates that cortico- and thalamostriatal synapses active when phasic changes in dopamine (increases or decreases) occur are selectively modified via long-term potentiation or depression. Transient changes in cholinergic activity (i.e., TAN pauses) also likely modulate dopamine-mediated synaptic plasticity, perhaps by providing a temporal window in which cortico- and thalamostriatal plasticity is most effective (Morris et al., 2004). The direction in which synaptic strength is modified depends on the precise timing of afferent input, as well as the dopamine-receptor profile of striatal neurons (Shen et al., 2008). D2 receptors have a higher affinity for dopamine than D1 receptors, raising the possibility that, at tonic dopamine levels, D2 receptors are largely occupied while D1 receptors are not. Phasic increases in dopamine would then leave the indirect pathway relatively unchanged, while modifying corticostriatal synapses in the direct pathway. Conversely, drops in dopamine concentration due to negative RPEs would leave already-vacant D1 receptors unoccupied, but D2 receptors would go from occupied to unoccupied. Thus, reinforcement learning from positive RPEs may take place primarily along the direct pathway, while independent learning from negative RPEs may take place along the indirect pathway. Support for this hypothesis comes from studies of implicit learning in patients with PD. When off medications, patients tend to learn more from omitted rewards; on medications, they tend to learn more from rewarded events (Frank, Seeberger, & O’reilly, 2004). While an attractive hypothesis, independent manipulation of the direct and indirect pathways by phasic dopamine increases and decreases remains to be proven (Dreyer, Herrik, Berg, & Hounsgaard, 2010; Wall et al., 2011).
3.2.3 Habit formation
Habits are motor or cognitive behaviors that are performed automatically, without regard to the expected outcome of the behavior. From a practical standpoint, there are two primary assays used to identify actions as habitual in experimental animals (Yin & Knowlton, 2006). In the first, the anticipated value of a specific action is modified. A subject may be given free access to reward prior to a “probe session,” devaluing successful task completion. For example, a rat trained to press a lever for a food reward may be sated prior to testing. If the lever-press behavior is “goal-directed,” the sated rat will respond less often than a hungry rat. A rat that responds habitually will press the lever whether hungry or full. Alternatively, a previously desired outcome may be associated with an aversive stimulus. For example, pairing lithium chloride injections (which induce nausea) with chocolate milk renders the previously rewarding outcome undesirable (Smith & Graybiel, 2013a). If the subject avoids (or at least does not actively pursue) a devalued outcome, the behavior is interpreted as “goal-directed.” If the subject continues to respond, the behavior is identified as a habit.
The second assay involves changing the “action–outcome” contingency of a task. In this case, the probability of reward given a response relative to the probability without that response is manipulated. This may be accomplished by increasing the probability of a rewarding event during free behavior, or decreasing the probability of reward after a response. Again, if subjects continue to respond to a cue in a manner that no longer increases the probability of reward (“stimulus–response” vs. “action–outcome” behavior), the behavior is considered habitual.
Initial stages of habit learning involve a goal-directed behavior, which becomes habitual following extended experience or training. The switch from goal-directed behavior to habit involves a transition in the neural activity of circuits controlling these behaviors (Smith & Graybiel, 2013b). Once a behavior (such as maze walking or lever pressing in rodents, or sequential eye movements in primates) becomes habitual, a “task-bracketing” activity pattern emerges such that striatal firing occurs prominently at the beginning and end of the task (Barnes, Kubota, Hu, Jin, & Graybiel, 2005; Jin, Tecuapetla, & Costa, 2014; Jin & Costa, 2010; Jog et al., 1999, #40056). This bracketing activity is thought to allow the striatum to “chunk” or crystallize the habitual action so it can be performed as a unit (Graybiel, 1998), allowing each sequence of actions within the habit to be marked as valuable and performed without extra oversight. Striatal task bracketing, however, is readily broken by “extinction” trials, when the behavior is extinguished in the absence of reward. Similar “task-bracketing” activity also evolves in infralimbic cortex, which is more tightly associated with the transition from goal-directed to habitual behavior (Smith & Graybiel, 2013a).
Experiments which lesion or inactivate specific brain regions demonstrate that both the dorsolateral striatum and prefrontal cortex are critical for habit formation. Lesions of dorsolateral striatum prior to training cause behavior that would otherwise have become habitual to remain goal-directed (Yin, Knowlton, & Balleine, 2004). Similar results were obtained by transiently inactivating dorsolateral striatum only during devaluation sessions (Yin, Knowlton,&Balleine, 2006). Conversely, lesions of dorsomedial striatum force animals from goal-directed to habitual behaviors (Yin, Ostlund, Knowlton, & Balleine, 2005). Similar dissociations have been found in cortex, where lesions or inactivation of infralimbic cortex promote goal-directedness (Coutureau & Killcross, 2003). The combination of task-bracketing activity and lesion/inactivation studies have led to a “dual operator” hypothesis of habit formation, in which infralimbic cortex and dorsolateral striatum cooperate to generate habitual behavior (Smith & Graybiel, 2013a). Interestingly, inactivation of infralimbic cortex after a new habit has replaced an old one caused rats to revert to the original habit, instead of abolishing habitual behavior altogether (Smith, Virkud, Deisseroth, & Graybiel, 2012).
In summary, dorsolateral striatum is critical to the formation and expression of habitual behavior, which may serve the adaptive purpose of freeing valuable cerebral resources. How the brain decides whether to operate in habitual or goal-directed mode remains an area of active research, but it is easy to imagine how dysfunction of such a habit system could result in tics or other compulsive behaviors.
3.2.4 Behavioral sequencing
Related to the concepts of action selection and habit formation is that of motor sequencing. Sequential movements of complex behavioral tasks have been studied in many species and have focused on tasks such as ordered button pressing (Kermadi & Joseph, 1995; Matsumoto, Hanakawa, Maki, Graybiel, & Kimura, 1999), joystick movements (Desmurget & Turner, 2010), and sequential eye movements in monkeys (Kermadi & Joseph, 1995; Lu, Matsuzawa, & Hikosaka, 2002). In rats, lever pressing (Yin, 2010) and grooming behavior (Berridge & Whishaw, 1992) have been commonly used. These studies, along with imaging studies in humans, have implicated the striatum in both the learning and execution of sequential movements. One must be careful, however, not to confuse a general deficit in motor function from a specific deficit in action sequencing.
One approach to assessing the performance of motor sequences independently of their acquisition is to test naturally occurring sequential behaviors, for example, rodent grooming. One grooming pattern, termed the “syntactic chain,” has received particular attention due to its highly stereotyped organization (Berridge & Whishaw, 1992). The syntactic chain is made up of at least 25 individual actions that are performed in a stereotypic and predictable order, which can be broken into constituent parts. Rats with striatal, but not cortical or cerebellar lesions, consistently fail to complete syntactic chains, and do not recover this ability over time (Berridge & Whishaw, 1992). Similarly, inactivating caudate/putamen in monkeys caused incorrect button-presses when monkeys attempted to reproduce previously learned sequences (Miyachi, Hikosaka, Miyashita, Karadi, & Rand, 1997).
Dopamine also is implicated in the execution of sequential movements. Monkeys treated with MPTP move slower to complete previously learned motor sequences (Matsumoto et al., 1999), and mice lacking D1 receptors are impaired in completing grooming chains (Cromwell, Berridge, Drago, & Levine, 1998). Patients with PD are also impaired in executing movement sequences, though in this case it is virtually impossible to separate learning from performance effects (Benecke, Rothwell, Dick, Day, & Marsden, 1987). In contrast, hyperdopaminergic mice (a model of OCD reviewed in Section 4) perform more frequent, behaviorally rigid grooming chains (Berridge et al., 2005). Further, cocaine and amphetamine, which both increase catecholamines including dopamine, induce repetitive, stereotypic behaviors in rodents (Wolgin, 2012). This is likely via a D1 receptor-dependent process, as D1 agonists induce stereotypic movements, but D2 agonists do not (Berridge & Aldridge, 2000a, 2000b; Taylor, Rajbhandari, Berridge, & Aldridge, 2010).
The above evidence is suggestive of a role for the striatum, in particular, nigrostriatal dopamine, in the expression of stereotyped movement sequences. If so, the striatum must convey sequence-specific signals to the rest of the brain via BG output nodes (GPi and SNr), which has been investigated in several studies. Silencing GPi with muscimol slows the performance of learned sequences, and this finding is consistent across studies (Turner & Desmurget, 2010). However, this appears to be a property of movement in general after pallidal inactivation, not just sequenced movements (Inase et al., 1996; Wenger et al., 1999). In a key experiment, Desmurget and Turner compared the performance of well-learned and random movement sequences after pallidal inactivation. Both types of sequence were performed slowly, but the overlearned sequence was still performed faster compared to the random sequence. Thus, the monkeys were still able to anticipate the next movement, suggesting that the BG are not required for the execution of learned motor sequences (Desmurget & Turner, 2010).
We have thus far addressed the nuanced role of the striatum in executing motor sequences. Much less controversial is the idea that the striatum is critical for acquiring motor sequences. Some of this evidence comes from the study of songbirds, which learn a highly stereotyped song early in life that ultimately becomes crystallized and relatively immutable. Lesions to the songbird homolog of the BG markedly impair song learning, but have minimal effect on performance of crystallized songs (Andalman & Fee, 2009; Brainard & Doupe, 2013). Lesion studies in mammals reveal similar results. Lesions of dorsolateral striatum impair sequence learning in mice, while lesions of dorsomedial striatum do not (Yin, 2010). Further, functional imaging studies have found enhanced striatal activity as motor sequences are learned (Doyon, 2008; Grafton, Hazeltine, & Ivry, 1995; Lehericy et al., 2005; Rauch et al., 1997; Seidler et al., 2005). Nigrostriatal dopamine specifically seems to play a role in sequence acquisition, as both humans (Frank et al., 2004; Kwak, Bohnen, Muller, Dayalu, & Seidler, 2013) and non-human primates (Matsumoto et al., 1999) with striatal dopamine depletion are impaired in the acquisition of new motor sequences. Perhaps most convincing, disruptions of BG output impair sequence acquisition (Brown et al., 2003; Obeso et al., 2009), but, as reviewed above, do not eliminate knowledge of prelearned sequences.
In summary, the BG, in general, and nigrostriatal dopamine specifically, play a central role in the acquisition of movement sequences. While it is clear that these structures also influence the expression of learned sequences, the sequences themselves do not seem to be stored within BG circuits.
4. DISEASE MECHANISMS AND MOUSE MODELS
There are three broad categories of criteria used to assess the relevance of animal models to human disease (Table 3.2). Face validity refers to the similarity between disease features and those exhibited by the animal model. Construct validity refers to similarity in pathophysiological mechanisms, and predictive validity refers to similarity of treatment efficacy (Albelda & Joel, 2012). The study of a model with any one type of validity may lead to new insights into disease mechanism, though the more categories the model matches, the easier it is to extrapolate findings to humans. Early OCD models provide an example in which the study of a model with face validity ultimately led to previously unknown pathophysiological mechanisms (Campbell et al., 1999; Greer & Capecchi, 2002; Powell, Newman, Pendergast, & Lewis, 1999). In the following section, we describe current efforts at modeling OCD-spectrum disorders, RTT, and primary dystonia, highlighting new findings as they relate to human disease.
Table 3.2.
Validity
| Disorder | Validity | |
|---|---|---|
| OCD-spectrum | Face | Ability to induce behaviors resembling compulsions or stereotypies. Repetitive, self-injurious grooming. Perseverative burying |
| Construct | Involvement of orbitofrontal cortex and basal ganglia | |
| Predictive | Response to SSRIs. Response to high-frequency stimulation of subthalamic nucleus or ventromedial striatum | |
| Rett syndrome | Face | Normal early development followed by developmental regression and motor abnormalities |
| Construct | Neuropathological abnormalities: smaller, more densely packed neurons | |
| Predictive | Unknown | |
| Dystonia | Face | Abnormal twisting movements, hyperactivity, action-induced abnormal movements |
| Construct | Deficient cortical and basal ganglia inhibition, maladaptive plasticity, sensory abnormalities, targeting genes known to be involved in human disease | |
| Predictive | Response to trihexyphenidyl or DBS |
5. OCD-SPECTRUM DISORDERS AND RODENT MODELS
As outlined in Section 2, dysfunction of a circuit including the orbitofrontal and anterior cingulate cortices, and ventral striatum are demonstrated repeatedly in functional imaging studies of OCD subjects (Graybiel & Rauch, 2000). Dysfunction of the orbitofrontal-BG network is considered the construct validity hallmark for OCD models. Other potential standards for construct validity include changes in neurochemical systems observed in imaging studies (e.g., dopaminergic, glutamatergic, and serotoninergic), though many of the imaging findings between different research groups are not consistent. Deep brain stimulation of the subthalamic nucleus or ventromedial striatum has been demonstrated effective in controlling OCD symptoms (Burdick, Goodman, & Foote, 2009; Greenberg et al., 2010, 2006; Lipsman, Neimat, & Lozano, 2007). Manipulation of these structures, for example, with optogenetic strategies, is therefore a strategy for demonstrating predictive validity, and may enable future investigations of the type of circuit modulation most effective in symptom control. Suppression of OCD-like behaviors by fluoxetine is the most commonly used pharmacological assay for predictive validity, and the specificity of this response is often addressed by showing that the norepinephrine transporter inhibitor desipramine is ineffective. Face validity is more difficult to assess. Behavioral assays include repetitive grooming or leaping, and perseverative object burying, which are not common human OCD behaviors. Measures of anxiety are useful ancillary methods, as anxiety is a common feature of OCD.
5.1. Spontaneous and circuit manipulation models
5.1.1 Optogenetic modeling of OCD
A study employing optogenetic methods provides direct evidence linking hyperactivity in the OCD circuit to persistent, repetitive behaviors (Ahmari et al., 2013). These investigators repeatedly activated ventromedial striatal terminals originating in orbitofrontal cortex and documented progressive increases in repetitive grooming. Acute stimulation of this circuit had no effect, nor did optogenetic stimulation of terminals originating from the motor or prelimbic cortices. The lack of effects of acute stimulation and persistence of repetitive grooming for up to 2 weeks following cessation of stimulation suggest activity in this circuit per se is not driving the OCD-like behavior. More durable alterations of synaptic strength or persistent alterations of function in a downstream structure may be involved. Regardless, this study provides strong causal evidence for hyperactivity of orbitofrontal striatal afferents in the generation of OCD-like behaviors.
5.1.2 Spontaneous stereotypy in deer mice
A large percentage of deer mice housed in laboratory colonies develop persistent stereotypies, including repetitive jumping, patterned running, and backward somersaulting. These repetitive behaviors occur spontaneously. Enriched housing conditions reduce the frequency of these behaviors (Powell et al., 1999). These deer mouse-specific stereotypies are distinct from analogous behaviors induced by dopamine agonists and are not exacerbated by treatment with these agents (Presti, Gibney, & Lewis, 2004; Presti, Powell, & Lewis, 2002). Several findings implicate abnormal corticostriatal activity in these behaviors. During repetitive rearing, striatal glutamate concentrations are elevated (Presti, Watson, Kennedy, Yang, & Lewis, 2004), striatal opioid concentrations are altered (Presti & Lewis, 2005), and intrastriatal administration of NMDA and D1 antagonists reduces these behaviors (Presti, Mikes, & Lewis, 2003). Metabolic imaging studies employing cytochrome oxidase histochemistry also implicate BG dysfunction in this model. Deer mice exhibiting high levels of stereotypies show decreased subthalamic nucleus cytochrome oxidase activity (Tanimura, King, Williams, & Lewis, 2011; Tanimura, Vaziri, & Lewis, 2010). Serotoninergic alterations are suggested by reduced density of striatal serotonin transporters (Wolmarans de, Brand, Stein, & Harvey, 2013), and selective serotonin-reuptake inhibitors (SSRIs) suppress these stereotypies (Korff, Stein, & Harvey, 2008). Deer mouse stereotypies exhibit significant face and predictive validity with suggestions of construct validity for OCD. The etiologic trigger causing these behaviors is unknown, however, limiting the utility of this model.
5.1.3 Sequential super-stereotypy in hyperdopaminergic mice
As described in Section 3, rodents exhibit a well-characterized stereotypic chain of grooming behaviors (Berridge, Fentress, & Parr, 1987), and investigators have dissected the neurobiological basis of these stereotypies by analyzing interventions that disrupt (Cromwell & Berridge, 1996; Cromwell et al., 1998) or enhance (Berridge & Aldridge, 2000a, 2000b; Deveney & Waddington, 1997) their expression. Grooming sequences exhibit an ordered chain of distinct movement phases (syntax) that is species specific. An important point is that such analyses not only assess the occurrence of grooming sequences but also quantify the rate of completion of the whole sequence after expression of the initial components. Enhanced sequence completion (super-stereotypy) is the putative homologue of OCD. Such studies strongly support a role for nigrostriatal neurotransmission in sequential behavior (Berridge et al., 2005; Cromwell et al., 1998). Pharmacologic studies indicated that super-stereotypy is driven by activation of D1 but not D2 receptors (Berridge & Aldridge, 2000a, 2000b). Berridge and colleagues (Berridge et al., 2005) examined “hyperdopaminergic” mice genetically modified to express ~10% of normal levels of the DAT, causing ~170% excess extracellular striatal dopamine concentrations (Zhuang et al., 2001) and altered corticostriatal neurotransmission (Wu, Cepeda, Zhuang, & Levine, 2007). These mice exhibit a clear super-stereotypy. This supports a role for nigrostriatal dysfunction in the pathogenesis of OCD-spectrum disorders and provides a potential mechanism underlying the development of overly rigid sequential behaviors. The results of this study are consistent with experiments that disrupt grooming behaviors (Cromwell et al., 1998) and those that induce stereotypies via pharmacological manipulation (Berridge & Aldridge, 2000a, 2000b; Deveney & Waddington, 1997). Considered together, these features mark DAT knockdown mice as a model with strong face validity for OCD-spectrum disorders.
5.1.4 “Genetic neurostimulatory” model
Functional imaging suggests that abnormal activity of corticostriate afferents is linked to OCD-spectrum behaviors. By selectively expressing a cholera toxin subunit enhancing G-protein signaling in cortical inputs to ventral striatum, Campbell and colleagues produced mice that exhibit a variety of repetitive behaviors, including repetitive leaping and nonaggressive biting of littermates. These mice expressed the “neurostimulatory” transgene selectively in layer 2 of piriform cortex, layers 2–3 of somatosensory cortex, and the intercalated nucleus of the amygdala. These cortical regions preferentially innervate ventral striatum and, in turn, the intercalated nucleus regulates outputs to the ventral striatum and prefrontal cortices (Campbell et al., 1999; McGrath, Campbell, & Burton, 1999). In this model, drugs that activate corticostriatal glutamate release exacerbate the OCD-like behaviors. This model demonstrates that chronic potentiation of cortical and limbic neurons, which likely increases excitatory inputs to the striatum, causes repetitive behavior (McGrath, Campbell, Parks, & Burton, 2000).
5.2. Gene-targeted models
5.2.1 Hoxb8 mutant mice
Mice null for the Hoxb8 homeobox transcription factor were the first genetic model of excessive grooming and self-injury (Greer & Capecchi, 2002). Subsequent reports of Hoxb8 inactivation confirmed this striking phenotype (Chen, Tvrdik, et al., 2010; Holstege et al., 2008). These mice spend at least twice as much time grooming, and initiate more syntactic grooming sequences under natural conditions, as well as following induction of grooming via water drops or misting.
