Abstract
Gpr161 (also known as RE2) is an orphan G protein-coupled receptor (GPCR) that is expressed during embryonic development in zebrafish. Determining its biological function has proven difficult due to lack of knowledge regarding its natural or synthetic ligands. Here, we show that targeted knockdown of gpr161 disrupts asymmetric gene expression in the lateral plate mesoderm, resulting in aberrant looping of the heart tube. This is associated with elevated Ca2+ levels in cells lining the Kupffer's vesicle and normalization of Ca2+ levels, by over-expression of ncx1 or pmca RNA, is able to partially rescue the cardiac looping defect in gpr161 knockdown embryos. Taken together, these data support a model in which gpr161 plays an essential role in left-right (L-R) patterning by modulating Ca2+ levels in the cells surrounding the Kupffer's vesicle.
Keywords: orphan GPCR, Ca2+, left-right asymmetry, cardiac looping, visceral asymmetry, zebrafish development
Introduction
G protein coupled receptors (GPCRs) are emerging as critical regulators of diverse developmental processes such as oocyte maturation (Romo et al., 2008), fertilization (Fraser et al., 2003), gastrulation (Lin et al., 2005), and organogenesis (Griffin et al., 2001; Kupperman et al., 2000; Leung et al., 2006; Offermanns et al., 1997; Ruppel et al., 2005; Scott et al., 2007; Zeng et al., 2007). However, there is limited information regarding the embryonic expression patterns and functions of the ~400 non-olfactory GPCRs identified in the human and mouse genomes (Fredriksson et al., 2003; Vassilatis et al., 2003). Zebrafish offer a number of advantages for undertaking this type of large-scale analysis (Amsterdam et al., 1999; Driever et al., 1996; Geisler et al., 2007; Haffter et al., 1996; Peterson et al., 2000). The expression patterns of the individual GPCRs can be quickly determined by whole mount in situ hybridization, while their functions can be rapidly surveyed by visual inspection of the knockdown phenotypes resulting from morpholino antisense oligonucleotide (MO) injection (Nasevicius and Ekker, 2000). Particularly relevant to this study, zebrafish are very suitable for studying cardiac development since the embryos develop externally and do not require blood circulation for their early development, thereby allowing even those embryos showing severe cardiac defects to be analyzed in detail. Understanding the molecular basis for such cardiac defects is important for long term prevention of congenital heart problems that represent the most common of all human birth defects and affect nearly 1% of the population.
The formation of a fully functional heart is a multi-step process (Yelon et al. 1999). It begins with the specification of the appropriate numbers of myocardial cells within the lateral plate mesoderm (LPM). As myocardial differentiation progresses, the bilateral clusters of myocardial cells migrate medially and merge at the embryonic midline. As myocardial elongation occurs, the heart tube undergoes a complex series of morphogenetic movements in which the tube initially jogs to the left at 24 hpf (hour-post-fertilization) and then loops to the right between 30 and 48 hpf to form the functional, two-chambered heart. In the present study, we exploited zebrafish as a model system to investigate the role of a newly discovered orphan GPCR in this process. Using a combination of whole mount in situ hybridization and antisense morpholino oligonucleotide (MO) knockdown approach, we show that gpr161 is normally expressed in the lateral plate mesoderm and that knockdown of its expression perturbs cardiac looping as the result of a defect in left-right (L-R) patterning. This study reveals an essential role for a GPCR in L-R patterning for the first time and also adds to a growing list of GPCRs known to have critical roles during development (Kupperman et al., 2000; Scott et al., 2007; Zeng et al., 2007).
Results
The orphan G protein coupled receptor 161 is expressed in the developing zebrafish embryos
The human orphan receptor RE2 was originally isolated from fetal brain (Genbank accession AF091890; http://www.ncbi.nlm.nih.gov) and was later renamed GPR161 as a member of the GPCR superfamily (reference sequence NM_153832). Following a large scale phylogenetic analysis, the human GPR161 was assigned to the δ group of the RHODOPSIN family within the purine receptor cluster that includes several known receptors that bind such diverse ligands as nucleotides, leukotrienes, and thrombin (Fredriksson et al, 2003). However, nearly ten years after its initial discovery, the natural ligand and biological function of the human GPR161 remain to be discovered.