Analysis of different lines of Hoxb8-null mice indicates varied mechanisms for the observed behaviors, highlighting the potential pitfalls of relying on face validity alone. The repetitive behaviors and hairless patches observed in an independent line of Hoxb8 mutants generated by Holstege and colleagues (Holstege et al., 2008) differ from those observed in the original line analyzed (Greer & Capecchi, 2002), as they appear to result primarily from a sensory abnormality (i.e., excessive itch). This important distinction derives from the fact that local lidocaine treatment alleviates excessive grooming in one line (Holstege et al., 2008) but has no effect on the original null mutant (Chen, Tvrdik, et al., 2010). The difference between these models is probably related to the different genetic strategies used for Hoxb8 inactivation. The original line (Chen, Tvrdik, et al., 2010; Greer & Capecchi, 2002) contains a nonsense codon in the first exon, while the later mutant was generated with a LacZ reporter knockin into the first exon (Holstege et al., 2008; van den Akker et al., 1999). The LacZ knockin allele may lead to a more complex phenotype by interfering with closely neighboring Hox genes (Chen, Tvrdik, et al., 2010). Both models demonstrate the previously unsuspected importance of Hoxb8 in the function of the adult nervous system. Indeed, the presence of hairless patches in the line that does not exhibit sensory abnormalities (Greer & Capecchi, 2002) suggests that Hoxb8 dysfunction may participate in the pathogenesis of trichotillomania, an OC-spectrum disorder characterized by compulsive pulling out of one’s own hair (but does not feature sensory abnormalities such as excessive itch; Franklin, Zagrabbe, & Benavides, 2011).
The behavioral phenotype of Hoxb8-null mice arises from an unexpected source: CNS microglia (Chen, Tvrdik, et al., 2010). While Hoxb8 is expressed widely throughout the adult CNS, including OCD circuit regions, expression is limited to microglia. Definitive proof comes from experiments in which grooming behavior abnormalities are recapitulated by conditionally deleting Hoxb8 from the hematopoietic system. Rescue of behavioral abnormalities in constitutive knockout mice follows transplants of wild-type bone marrow. Conversely, some control mice receiving marrow transplants from Hoxb8 mutant mice develop hairless patches (Chen, Tvrdik, et al., 2010). Chen et al. suggest several possible mechanisms for microglial dysfunction disrupting OCD circuit function, including regulation of developmental neuronal death, cytokine-mediated stimulation or inhibition of neuronal activity, and the stabilization and maintenance of neural networks. Studies to date do not demonstrate directly any abnormalities of the putative frontostriatal OCD circuit. These findings fall within a larger literature linking immune dysfunction to neuropsychiatric syndromes (Hyman, 2010) and illustrate the unexpected and complex mechanisms that may occur in models identified by face validity.
5.2.2 SAPAP3 mutant mice
SAPAP3 (SAP90/PSD95-associated protein 3, or DLGAP3, or GKAP) is a member of the SAPAP family of postsynaptic scaffolding proteins interacting with PSD95 and Shank protein families (Kim et al., 1997; Takeuchi et al., 1997) at the postsynaptic excitatory synapse. These proteins form scaffolding complexes providing key regulation of postsynaptic excitatory signaling via trafficking and targeting of interacting proteins in the postsynaptic membrane. SAPAP proteins are widely expressed in the brain, and SAPAP3 is particularly enriched in the striatum (Welch, Wang, & Feng, 2004).
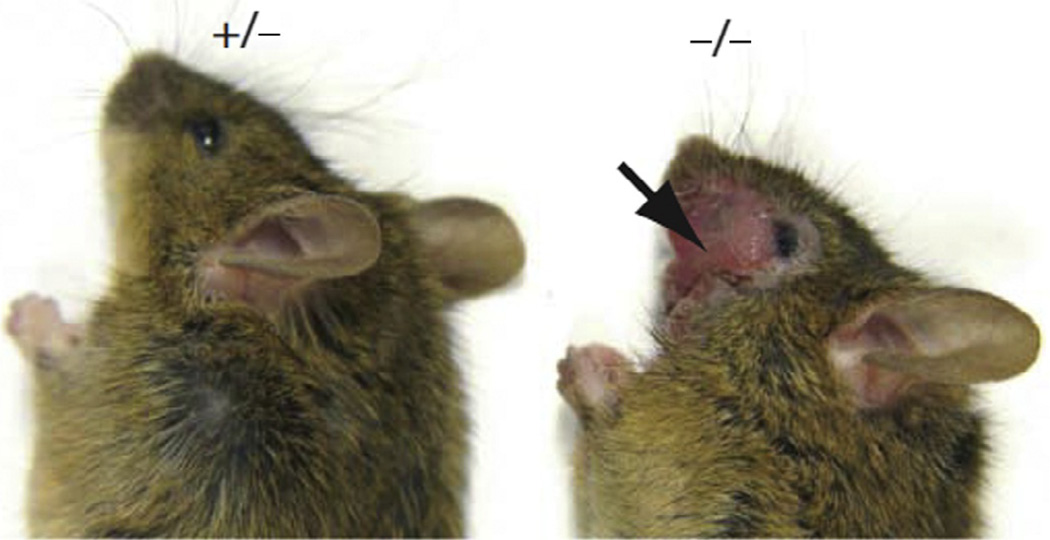
SAPAP3-null mice exhibit a striking phenotype. These animals are initially indistinguishable from littermate controls, but by 4–6 months develop skin lesions of the head, neck, and snout (Welch et al., 2007) (Fig. 3.2). Increased number and duration of grooming bouts and increased anxiety characterize the 100% penetrant behavioral phenotype. All aspects of this OCD-like syndrome are suppressed by chronic treatment with fluoxetine. Further, pathological grooming was significantly reduced following striatal reexpression of SAPAP3 using lentivirus vectors. Consistent with a critical role for corticostriatal neurotransmission, SAPAP3-null mice exhibit specific reductions in excitatory postsynaptic field potentials and subtle defects in the structure of excitatory synapse postsynaptic complexes (Welch et al., 2007). Loss of SAPAP3 reduces AMPA receptor-mediated corticostriatal excitatory transmission in MSNs by enhancing receptor endocytosis through an mGluR5-dependent mechanism (Wan, Feng, & Calakos, 2011), suggesting that SAPAP3 normally functions to inhibit mGluR5- mediated endocytosis of AMPA receptors (Welch et al., 2007). Importantly, these effects appear relatively specific for the corticostriatal circuit, as thalamostriatal synaptic function remains intact in SAPAP3-null mice, possibly because of the expression of SAPAP4 in thalamostriatal synapses (Wan et al., 2013). These findings further support a role for dysfunctional excitatory corticostriatal neurotransmission in the pathogenesis of OCD.
Figure 3.2.
SAPAP3−/− mice exhibit facial lesions due to self-injurious grooming. Figure reprinted with permission from Macmillan Publishers Ltd.: [Nature] (Welch et al., 2007), copyright 2007.
Work from Xu and colleagues adds another piece to the SAPAP3 puzzle, demonstrating a functional interaction between SAPAP3 and the melanocortin 4 receptor (MC4R) (Xu et al., 2013). MC4R activation causes AMPAR endocytosis, and MC4R may also function in ventral striatal D1-bearing neurons to mediate behavioral responses to stress and procedural learning (Cui et al., 2012; Lim, Huang, Grueter, Rothwell, & Malenka, 2012). Loss of MC4R suppresses the compulsive behavior of SAPAP3 KO mice and, conversely, deletion of SAPAP3 reverses the metabolic abnormalities of MC4R-null mice. These effects were accompanied by normalization of striatal AMPAR-mediated excitatory field potentials. Whereas antagonism of MCR4 function did not affect grooming behavior in wild-type mice, chronic MC4R antagonism reduced grooming time and the number of grooming bouts in SAPAP3 KO mice, establishing a role for this receptor in the mature CNS. By injecting Cre-expressing viruses into SAPAP3-null mice carrying floxed MC4R alleles, Xu and colleagues confirmed that these effects resulted from MC4R function in the orbitofrontal cortical—ventral striatal circuit.
Additional work implicates defects in inhibitory neurotransmission as well, showing that SAPAP3-null mice have significantly fewer parvalbumin-positive FSIs, and lack the learning-related tuning of MSNs that requires FSI function. The loss of FSIs in this mouse model mimics the observed loss of FSI in TS (Kalanithi et al., 2005; Kataoka et al., 2010). Optogenetic stimulation of orbitofrontal cortical fibers that terminate on FSIs rescues the electrophysiological and behavioral phenotypes, further supporting a role for these inhibitory interneurons (Burguiere, Monteiro, Feng, & Graybiel, 2013).
Considered together, these findings support the face, construct, and predictive validity of SAPA3-null mice as an OCD model. The multiple strengths of SAPAP3 as a model of OCD led human subject investigators to explore a role for SAPAP3 dysfunction as a cause of human OCD. Several preliminary studies have identified sequence variants in SAPAP3 in patients with OCD and related grooming disorders (Bienvenu et al., 2009; Boardman et al., 2011; Crane et al., 2011; Zuchner et al., 2009). Future studies in larger patient populations will be required to determine the contribution of SAPAP3 as a cause or risk factor for human OCD-spectrum disorders.
5.2.3 SLITRK-null mice
Two models of OCD are based on findings of an association of sequence variants in SLITRK1, a member of the SLITRK family of integral membrane proteins. The SLITRK family is so named because of similarities with the SLIT family (extracellular LRR repeats) and Trk tyrosine kinase receptors (intracellular tyrosine kinase domain). SLITRK1 sequence variants are associated with both TS and OCD (Abelson et al., 2005; Karagiannidis et al., 2012; Ozomaro et al., 2013), though some have questioned this association (e.g., Keen-Kim et al., 2006). Although the association of SLITRK variants with TS and OCD is highly controversial, these proteins are rational candidates for neurodevelopmental disease, as they are developmentally regulated, linked to neurite outgrowth (Aruga & Mikoshiba, 2003), and are widely expressed in CNS neurons (Beaubien & Cloutier, 2009; Stillman et al., 2009).
Slitrk1-null mice exhibit significantly increased “anxiety-like behaviors” in the elevated plus maze and open-field tasks, and increased freezing in a fear-conditioning task (Katayama et al., 2010). Norepinephrine levels in prefrontal cortex and norepinephrine metabolite levels in nucleus accumbens are modestly elevated, but the relationship of these findings to the behavioral phenotype is unclear. The administration of clonidine attenuates the mutant phenotype while not affecting the behavior of wild type mice. Clinical reports suggest that clonidine is successful in treating TS (Cohen, Detlor, Young, & Shaywitz, 1980; Cohen, Young, Nathanson, & Shaywitz, 1979; Robertson, 2000) and comorbid ADHD (Rizzo, Gulisano, Cali, & Curatolo, 2013; Robertson, 2006), but no conclusive clinical trial demonstrates its effectiveness. The strength of this model remains uncertain due to its limited characterization and the absence of stronger evidence of construct and predictive validity.
While no associations with TS or OCD are published for SLITRK5, deletion of this gene in mice generates a model with face, construct, and predictive validity. Slitrk5-null mice appear normal and perform similarly to littermate controls on several motor tasks, but show increased measures of anxiety. At approximately 3 months of age, these mice exhibit pathologically increased grooming and resultant facial lesions reminiscent of those seen in SAPAP3 and Hoxb8-null mice. Heterozygous null mice also develop increased grooming and facial lesions, but approximately 4–6 months later than in the null animals. The repetitive and anxiety-related behavior is nearly extinguished following chronic treatment with fluoxetine. In null mice, FosB immunoreactivity is selectively decreased in the orbitofrontal cortex, indicating a relatively specific functional defect in the OCD circuit. Electrophysiological, morphological, and protein abundance studies of glutamatergic receptors revealed additional abnormalities in this circuit. Considered together, these studies provide strong support for the role of Slitrk5, and perhaps other family members, in the pathogenesis of OCD spectrum symptoms. The construct and predictive validity of this model suggest that it would be fruitful to search for Slitrk5 sequence alterations in patients with OCD and Tourettism.
5.2..4 Histidine decarboxylase-deficient mice
Genome-wide association studies have not identified risk factors that apply broadly to TS (Scharf et al., 2013). A small number of families exist that transmit TS with Mendelian frequency, however, allowing for an alternative strategy for the identification of potential pathways and disease mechanisms. A family carrying the nonsense mutation (W317X) in the HDC gene encoding histidine decarboxylase shows autosomal dominant transmission of TS (Ercan-Sencicek et al., 2010). Histidine decarboxylase is responsible for the synthesis of histamine from histidine (Schwartz, Arrang, Garbarg, Pollard, & Ruat, 1991). This mutation has not been identified outside of this index family, but additional studies identify HDC polymorphisms (Karagiannidis et al., 2013) and implicate histaminergic pathway abnormalities (Fernandez et al., 2012) in TS.
The W317X mutation truncates the HDC protein, abolishing its enzymatic activity (Ercan-Sencicek et al., 2010). While this suggests haploinsufficiency (Fleming & Wang, 2003), enzymatic assays indicate a dominant negative effect of the mutant protein (Ercan-Sencicek et al., 2010). In a recent study, Castellan Baldan et al. (2014) studied Hdc-null mice, which contained undetectable tissue histamine levels. These mice appear normal in open-field testing and do not exhibit motor stereotypies. Following a single dose of amphetamine, however, Hdc-null mice develop a significantly greater number of motor stereotypies, such as repetitive focused sniffing and orofacial movements. Hdc heterozygotes developed similarly abnormal stereotypies when administered a higher dose of amphetamine. Intracerebroventricular histamine pretreatment fully eliminated amphetamine-induced stereotypies in Hdc-null mice, confirming a direct role for histamine deficiency in the behavioral abnormalities. Similar to patients carrying the HDC W317X mutation, Hdc-null mice showed reduced auditory prepulse inhibition. No abnormalities of anxiety or excessive grooming were observed in these mice, though syntactic grooming was not examined.
Several experiments suggest that the behavioral effects of histamine deficiency act via altered dopamine function. Histamine infusion into Hdc-null mice significantly decreased striatal dopamine levels, consistent with a previously hypothesized negative regulation of dopamine by histamine (Schlicker, Malinowska, Kathmann, & Gothert, 1994). Fos staining of amphetamine-treated animals showed a pattern of abnormality consistent with enhanced dopamine signaling, and receptor-binding studies demonstrated dysregulation of D2 and D3 receptors. These findings are similar to those seen in PET studies of patients carrying the HDC W317X mutation, using a high-affinity D2 and D3 receptor agonist tracer (11C-PHNO). Amphetamine-induced stereotypies were attenuated by pretreatment with dopamine D2 antagonists. While this implies predictive validity for TS, D2 antagonists reduce total movement, which may confound these results.
Considering the recent associations of the histamine pathway with TS (Ercan-Sencicek et al., 2010; Fernandez et al., 2012; Karagiannidis et al., 2013), the Hdc mutant mouse is an attractive model of TS. While overt stereotypies are not observed, altered prepulse inhibition and psychostimulant-induced repetitive movements represent a moderate level of face validity (Castellan Baldan et al., 2014). Importantly, these mice contain a high degree of construct and potential predictive validity, supporting their relevance to TS. These findings warrant future studies of histaminergic alterations in a larger population of TS patients.
Despite the high heritability of TS and the large number of genetic studies of OC-spectrum disorders, there is limited success in reproducibly finding disease-related genes. This highlights the complex nature of OC-spectrum genetics. Future analysis of animal models with high face, construct, and predictive validity may shed light on important disease genes of interest. Like the present models employing optogenetic targeting, spontaneous behaviors, or gene-targeted approaches, keen -observation of repetitive behaviors and systems-level pathologies will be important. A potential translation from animal models to human disorders in the context of BG dysfunction may allow for further understanding of these complex disorders.
6. RETT SYNDROME AND MOUSE MODELS
RTT is a devastating neurobehavioral syndrome of early childhood. As described in Section 2, the illness affects girls essentially exclusively who, among other symptoms, develop characteristic stereotypic handwringing movements. The causative protein, MeCP2 (Amir et al., 1999; Van den Veyver & Zoghbi, 2000; Wan et al., 1999) was little studied but known to be a transcriptional repressor prior to its connection to RTT (Jones et al., 1998; Lewis et al., 1992; Nan et al., 1998). As reviewed below, our understanding of MeCP2 function has vastly increased since the initial reports linking it to RTT.
6.1. Loss of MeCP2 function in neurons recapitulates the Rett syndrome phenotype
A mouse genetic study prior to these discoveries (Tate, Skarnes, & Bird, 1996) reported that Mecp2-null chimeric mice exhibited developmental defects and embryonic lethality, concluding that MeCP2 was essential for embryonic development. Subsequent investigators succeeded in generating viable Mecp2-null mice, leading to the suggestion that the original strategy also disrupted genes surrounding Mecp2 (Guy, Hendrich, Holmes, Martin, & Bird, 2001). The initial two groups reporting viable MeCP2-null mice recovered them in Mendelian frequencies (Chen, Akbarian, Tudor, & Jaenisch, 2001; Guy et al., 2001). These mutants do not differ from their littermate controls at birth, but develop progressive neurological deterioration leading to death by 7–10 weeks of age. Behavioral abnormalities include stiff, uncoordinated gait, reduced spontaneous movement, and both hindlimb and forelimb clasping. No histopathological abnormalities were identified in the brain or other organs. Both groups also demonstrated that conditional deletion of MeCP2 from the CNS caused a phenotype indistinguishable from the germline mutants, establishing distinctive CNS requirements for MeCP2 function.
Both groups also observed that heterozygous null female mice—a genocopy of the human disease—display a milder behavioral phenotype and histopathological abnormalities. These mice appeared normal until 3–4 months of age, after which they became hypoactive and ataxic (Chen et al., 2001; Guy et al., 2001), developing respiratory abnormalities similar to those observed in the human disease (Guy et al., 2001). Chen and colleagues found the behavioral abnormalities were accompanied by subtle histochemical abnormalities, including smaller, more densely packed neurons in the cortex, cerebellum, and hippocampus. Female mice with heterozygous deletions confined to the CNS (using Nestin-Cre) displayed a similar phenotype, in agreement with the complete loss-of-function studies. Nestin-Cre causes deletion from neurons and glia, so Chen and colleagues further dissected the responsible cell type by using CamKII-Cre to delete MeCP2 selectively from postmitotic neurons. These mice developed behavioral and histopathological abnormalities similar to heterozygous null females. These findings established MeCP2 mutant mice as a robust model of RTT with construct and face validity, and identified neuronal cell autonomous dysfunction of MeCP2 as key to disease pathogenesis. The unique neuronal requirement for MeCP2 was surprising, considering its ubiquitous expression and previous work implicating its function as a general regulator of transcription.
A subsequent RTT model was developed by mimicking a human RTT mutation that results in a truncated MeCP2 protein (Shahbazian et al., 2002). These mice appear normal up until 6 weeks of age, when they develop progressive motor abnormalities similar to those described in previous studies (Chen et al., 2001; Guy et al., 2001). They also developed increased anxiety (McGill et al., 2006). In contrast to germline MeCP2-null mice, these animals typically live to at least 1 year of age, and the truncated protein localizes to heterochromatin domains normally, suggesting it retains some function (Shahbazian et al., 2002).
Mutations in MECP2 are linked to several other neurological diseases, including MECP2 duplication syndrome, severe neonatal encephalopathy, and PPM-X (X-linked syndrome of psychosis, pyramidal signs, and macroorchidism) syndrome (Gonzales & LaSalle, 2010; Villard, 2007). Of these, MECP2 duplication syndrome, characterized by seizures, intellectual disability, and developmental regression, has been modeled in mice (Collins et al., 2004). In this model, approximately twofold MECP2 overexpression initially improved performance in motor and contextual learning paradigms and enhanced synaptic plasticity. By 5–6 months of age, however, these animals became hypoactive and developed reduced seizure threshold and subtle histopathological abnormalities (Bodda et al., 2013; Jiang et al., 2013; Marshak, Meynard, De Vries, Kidane, & Cohen-Cory, 2012; McGill et al., 2006; Na et al., 2012). This work emphasizes that neurons are sensitive to even modest changes in MeCP2 function and is consistent with the distinct neurological syndromes linked to various MECP2 mutations.
The facts that RTT mice initially appear normal, and that an RTT syndrome can be modeled by MeCP2 deletion in postnatal neurons, argues against the notion that initial brain formation is abnormal in RTT. Rather, this work pointed to a role for MeCP2 in maturing neurons, and potentially in adult neurons—a possibility with important implications for potential treatment strategies. To explore this issue, several investigators examined the ability of MeCP2 reexpression at various ages to rescue RTT model phenotypes, and the effects of inducing MeCP2 loss-of-function in the mature CNS (Cheval et al., 2012; McGraw, Samaco, & Zoghbi, 2011; Nguyen et al., 2012). Reexpression of MeCP2 in early postnatal or adult RTT mice rescues behavioral (Giacometti, Luikenhuis, Beard, & Jaenisch, 2007; Guy, Gan, Selfridge, Cobb, & Bird, 2007) and electrophysiological (Guy et al., 2007) phenotypes of MeCP2-null mice, even when reexpression was induced following phenotype onset (Guy et al., 2007). This work strongly implied an ongoing role for MeCP2 in adult neurons. Several groups have confirmed the importance of MeCP2 in mature neuronal function, finding that reducing MeCP2 function in mature mice recapitulates the core RTT phenotypes seen following germline or early conditional deletion (Cheval et al., 2012; McGraw et al., 2011; Nguyen et al., 2012). Surprisingly, this requirement for MeCP2 was present even when deleted at 30–45 weeks of age, when a ~80% reduction was lethal (Cheval et al., 2012). Adult MeCP2 deletion causes brain shrinkage and increased neuronal density, characteristics of human RTT (Nguyen et al., 2012). Brain shrinkage is accompanied by dramatic morphological changes in the hippocampus, including severe retraction and reductions in dendritic spines of pyramidal neurons, and less complex astrocyte morphology. While mRNA levels for a number of inhibitory and excitatory synaptic proteins are normal in these mutants, the proteins themselves were significantly decreased. This led to the suggestion that MeCP2 loss-of-function disrupts posttranslational regulation of key synaptic components. A critical implication of these findings is that treatment of RTT will likely require lifetime therapy.
6.2. Gene Expression and electrophysiological abnormalities in RTT: Insights from mouse models
Considerable effort is focused on using the RTT models described above to identify key pathophysiological events of potential value for RTT therapy. Given the role of MeCP2 as a transcriptional repressor (Jones et al., 1998; Lewis et al., 1992; Nan et al., 1998), these studies have focused on identifying genes dysregulated by MeCP2 dysfunction, and the electrophysiological consequences of these changes.
Global transcriptional profiling of forebrains from asymptomatic, early symptomatic, or late symptomatic MeCP2-null mice does not show robust changes in gene expression, even in mice with overt phenotypes (Tudor, Akbarian, Chen, & Jaenisch, 2002). This lack of differential gene expression contrasts with postmortem studies of brains from RTT patients, which show striking changes in expression patterns, including increased levels of glial transcripts and decreased levels of presynaptic neuron-specific mRNAs (Colantuoni et al., 2001). These differences may reflect the fact that human postmortem studies of brains from RTT subject show gliosis and synapse loss, features not observed in mouse models. While it is possible that MeCP2-null mice have gene expression changes below the limits of detection, Tudor et al. suggest several possible biological explanations for their negative findings, including compensation by other methyl-binding proteins, transcriptional dysregulation in a small subset of cells, or the presence of only very subtle changes to expression levels in a highly vulnerable population of cells. These authors also raised the possibility that MeCP2 does not act solely as a transcriptional regulator, and that the observed changes in gene expression are secondary to some other function of MeCP2 (Tudor et al., 2002).
Other investigators identified specific transcriptional abnormalities in Mecp2 mutant mice. Both targeted and broad analyses have been performed. The gene encoding Uqcrc1, for example, a component of the mitochondrial respiratory chain, was over expressed in MeCP2-null mice during the period that abnormal behaviors developed (Kriaucionis et al., 2006). Because subtle region-specific changes may be masked in whole-tissue analysis, some groups have performed gene expression analysis in specific brain regions. Thousands of altered genes were identified in the hypothalamus or cerebellum of mice lacking or overexpressing MeCP2 (Ben-Shachar, Chahrour, Thaller, Shaw, & Zoghbi, 2009; Chahrour et al., 2008). Approximately 85% of these genes were activated rather than repressed by MeCP2. This study highlights the heterogeneity of MeCP2 actions in different brain regions. In the hypothalamus and cerebellum, genes activated and repressed by MeCP2 fell into several functional categories, with enrichment for genes encoding neuropeptides and G protein-coupled receptors (Ben-Shachar et al., 2009; Chahrour et al., 2008).
Following the development of behavioral abnormalities, striata of MeCP2-null mice show reduced levels of genes encoding protein products involved in ion and chemical homeostasis, and increased levels of genes encoding proteins functioning in transcriptional regulation, development, and differentiation (Zhao, Goffin, Johnson, & Zhou, 2013). Bioinformatic analysis of differentially expressed genes in striatum, hippocampus, and cerebellum suggest a heterogeneous role for MeCP2 in these tissues (Zhao, Goffin, et al., 2013). This phenomenon is not specific to MeCP2 deletion during development, as alterations to gene expression also occur following deletion in adulthood (McGraw et al., 2011).
In different tissues, findings implicating alterations in both excitatory and inhibitory neurotransmission are described, but the specific transcriptional changes responsible for these effects are elusive. GABAergic neurotransmission, for example, was differentially affected in two different thalamic nuclei in MeCP2-null mice (Zhang, Zak, & Liu, 2010). MeCP2 appears to be enriched in cortical GABAergic compared to non-GABAergic neurons (Chao et al., 2010), and selective dysfunction of GABAergic neurotransmission is the most commonly reported electrophysiological abnormality in MeCP2-null mice. Decreases in GABA (but not glycine) release, reductions in the vesicular inhibitory transporter, and alterations in postsynaptic GABAergic receptors are found in the ventrolateral medulla of MeCP2-null mice (Medrihan et al., 2008). IPSP-based rhythmic activity is reduced in hippocampal slices from these animals (Zhang, He, Jugloff, & Eubanks, 2008). Conditional deletion of MeCP2 from all CNS GABAergic and glycine neurons (using a newly generated vesicular inhibitory amino acid transporter “Viaat”-Cre line) provided a particularly compelling link between disrupted inhibitory neurotransmission and phenotypes characteristic of germline MeCP2-null mice (Chao et al., 2010). Similar to the phenotype of germline MeCP2-null mice, deletion of MeCP2 with Viaat-Cre produced initially normal appearing mice that at ~5 weeks developed progressive motor dysfunction, repetitive stereotypies, and increased grooming and consequent skin lesions. These animals exhibited clear abnormalities in GABAergic neurotransmission, including decreased levels of GAD1, GAD2, and GABA, accompanied by a decrease in GABA release and network hyperexcitability (Chao et al., 2010).
The repetitive stereotypies in “Viaat-Cre” mice are of particular interest, as compulsive-like repetitive behaviors and stereotypic hand wringing occur in RTT subjects. This aspect of the Viaat-cre/MeCP2 line indicated an overlap with OCD-spectrum mouse models and, along with motor dysfunction phenotypes, suggested that dysfunction of forebrain structures including ventral striatum could underlie the behaviors. Chao and colleagues tested this possibility by conditionally deleting MeCP2 with Dlx5/6-Cre mice that causes recombination in all forebrain GABAergic and cholinergic neurons. Consistent with a role in the OCD circuit, Dlx5/6 conditional MeCP2-null mice showed repetitive behaviors, impaired motor coordination, and increased prepulse inhibition.
While the effects of MeCP2 loss-of-function appear to predominantly affect GABAergic neurotransmission, disruption of glutamatergic signaling has also been observed. In MeCP2-null cortex, reductions of glutamatergic neurotransmission were identified, including reduced spontaneous firing of layer 5 somatosensory pyramidal neurons (Dani et al., 2005) and decreased numbers of hippocampal glutamatergic synapses (Chao, Zoghbi, & Rosenmund, 2007). The findings in cortex reflected a reduced excitatory and increased inhibitory total drive onto glutamatergic neurons, rather than changes in intrinsic excitability. The effects onglutamatergic synapse formation were bidirectional, with an increase in synapse number in MeCP2 overexpressing mice, consistent with the clinical consequences of MECP2 gene duplication.
7. PRIMARY DYSTONIA
Modeling primary dystonia is challenging, because manipulation of genes that cause the disease have not, in general, produced models with overt dystonic movements. Further complicating this situation, dystonialike twisting may be caused by neural insults not thought relevant to causes of human dystonia. For example, abnormal twisting movements can be caused by disrupting the vestibular nuclei (Burke & Fahn, 1983), structures not believed to participate in the pathophysiology of human dystonia. While “behavioral” models of dystonia have face validity and may lead to novel insights into motor circuitry, particular care must be taken in interpreting the findings from models not based upon causes of human dystonia (i.e., models lacking construct validity).
Several models of secondary dystonia exhibit prolonged abnormal twisting movements consistent with human dystonias (Burke & Karanas, 1990). Thus far, however, none of the mouse models based on the manipulation of primary dystonia-causing genes exhibit dystonic-like movements. Instead, such models (most commonly Tor1a) have typically resulted in relatively subtle abnormalities on motor tasks (e.g., beam walking or rotarod) (Dang, Yokoi, Pence, & Li, 2006; Sciamanna, Hollis, et al., 2012; Yokoi, Dang, Li, Standaert, & Li, 2011; Yokoi, Dang, Mitsui, Li, & Li, 2008). In contrast, a recent rat TOR1A transgenic model is reported to exhibit hindlimb twisting during the tail-suspension test (Grundmann et al., 2012). Another feature suggested to establish face validity is the abnormal co-contraction of agonist and antagonist muscles that characterize dystonia. However, this measure is not specific for dystonic movements (Tanabe, Kim, Alagem, & Dauer, 2009).
Clinical electrophysiological studies of dystonic patients highlight several features potentially valuable for assessing the face and construct validity of dystonia models, including deficient intracortical inhibition, maladaptive cortical plasticity, and abnormal sensory function. Hallett presented an attractive model in which subcortical (e.g., BG) abnormalities result in sensorimotor cortex dysfunction that is the proximate cause of dystonic movements. Deficient inhibitory function is arguably the most consistently identified pathophysiological phenotype in dystonia (Hallett, 2011). This abnormality has been identified in genetic forms of childhood dystonia as well as idiopathic adult-onset disease. It is, however, not limited to brain regions subserving affected body parts, so appears to be a general phenomenon of the dystonic CNS that is perhaps necessary but not sufficient to produce dystonia (Berardelli, Rothwell, Day, & Marsden, 1985; Berardelli et al., 1998; Hallett, 2011; Nakashima et al., 1989; Panizza, Lelli, Nilsson, & Hallett, 1990; Tisch et al., 2006). Abnormal synaptic plasticity is another pathophysiological feature observed in a range of subjects with primary dystonia; this has been proposed as a key pathophysiological mechanism in dystonia (Edwards, Huang, Mir, Rothwell, & Bhatia, 2006; Peterson, Sejnowski, & Poizner, 2010; Quartarone, Classen, Morgante, Rosenkranz, & Hallett, 2009; Quartarone & Pisani, 2011; Quartarone, Siebner, & Rothwell, 2006). The occurrence of “task-specific” dystonias that arise in body parts of subjects used to perform highly trained stereotyped tasks, such as the fingers of musicians or telegraph operators, or the vocal cords of auctioneers, provides conceptual support for the notion that dystonia may represent maladaptive plasticity (Ferguson, 1971; Frucht, 2004; Scolding, Smith, Sturman, Brookes, & Lees, 1995). Indeed, impaired plasticity at striatal synapses has been a strong feature of several dystonia animal models (Bonsi et al., 2008; Martella et al., 2009; Peterson et al., 2010; Sciamanna et al., 2012). Dystonia patients show abnormalities of sensory processing and the organization of the somatosensory cortex is disrupted in the dystonic brain. (Bara-Jimenez, Catalan, Hallett, & Gerloff, 1998; Bara-Jimenez et al., 1998; Scontrini et al., 2009; Quartarone & Hallett, 2013). The demonstration of related sensory abnormalities in models of dystonia would therefore also serve as a measure of construct validity.
An emerging neuroimaging literature documenting relatively subtle structural abnormalities of the dystonic brain is challenging traditional views that primary dystonias lack structural pathologies and provides another opportunity to identify phenotypes shared by human subjects and mouse models (Ramdhani & Simonyan, 2013). This work includes voxel-based morphometry studies documenting differences in the size of various motor circuit components and diffusion tensor imaging pointing to microstructural abnormalities in key motor tracts. Neuroimaging has been used to provide support for the construct validity of a mouse model of DYT1 dystonia, as detailed below.
The most commonly used measure of predictive validity is the response of various phenotypes to the acute administration of the anticholinergic compound trihexyphenidyl. This is based on the fact that muscarinic anticholinergic medications are the most commonly used pharmacological treatment for primary dystonia (Burke & Fahn, 1985a, 1985b; Burke, Fahn, & Marsden, 1986; Fahn, 1983). Anticholinergic therapy is most practical and effective in younger patients, who can tolerate higher doses (Burke et al., 1986) and can be treated quickly after symptom onset (Greene, Shale, & Fahn, 1988). While undoubtedly effective in some patients, open trials and double-blind studies indicate that a substantial proportion of patients do not obtain long-term benefit, and dose-limiting side effects are common. Anticholinergic treatment was not beneficial in nearly 40% of subjects with childhood-onset dystonia, and only a minority of adult subjects experience long-term benefit from these medications (Burke & Fahn, 1985b; Fahn, 1983). Cognitive side effects such as memory loss and confusion are common in older adults (Burke et al., 1986; Taylor, Lang, Saint- Cyr, Riley, & Ranawaya, 1991). The use of anticholinergics as the “gold standard” of predictive validity for dystonia models may therefore be misleading. Other medications used to treat dystonia, including baclofen (GABAB receptor agonist) and benzodiazepines (enhance GABAA-mediated neurotransmission), may also be valuable for predictive validity studies. However, the low efficacy of these compounds precludes their use as a sole measure of predictive validity.
Whatever pharmacological strategy is employed, it is important to recall that chronic administration of medication, and even deep brain stimulation, is typically required for symptomatic improvement of dystonia. For example, weeks are typically required to observe benefit following DBS (Krauss, 2002) and benefits may continue to accrue over months (Vercueil, Krack, & Pollak, 2002; Vidailhet et al., 2005). This pattern contrasts sharply to that seen in PD and essential tremor, which respond over seconds to days to deep brain stimulation (Wichmann & Delong, 2006). These observations underscore the importance of not relying on acute responses to treatment interventions when assessing the predictive validity of dystonia models.
7.1. Early “phenotypic” models
Prior to the discovery of mutations that cause primary dystonia in humans, investigators relied on mutants with abnormal twisting movements that arose spontaneously in rodent colonies. Perhaps, the earliest example is the dystonia musculorum mouse (Duchen et al., 1964). This model is of uncertain significance to primary dystonia, because it exhibits progressive peripheral axonal neuropathy including nerve fiber loss in sensory ganglia, and degenerative changes in the spinal cord and brainstem (Duchen et al., 1964). The most notable spontaneous models that, like primary dystonia, do not develop overt histopathology, are the dystonic (dt) rat (Lorden, McKeon, Baker, Cox, & Walkley, 1984), dystonic dt/sz hamster (Loscher et al., 1989), and tottering mice (Neychev, Fan, Mitev, Hess, & Jinnah, 2008). These models, recently reviewed elsewhere (Wilson & Hess, 2013), provide a wealth of information on motor circuit function, and, in the case of the dystonic rat and tottering mice, highlight the role of hindbrain structures in abnormal twisting movements. The mutation responsible for the dystonic hamster is unknown, while the dystonic rat is caused by loss-of-function of the atcay gene. In humans, mutations in this gene cause “Cayman” ataxia, a syndrome of apparently static cerebellar ataxia and psychomotor retardation that may include dystonia (Bomar et al., 2003; Xiao & Ledoux, 2005). The apparently complex phenotype of Cayman ataxia and the paucity of clinical description of the Cayman ataxia phenotype make it difficult to determine which features of the dystonic rat and dystonic mice are relevant to primary dystonia. This highlights the challenge of studying “phenotypic” models of dystonia identified from face validity alone. The remainder of this section will therefore focus on models based on genes that cause human primary dystonia.
7.2. DYT1 dystonia
DYT1 dystonia was the first form of primary dystonia for which a causative gene was identified. This disease is caused by a dominantly inherited mutation in the TOR1A gene encoding the protein torsinA (Ozelius et al., 1997). All patients have the same mutation: an in-frame 3 base-pair deletion that removes a single glutamic acid (hence, “ΔE”). TorsinA is a member of the AAA + family of proteins (ATPases associated with a variety of cellular activities). These chaperone-like proteins typically function as oligomers and use ATP hydrolysis to disassemble protein complexes and alter protein conformations (Neuwald, Aravind, Spouge, & Koonin, 1999). TorsinA is localized to the endoplasmic reticular/nuclear membrane compartment (Kustedjo, Bracey, & Cravatt, 2000). While the function of torsinA in this compartment is not fully understood, several findings support its involvement in protein quality control. Biochemical studies show that the ΔE mutation impairs the ATPase activity of torsinA by inhibiting binding to proteins required for its enzymatic activity (LAP1 and LULL1) (Zhao, Brown, Chase, Eisele, & Schlieker, 2013). One consequence of inhibiting torsinA function is to enhance its concentration in the nuclear membrane where it interacts with several nuclear membrane-localized proteins (Goodchild & Dauer, 2004, 2005). There are numerous examples of cell biological effects observed when overexpressing wild-type torsinA that are reduced or abolished by the ΔE mutation (e.g., ;Caldwell et al., 2003 Chen, Burdette, et al., 2010; Hewett et al., 2007; Torres, Sweeney, Beaulieu, Shashidharan, & Caron, 2004). Among these is inhibited trafficking of polytopic membrane-bound proteins, including the DAT (Torres et al., 2004). Protein processing through the secretory pathway is defective in fibroblasts derived from subjects with DYT1 dystonia and torsinA-null mice (Hewett et al., 2007). TorsinA was reported to participate in the protein “quality control” functions of the ER (Nery et al., 2011) and to interact with synaptic vesicle membrane proteins (Granata, Watson, Collinson, Schiavo, & Warner, 2008; Kakazu, Koh, Ho, Gonzalez-Alegre, & Harata, 2012).
7.2.1 Transgenic models
Several transgenic rodent models of DYT1 dystonia were generated by broadly overexpressing DYT1 mutant human torsinA (Grundmann et al., 2012, 2007; Sharma et al., 2005; Shashidharan et al., 2005). Of these models, two are reported to display overt twisting behavior during tail suspension (Grundmann et al., 2012; Sharma et al., 2005), whereas others exhibit statistical differences in measures of beam walking, pawprint analysis, and motor learning on the rotarod (Grundmann et al., 2007; Sharma et al., 2005). Expressing DYT1 mutant human torsinA selectively in dopaminergic neurons recapitulates statistically significant abnormalities in beam walking seen in other models, but does not cause dystonic-like twisting movements (Page et al., 2010). The reasons for the discrepancies in the behavioral phenotypes of the broadly expressing models are not clear, but may result—at least in part—from differences in the pattern, expression levels, and insertion sites of the transgenes. For example, Sharma and colleagues did not generate independent lines of transgenic mice overexpressing levels of the DYT1 transgene similar to the hMT1 line (Sharma et al., 2005), as is typical in the generation of transgenic models. This raises the possibility that some features of this widely studied line (Balcioglu et al., 2007; Hewett, Johanson, Sharma, Standaert, & Balcioglu, 2010; Napolitano et al., 2010; Pisani et al., 2006; Sciamanna et al., 2009, 2011; Sharma et al., 2005; Zhao, DeCuypere, & LeDoux, 2008) may result from insertional effects of the transgene, rather than the actions of DYT1 mutant torsinA protein.