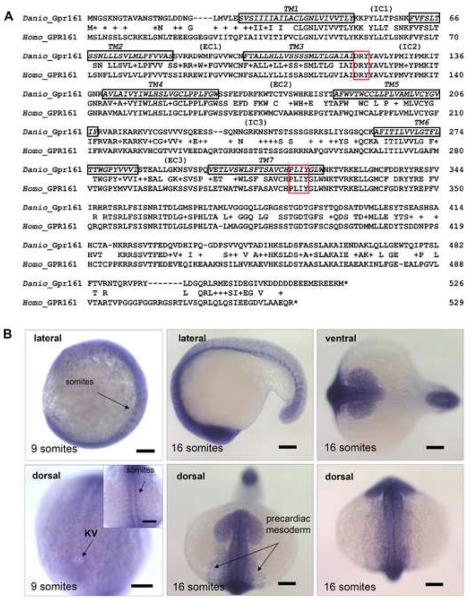
To search for the zebrafish ortholog, we used the human GPR161 gene to query the zebrafish genomic sequence databases and identified several contigs containing portions of the candidate gene. We subsequently generated a full length zebrafish gpr161 cDNA by reverse transcription-polymerase chain reaction (RT-PCR) for further analysis. Translation of the open reading frame predicted a 526-amino acid protein that showed 77% overall similarity to the human GPR161 protein. Since the RHODOPSIN family members typically bind their ligands via their seven transmembrane (7TM) domains (Schwartz et al, 2006), sequence alignments of the 7TM domains are often more insightful for making cross-species comparisons (Fredriksson and Schioth 2005). Using the hidden Markov model (Krogh et al, 2001) (TMHMM Server v. 2.0, http://www.cbs.dtu.dk/services/TMHMM/) to identify the 7TM domains (Fig. 1A; Supplementary Figs. 1A–C), the 7TM regions of the zebrafish Gpr161 protein showed a remarkable 86% overall similarity to the human GPR161 protein, providing evidence of their close evolutionary relationship and further suggesting their ligand binding function has been conserved from fish to man. Further comparison of the two protein sequences revealed other obvious similarities (Fig. 1A; Supplementary Figs. 1A–C). The most conserved regions were found on the inner face of the cell membrane, including the intracellular IC2 loop (100% similarity); the DRY motif at the border between TM3 and IC2 loop; the IC3 domain (71% homology); the PxxY motif in the TM7 domain, and the proximal portion of the C-terminal tail (84% similarity). Such high sequence homology between these proteins, particularly in the IC loops and the proximal portion of the C-terminal tail, indicated that the G protein coupling function of the predicted zebrafish and human GPR161 proteins has been evolutionarily conserved. By contrast, the more diverse regions were located on the outer face of the cell membrane, including the N-terminus and the three extracellular EC loops. Presumably, there was no strong evolutionary pressure to conserve these sequences since they are not predicted to mediate ligand binding or G protein activation (Möller et al, 2001). Thus, extensive sequence comparisons suggest the zebrafish gene is the ortholog of the human GPR161 gene (Fig. 1A; Supplementary Figs. 1A–C). This is further supported by phylogenetic analysis within the purine receptor cluster branch (Fredriksson et al, 2003) (Supplementary Fig. 2A).
Fig. 1.
Sequence comparison and in situ expression analysis of zebrafish gpr161. (A) Genbank accession no. of human GPR161: NM_007369 (Homo); zebrafish gpr161: EU090912 (Danio). 7TM (black), DRY (red) and PxxY (red) motifs were boxed. (B) gpr161 expression in the developing embryos by whole mount in situ hybridization. Inset showed the expression in developing somites and expression surrounding the Kupffer's vesicle (KV) near the tail bud region at 9 somite stage. All scale bars were 100μm.
Much can be learned about the possible functions of GPR161 from examination of its expression profile. ESTs for human GPR161 were identified in brain, colon, heart, lung, prostate, salivary gland, skin, mouth and uterus libraries (NCBI Expression Profile of Unigene Hs.632453 at http://www.ncbi.nlm.nih.gov/UniGene/). Using a combination of RT-PCR analysis (Supplementary Fig. 2B) and whole mount in situ hybridization (Fig. 1B; Supplementary Fig. 2C), we showed that zebrafish gpr161 transcripts were expressed throughout embryonic development. At earlier stages (9- to 16-somites), gpr161 transcripts were broadly expressed with specific staining observed in the developing nervous system, somites, and precardiac mesoderm (Fig. 1B; compare to negative control in Supplementary Fig. 2D). At later stages (1- to 3-days post-fertilization (dpf)), transcripts became more localized within the dorsal diencephalon, the otic vesicles, and the fin buds (Supplementary Fig. 2C). This dynamic expression pattern suggests important roles for zebrafish gpr161 throughout embryonic development.