Most studies of these transgenic models focused on dopaminergic and cholinergic signaling, the disruption of which are implicated in other forms of dystonia (e.g., dopa-responsive dystonia, tardive dystonias, and dystonic dyskinesias in PD) and by the response of dystonic symptoms to anticholinergic medication. Studies assessing baseline levels and evoked release of dopamine report conflicting results. Some find abnormal basal levels of dopamine but no changes to transporter or receptor activity (Grundmann et al., 2007; Shashidharan et al., 2005), while others find normal levels of dopamine at baseline, but abnormalities following dopamine-modulating drugs or differences in metabolite turnover (Balcioglu et al., 2007; Hewett et al., 2010; Page et al., 2010; Zhao et al., 2008). The study by Page and colleagues is notable for utilizing fast-scan cyclic voltammetry, a method capable of distinguishing neurotransmitter release and subsequent uptake. These authors found deficient dopamine release, whereas DAT-mediated dopamine uptake was unaffected. Because this model overexpresses DYT1 mutant torsinA selectively in dopaminergic neurons, it is unclear whether the ability to disrupt neurotransmitter release is specific to these cells, or whether torsinA can similarly affect other neuronal classes.
A series of studies by Pisani and colleagues, primarily utilizing the hMT1 line of DYT1 transgenic mice (Sharma et al., 2005) implicates abnormal function of striatal cholinergic interneurons in the pathogenesis of primary dystonia. These investigators demonstrate a paradoxical effect of D2 receptor stimulation on cholinergic interneuron firing, causing these cells to increase their firing rate (Pisani et al., 2006). In contrast, D2 receptor stimulation in control or transgenic mice overexpressing wild-type human torsinA does not cause detectable changes in cholinergic interneuron firing. Subsequent work on this model (Napolitano et al., 2010) pointed to postsynaptic D2 receptors as the site of this phenomenon. Conditionally deleting torsinA in cholinergic neurons recapitulated this paradoxical D2 effect, suggesting that torsinA dysfunction disrupts D2 receptor function on cholinergic interneurons in a cell autonomous fashion (Sciamanna, Tassone, et al., 2012). While the exact mechanism of D2 receptor dysregulation is unclear, abnormal interactions with N-type calcium channels and impaired coupling with RGS9 was suggested (Pisani et al., 2006). Additional work suggests that the D2 receptor dysfunction is responsible for the deficient “pause” response of cholinergic interneurons in hMT1 mice; this deficient pause is proposed to cause an imbalance in muscarinic M1 and M2 receptor stimulation that disrupts corticostriatal neurotransmission (Martella et al., 2009; Sciamanna, Tassone, et al., 2012). Similar abnormalities in corticostriatal neurotransmission are found in the transgenic rat model of DYT1 dystonia (Grundmann et al., 2012). Features of disrupted corticostriatal neurotransmission, including impaired long-term depression and synaptic depotentiation, are rescued by anticholinergic treatment. However, the relationship of these abnormalities to dystonic movements remains unclear, as they are present long before behavioral abnormalities appear (Sciamanna et al., 2011), and are present in rodents with and without abnormal twisting movements (Grundmann et al., 2012; Sciamanna, Tassone, et al., 2012; Sharma et al., 2005).
7.2.2 Gene-targeted Tor1a mutant mice
Two groups (Dang et al., 2006; Goodchild, Kim, & Dauer, 2005) used gene targeting to inactivate or “knockin” the DYT1 mutation into the Tor1a locus, and report similar effects. Both groups found that torsinA null and homozygous knockin mice are born in Mendelian ratios and appear morphologically normal, but die for unclear reasons during the first postnatal day. Goodchild and colleagues (Goodchild et al., 2005) identified morphological abnormalities in both torsinA null and homozygous knockin mice. They also observed decreased steady-state levels of mutant torsinA protein in the knockin mice, as well as skin fibroblasts derived from DYT1 subjects. Additional findings in torsinA-null mice indicate that neurons have a unique requirement for torsinA function. The nuclear membrane abnormalities observed in knockout and homozygous knockin lines occur exclusively in neurons, even though torsinA is expressed widely. In addition, these nuclear membrane abnormalities emerge in postmigratory maturing neurons, pointing to a specific role for torsinA in neuronal maturation. These findings are evocative of the neurodevelopmental signature of DYT1 dystonia. Considered together, these observations demonstrate that the DYT1 mutation impairs torsinA function, and highlight the importance of torsinA loss-of-function studies in advancing understanding the pathogenesis of DYT1 dystonia.
In contrast to the above findings, heterozygous mice that carry one DYT1 allele (a genocopy of human DYT1 subjects) are normally viable and do not exhibit overt motor abnormalities (Dang et al., 2005; Goodchild et al., 2005; Tanabe, Martin, & Dauer, 2012). These mice show DTI abnormalities similar to those seen in nonmanifesting DYT1 carriers, suggesting that they model the “nonmanifesting” carrier state (Ulug et al., 2011). Nonmanifesting DYT1 subjects exhibit metabolic and psychophysical abnormalities (Carbon et al., 2011, 2008; Carbon, Trost, Ghilardi, & Eidelberg, 2004; Ghilardi et al., 2003), which may in part be caused by the DTI observable microstructural and PET observable metabolic changes seen in humans and in these mice. Potential neuropathological and electrophysiological substrates of these findings are described in DYT1 heterozygous mice. These include subtle changes in cell size and synaptic structure in the striatum and cerebellum (Song et al., 2013), as well as abnormalities in synaptic vesicle recycling and calcium homeostasis (Iwabuchi, Kakazu, Koh, & Harata, 2013; Kakazu, Koh, Ho, et al., 2012; Kakazu, Koh, Iwabuchi, Gonzalez-Alegre, & Harata, 2012).
The early lethality of germline null mutants led investigators to conditionally delete torsinA in brain regions and cell types implicated in the disease. This work has focused on conditional deletion in cortex, striatum, and cerebellum (Sciamanna, Hollis, et al., 2012; Yokoi et al., 2011, 2008; Zhang et al., 2011). None of these models exhibit overt twisting movements. Nevertheless, conditional deletion of torsinA did alter motor function and produce cellular abnormalities. Deletion from cholinergic neurons (using ChAT-Cre) caused the D2-related electrophysiological abnormalities described above (Sciamanna, Hollis, et al., 2012). Conditional deletion from cortical glutamatergic neurons (using Emx1-Cre) caused hyperactivity and altered performance on beam walking, but did not disrupt dopamine metabolism or cortical barrel field patterning (Yokoi et al., 2008). Striatal specific torsinA-null mice (using Rgs9-Cre) also showed beam-walking deficits but were normal in open-field testing (Yokoi et al., 2011). These animals had reductions in striatal D2 receptors but did not exhibit abnormalities in dopamine metabolism. Subtle abnormalities of dendritic morphology were seen in cerebellar Purkinje cells following conditional deletion using Pcp2-Cre, but neither behavioral nor neurochemical studies were performed on these mutants (Zhang et al., 2011). The lack of overt phenotype in these conditional mutants is consistent with the possibility that DYT1 dystonia is a disorder requiring the simultaneous disruption of multiple motor circuit nodes for phenotypic expression. This notion has been emphasized previously (Tanabe et al., 2009) and is supported by the occurrence of dystonia following lesions to several different brain regions (Neychev et al., 2008; Prudente, Hess, & Jinnah, 2013). DYT1 dystonia is a neurodevelopmental disease (Tanabe et al., 2009), so torsinA deletion in these models may have occurred after the critical period for torsinA function has passed.
8. CONCLUDING REMARKS
Assisted by progress in identifying disease-related mutations and their phenotypic features, the expanding power of targeted mutations in rodents, and more sophisticated understanding of BG function, there has been remarkable progress in animal modeling of BG neurodevelopmental diseases in recent years. A better understanding of BG circuits and functions has allowed for the development of a large variety of mouse models, including spontaneously arising, transgenic, gene-targeted, and circuit manipulation models. Each of these approaches exhibits different levels of face, construct, and predictive validity, and provides unique benefits for understanding striatal-based disorders. Unfortunately, each model also exhibits specific drawbacks as they relate to human pathophysiological conditions. Considering each model in the context of the overall field will provide further insight into the function of these circuits in health and disease, and potential insight into novel therapeutic targets.
REFERENCES
- Abelson JF, Kwan KY, O’Roak BJ, Baek DY, Stillman AA, Morgan TM, et al. Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science. 2005;310:317–320. doi: 10.1126/science.1116502. [DOI] [PubMed] [Google Scholar]
- Ackermans L, Kuhn J, Neuner I, Temel Y, Visser-Vandewalle V. Surgery for Tourette syndrome. World Neurosurgery. 2013;80:S29.e15–S29.e22. doi: 10.1016/j.wneu.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Adler A, Katabi S, Finkes I, Prut Y, Bergman H. Different correlation patterns of cholinergic and GABAergic interneurons with striatal projection neurons. Frontiers in Systems Neuroscience. 2013;7:47. doi: 10.3389/fnsys.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, et al. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340:1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, et al. Phenomenology and classification of dystonia: A consensus update. Movement Disorders. 2013;28:863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albelda N, Joel D. Animal models of obsessive-compulsive disorder: Exploring pharmacology and neural substrates. Neuroscience & Biobehavioral Reviews. 2012;36:47–63. doi: 10.1016/j.neubiorev.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Albin RL, Koeppe RA, Bohnen NI, Nichols TE, Meyer P, Wernette K, et al. Increased ventral striatal monoaminergic innervation in Tourette syndrome. Neurology. 2003;61:310–315. doi: 10.1212/01.wnl.0000076181.39162.fc. [DOI] [PubMed] [Google Scholar]
- Albin RL, Koeppe RA, Wernette K, Zhuang W, Nichols T, Kilbourn MR, et al. Striatal [11C]dihydrotetrabenazine and [11C]methylphenidate binding in Tourette syndrome. Neurology. 2009;72:1390–1396. doi: 10.1212/WNL.0b013e3181a187dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in Neurosciences. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature Genetics. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Andalman AS, Fee MS. A basal ganglia-forebrain circuit in the songbird biases motor output to avoid vocal errors. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12518–12523. doi: 10.1073/pnas.0903214106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M. Responses of tonically active neurons in the primate’s striatum undergo systematic changes during behavioral sensorimotor conditioning. The Journal of Neuroscience. 1994;14:3969–3984. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss HU, Bressman SB, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. The Journal of Neuroscience. 2009;29:9740–9747. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J, Pineda M, Aibar E, Gean E, Monros E. Classic Rett syndrome in a boy as a result of somatic mosaicism for a MECP2 mutation. Annals of Neurology. 2001;50:692. doi: 10.1002/ana.1272. [DOI] [PubMed] [Google Scholar]
- Aruga J, Mikoshiba K. Identification and characterization of Slitrk, a novel neuronal transmembrane protein family controlling neurite outgrowth. Molecular and Cellular Neurosciences. 2003;24:117–129. doi: 10.1016/s1044-7431(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Ma Y, Okulski J, Dhawan V, Chaly T, Carbon M, et al. Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation. Neurology. 2005;64:347–349. doi: 10.1212/01.WNL.0000149764.34953.BF. [DOI] [PubMed] [Google Scholar]
- Balcioglu A, Kim MO, Sharma N, Cha JH, Breakefield XO, Standaert DG. Dopamine release is impaired in a mouse model of DYT1 dystonia. Journal of Neurochemistry. 2007;102:783–788. doi: 10.1111/j.1471-4159.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- Bara-Jimenez W, Catalan MJ, Hallett M, Gerloff C. Abnormal somatosensory homunculus in dystonia of the hand. Annals of Neurology. 1998;44:828–831. doi: 10.1002/ana.410440520. [DOI] [PubMed] [Google Scholar]
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- Beaubien F, Cloutier JF. Differential expression of Slitrk family members in the mouse nervous system. Developmental Dynamics. 2009;238:3285–3296. doi: 10.1002/dvdy.22160. [DOI] [PubMed] [Google Scholar]
- Beeler JA, Frank MJ, McDaid J, Alexander E, Turkson S, Bernandez MS, et al. A role for dopamine-mediated learning in the pathophysiology and treatment of Parkinson’s disease. Cell Reports. 2012;2:1747–1761. doi: 10.1016/j.celrep.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD. Disturbance of sequential movements in patients with Parkinson’s disease. Brain. 1987;110:361–379. doi: 10.1093/brain/110.2.361. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Bolam JP. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience. 1994;62:707–719. doi: 10.1016/0306-4522(94)90471-5. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar S, Chahrour M, Thaller C, Shaw CA, Zoghbi HY. Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Human Molecular Genetics. 2009;18:2431–2442. doi: 10.1093/hmg/ddp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Day BL, Marsden CD. Pathophysiology of blepharospasm and oromandibular dystonia. Brain. 1985;108:593–608. doi: 10.1093/brain/108.3.593. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121:1195–1212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- Berke JD. Uncoordinated firing rate changes of striatal fast-spiking interneurons during behavioral task performance. The Journal of Neuroscience. 2008;28:10075–10080. doi: 10.1523/JNEUROSCI.2192-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD. Functional properties of striatal fast-spiking interneurons. Frontiers in Systems Neuroscience. 2011;5:45. doi: 10.3389/fnsys.2011.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Aldridge JW. Super-stereotypy I: Enhancement of a complex movement sequence by systemic dopamine D1 agonists. Synapse. 2000a;37:194–204. doi: 10.1002/1098-2396(20000901)37:3<194::AID-SYN3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Aldridge JW. Super-stereotypy II: Enhancement of a complex movement sequence by intraventricular dopamine D1 agonists. Synapse. 2000b;37:205–215. doi: 10.1002/1098-2396(20000901)37:3<205::AID-SYN4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Aldridge JW, Houchard KR, Zhuang X. Sequential super-stereotypy of an instinctive fixed action pattern in hyper-dopaminergic mutant mice: A model of obsessive compulsive disorder and Tourette’s. BMC Biology. 2005;3:4. doi: 10.1186/1741-7007-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Fentress JC, Parr H. Natural syntax rules control action sequence of rats. Behavioural Brain Research. 1987;23:59–68. doi: 10.1016/0166-4328(87)90242-7. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Whishaw IQ. Cortex, striatum and cerebellum: Control of serial order in a grooming sequence. Experimental Brain Research. 1992;90:275–290. doi: 10.1007/BF00227239. [DOI] [PubMed] [Google Scholar]
- Berthier ML, Kulisevsky J, Gironell A, Heras JA. Obsessive-compulsive disorder associated with brain lesions: Clinical phenomenology, cognitive function, and anatomic correlates. Neurology. 1996;47:353–361. doi: 10.1212/wnl.47.2.353. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Booth PA, Eaton SA, Bolam JP. Selective innervation of neostriatal interneurons by a subclass of neuron in the globus pallidus of the rat. The Journal of Neuroscience. 1998;18:9438–9452. doi: 10.1523/JNEUROSCI.18-22-09438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharucha KJ, Sethi KD. Tardive tourettism after exposure to neuroleptic therapy. Movement Disorders. 1995;10:791–793. doi: 10.1002/mds.870100613. [DOI] [PubMed] [Google Scholar]
- Bienvenu OJ, Wang Y, Shugart YY, Welch JM, Grados MA, Fyer AJ, et al. Sapap3 and pathological grooming in humans: Results from the OCD collaborative genetics study. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009;150B:710–720. doi: 10.1002/ajmg.b.30897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Leckman JF, Zhu H, Peterson BS. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65:1253–1258. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Peterson BS, Scahill L, Otka J, Katsovich L, Zhang H, et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Archives of Pediatrics & Adolescent Medicine. 2006;160:65–69. doi: 10.1001/archpedi.160.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomstedt P, Sjoberg RL, Hansson M, Bodlund O, Hariz MI. Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurgery. 2013;80:e245–e253. doi: 10.1016/j.wneu.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Flaherty AW, Choi JK, Hochberg FH, Greve DN, Bonmassar G, et al. Basal ganglia activity remains elevated after movement in focal hand dystonia. Annals of Neurology. 2004;55:744–748. doi: 10.1002/ana.20108. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Kuster JK, Woodman SC, Kirlic N, Makhlouf ML, Multhaupt-Buell TJ, et al. Evidence for altered basal ganglia-brainstem connections in cervical dystonia. PLoS One. 2012;7:e31654. doi: 10.1371/journal.pone.0031654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue ME, Naidu S, Johnston MV. Altered development of glutamate and GABA receptors in the basal ganglia of girls with Rett syndrome. Experimental Neurology. 1999;156:345–352. doi: 10.1006/exnr.1999.7030. [DOI] [PubMed] [Google Scholar]
- Boardman L, van der Merwe L, Lochner C, Kinnear CJ, Seedat S, Stein DJ, et al. Investigating SAPAP3 variants in the etiology of obsessive-compulsive disorder and trichotillomania in the South African white population. Comprehensive Psychiatry. 2011;52:181–187. doi: 10.1016/j.comppsych.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Bodda C, Tantra M, Mollajew R, Arunachalam JP, Laccone FA, Can K, et al. Mild overexpression of Mecp2 in mice causes a higher susceptibility toward seizures. The American Journal of Pathology. 2013;183:195–210. doi: 10.1016/j.ajpath.2013.03.019. [DOI] [PubMed] [Google Scholar]
- Bomar JM, Benke PJ, Slattery EL, Puttagunta R, Taylor LP, Seong E, et al. Mutations in a novel gene encoding a CRAL-TRIO domain cause human Cayman ataxia and ataxia/dystonia in the jittery mouse. Nature Genetics. 2003;35:264–269. doi: 10.1038/ng1255. [DOI] [PubMed] [Google Scholar]
- Bonsi P, Martella G, Cuomo D, Platania P, Sciamanna G, Bernardi G, et al. Loss of muscarinic autoreceptor function impairs long-term depression but not long-term potentiation in the striatum. The Journal of Neuroscience. 2008;28:6258–6263. doi: 10.1523/JNEUROSCI.1678-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Translating birdsong: Songbirds as a model for basic and applied medical research. Annual Review of Neuroscience. 2013;36:489–517. doi: 10.1146/annurev-neuro-060909-152826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasic JR, Bibat G, Kumar A, Zhou Y, Hilton J, Yablonski ME, et al. Correlation of the vesicular acetylcholine transporter densities in the striata to the clinical abilities of women with Rett syndrome. Synapse. 2012;66:471–482. doi: 10.1002/syn.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressman SB, Sabatti C, Raymond D, de Leon D, Klein C, Kramer PL, et al. The DYT1 phenotype and guidelines for diagnostic testing. Neurology. 2000;54:1746–1752. doi: 10.1212/wnl.54.9.1746. [DOI] [PubMed] [Google Scholar]
- Broen M, Duits A, Visser-Vandewalle V, Temel Y, Winogrodzka A. Impulse control and related disorders in Parkinson’s disease patients treated with bilateral subthalamic nucleus stimulation: A review. Parkinsonism & Related Disorders. 2011;17:413–417. doi: 10.1016/j.parkreldis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Bronfeld M, Bar-Gad I. Tic disorders: What happens in the basal ganglia? The Neuroscientist. 2013;19:101–108. doi: 10.1177/1073858412444466. [DOI] [PubMed] [Google Scholar]
- Brown RG, Jahanshahi M, Limousin-Dowsey P, Thomas D, Quinn NP, Rothwell JC. Pallidotomy and incidental sequence learning in Parkinson’s disease. Neuroreport. 2003;14:21–24. doi: 10.1097/00001756-200301200-00004. [DOI] [PubMed] [Google Scholar]
- Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. The European Journal of Neuroscience. 2011;34:1997–2006. doi: 10.1111/j.1460-9568.2011.07914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VJ, Robbins TW. Elementary processes of response selection mediated by distinct regions of the striatum. The Journal of Neuroscience. 1989;9:3760–3765. doi: 10.1523/JNEUROSCI.09-11-03760.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick A, Goodman WK, Foote KD. Deep brain stimulation for refractory obsessive-compulsive disorder. Frontiers in Bioscience (Landmark Edition) 2009;14:1880–1890. doi: 10.2741/3348. [DOI] [PubMed] [Google Scholar]
- Burguiere E, Monteiro P, Feng G, Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science. 2013;340:1243–1246. doi: 10.1126/science.1232380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Fahn S. Chlorpromazine methiodide acts at the vestibular nuclear complex to induce barrel rotation in the rat. Brain Research. 1983;288:273–281. doi: 10.1016/0006-8993(83)90103-8. [DOI] [PubMed] [Google Scholar]
- Burke RE, Fahn S. Pharmacokinetics of trihexyphenidyl after short-term and long-term administration to dystonic patients. Annals of Neurology. 1985a;18:35–40. doi: 10.1002/ana.410180107. [DOI] [PubMed] [Google Scholar]
- Burke RE, Fahn S. Serum trihexyphenidyl levels in the treatment of torsion dystonia. Neurology. 1985b;35:1066–1069. doi: 10.1212/wnl.35.7.1066. [DOI] [PubMed] [Google Scholar]
- Burke RE, Fahn S, Marsden CD. Torsion dystonia: A double-blind, prospective trial of high-dosage trihexyphenidyl. Neurology. 1986;36:160–164. doi: 10.1212/wnl.36.2.160. [DOI] [PubMed] [Google Scholar]
- Burke RE, Karanas AL. Quantitative morphological analysis of striatal cholinergic neurons in perinatal asphyxia. Annals of Neurology. 1990;27:81–88. doi: 10.1002/ana.410270113. [DOI] [PubMed] [Google Scholar]
- Caldwell GA, Cao S, Sexton EG, Gelwix CC, Bevel JP, Caldwell KA. Suppression of polyglutamine-induced protein aggregation in Caenorhabditis elegans by torsin proteins. Human Molecular Genetics. 2003;12:307–319. doi: 10.1093/hmg/ddg027. [DOI] [PubMed] [Google Scholar]
- Campbell KM, de Lecea L, Severynse DM, Caron MG, McGrath MJ, Sparber SB, et al. OCD-Like behaviors caused by a neuropotentiating transgene targeted to cortical and limbic D1+ neurons. The Journal of Neuroscience. 1999;19:5044–5053. doi: 10.1523/JNEUROSCI.19-12-05044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon CM, Palmiter RD. Reward without dopamine. The Journal of Neuroscience. 2003;23:10827–10831. doi: 10.1523/JNEUROSCI.23-34-10827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Argyelan M, Ghilardi MF, Mattis P, Dhawan V, Bressman S, et al. Impaired sequence learning in dystonia mutation carriers: A genotypic effect. Brain. 2011;134:1416–1427. doi: 10.1093/brain/awr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Ghilardi MF, Argyelan M, Dhawan V, Bressman SB, Eidelberg D. Increased cerebellar activation during sequence learning in DYT1 carriers: An equiperformance study. Brain. 2008;131:146–154. doi: 10.1093/brain/awm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Trost M, Ghilardi MF, Eidelberg D. Abnormal brain networks in primary torsion dystonia. Advances in Neurology. 2004;94:155–161. [PubMed] [Google Scholar]
- Carli M, Evenden JL, Robbins TW. Depletion of unilateral striatal dopamine impairs initiation of contralateral actions and not sensory attention. Nature. 1985;313:679–682. doi: 10.1038/313679a0. [DOI] [PubMed] [Google Scholar]
- Carter JC, Lanham DC, Pham D, Bibat G, Naidu S, Kaufmann WE. Selective cerebral volume reduction in Rett syndrome: A multiple-approach MR imaging study. AJNR. American Journal of Neuroradiology. 2008;29:436–441. doi: 10.3174/ajnr.A0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Naidu S, Goldberg TE, Moser HW, Khoromi S, Kumar A, et al. Quantitative magnetic resonance imaging in Rett syndrome. The Journal of Neuropsychiatry and Clinical Neurosciences. 1991;3:66–72. doi: 10.1176/jnp.3.1.66. [DOI] [PubMed] [Google Scholar]
- Castellan Baldan L, Williams KA, Gallezot JD, Pogorelov V, Rapanelli M, Crowley M, et al. Histidine decarboxylase deficiency causes Tourette syndrome: Parallel findings in humans and mice. Neuron. 2014;81:77–90. doi: 10.1016/j.neuron.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Rickards H. The psychopathological spectrum of Gilles de la Tourette syndrome. Neuroscience & Biobehavioral Reviews. 2013;37:1008–1015. doi: 10.1016/j.neubiorev.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Seri S. Tourette’s syndrome. BMJ. 2013;347:f4964. doi: 10.1136/bmj.f4964. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PL, Nutt JG, Holford NH. Modeling the short- and long-duration responses to exogenous levodopa and to endogenous levodopa production in Parkinson’s disease. Journal of Pharmacokinetics and Pharmacodynamics. 2004;31:243–268. doi: 10.1023/b:jopa.0000039566.75368.59. [DOI] [PubMed] [Google Scholar]
- Chang HT, Kita H. Interneurons in the rat striatum: Relationships between parvalbumin neurons and cholinergic neurons. Brain Research. 1992;574:307–311. doi: 10.1016/0006-8993(92)90830-3. [DOI] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HT, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron. 2007;56:58–65. doi: 10.1016/j.neuron.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nature Genetics. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- Chen P, Burdette AJ, Porter JC, Ricketts JC, Fox SA, Nery FC, et al. The early-onset torsion dystonia-associated protein, torsinA, is a homeostatic regulator of endoplasmic reticulum stress response. Human Molecular Genetics. 2010;19:3502–3515. doi: 10.1093/hmg/ddq266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SK, Tvrdik P, Peden E, Cho S, Wu S, Spangrude G, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheval H, Guy J, Merusi C, De Sousa D, Selfridge J, Bird A. Postnatal inactivation reveals enhanced requirement for MeCP2 at distinct age windows. Human Molecular Genetics. 2012;21:3806–3814. doi: 10.1093/hmg/dds208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiron C, Bulteau C, Loc’h C, Raynaud C, Garreau B, Syrota A, et al. Dopaminergic D2 receptor SPECT imaging in Rett syndrome: Increase of specific binding in striatum. Journal of Nuclear Medicine. 1993;34:1717–1721. [PubMed] [Google Scholar]
- Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2012;108:2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary DR, Raslan AM, Rubin JE, Bahgat D, Viswanathan A, Heinricher MM, et al. Deep brain stimulation entrains local neuronal firing in human globus pallidus internus. Journal of Neurophysiology. 2013;109:978–987. doi: 10.1152/jn.00420.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DJ, Detlor J, Young JG, Shaywitz BA. Clonidine ameliorates Gilles de la Tourette syndrome. Archives of General Psychiatry. 1980;37:1350–1357. doi: 10.1001/archpsyc.1980.01780250036004. [DOI] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-typespecific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SC, Leckman JF, Bloch MH. Clinical assessment of Tourette syndrome and tic disorders. Neuroscience & Biobehavioral Reviews. 2013;37:997–1007. doi: 10.1016/j.neubiorev.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DJ, Young JG, Nathanson JA, Shaywitz BA. Clonidine in Tourette’s syndrome. Lancet. 1979;2:551–553. doi: 10.1016/s0140-6736(79)91614-3. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Jeon OH, Hyder K, Chenchik A, Khimani AH, Narayanan V, et al. Gene expression profiling in postmortem Rett syndrome brain: Differential gene expression and patient classification. Neurobiology of Disease. 2001;8:847–865. doi: 10.1006/nbdi.2001.0428. [DOI] [PubMed] [Google Scholar]
- Collins AL, Levenson JM, Vilaythong AP, Richman R, Armstrong DL, Noebels JL, et al. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Human Molecular Genetics. 2004;13:2679–2689. doi: 10.1093/hmg/ddh282. [DOI] [PubMed] [Google Scholar]
- Cordes M, Snow BJ, Cooper S, Schulzer M, Pate BD, Ruth TJ, et al. Age-dependent decline of nigrostriatal dopaminergic function: A positron emission tomographic study of grandparents and their grandchildren. Annals of Neurology. 1994;36:667–670. doi: 10.1002/ana.410360420. [DOI] [PubMed] [Google Scholar]
- Coutureau E, Killcross S. Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behavioural Brain Research. 2003;146:167–174. doi: 10.1016/j.bbr.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Cragg SJ. Meaningful silences: How dopamine listens to the ACh pause. Trends in Neurosciences. 2006;29:125–131. doi: 10.1016/j.tins.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Crane J, Fagerness J, Osiecki L, Gunnell B, Stewart SE, Pauls DL, et al. Family-based genetic association study of DLGAP3 in Tourette syndrome. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2011;156B:108–114. doi: 10.1002/ajmg.b.31134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden JR, Graybiel AM. Basal ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Frontiers in Neuroanatomy. 2011;5:59. doi: 10.3389/fnana.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC. Implementation of action sequences by a neostriatal site: A lesion mapping study of grooming syntax. The Journal of Neuroscience. 1996;16:3444–3458. doi: 10.1523/JNEUROSCI.16-10-03444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC, Drago J, Levine MS. Action sequencing is impaired in D1A-deficient mutant mice. The European Journal of Neuroscience. 1998;10:2426–2432. doi: 10.1046/j.1460-9568.1998.00250.x. [DOI] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Mason BL, Lee C, Nishi A, Elmquist JK, Lutter M. Melanocortin 4 receptor signaling in dopamine 1 receptor neurons is required for procedural memory learning. Physiology & Behavior. 2012;106:201–210. doi: 10.1016/j.physbeh.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang MT, Yokoi F, McNaught KS, Jengelley TA, Jackson T, Li J, et al. Generation and characterization of Dyt1 DeltaGAG knock-in mouse as a model for early-onset dystonia. Experimental Neurology. 2005;196:452–463. doi: 10.1016/j.expneurol.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Dang MT, Yokoi F, Pence MA, Li Y. Motor deficits and hyperactivity in Dyt1 knockdown mice. Neuroscience Research. 2006;56:470–474. doi: 10.1016/j.neures.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayalu P, Chou KL. Antipsychotic-induced extrapyramidal symptoms and their management. Expert Opinion on Pharmacotherapy. 2008;9:1451–1462. doi: 10.1517/14656566.9.9.1451. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Bottani A, Bouchardy I, Fluss J, Antonarakis SE, Haenggeli CA, et al. MECP2 mutant allele in a boy with Rett syndrome and his unaffected heterozygous mother. Brain Dev. 2007;29:47–50. doi: 10.1016/j.braindev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Delmaire C, Vidailhet M, Elbaz A, Bourdain F, Bleton JP, Sangla S, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer’s cramp. Neurology. 2007;69:376–380. doi: 10.1212/01.wnl.0000266591.49624.1a. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends in Neurosciences. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Deng H, Gao K, Jankovic J. The genetics of Tourette syndrome. Nature Reviews Neurology. 2012;8:203–213. doi: 10.1038/nrneurol.2012.26. [DOI] [PubMed] [Google Scholar]
- Denys D, de Vries F, Cath D, Figee M, Vulink N, Veltman DJ, et al. Dopa-minergic activity in Tourette syndrome and obsessive-compulsive disorder. European Neuropsychopharmacology. 2013;23(11):1423–1431. doi: 10.1016/j.euroneuro.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Turner RS. Motor sequences and the basal ganglia: Kinematics, not habits. The Journal of Neuroscience. 2010;30:7685–7690. doi: 10.1523/JNEUROSCI.0163-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveney AM, Waddington JL. Psychopharmacological distinction between novel full-efficacy “D1-like” dopamine receptor agonists. Pharmacology, Biochemistry and Behavior. 1997;58:551–558. doi: 10.1016/s0091-3057(97)00248-7. [DOI] [PubMed] [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron. 2010;67:294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolce A, Ben-Zeev B, Naidu S, Kossoff EH. Rett syndrome and epilepsy: An update for child neurologists. Pediatric Neurology. 2013;48:337–345. doi: 10.1016/j.pediatrneurol.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Doyon J. Motor sequence learning and movement disorders. Current Opinion in Neurology. 2008;21:478–483. doi: 10.1097/WCO.0b013e328304b6a3. [DOI] [PubMed] [Google Scholar]
- Draganski B, Thun-Hohenstein C, Bogdahn U, Winkler J, May A. “Motor circuit” gray matter changes in idiopathic cervical dystonia. Neurology. 2003;61:1228–1231. doi: 10.1212/01.wnl.0000094240.93745.83. [DOI] [PubMed] [Google Scholar]
- Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. Influence of phasic and tonic dopamine release on receptor activation. The Journal of Neuroscience. 2010;30:14273–14283. doi: 10.1523/JNEUROSCI.1894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen LW, Strich SJ, Falconer DS. Clinical and pathological studies of an hereditary neuropathy in mice (dystonia musculorum) Brain. 1964;87:367–378. doi: 10.1093/brain/87.2.367. [DOI] [PubMed] [Google Scholar]
- Dunn HG, MacLeod PM. Rett syndrome: Review of biological abnormalities. The Canadian Journal of Neurological Sciences. 2001;28:16–29. doi: 10.1017/s0317167100052513. [DOI] [PubMed] [Google Scholar]
- Dunn HG, Stoessl AJ, Ho HH, MacLeod PM, Poskitt KJ, Doudet DJ, et al. Rett syndrome: Investigation of nine patients, including PET scan. The Canadian Journal of Neurological Sciences. 2002;29:345–357. doi: 10.1017/s0317167100002213. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Huang YZ, Mir P, Rothwell JC, Bhatia KP. Abnormalities in motor cortical plasticity differentiate manifesting and nonmanifesting DYT1 carriers. Movement Disorders. 2006;21:2181–2186. doi: 10.1002/mds.21160. [DOI] [PubMed] [Google Scholar]
- Egger K, Mueller J, Schocke M, Brenneis C, Rinnerthaler M, Seppi K, et al. Voxel based morphometry reveals specific gray matter changes in primary dystonia. Movement Disorders. 2007;22:1538–1542. doi: 10.1002/mds.21619. [DOI] [PubMed] [Google Scholar]
- Ellens DJ, Leventhal DK. Review: Electrophysiology of basal ganglia and cortex in models of Parkinson disease. Journal of Parkinson’s Disease. 2013;3:241–254. doi: 10.3233/JPD-130204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English DF, Ibanez-Sandoval O, Stark E, Tecuapetla F, Buzsaki G, Deisseroth K, et al. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nature Neuroscience. 2012;15:123–130. doi: 10.1038/nn.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan-Sencicek AG, Stillman AA, Ghosh AK, Bilguvar K, O’Roak BJ, Mason CE, et al. L-histidine decarboxylase and Tourette’s syndrome. The New England Journal of Medicine. 2010;362:1901–1908. doi: 10.1056/NEJMoa0907006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S. High dosage anticholinergic therapy in dystonia. Neurology. 1983;33:1255–1261. doi: 10.1212/wnl.33.10.1255. [DOI] [PubMed] [Google Scholar]
- Felling RJ, Singer HS. Neurobiology of tourette syndrome: Current status and need for further investigation. The Journal of Neuroscience. 2011;31:12387–12395. doi: 10.1523/JNEUROSCI.0150-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson D. An Australian study of telegraphists’ cramp. British Journal of Industrial Medicine. 1971;28:280–285. doi: 10.1136/oem.28.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez TV, Sanders SJ, Yurkiewicz IR, Ercan-Sencicek AG, Kim YS, Fishman DO, et al. Rare copy number variants in Tourette syndrome disrupt genes in histaminergic pathways and overlap with autism. Biological Psychiatry. 2012;71:392–402. doi: 10.1016/j.biopsych.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald PM, Jankovic J, Glaze DG, Schultz R, Percy AK. Extrapyramidal involvement in Rett’s syndrome. Neurology. 1990;40:293–295. doi: 10.1212/wnl.40.2.293. [DOI] [PubMed] [Google Scholar]
- FitzGerald PM, Jankovic J, Percy AK. Rett syndrome and associated movement disorders. Movement Disorders. 1990;5:195–202. doi: 10.1002/mds.870050303. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JV, Wang TC. The production of 53–55-kDa isoforms is not required for rat L-histidine decarboxylase activity. The Journal of Biological Chemistry. 2003;278:686–694. doi: 10.1074/jbc.M210718200. [DOI] [PubMed] [Google Scholar]
- Fontaine D, Mattei V, Borg M, von Langsdorff D, Magnie MN, Chanalet S, et al. Effect of subthalamic nucleus stimulation on obsessive-compulsive disorder in a patient with Parkinson disease. Case report. Journal of Neurosurgery. 2004;100:1084–1086. doi: 10.3171/jns.2004.100.6.1084. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O’reilly RC. By carrot or by stick: Cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Franklin ME, Zagrabbe K, Benavides KL. Trichotillomania and its treatment: A review and recommendations. Expert Review of Neurotherapeutics. 2011;11:1165–1174. doi: 10.1586/ern.11.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC. Control of basal ganglia output by direct and indirect pathway projection neurons. The Journal of Neuroscience. 2013;33:18531–18539. doi: 10.1523/JNEUROSCI.1278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frucht SJ. Focal task-specific dystonia in musicians. Advances in Neurology. 2004;94:225–230. [PubMed] [Google Scholar]
- Fukuda T. Network architecture of gap junction-coupled neuronal linkage in the striatum. The Journal of Neuroscience. 2009;29:1235–1243. doi: 10.1523/JNEUROSCI.4418-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y, Hornykiewicz O, Fahn S, Kish SJ. Striatal dopamine in early-onset primary torsion dystonia with the DYT1 mutation. Neurology. 2000;54:1193–1195. doi: 10.1212/wnl.54.5.1193. [DOI] [PubMed] [Google Scholar]
- Gage GJ, Stoetzner CR, Wiltschko AB, Berke JD. Selective activation of striatal fast-spiking interneurons during choice execution. Neuron. 2010;67:466–479. doi: 10.1016/j.neuron.2010.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraux G, Bauer A, Hanakawa T, Wu T, Kansaku K, Hallett M. Changes in brain anatomy in focal hand dystonia. Annals of Neurology. 2004;55:736–739. doi: 10.1002/ana.20113. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annual Review of Neuroscience. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilardi MF, Carbon M, Silvestri G, Dhawan V, Tagliati M, Bressman S, et al. Impaired sequence learning in carriers of the DYT1 dystonia mutation. Annals of Neurology. 2003;54:102–109. doi: 10.1002/ana.10610. [DOI] [PubMed] [Google Scholar]
- Giacometti E, Luikenhuis S, Beard C, Jaenisch R. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1931–1936. doi: 10.1073/pnas.0610593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ, Marsden CD. Pathological report of four patients presenting with cranial dystonias. Movement Disorders. 1988;3:211–221. doi: 10.1002/mds.870030305. [DOI] [PubMed] [Google Scholar]