Genetic manipulation of zebrafish gpr161 disrupts embryonic development
To investigate how zebrafish gpr161 may function in embryonic development, we used a morpholino antisense oligonucleotide (MO) approach to knockdown gpr161 in developing zebrafish embryos (Nasevicius and Ekker, 2000; Leung et al, 2006). To confirm the sequence specificity of any observed defects, two morpholinos targeted against different regions of the 5'UTR of the zebrafish gpr161 mRNA were used (Supplementary Fig. 3A). As validated by the in vitro translation assay, both gpr161-MOs (MO#24 and MO#36) inhibited expression of the zebrafish Gpr161 protein in a dose dependent fashion (Supplementary Figs. 3B, C). By contrast, a 5-base mismatch control MO (MO#28) failed to inhibit Gpr161 protein expression, attesting to the efficacy and sequence specificity of the knockdown approach (Supplementary Figs. 3B, C).
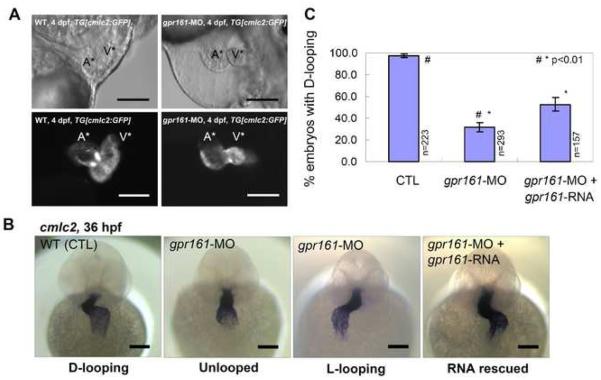
Following injection of either gpr161-MO (MO#24 or MO#36), transgenic embryos exhibiting heart specific fluorescence (TG[cmlc:GFP]) were collected from different stages and screened for morphological, molecular, and functional defects. This analysis revealed that the majority of the zebrafish gpr161 knockdown embryos exhibited pericardial edema and improper juxtaposition of the atrium and ventricle at 4 dpf (Fig. 2A). Attesting to specificity, the same phenotype was observed with both gpr161-MOs (Supplementary Fig. 3D). To investigate the underlying basis for this phenotype, we examined the expression of the cardiac specific marker, cardiac myosin light chain 2 (cmlc2) (Yelon et al, 1999), in zebrafish gpr161 knockdown embryos (Fig. 2B). In control embryos, the cmlc2 expressing cells form a linear heart tube around 25 hpf (data not shown) and the heart tube undergoes chamber maturation and normal looping of the ventricle to the right (D-looping) around 36 hpf (Fig. 2B). By contrast, aberrant looping of the ventricle was observed in a significant portion of gpr161 knockdown embryos (Fig. 2B). Quantitative analysis of the observed phenotypes showed that 67.6% of gpr161 knockdown embryos exhibited either no looping or abnormal looping of the ventricle to the left (L-looping), in contrast to 97.8% of the control embryos that underwent normal D-looping (Fig. 2C). Indicating an actual defect rather than a delay, the cardiac looping defect was still observed at 4 dpf (Fig. 2A). To further confirm that absent or aberrant L-looping was due to knockdown of zebrafish gpr161, we showed that the defect could be partially rescued by co-injection of gpr161-MO along with a morpholino resistant form of the zebrafish gpr161-RNA (open-reading frame without the 5' UTR targeted by the morpholino). Quantitative analysis showed that 52.2% of the embryos co-injected with gpr161-MO and -RNA displayed proper D-looping, compared to 32.4% injected with gpr161-MO alone (MO#36 at 5' UTR) (Fig. 2C; Supplementary Table 1). Consistent with the literature for other zebrafish genes (Ebert et al, 2005; Heisenberg et al, 2000), the lack of complete rescue is likely due to the inability of the injected gpr161-RNA to fully recapitulate the spatial and temporal expression of the endogenous gene. Collectively, these results indicate that zebrafish gpr161 plays a critical role in cardiac looping.