- Gittis AH, Leventhal DK, Fensterheim BA, Pettibone JR, Berke JD, Kreitzer AC. Selective inhibition of striatal fast-spiking interneurons causes dyskinesias. The Journal of Neuroscience. 2011;31:15727–15731. doi: 10.1523/JNEUROSCI.3875-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Nelson AB, Thwin MT, Palop JJ, Kreitzer AC. Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. The Journal of Neuroscience. 2010;30:2223–2234. doi: 10.1523/JNEUROSCI.4870-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW. Understanding dopamine and reinforcement learning: The dopamine reward prediction error hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl. 3):15647–15654. doi: 10.1073/pnas.1014269108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JH, Farries MA, Fee MS. Basal ganglia output to the thalamus: Still a paradox. Trends in Neurosciences. 2013;36:695–705. doi: 10.1016/j.tins.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JH, Fee MS. A cortical motor nucleus drives the basal ganglia-recipient thalamus in singing birds. Nature Neuroscience. 2012;15:620–627. doi: 10.1038/nn.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Reynolds JN. Spontaneous firing and evoked pauses in the tonically active cholinergic interneurons of the striatum. Neuroscience. 2011;198:27–43. doi: 10.1016/j.neuroscience.2011.08.067. [DOI] [PubMed] [Google Scholar]
- Gonzales ML, LaSalle JM. The role of MeCP2 in brain development and neurodevelopmental disorders. Current Psychiatry Reports. 2010;12:127–134. doi: 10.1007/s11920-010-0097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild RE, Dauer WT. Mislocalization to the nuclear envelope: An effect of the dystonia-causing torsinA mutation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:847–852. doi: 10.1073/pnas.0304375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild RE, Dauer WT. The AAA+ protein torsinA interacts with a conserved domain present in LAP1 and a novel ER protein. The Journal of Cell Biology. 2005;168:855–862. doi: 10.1083/jcb.200411026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild RE, Kim CE, Dauer WT. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron. 2005;48:923–932. doi: 10.1016/j.neuron.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Gordon N. Pantothenate kinase-associated neurodegeneration (Hallervorden-Spatz syndrome) European Journal of Paediatric Neurology. 2002;6:243–247. doi: 10.1053/ejpn.2002.0606. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry R. Functional mapping of sequence learning in normal humans. Journal of Cognitive Neuroscience. 1995;7:497–510. doi: 10.1162/jocn.1995.7.4.497. [DOI] [PubMed] [Google Scholar]
- Granata A, Watson R, Collinson LM, Schiavo G, Warner TT. The dystonia-associated protein torsinA modulates synaptic vesicle recycling. The Journal of Biological Chemistry. 2008;283:7568–7579. doi: 10.1074/jbc.M704097200. [DOI] [PubMed] [Google Scholar]
- Graveland GA, DiFiglia M. The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Research. 1985;327:307–311. doi: 10.1016/0006-8993(85)91524-0. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiology of Learning and Memory. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Baughman RW, Eckenstein F. Cholinergic neuropil of the striatum observes striosomal boundaries. Nature. 1986;323:625–627. doi: 10.1038/323625a0. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Gabriels LA, Malone DAJ, Rezai AR, Friehs GM, Okun MS, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: Worldwide experience. Molecular Psychiatry. 2010;15:64–79. doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- Greene P, Shale H, Fahn S. Analysis of open-label trials in torsion dystonia using high dosages of anticholinergics and other drugs. Movement Disorders. 1988;3:46–60. doi: 10.1002/mds.870030107. [DOI] [PubMed] [Google Scholar]
- Greer JM, Capecchi MR. Hoxb8 is required for normal grooming behavior in mice. Neuron. 2002;33:23–34. doi: 10.1016/s0896-6273(01)00564-5. [DOI] [PubMed] [Google Scholar]
- Grillner S, Robertson B, Stephenson-Jones M. The evolutionary origin of the vertebrate basal ganglia and its role in action selection. The Journal of Physiology. 2013;591:5425–5431. doi: 10.1113/jphysiol.2012.246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann K, Glockle N, Martella G, Sciamanna G, Hauser TK, Yu L, et al. Generation of a novel rodent model for DYT1 dystonia. Neurobiology of Disease. 2012;47:61–74. doi: 10.1016/j.nbd.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Grundmann K, Reischmann B, Vanhoutte G, Hubener J, Teismann P, Hauser TK, et al. Overexpression of human wildtype torsinA and human DeltaGAG torsinA in a transgenic mouse model causes phenotypic abnormalities. Neurobiology of Disease. 2007;27:190–206. doi: 10.1016/j.nbd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Kuwajima M, Mayhill E, Rebec GV. Behavior-related changes in the activity of substantia nigra pars reticulata neurons in freely moving rats. Brain Research. 1999;845:68–76. doi: 10.1016/s0006-8993(99)01932-0. [DOI] [PubMed] [Google Scholar]
- Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nature Genetics. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. The Journal of Neuroscience. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg B, Hanefeld F, Percy A, Skjeldal O. An update on clinically applicable diagnostic criteria in Rett syndrome. Comments to Rett Syndrome Clinical Criteria Consensus Panel Satellite to European Paediatric Neurology Society Meeting, Baden Baden, Germany, 11 September 2001. European Journal of Paediatric Neurology. 2002;6:293–297. doi: 10.1053/ejpn.2002.0612. [DOI] [PubMed] [Google Scholar]
- Hallett M. Neurophysiology of dystonia: The role of inhibition. Neurobiology of Disease. 2011;42:177–184. doi: 10.1016/j.nbd.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JC, Wong DF, Wagner HNJ, Rett A, Naidu S, Dannals RF, et al. Positron emission tomographic study of D2 dopamine receptor binding and CSF biogenic amine metabolites in Rett syndrome. American Journal of Medical Genetics Supplement. 1986;1:201–210. doi: 10.1002/ajmg.1320250523. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, Lopez-Sola M, Hernandez-Ribas R, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Archives of General Psychiatry. 2009;66:1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- Hewett J, Johanson P, Sharma N, Standaert D, Balcioglu A. Function of dopamine transporter is compromised in DYT1 transgenic animal model in vivo. Journal of Neurochemistry. 2010;113:228–235. doi: 10.1111/j.1471-4159.2010.06590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett JW, Tannous B, Niland BP, Nery FC, Zeng J, Li Y, et al. Mutant torsinA interferes with protein processing through the secretory pathway in DYT1 dystonia cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7271–7276. doi: 10.1073/pnas.0701185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth J, Blackwell KT, Kotaleski JH. Gap junctions between striatal fast-spiking interneurons regulate spiking activity and synchronization as a function of cortical activity. The Journal of Neuroscience. 2009;29:5276–5286. doi: 10.1523/JNEUROSCI.6031-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Sotak BN, Palmiter RD. Morphine reward in dopamine-deficient mice. Nature. 2005;438:854–857. doi: 10.1038/nature04172. [DOI] [PubMed] [Google Scholar]
- Holmes AL, Forcelli PA, DesJardin JT, Decker AL, Teferra M, West EA, et al. Superior colliculus mediates cervical dystonia evoked by inhibition of the substantia nigra pars reticulata. The Journal of Neuroscience. 2012;32:13326–13332. doi: 10.1523/JNEUROSCI.2295-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege JC, de Graaff W, Hossaini M, Cardona Cano S, Jaarsma D, van den Akker E, et al. Loss of Hoxb8 alters spinal dorsal laminae and sensory responses in mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6338–6343. doi: 10.1073/pnas.0802176105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton JL, Schneider SA, Ganesharajah T, Gandhi S, Strand C, Shashidharan P, et al. Neuropathology of primary adult-onset dystonia. Neurology. 2008;70:695–699. doi: 10.1212/01.wnl.0000302175.76229.f0. [DOI] [PubMed] [Google Scholar]
- Horak FB, Anderson ME. Influence of globus pallidus on arm movements in monkeys. I. Effects of kainic acid-induced lesions. Journal of Neurophysiology. 1984;52:290–304. doi: 10.1152/jn.1984.52.2.290. [DOI] [PubMed] [Google Scholar]
- Humphries MD, Wood R, Gurney K. Dopamine-modulated dynamic cell assemblies generated by the GABAergic striatal microcircuit. Neural Networks. 2009;22:1174–1188. doi: 10.1016/j.neunet.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Stacey ME, Coppola R, Handel SF, Rickler KC, Weinberger DR. Cerebral morphometric abnormalities in Tourette’s syndrome: A quantitative MRI study of monozygotic twins. Neurology. 1995;45:1176–1182. doi: 10.1212/wnl.45.6.1176. [DOI] [PubMed] [Google Scholar]
- Hyman SE. A bone to pick with compulsive behavior. Cell. 2010;141:752–754. doi: 10.1016/j.cell.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Inase M, Buford JA, Anderson ME. Changes in the control of arm position, movement, and thalamic discharge during local inactivation in the globus pallidus of the monkey. Journal of Neurophysiology. 1996;75:1087–1104. doi: 10.1152/jn.1996.75.3.1087. [DOI] [PubMed] [Google Scholar]
- Iwabuchi S, Kakazu Y, Koh JY, Harata NC. Abnormal cytoplasmic calcium dynamics in central neurons of a dystonia mouse model. Neuroscience Letters. 2013;548:61–66. doi: 10.1016/j.neulet.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Ashizawa T. Tourettism associated with Huntington’s disease. Movement Disorders. 1995;10:103–105. doi: 10.1002/mds.870100116. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Rett syndrome—An update. Journal of Neural Transmission. 2003;110:681–701. doi: 10.1007/s00702-003-0822-z. [DOI] [PubMed] [Google Scholar]
- Jellinger K, Armstrong D, Zoghbi HY, Percy AK. Neuropathology of Rett syndrome. Acta Neuropathologica. 1988;76:142–158. doi: 10.1007/BF00688098. [DOI] [PubMed] [Google Scholar]
- Jiang M, Ash RT, Baker SA, Suter B, Ferguson A, Park J, et al. Dendritic arborization and spine dynamics are abnormal in the mouse model of MECP2 duplication syndrome. The Journal of Neuroscience. 2013;33:19518–19533. doi: 10.1523/JNEUROSCI.1745-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Costa RM. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature. 2010;466:457–462. doi: 10.1038/nature09263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Tecuapetla F, Costa RM. Basal ganglia subcircuits distinctively encode the parsing and concatenation of action sequences. Nature Neuroscience. 2014;17(3):423–430. doi: 10.1038/nn.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Weiner I. The organization of the basal ganglia-thalamocortical circuits: Open interconnected rather than closed segregated. Neuroscience. 1994;63:363–379. doi: 10.1016/0306-4522(94)90536-3. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nature Genetics. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- Kakazu Y, Koh JY, Ho KW, Gonzalez-Alegre P, Harata NC. Synaptic vesicle recycling is enhanced by torsinA that harbors the DYT1 dystonia mutation. Synapse. 2012;66:453–464. doi: 10.1002/syn.21534. [DOI] [PubMed] [Google Scholar]
- Kakazu Y, Koh JY, Iwabuchi S, Gonzalez-Alegre P, Harata NC. Miniature release events of glutamate from hippocampal neurons are influenced by the dystonia-associated protein torsinA. Synapse. 2012;66:807–822. doi: 10.1002/syn.21571. [DOI] [PubMed] [Google Scholar]
- Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannidis I, Dehning S, Sandor P, Tarnok Z, Rizzo R, Wolanczyk T, et al. Support of the histaminergic hypothesis in Tourette syndrome: Association of the histamine decarboxylase gene in a large sample of families. Journal of Medical Genetics. 2013;50:760–764. doi: 10.1136/jmedgenet-2013-101637. [DOI] [PubMed] [Google Scholar]
- Karagiannidis I, Rizzo R, Tarnok Z, Wolanczyk T, Hebebrand J, Nothen MM, et al. Replication of association between a SLITRK1 haplotype and Tourette syndrome in a large sample of families. Molecular Psychiatry. 2012;17:665–668. doi: 10.1038/mp.2011.151. [DOI] [PubMed] [Google Scholar]
- Karimi M, Moerlein SM, Videen TO, Luedtke RR, Taylor M, Mach RH, et al. Decreased striatal dopamine receptor binding in primary focal dystonia: A D2 or D3 defect? Movement Disorders. 2011;26:100–106. doi: 10.1002/mds.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Moerlein SM, Videen TO, Su Y, Flores HP, Perlmutter JS. Striatal dopamine D1-like receptor binding is unchanged in primary focal dystonia. Movement Disorders. 2013;28(14):2002–2006. doi: 10.1002/mds.25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. The Journal of Comparative Neurology. 2010;518:277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama K, Yamada K, Ornthanalai VG, Inoue T, Ota M, Murphy NP, et al. Slitrk1-deficient mice display elevated anxiety-like behavior and noradrenergic abnormalities. Molecular Psychiatry. 2010;15:177–184. doi: 10.1038/mp.2008.97. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Large aspiny cells in the matrix of the rat neostriatum in vitro: Physiological identification, relation to the compartments and excitatory postsynaptic currents. Journal of Neurophysiology. 1992;67:1669–1682. doi: 10.1152/jn.1992.67.6.1669. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: Chemical, physiological and morphological characterization. Trends in Neurosciences. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Keen-Kim D, Mathews CA, Reus VI, Lowe TL, Herrera LD, Budman CL, et al. Overrepresentation of rare variants in a specific ethnic group may confuse interpretation of association analyses. Human Molecular Genetics. 2006;15:3324–3328. doi: 10.1093/hmg/ddl408. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The cortico-striate projection in the monkey. Brain. 1970;93:525–546. doi: 10.1093/brain/93.3.525. [DOI] [PubMed] [Google Scholar]
- Kermadi I, Joseph JP. Activity in the caudate nucleus of monkey during spatial sequencing. Journal of Neurophysiology. 1995;74:911–933. doi: 10.1152/jn.1995.74.3.911. [DOI] [PubMed] [Google Scholar]
- Kim E, Naisbitt S, Hsueh YP, Rao A, Rothschild A, Craig AM, et al. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. The Journal of Cell Biology. 1997;136:669–678. doi: 10.1083/jcb.136.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kosaka T, Heizmann CW. Parvalbumin-immunoreactive neurons in the rat neostriatum: A light and electron microscopic study. Brain Research. 1990;536:1–15. doi: 10.1016/0006-8993(90)90002-s. [DOI] [PubMed] [Google Scholar]
- Kitt CA, Wilcox BJ. Preliminary evidence for neurodegenerative changes in the substantia nigra of Rett syndrome. Neuropediatrics. 1995;26:114–118. doi: 10.1055/s-2007-979739. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Gratton A. Electrochemical monitoring of extracellular dopamine in nucleus accumbens of rats lever-pressing for food. Brain Research. 1994;652:225–234. doi: 10.1016/0006-8993(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nature Neuroscience. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Korff S, Stein DJ, Harvey BH. Stereotypic behaviour in the deer mouse: Pharmacological validation and relevance for obsessive compulsive disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32:348–355. doi: 10.1016/j.pnpbp.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Krauss JK. Deep brain stimulation for dystonia in adults. Overview and developments. Stereotactic and Functional Neurosurgery. 2002;78:168–182. doi: 10.1159/000068963. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature Neuroscience. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC. Physiology and pharmacology of striatal neurons. Annual Review of Neuroscience. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- Kriaucionis S, Paterson A, Curtis J, Guy J, Macleod N, Bird A. Gene expression analysis exposes mitochondrial abnormalities in a mouse model of Rett syndrome. Molecular and Cellular Biology. 2006;26:5033–5042. doi: 10.1128/MCB.01665-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Dependence of GABAergic synaptic areas on the interneuron type and target size. The Journal of Neuroscience. 2000;20:375–386. doi: 10.1523/JNEUROSCI.20-01-00375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulisevsky J, Marti MJ, Ferrer I, Tolosa E. Meige syndrome: Neuropathology of a case. Movement Disorders. 1988;3:170–175. doi: 10.1002/mds.870030209. [DOI] [PubMed] [Google Scholar]
- Kurlan R. Clinical practice. Tourette’s syndrome. The New England Journal of Medicine. 2010;363:2332–2338. doi: 10.1056/NEJMcp1007805. [DOI] [PubMed] [Google Scholar]
- Kustedjo K, Bracey MH, Cravatt BF. Torsin A and its torsion dystonia-associated mutant forms are lumenal glycoproteins that exhibit distinct subcellular localizations. The Journal of Biological Chemistry. 2000;275:27933–27939. doi: 10.1074/jbc.M910025199. [DOI] [PubMed] [Google Scholar]
- Kwak Y, Bohnen NI, Muller ML, Dayalu P, Seidler RD. Striatal denervation pattern predicts levodopa effects on sequence learning in Parkinson’s disease. Journal of Motor Behavior. 2013;45:423–429. doi: 10.1080/00222895.2013.817380. [DOI] [PubMed] [Google Scholar]
- Kwak CH, Jankovic J. Tourettism and dystonia after subcortical stroke. Movement Disorders. 2002;17:821–825. doi: 10.1002/mds.10207. [DOI] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, Pennartz CM. Fast-spiking interneurons of the rat ventral striatum: Temporal coordination of activity with principal cells and responsiveness to reward. The European Journal of Neuroscience. 2010;32:494–508. doi: 10.1111/j.1460-9568.2010.07293.x. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Benali H, Van de Moortele PF, Pelegrini-Issac M, Waechter T, Ugurbil K, et al. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekman A, Witt-Engerstrom I, Gottfries J, Hagberg BA, Percy AK, Svennerholm L. Rett syndrome: Biogenic amines and metabolites in postmortem brain. Pediatric Neurology. 1989;5:357–362. doi: 10.1016/0887-8994(89)90049-0. [DOI] [PubMed] [Google Scholar]
- Leventhal DK, Stoetzner CR, Abraham R, Pettibone J, DeMarco K, Berke JD. Dissociable effects of dopamine on learning and performance within sensorimotor striatum. Basal Ganglia. 2014 doi: 10.1016/j.baga.2013.11.001. in press. http://dx.doi.org/10.1016/j.baga.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Meehan RR, Henzel WJ, Maurer-Fogy I, Jeppesen P, Klein F, et al. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69:905–914. doi: 10.1016/0092-8674(92)90610-o. [DOI] [PubMed] [Google Scholar]
- Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limousin P, Brown RG, Jahanshahi M, Asselman P, Quinn NP, Thomas D, et al. The effects of posteroventral pallidotomy on the preparation and execution of voluntary hand and arm movements in Parkinson’s disease. Brain. 1999;122:315–327. doi: 10.1093/brain/122.2.315. [DOI] [PubMed] [Google Scholar]
- Lipsman N, Neimat JS, Lozano AM. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: The search for a valid target. Neurosurgery. 2007;61:1–11. doi: 10.1227/01.neu.0000279719.75403.f7. discussion 11. [DOI] [PubMed] [Google Scholar]
- Lo CC, Wang XJ. Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nature Neuroscience. 2006;9:956–963. doi: 10.1038/nn1722. [DOI] [PubMed] [Google Scholar]
- Lorden JF, McKeon TW, Baker HJ, Cox N, Walkley SU. Characterization of the rat mutant dystonic (dt): A new animal model of dystonia musculorum deformans. The Journal of Neuroscience. 1984;4:1925–1932. doi: 10.1523/JNEUROSCI.04-08-01925.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz MT. Recognition and treatment of neurologic Wilson’s disease. Seminars in Neurology. 2012;32:538–543. doi: 10.1055/s-0033-1334476. [DOI] [PubMed] [Google Scholar]
- Loscher W, Fisher JEJ, Schmidt D, Fredow G, Honack D, Iturrian WB. The sz mutant hamster: A genetic model of epilepsy or of paroxysmal dystonia? Movement Disorders. 1989;4:219–232. doi: 10.1002/mds.870040304. [DOI] [PubMed] [Google Scholar]
- Lu X, Matsuzawa M, Hikosaka O. A neural correlate of oculomotor sequences in supplementary eye field. Neuron. 2002;34:317–325. doi: 10.1016/s0896-6273(02)00657-8. [DOI] [PubMed] [Google Scholar]
- Luk KC, Sadikot AF. GABA promotes survival but not proliferation of parvalbumin-immunoreactive interneurons in rodent neostriatum: An in vivo study with stereology. Neuroscience. 2001;104:93–103. doi: 10.1016/s0306-4522(01)00038-0. [DOI] [PubMed] [Google Scholar]
- Mallet N, Micklem BR, Henny P, Brown MT, Williams C, Bolam JP, et al. Dichotomous organization of the external globus pallidus. Neuron. 2012;74:1075–1086. doi: 10.1016/j.neuron.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark MH, Sage JI, Dickson DW, Heikkila RE, Manzino L, Schwarz KO, et al. Meige syndrome in the spectrum of Lewy body disease. Neurology. 1994;44:1432–1436. doi: 10.1212/wnl.44.8.1432. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Obeso JA, Zarranz JJ, Lang AE. The anatomical basis of symptomatic hemidystonia. Brain. 1985;108:463–483. doi: 10.1093/brain/108.2.463. [DOI] [PubMed] [Google Scholar]
- Marshak S, Meynard MM, De Vries YA, Kidane AH, Cohen-Cory S. Cell-autonomous alterations in dendritic arbor morphology and connectivity induced by overexpression of MeCP2 in Xenopus central neurons in vivo. PLoS One. 2012;7:e33153. doi: 10.1371/journal.pone.0033153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella G, Tassone A, Sciamanna G, Platania P, Cuomo D, Viscomi MT, et al. Impairment of bidirectional synaptic plasticity in the striatum of a mouse model of DYT1 dystonia: Role of endogenous acetylcholine. Brain. 2009;132:2336–2349. doi: 10.1093/brain/awp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama T, Matsuo M, Jing JJ, Tabara Y, Kitsuki K, Yamagata H, et al. Classic Rett syndrome in a boy with R133C mutation of MECP2. Brain Dev. 2005;27:439–442. doi: 10.1016/j.braindev.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Mathai A, Smith Y. The corticostriatal and corticosubthalamic pathways: Two entries, one target. So what? Frontiers in Systems Neuroscience. 2011;5:64. doi: 10.3389/fnsys.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto N, Hanakawa T, Maki S, Graybiel AM, Kimura M. Role of [corrected] nigrostriatal dopamine system in learning to perform sequential motor tasks in a predictive manner. Journal of Neurophysiology. 1999;82:978–998. doi: 10.1152/jn.1999.82.2.978. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto N, Minamimoto T, Graybiel AM, Kimura M. Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. Journal of Neurophysiology. 2001;85:960–976. doi: 10.1152/jn.2001.85.2.960. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, Zoghbi HY. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18267–18272. doi: 10.1073/pnas.0608702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath MJ, Campbell KM, Burton FH. The role of cognitive and affective processing in a transgenic mouse model of cortical-limbic neuropotentiated compulsive behavior. Behavioral Neuroscience. 1999;113:1249–1256. doi: 10.1037//0735-7044.113.6.1249. [DOI] [PubMed] [Google Scholar]
- McGrath MJ, Campbell KM, Parks CR, Burton FH. Glutamatergic drugs exacerbate symptomatic behavior in a transgenic model of comorbid Tourette’s syndrome and obsessive-compulsive disorder. Brain Research. 2000;877:23–30. doi: 10.1016/s0006-8993(00)02646-9. [DOI] [PubMed] [Google Scholar]
- McGraw CM, Samaco RC, Zoghbi HY. Adult neural function requires MeCP2. Science. 2011;333:186. doi: 10.1126/science.1206593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaught KS, Kapustin A, Jackson T, Jengelley TA, Jnobaptiste R, Shashidharan P, et al. Brainstem pathology in DYT1 primary torsion dystonia. Annals of Neurology. 2004;56:540–547. doi: 10.1002/ana.20225. [DOI] [PubMed] [Google Scholar]
- Medrihan L, Tantalaki E, Aramuni G, Sargsyan V, Dudanova I, Missler M, et al. Early defects of GABAergic synapses in the brain stem of a MeCP2 mouse model of Rett syndrome. Journal of Neurophysiology. 2008;99:112–121. doi: 10.1152/jn.00826.2007. [DOI] [PubMed] [Google Scholar]
- Mehanna R, Jankovic J. Movement disorders in cerebrovascular disease. Lancet Neurology. 2013;12:597–608. doi: 10.1016/S1474-4422(13)70057-7. [DOI] [PubMed] [Google Scholar]
- Mink JW. The Basal Ganglia and involuntary movements: Impaired inhibition of competing motor patterns. Archives of Neurology. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT. Basal ganglia motor control. II. Late pallidal timing relative to movement onset and inconsistent pallidal coding of movement parameters. Journal of Neurophysiology. 1991a;65:301–329. doi: 10.1152/jn.1991.65.2.301. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT. Basal ganglia motor control. III. Pallidal ablation: Normal reaction time, muscle cocontraction, and slow movement. Journal of Neurophysiology. 1991b;65:330–351. doi: 10.1152/jn.1991.65.2.330. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Miyashita K, Karadi Z, Rand MK. Differential roles of monkey striatum in learning of sequential hand movement. Experimental Brain Research. 1997;115:1–5. doi: 10.1007/pl00005669. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Paladini CA. Dynamic regulation of midbrain dopamine neuron activity: Intrinsic, synaptic, and plasticity mechanisms. Neuroscience. 2011;198:95–111. doi: 10.1016/j.neuroscience.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Murakami JW, Courchesne E, Haas RH, Press GA, Yeung-Courchesne R. Cerebellar and cerebral abnormalities in Rett syndrome: A quantitative MR analysis. AJR. American Journal of Roentgenology. 1992;159:177–183. doi: 10.2214/ajr.159.1.1609693. [DOI] [PubMed] [Google Scholar]
- Na ES, Nelson ED, Adachi M, Autry AE, Mahgoub MA, Kavalali ET, et al. A mouse model for MeCP2 duplication syndrome: MeCP2 overexpression impairs learning and memory and synaptic transmission. The Journal of Neuroscience. 2012;32:3109–3117. doi: 10.1523/JNEUROSCI.6000-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Rothwell JC, Day BL, Thompson PD, Shannon K, Marsden CD. Reciprocal inhibition between forearm muscles in patients with writer’s cramp and other occupational cramps, symptomatic hemidystonia and hemiparesis due to stroke. Brain. 1989;112:681–697. doi: 10.1093/brain/112.3.681. [DOI] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- Napolitano F, Pasqualetti M, Usiello A, Santini E, Pacini G, Sciamanna G, et al. Dopamine D2 receptor dysfunction is rescued by adenosine A2A receptor antagonism in a model of DYT1 dystonia. Neurobiology of Disease. 2010;38:434–445. doi: 10.1016/j.nbd.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanaswamy JC, Viswanath B, Veshnal Cherian A, Bada Math S, Kandavel T, Janardhan Reddy YC. Impact of age of onset of illness on clinical phenotype in OCD. Psychiatry Research. 2012;200:554–559. doi: 10.1016/j.psychres.2012.03.037. [DOI] [PubMed] [Google Scholar]
- Nery FC, Armata IA, Farley JE, Cho JA, Yaqub U, Chen P, et al. TorsinA participates in endoplasmic reticulum-associated degradation. Nature Communications. 2011;2:393. doi: 10.1038/ncomms1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Research. 1999;9:27–43. [PubMed] [Google Scholar]
- Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–2509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MV, Du F, Felice CA, Shan X, Nigam A, Mandel G, et al. MeCP2 is critical for maintaining mature neuronal networks and global brain anatomy during late stages of postnatal brain development and in the mature adult brain. The Journal of Neuroscience. 2012;32:10021–10034. doi: 10.1523/JNEUROSCI.1316-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, Jahanshahi M, Alvarez L, Macias R, Pedroso I, Wilkinson L, et al. What can man do without basal ganglia motor output? The effect of combined unilateral subthalamotomy and pallidotomy in a patient with Parkinson’s disease. Experimental Neurology. 2009;220:283–292. doi: 10.1016/j.expneurol.2009.08.030. [DOI] [PubMed] [Google Scholar]
- Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nature Genetics. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- Ozomaro U, Cai G, Kajiwara Y, Yoon S, Makarov V, Delorme R, et al. Characterization of SLITRK1 variation in obsessive-compulsive disorder. PLoS One. 2013;8:e70376. doi: 10.1371/journal.pone.0070376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Bao L, Andre P, Pelta-Heller J, Sluzas E, Gonzalez-Alegre P, et al. Cell-autonomous alteration of dopaminergic transmission by wild type and mutant (DeltaE) TorsinA in transgenic mice. Neurobiology of Disease. 2010;39:318–326. doi: 10.1016/j.nbd.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizza M, Lelli S, Nilsson J, Hallett M. H-reflex recovery curve and reciprocal inhibition of H-reflex in different kinds of dystonia. Neurology. 1990;40:824–828. doi: 10.1212/wnl.40.5.824. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Research Brain Research Reviews. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Paudel R, Hardy J, Revesz T, Holton JL, Houlden H. Review: Genetics and neuropathology of primary pure dystonia. Neuropathology and Applied Neurobiology. 2012;38:520–534. doi: 10.1111/j.1365-2990.2012.01298.x. [DOI] [PubMed] [Google Scholar]
- Pauls DL. The genetics of obsessive compulsive disorder: A review of the evidence. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2008;148C:133–139. doi: 10.1002/ajmg.c.30168. [DOI] [PubMed] [Google Scholar]
- Perlmutter JS, Stambuk MK, Markham J, Black KJ, McGee-Minnich L, Jankovic J, et al. Decreased [18F]spiperone binding in putamen in idiopathic focal dystonia. The Journal of Neuroscience. 1997;17:843–850. doi: 10.1523/JNEUROSCI.17-02-00843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DA, Sejnowski TJ, Poizner H. Convergent evidence for abnormal striatal synaptic plasticity in dystonia. Neurobiology of Disease. 2010;37:558–573. doi: 10.1016/j.nbd.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, et al. Basal Ganglia volumes in patients with Gilles de la Tourette syndrome. Archives of General Psychiatry. 2003;60:415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends in Neurosciences. 2007;30:545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Pisani A, Martella G, Tscherter A, Bonsi P, Sharma N, Bernardi G, et al. Altered responses to dopaminergic D2 receptor activation and N-type calcium currents in striatal cholinergic interneurons in a mouse model of DYT1 dystonia. Neurobiology of Disease. 2006;24:318–325. doi: 10.1016/j.nbd.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Powell SB, Newman HA, Pendergast JF, Lewis MH. A rodent model of spontaneous stereotypy: Initial characterization of developmental, environmental, and neurobiological factors. Physiology & Behavior. 1999;66:355–363. doi: 10.1016/s0031-9384(98)00303-5. [DOI] [PubMed] [Google Scholar]
- Presti MF, Gibney BC, Lewis MH. Effects of intrastriatal administration of selective dopaminergic ligands on spontaneous stereotypy in mice. Physiology & Behavior. 2004;80:433–439. doi: 10.1016/j.physbeh.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Presti MF, Lewis MH. Striatal opioid peptide content in an animal model of spontaneous stereotypic behavior. Behavioural Brain Research. 2005;157:363–368. doi: 10.1016/j.bbr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Presti MF, Mikes HM, Lewis MH. Selective blockade of spontaneous motor stereotypy via intrastriatal pharmacological manipulation. Pharmacology, Biochemistry and Behavior. 2003;74:833–839. doi: 10.1016/s0091-3057(02)01081-x. [DOI] [PubMed] [Google Scholar]
- Presti MF, Powell SB, Lewis MH. Dissociation between spontaneously emitted and apomorphine-induced stereotypy in Peromyscus maniculatus bairdii. Physiology & Behavior. 2002;75:347–353. doi: 10.1016/s0031-9384(02)00641-8. [DOI] [PubMed] [Google Scholar]
- Presti MF, Watson CJ, Kennedy RT, Yang M, Lewis MH. Behavior-related alterations of striatal neurochemistry in a mouse model of stereotyped movement disorder. Pharmacology, Biochemistry and Behavior. 2004;77:501–507. doi: 10.1016/j.pbb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Priori A, Giannicola G, Rosa M, Marceglia S, Servello D, Sassi M, et al. Deep brain electrophysiological recordings provide clues to the pathophysiology of Tourette syndrome. Neuroscience & Biobehavioral Reviews. 2013;37:1063–1068. doi: 10.1016/j.neubiorev.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Prudente CN, Hess EJ, Jinnah HA. Dystonia as a network disorder: What is the role of the cerebellum? Neuroscience. 2013;260C:23–35. doi: 10.1016/j.neuroscience.2013.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartarone A, Classen J, Morgante F, Rosenkranz K, Hallett M. Consensus paper: Use of transcranial magnetic stimulation to probe motor cortex plasticity in dystonia and levodopa-induced dyskinesia. Brain Stimulation. 2009;2:108–117. doi: 10.1016/j.brs.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Hallett M. Emerging concepts in the physiological basis of dystonia. Movement Disorders. 2013;28:958–967. doi: 10.1002/mds.25532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartarone A, Pisani A. Abnormal plasticity in dystonia: Disruption of synaptic homeostasis. Neurobiology of Disease. 2011;42:162–170. doi: 10.1016/j.nbd.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Siebner HR, Rothwell JC. Task-specific hand dystonia: Can too much plasticity be bad for you? Trends in Neurosciences. 2006;29:192–199. doi: 10.1016/j.tins.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Hanley JJ, Deniau JM, Bolam JP. Synaptic convergence of motor and somatosensory cortical afferents onto GABAergic interneurons in the rat striatum. The Journal of Neuroscience. 2002;22:8158–8169. doi: 10.1523/JNEUROSCI.22-18-08158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdhani RA, Simonyan K. Primary dystonia: Conceptualizing the disorder through a structural brain imaging lens. Tremor and Other Hyperkinetic Movements (New York) 2013;3:1–11. doi: 10.7916/D8H70DJ7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Frank MJ. Reinforcement-based decision making in corticostriatal circuits: Mutual constraints by neurocomputational and diffusion models. Neural Computation. 2012;24:1186–1229. doi: 10.1162/NECO_a_00270. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Savage CR, Curran T, Kendrick A, Brown HD, et al. Striatal recruitment during an implicit sequence learning task as measured by functional magnetic resonance imaging. Human Brain Mapping. 1997;5:124–132. [PubMed] [Google Scholar]
- Redgrave P, Vautrelle N, Reynolds JN. Functional properties of the basal ganglia’s re-entrant loop architecture: Selection and reinforcement. Neuroscience. 2011;198:138–151. doi: 10.1016/j.neuroscience.2011.07.060. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Faruque F, Naidu S, Abrams M, Beaty T, Bryan RN, et al. Neuroanatomy of Rett syndrome: A volumetric imaging study. Annals of Neurology. 1993;34:227–234. doi: 10.1002/ana.410340220. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Iivanainen M, Metsahonkala L, Vainionpaa L, Paakkonen L, Nagren K, et al. Striatal dopaminergic system in dopa-responsive dystonia: A multi-tracer PET study shows increased D2 receptors. Journal of Neural Transmission. 2004;111:59–67. doi: 10.1007/s00702-003-0053-3. [DOI] [PubMed] [Google Scholar]
- Rizzo R, Gulisano M, Cali PV, Curatolo P. Tourette syndrome and comorbid ADHD: Current pharmacological treatment options. European Journal of Paediatric Neurology. 2013;17:421–428. doi: 10.1016/j.ejpn.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Robertson MM. Tourette syndrome, associated conditions and the complexities of treatment. Brain. 2000;123(3):425–462. doi: 10.1093/brain/123.3.425. [DOI] [PubMed] [Google Scholar]
- Robertson MM. Attention deficit hyperactivity disorder, tics and Tourette’s syndrome: The relationship and treatment implications. A commentary. European Child & Adolescent Psychiatry. 2006;15:1–11. doi: 10.1007/s00787-006-0505-z. [DOI] [PubMed] [Google Scholar]
- Robertson MM, Eapen V, Cavanna AE. The international prevalence, epidemiology, and clinical phenomenology of Tourette syndrome: A cross-cultural perspective. Journal of Psychosomatic Research. 2009;67:475–483. doi: 10.1016/j.jpsychores.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Robinson S, Sotak BN, During MJ, Palmiter RD. Local dopamine production in the dorsal striatum restores goal-directed behavior in dopamine-deficient mice. Behavioral Neuroscience. 2006;120:196–200. doi: 10.1037/0735-7044.120.1.000. [DOI] [PubMed] [Google Scholar]
- Roessner V, Overlack S, Schmidt-Samoa C, Baudewig J, Dechent P, Rothenberger A, et al. Increased putamen and callosal motor subregion in treatment-naive boys with Tourette syndrome indicates changes in the bihemispheric motor network. The Journal of Child Psychology and Psychiatry. 2011;52:306–314. doi: 10.1111/j.1469-7610.2010.02324.x. [DOI] [PubMed] [Google Scholar]
- Rubin JE, McIntyre CC, Turner RS, Wichmann T. Basal ganglia activity patterns in parkinsonism and computational modeling of their downstream effects. The European Journal of Neuroscience. 2012;36:2213–2228. doi: 10.1111/j.1460-9568.2012.08108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo G, Nieus TR, Maggi S, Taverna S. Dynamics of action potential firing in electrically connected striatal fast-spiking interneurons. Frontiers in Cellular Neuroscience. 2013;7:209. doi: 10.3389/fncel.2013.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadikot AF, Rymar VV. The primate centromedian-parafascicular complex: Anatomical organization with a note on neuromodulation. Brain Research Bulletin. 2009;78:122–130. doi: 10.1016/j.brainresbull.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Sarvet B. Childhood obsessive-compulsive disorder. Pediatrics in Review. 2013;34:19–27. doi: 10.1542/pir.34-1-19. quiz 28. [DOI] [PubMed] [Google Scholar]
- Scharf JM, Yu D, Mathews CA, Neale BM, Stewart SE, Fagerness JA, et al. Genome-wide association study of Tourette’s syndrome. Molecular Psychiatry. 2013;18:721–728. doi: 10.1038/mp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker E, Malinowska B, Kathmann M, Gothert M. Modulation of neurotransmitter release via histamine H3 heteroreceptors. Fundamental & Clinical Pharmacology. 1994;8:128–137. doi: 10.1111/j.1472-8206.1994.tb00789.x. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Leventhal DK, Mallet N, Chen F, Berke JD. Canceling actions involves a race between basal ganglia pathways. Nature Neuroscience. 2013;16:1118–1124. doi: 10.1038/nn.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Updating dopamine reward signals. Current Opinion in Neurobiology. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. The Journal of Neuroscience. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schulz JM, Reynolds JN. Pause and rebound: Sensory control of cholinergic signaling in the striatum. Trends in Neurosciences. 2013;36:41–50. doi: 10.1016/j.tins.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Arrang JM, Garbarg M, Pollard H, Ruat M. Histaminergic transmission in the mammalian brain. Physiological Reviews. 1991;71:1–51. doi: 10.1152/physrev.1991.71.1.1. [DOI] [PubMed] [Google Scholar]