Fig. 2.
gpr161 knockdown disrupts cardiac looping morphogenesis. (A) Lateral view of zebrafish hearts at 4 dpf. Control and gpr161 knockdown in transgenic zebrafish embryos with cardiac specific GFP, TG[cmlc2:GFP], showing images of bright field (top panels) and fluorescent (bottom panels). A* labelled atrium, V* labelled ventricle. (B) Cardiac looping morphogenesis marked by cmlc2 expression in control (D-looping), gpr161 knockdown (unlooped and L-looping) and RNA rescued embryos. All scale bars were 100μm. (C) Graphical summary of gpr161 knockdown disrupted normal cardiac D-looping morphogenesis at 36 hpf. Calculation as % of embryos with D-looping in control (97.8±1.3%; n=223), embryos injected with gpr161 morpholino (MO#36, 32.4±4.1%; n=293) and RNA rescue (gpr161 RNA without 5'UTR; 52.2±3.4%; n=157), n was number of total embryos and results were from 4 injection experiments. Error bars were ± SEM. t-test * and # indicated statistical significance, p<0.01.
Knockdown of zebrafish gpr161 disrupts L-R asymmetry
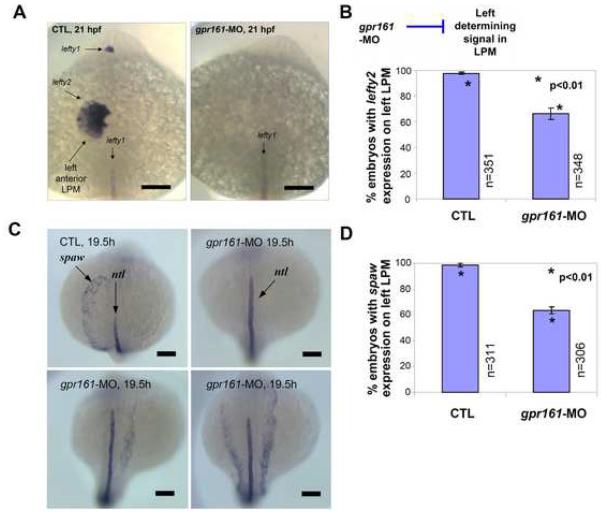
Failure to undergo cardiac looping is frequently the result of a defect in L-R patterning (Chen et al, 1997). One model for establishment of the L-R axis suggests the involvement of a ciliated organ known as the embryonic node in mice (Hirokawa et al, 2006) or Kupffer's vesicle in zebrafish (Oishi et al, 2006). Prior to formation of the heart tube, this organ is thought to produce a signal that leads to left-sided expression of several genes such as lefty2 and southpaw (spaw) in the lateral plate mesoderm (LPM) in close proximity to the developing precardiac mesoderm (Essner et al, 2005; Chocron et al, 2007). To determine if loss of zebrafish gpr161 disrupts L-R asymmetry, we first examined the expression of the nodal antagonists lefty1 and lefty2 at 21 hpf. In control embryos, left-sided expression of lefty1 (dorsal diencephalon) and lefty2 (LPM) was detected in 99.3% of cases (Figs. 3A, B; Supplementary Table 2). By contrast, the left-sided expression of lefty1 and lefty2 was almost completely suppressed in 31.6% of gpr161 knockdown embryos and was aberrant in 1.4% of embryos with bilateral or right-sided expression of lefty2 in the LPM. Knockdown of spaw has been reported to perturb left-sided expression of lefty2 and to disrupt cardiac looping in zebrafish embryos (Long et al., 2003). To determine if spaw expression was altered in gpr161 knockdown embryos, we examined the expression of spaw at an even earlier stage of somitogenesis (19.5 hpf). In control embryos, left-sided expression of spaw was detected in 98.7% of cases (Fig 3C, D; Supplementary Table 3). By contrast, the left-sided expression of spaw was completely suppressed in 30.4% of gpr161 knockdown embryos and was aberrant in 6.2% of knockdown embryos with either bilateral or right-sided expression of spaw. Demonstrating altered spaw or lefty2 expression was not due to a general disruption of the LPM, we showed that bilateral expression of nkx2.5 (Chen and Fishman, 1996) in the pre-cardiac mesoderm was not affected in gpr161 knockdown embryos (Supplementary Fig. 4A). Also, ruling out a general disruption of the dorsal midline structures, we demonstrated that midline expression of no tail (ntl) (arrow in Fig. 3C) and lefty 1 (arrow in Fig. 3A) were not altered in gpr161 knockdown embryos. Collectively, these results demonstrate that L-R patterning in the LPM is specifically disrupted in the majority of gpr161 knockdown embryos.
Fig. 3.
gpr161 knockdown disrupts L-R identity in lateral plate mesoderm. (A) lefty2 expression in the left anterior lateral plate mesoderm (LPM) and lefty1 expression in the left diencephalon were disrupted in gpr161 knockdown embryos. (B) Graphical summary of control (99.3±1.1%; n=351), gpr161 morpholino (MO#24, 67.0±4.7%; n=348) and t-test (p<0.01) results were from 11 injection experiments. (C) spaw expression in the left anterior lateral plate mesoderm (LPM) at 19.5 hpf in control and gpr161 knockdown embryos. ntl expression marked the midline as a reference. (D) Graphical summary of spaw expression in control (98.7±1.4%; n=311), gpr161 morpholino (MO#24, 63.4±2.7%; n=306) and t-test (p<0.01) results were from 13 injection experiments. Error bars were ± SEM. All scale bars were 100μm.
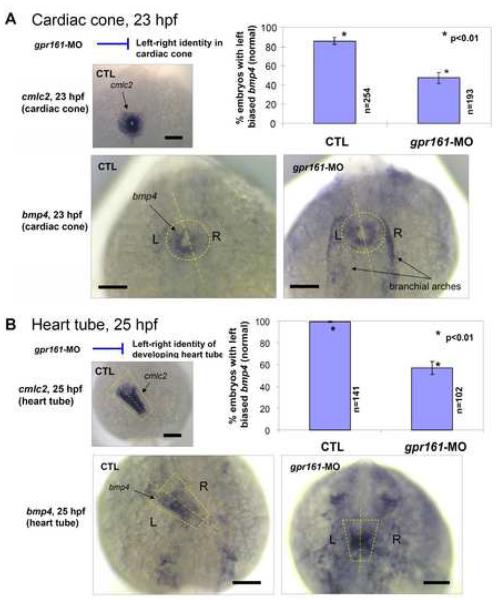
The bmp4 gene, which encodes bone morphogenetic protein 4, is used as molecular marker of L-R asymmetry in the developing heart tube (Chen et al, 1997; Schilling et al, 1999). At 19 hpf (20 somite stage), bmp4 is uniformly expressed in the cardiac cone. However, at 20 hpf (22 somite stage), the pattern of bmp4 expression becomes asymmetric, with left-sided expression predominating over the right, immediately prior to the leftward jogging of the heart tube and subsequent looping to the right (Chen et al, 1997). As expected, at 23 hpf, 85.8% of control embryos preferentially expressed bmp4 on the left side of the cardiac cone (Fig. 4A). By contrast, only 47.5% of gpr161 knockdown embryos showed this pattern, with the rest of the embryos exhibiting similar levels of bmp4 expression on both sides of the cardiac cone (Fig. 4A; Supplementary Table 4). Likewise, at 25-hpf, 99.5% of control embryos preferentially expressed bmp4 on the left side of the developing heart tube compared to only 56.9% of gpr161 knockdown embryos (Fig. 4B; Supplementary Table 4). Collectively, these results indicate that L-R patterning in the developing heart tube is perturbed in the majority of gpr161 knockdown embryos. Given the importance of L-R asymmetry for proper positioning of the heart tube, a primary defect in this process is the most likely explanation for the cardiac defect that was observed in gpr161 knockdown embryos.
Fig. 4.
(A) bmp4 expression was predominantly on the left side of the cardiac cone in comparison to the uniform expression of cmlc2 (inset) was disrupted in gpr161 knockdown embryos. Graphical summary of control (85.8±3.7%; n=254), gpr161 knockdown (MO#24, 47.5±5.7%; n=193) and t-test (p<0.01) results were from 7 injection experiments. (B) bmp4 expression was predominantly on the left side of the heart tube in comparison to the uniform expression of cmlc2 (inset) was disrupted in gpr161 knockdown embryos. Graphical summary of control (99.5±1.3%; n=141), gpr161 knockdown (MO#24, 56.9±5.9%; n=102) and t-test (p<0.01) results were from 4 injection experiments. Dashed lines were drawn over the cardiac cone and heart tube and the midline of the structures. n was number of total embryos. Error bars were ± SEM. All scale bars were 100μm.