- Sciamanna G, Bonsi P, Tassone A, Cuomo D, Tscherter A, Viscomi MT, et al. Impaired striatal D2 receptor function leads to enhanced GABA transmission in a mouse model of DYT1 dystonia. Neurobiology of Disease. 2009;34:133–145. doi: 10.1016/j.nbd.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciamanna G, Hollis R, Ball C, Martella G, Tassone A, Marshall A, et al. Cholinergic dysregulation produced by selective inactivation of the dystonia-associated protein torsinA. Neurobiology of Disease. 2012;47:416–427. doi: 10.1016/j.nbd.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciamanna G, Tassone A, Mandolesi G, Puglisi F, Ponterio G, Martella G, et al. Cholinergic dysfunction alters synaptic integration between thalamostriatal and corticostriatal inputs in DYT1 dystonia. The Journal of Neuroscience. 2012;32:11991–12004. doi: 10.1523/JNEUROSCI.0041-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciamanna G, Tassone A, Martella G, Mandolesi G, Puglisi F, Cuomo D, et al. Developmental profile of the aberrant dopamine D2 receptor response in striatal cholinergic interneurons in DYT1 dystonia. PLoS One. 2011;6:e24261. doi: 10.1371/journal.pone.0024261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolding NJ, Smith SM, Sturman S, Brookes GB, Lees AJ. Auctioneer’s jaw: A case of occupational oromandibular hemidystonia. Movement Disorders. 1995;10:508–509. doi: 10.1002/mds.870100418. [DOI] [PubMed] [Google Scholar]
- Scontrini A, Conte A, Defazio G, Fiorio M, Fabbrini G, Suppa A, et al. Somatosensory temporal discrimination in patients with primary focal dystonia. Journal of Neurology, Neurosurgery and Psychiatry. 2009;80:1315–1319. doi: 10.1136/jnnp.2009.178236. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Purushotham A, Kim SG, Ugurbil K, Willingham D, Ashe J. Neural correlates of encoding and expression in implicit sequence learning. Experimental Brain Research. 2005;165:114–124. doi: 10.1007/s00221-005-2284-z. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. The Journal of Neuroscience. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, et al. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- Sharma N, Baxter MG, Petravicz J, Bragg DC, Schienda A, Standaert DG, et al. Impaired motor learning in mice expressing torsinA with the DYT1 dystonia mutation. The Journal of Neuroscience. 2005;25:5351–5355. doi: 10.1523/JNEUROSCI.0855-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashidharan P, Sandu D, Potla U, Armata IA, Walker RH, McNaught KS, et al. Transgenic mouse model of early-onset DYT1 dystonia. Human Molecular Genetics. 2005;14:125–133. doi: 10.1093/hmg/ddi012. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidibe M, Smith Y. Thalamic inputs to striatal interneurons in monkeys: Synaptic organization and co-localization of calcium binding proteins. Neuroscience. 1999;89:1189–1208. doi: 10.1016/s0306-4522(98)00367-4. [DOI] [PubMed] [Google Scholar]
- Singer HS, Szymanski S, Giuliano J, Yokoi F, Dogan AS, Brasic JR, et al. Elevated intrasynaptic dopamine release in Tourette’s syndrome measured by PET. The American Journal of Psychiatry. 2002;159:1329–1336. doi: 10.1176/appi.ajp.159.8.1329. [DOI] [PubMed] [Google Scholar]
- Smith KS, Graybiel AM. A dual operator view of habitual behavior reflecting cortical and striatal dynamics. Neuron. 2013a;79:361–374. doi: 10.1016/j.neuron.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Graybiel AM. Using optogenetics to study habits. Brain Research. 2013b;1511:102–114. doi: 10.1016/j.brainres.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Virkud A, Deisseroth K, Graybiel AM. Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18932–18937. doi: 10.1073/pnas.1216264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CH, Bernhard D, Bolarinwa C, Hess EJ, Smith Y, Jinnah HA. Subtle microstructural changes of the striatum in a DYT1 knock-in mouse model of dystonia. Neurobiology of Disease. 2013;54:362–371. doi: 10.1016/j.nbd.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman AA, Krsnik Z, Sun J, Rasin MR, State MW, Sestan N, et al. Developmentally regulated and evolutionarily conserved expression of SLITRK1 in brain circuits implicated in Tourette syndrome. The Journal of Comparative Neurology. 2009;513:21–37. doi: 10.1002/cne.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam B, Naidu S, Reiss AL. Neuroanatomy in Rett syndrome: Cerebral cortex and posterior fossa. Neurology. 1997;48:399–407. doi: 10.1212/wnl.48.2.399. [DOI] [PubMed] [Google Scholar]
- Sullivan MA, Chen H, Morikawa H. Recurrent inhibitory network among striatal cholinergic interneurons. The Journal of Neuroscience. 2008;28:8682–8690. doi: 10.1523/JNEUROSCI.2411-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, Hata Y, Hirao K, Toyoda A, Irie M, Takai Y. SAPAPs. A family of PSD-95/SAP90-associated proteins localized at postsynaptic density. The Journal of Biological Chemistry. 1997;272:11943–11951. doi: 10.1074/jbc.272.18.11943. [DOI] [PubMed] [Google Scholar]
- Tanabe LM, Kim CE, Alagem N, Dauer WT. Primary dystonia: Molecules and mechanisms. Nature Reviews Neurology. 2009;5:598–609. doi: 10.1038/nrneurol.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe LM, Martin C, Dauer WT. Genetic background modulates the phenotype of a mouse model of DYT1 dystonia. PLoS One. 2012;7:e32245. doi: 10.1371/journal.pone.0032245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CC, Root DH, Duke DC, Zhu Y, Teixeria K, Ma S, et al. Decreased firing of striatal neurons related to licking during acquisition and overtraining of a licking task. The Journal of Neuroscience. 2009;29:13952–13961. doi: 10.1523/JNEUROSCI.2824-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura Y, King MA, Williams DK, Lewis MH. Development of repetitive behavior in a mouse model: Roles of indirect and striosomal basal ganglia pathways. International Journal of Developmental Neuroscience. 2011;29:461–467. doi: 10.1016/j.ijdevneu.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura Y, Vaziri S, Lewis MH. Indirect basal ganglia pathway mediation of repetitive behavior: Attenuation by adenosine receptor agonists. Behavioural Brain Research. 2010;210:116–122. doi: 10.1016/j.bbr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate P, Skarnes W, Bird A. The methyl-CpG binding protein MeCP2 is essential for embryonic development in the mouse. Nature Genetics. 1996;12:205–208. doi: 10.1038/ng0296-205. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Lang AE, Saint-Cyr JA, Riley DE, Ranawaya R. Cognitive processes in idiopathic dystonia treated with high-dose anticholinergic therapy: Implications for treatment strategies. Clinical Neuropharmacology. 1991;14:62–77. doi: 10.1097/00002826-199102000-00005. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Rajbhandari AK, Berridge KC, Aldridge JW. Dopamine receptor modulation of repetitive grooming actions in the rat: Potential relevance for Tourette syndrome. Brain Research. 2010;1322:92–101. doi: 10.1016/j.brainres.2010.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM. GABAergic interneurons of the striatum. In: Steiner H, Tseng K, editors. Handbook of basal ganglia structure and function. Oxford: Elsevier B.V.; 2010. pp. 151–166. [Google Scholar]
- Tepper JM, Tecuapetla F, Koos T, Ibanez-Sandoval O. Heterogeneity and diversity of striatal GABAergic interneurons. Frontiers in Neuroanatomy. 2010;4:150. doi: 10.3389/fnana.2010.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas TM, Smith Y, Levey AI, Hersch SM. Cortical inputs to m2-immunoreactive striatal interneurons in rat and monkey. Synapse. 2000;37:252–261. doi: 10.1002/1098-2396(20000915)37:4<252::AID-SYN2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Tisch S, Limousin P, Rothwell JC, Asselman P, Quinn N, Jahanshahi M, et al. Changes in blink reflex excitability after globus pallidus internus stimulation for dystonia. Movement Disorders. 2006;21:1650–1655. doi: 10.1002/mds.20899. [DOI] [PubMed] [Google Scholar]
- Torres GE, Sweeney AL, Beaulieu JM, Shashidharan P, Caron MG. Effect of torsinA on membrane proteins reveals a loss of function and a dominant-negative phenotype of the dystonia-associated DeltaE-torsinA mutant. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15650–15655. doi: 10.1073/pnas.0308088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trender-Gerhard I, Sweeney MG, Schwingenschuh P, Mir P, Edwards MJ, Gerhard A, et al. Autosomal-dominant GTPCH1-deficient DRD: Clinical characteristics and long-term outcome of 34 patients. Journal of Neurology, Neurosurgery and Psychiatry. 2009;80:839–845. doi: 10.1136/jnnp.2008.155861. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor M, Akbarian S, Chen RZ, Jaenisch R. Transcriptional profiling of a mouse model for Rett syndrome reveals subtle transcriptional changes in the brain. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15536–15541. doi: 10.1073/pnas.242566899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RS, Desmurget M. Basal ganglia contributions to motor control: A vigorous tutor. Current Opinion in Neurobiology. 2010;20:704–716. doi: 10.1016/j.conb.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulug AM, Vo A, Argyelan M, Tanabe L, Schiffer WK, Dewey S, et al. Cerebellothalamocortical pathway abnormalities in torsinA DYT1 knock-in mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6638–6643. doi: 10.1073/pnas.1016445108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Research. 1970;24:485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- van den Akker E, Reijnen M, Korving J, Brouwer A, Meijlink F, Deschamps J. Targeted inactivation of Hoxb8 affects survival of a spinal ganglion and causes aberrant limb reflexes. Mechanisms of Development. 1999;89:103–114. doi: 10.1016/s0925-4773(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Van den Veyver IB, Zoghbi HY. Methyl-CpG-binding protein 2 mutations in Rett syndrome. Current Opinion in Genetics & Development. 2000;10:275–279. doi: 10.1016/s0959-437x(00)00083-6. [DOI] [PubMed] [Google Scholar]
- Vandewalle V, van der Linden C, Groenewegen HJ, Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet. 1999;353:724. doi: 10.1016/s0140-6736(98)05964-9. [DOI] [PubMed] [Google Scholar]
- Vercueil L, Krack P, Pollak P. Results of deep brain stimulation for dystonia: A critical reappraisal. Movement Disorders. 2002;17(Suppl. 3):S89–S93. doi: 10.1002/mds.10148. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Jutras MF, Grabli D, Roze E. Deep brain stimulation for dystonia. Journal of Neurology, Neurosurgery and Psychiatry. 2013;84:1029–1042. doi: 10.1136/jnnp-2011-301714. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. The New England Journal of Medicine. 2005;352:459–467. doi: 10.1056/NEJMoa042187. [DOI] [PubMed] [Google Scholar]
- Villard L. MECP2 mutations in males. Journal of Medical Genetics. 2007;44:417–423. doi: 10.1136/jmg.2007.049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard L, Kpebe A, Cardoso C, Chelly PJ, Tardieu PM, Fontes M. Two affected boys in a Rett syndrome family: Clinical and molecular findings. Neurology. 2000;55:1188–1193. doi: 10.1212/wnl.55.8.1188. [DOI] [PubMed] [Google Scholar]
- Walker RH, Brin MF, Sandu D, Good PF, Shashidharan P. TorsinA immunoreactivity in brains of patients with DYT1 and non-DYT1 dystonia. Neurology. 2002;58:120–124. doi: 10.1212/wnl.58.1.120. [DOI] [PubMed] [Google Scholar]
- Wall VZ, Parker JG, Fadok JP, Darvas M, Zweifel L, Palmiter RD. A behavioral genetics approach to understanding D1 receptor involvement in phasic dopamine signaling. Molecular and Cellular Neurosciences. 2011;46:21–31. doi: 10.1016/j.mcn.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Ade KK, Caffall Z, Ilcim Ozlu M, Eroglu C, Feng G, et al. Circuit-selective striatal synaptic dysfunction in the Sapap3 knockout mouse model of obsessive-compulsive disorder. Biological Psychiatry. 2013;75(8):623–630. doi: 10.1016/j.biopsych.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Feng G, Calakos N. Sapap3 deletion causes mGluR5-dependent silencing of AMPAR synapses. The Journal of Neuroscience. 2011;31:16685–16691. doi: 10.1523/JNEUROSCI.2533-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M, Lee SS, Zhang X, Houwink-Manville I, Song HR, Amir RE, et al. Rett syndrome and beyond: Recurrent spontaneous and familial MECP2 mutations at CpG hotspots. The American Journal of Human Genetics. 1999;65:1520–1529. doi: 10.1086/302690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Munoz DP. Saccade suppression by electrical microstimulation in monkey caudate nucleus. The Journal of Neuroscience. 2010;30:2700–2709. doi: 10.1523/JNEUROSCI.5011-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JM, Wang D, Feng G. Differential mRNA expression and protein localization of the SAP90/PSD-95-associated proteins (SAPAPs) in the nervous system of the mouse. The Journal of Comparative Neurology. 2004;472:24–39. doi: 10.1002/cne.20060. [DOI] [PubMed] [Google Scholar]
- Wenger KK, Musch KL, Mink JW. Impaired reaching and grasping after focal inactivation of globus pallidus pars interna in the monkey. Journal of Neurophysiology. 1999;82:2049–2060. doi: 10.1152/jn.1999.82.5.2049. [DOI] [PubMed] [Google Scholar]
- Wenk GL. Alterations in dopaminergic function in Rett syndrome. Neuropediatrics. 1995;26:123–125. doi: 10.1055/s-2007-979741. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Mobley SL. Choline acetyltransferase activity and vesamicol binding in Rett syndrome and in rats with nucleus basalis lesions. Neuroscience. 1996;73:79–84. doi: 10.1016/0306-4522(96)00019-x. [DOI] [PubMed] [Google Scholar]
- Wenk GL, O’Leary M, Nemeroff CB, Bissette G, Moser H, Naidu S. Neurochemical alterations in Rett syndrome. Brain Research. Developmental Brain Research. 1993;74:67–72. doi: 10.1016/0165-3806(93)90084-n. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Kolb B. Decortication abolishes place but not cue learning in rats. Behavioural Brain Research. 1984;11:123–134. doi: 10.1016/0166-4328(84)90135-9. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Delong MR. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron. 2006;52:197–204. doi: 10.1016/j.neuron.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Wilson BK, Hess EJ. Animal models for dystonia. Movement Disorders. 2013;28:982–989. doi: 10.1002/mds.25526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SS, Jones DW, Knable MB, Gorey JG, Lee KS, Hyde TM, et al. Tourette syndrome: Prediction of phenotypic variation in monozygotic twins by caudate nucleus D2 receptor binding. Science. 1996;273:1225–1227. doi: 10.1126/science.273.5279.1225. [DOI] [PubMed] [Google Scholar]
- Wolgin DL. Amphetamine stereotypy, the basal ganglia, and the “selection problem”. Behavioural Brain Research. 2012;231:297–308. doi: 10.1016/j.bbr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Wolmarans de W, Brand L, Stein DJ, Harvey BH. Reappraisal of spontaneous stereotypy in the deer mouse as an animal model of obsessive-compulsive disorder (OCD): Response to escitalopram treatment and basal serotonin transporter (SERT) density. Behavioural Brain Research. 2013;256:545–553. doi: 10.1016/j.bbr.2013.08.049. [DOI] [PubMed] [Google Scholar]
- Wong DF, Ricaurte G, Grunder G, Rothman R, Naidu S, Singer H, et al. Dopamine transporter changes in neuropsychiatric disorders. Advances in Pharmacology. 1998;42:219–223. doi: 10.1016/s1054-3589(08)60732-2. [DOI] [PubMed] [Google Scholar]
- Worbe Y, Baup N, Grabli D, Chaigneau M, Mounayar S, McCairn K, et al. Behavioral and movement disorders induced by local inhibitory dysfunction in primate striatum. Cerebral Cortex. 2009;19:1844–1856. doi: 10.1093/cercor/bhn214. [DOI] [PubMed] [Google Scholar]
- Worbe Y, Mallet L, Golmard JL, Behar C, Durif F, Jalenques I, et al. Repetitive behaviours in patients with Gilles de la Tourette syndrome: Tics, compulsions, or both? PLoS One. 2010;5:e12959. doi: 10.1371/journal.pone.0012959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Cepeda C, Zhuang X, Levine MS. Altered corticostriatal neurotransmission and modulation in dopamine transporter knock-down mice. Journal of Neurophysiology. 2007;98:423–432. doi: 10.1152/jn.00971.2006. [DOI] [PubMed] [Google Scholar]
- Xiao J, Ledoux MS. Caytaxin deficiency causes generalized dystonia in rats. Brain Research Molecular Brain Research. 2005;141:181–192. doi: 10.1016/j.molbrainres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Xu P, Grueter BA, Britt JK, McDaniel L, Huntington PJ, Hodge R, et al. Double deletion of melanocortin 4 receptors and SAPAP3 corrects compulsive behavior and obesity in mice. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10759–10764. doi: 10.1073/pnas.1308195110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian EH, Van Hoesen GW. Cortico-striate projections in the rhesus monkey: The organization of certain cortico-caudate connections. Brain Research. 1978;139:43–63. doi: 10.1016/0006-8993(78)90059-8. [DOI] [PubMed] [Google Scholar]
- Yin HH. The sensorimotor striatum is necessary for serial order learning. The Journal of Neuroscience. 2010;30:14719–14723. doi: 10.1523/JNEUROSCI.3989-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature Reviews Neuroscience. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. The European Journal of Neuroscience. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behavioural Brain Research. 2006;166:189–196. doi: 10.1016/j.bbr.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. The European Journal of Neuroscience. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- Yokoi F, Dang MT, Li J, Standaert DG, Li Y. Motor deficits and decreased striatal dopamine receptor 2 binding activity in the striatum-specific Dyt1 conditional knockout mice. PLoS One. 2011;6:e24539. doi: 10.1371/journal.pone.0024539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi F, Dang MT, Mitsui S, Li J, Li Y. Motor deficits and hyperactivity in cerebral cortex-specific Dyt1 conditional knockout mice. Journal of Biochemistry. 2008;143:39–47. doi: 10.1093/jb/mvm191. [DOI] [PubMed] [Google Scholar]
- Zhang L, He J, Jugloff DG, Eubanks JH. The MeCP2-null mouse hippocampus displays altered basal inhibitory rhythms and is prone to hyperexcitability. Hippocampus. 2008;18:294–309. doi: 10.1002/hipo.20389. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yokoi F, Jin YH, DeAndrade MP, Hashimoto K, Standaert DG, et al. Altered dendritic morphology of Purkinje cells in Dyt1 DeltaGAG knock-in and purkinje cell-specific Dyt1 conditional knockout mice. PLoS One. 2011;6:e18357. doi: 10.1371/journal.pone.0018357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Zak JD, Liu H. MeCP2 is required for normal development of GABAergic circuits in the thalamus. Journal of Neurophysiology. 2010;103:2470–2481. doi: 10.1152/jn.00601.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Brown RS, Chase AR, Eisele MR, Schlieker C. Regulation of Torsin ATPases by LAP1 and LULL1. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E1545–E1554. doi: 10.1073/pnas.1300676110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, DeCuypere M, LeDoux MS. Abnormal motor function and dopamine neurotransmission in DYT1 DeltaGAG transgenic mice. Experimental Neurology. 2008;210:719–730. doi: 10.1016/j.expneurol.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YT, Goffin D, Johnson BS, Zhou Z. Loss of MeCP2 function is associated with distinct gene expression changes in the striatum. Neurobiology of Disease. 2013;59:257–266. doi: 10.1016/j.nbd.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, et al. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchner S, Wendland JR, Ashley-Koch AE, Collins AL, Tran-Viet KN, Quinn K, et al. Multiple rare SAPAP3 missense variants in trichotillomania and OCD. Molecular Psychiatry. 2009;14:6–9. doi: 10.1038/mp.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig RM, Hedreen JC, Jankel WR, Casanova MF, Whitehouse PJ, Price DL. Pathology in brainstem regions of individuals with primary dystonia. Neurology. 1988;38:702–706. doi: 10.1212/wnl.38.5.702. [DOI] [PubMed] [Google Scholar]