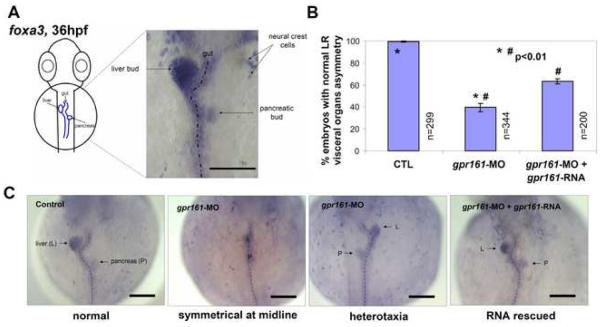
In addition to the heart, morphological L-R asymmetry of the visceral organs is also observed in zebrafish embryos (Horne-Badovinac et al., 2003). Between 26 and 30 hpf, the gut primordium starts to loop, with the liver bud curving to the left and the pancreatic bud occupying an asymmetric position on the right side of the gut. To determine if L-R asymmetry of the visceral organs is affected in gpr161 knockdown embryos, we used a pan-endodermal marker foxa3, which marks the developing gut primordium, including the liver and pancreatic buds (Odenthal and Nusslein-Volhard, 1998; Horne-Badovinac et al., 2003). As expected, 99.3% of control embryos exhibited a leftward budding of the liver and a rightward budding of the pancreas at 36 hpf (Fig 5A, B). By contrast, the majority of gpr161 knockdown embryos failed to show normal L-R asymmetry of the visceral organs, with 49.1% embryos exhibiting symmetrical placement of the liver and pancreas with respect to the midline and 11.4% embryos showing heterotaxic phenotypes (Figs. 5B, C; Supplementary Table 5). RNA rescue experiments showed that gpr161-RNA can suppress the visceral organ defects from 60.4% to 36.5% in gpr161 knockdown embryos (Figs. 5B, C; Supplementary Table 5). The L-R orientation of brain, heart, and gut is thought to be regulated by distinct pathways affecting different steps along the anterior-posterior axis of the embryos (Bisgrove et al, 2000). Since gpr161 knockdown embryos showed severe reduction of lefty2 and spaw expression and exhibited defects in both cardiac looping and visceral organ asymmetry, our results suggest that gpr161 plays a critical role in the establishment of L-R asymmetry at an early step.
Fig. 5.
L-R asymmetry of visceral organs in zebrafish embryos. (A) foxa3 expression marks the gut primordium at 36 hpf showing the liver bud (left) and pancreatic bud (right) in control embryo. (B) Graphical summary of normal visceral asymmetry in control (97.8±1.3%), gpr161 knockdown (32.4±4.1%) and RNA rescued (52.2±3.4%) embryos. t-test (p<0.01) results were from 6 injection experiments. n was number of total embryos. Error bars were ± SEM. (C) foxa3 expression of liver and pancreatic buds in normal L-R asymmetric position in control, symmetric or heterotaxia in gpr161 knockdown and normal L-R asymmetry in RNA rescued embryos. All scale bars were 100μm.
gpr161 knockdown perturbs Ca2+ handling surrounding the Kupffer's vesicle
Several recent studies suggest that L-R asymmetry may be controlled by a ciliated organ called the embryonic node in mice, Hensen's node in chick, or Kupffer's vesicle in zebrafish (Hirokawa et al, 2006; Oishi et al, 2006). In the latter, motile cilia are thought to generate a leftward nodal flow that stimulates sensory cilia on cells lining the Kuppfer's vesicle (Delmas 2004; Nauli and Zhou 2004; Tabin and Vogan 2003). This process is thought to establish an asymmetric Ca2+ signal that produces the asymmetric gene expression (Essner et al, 2005; McGrath et al, 2003; Sarmah et al, 2005). Consistent with a critical role for these cilia, mutations or knockdown of components such as dynein have been linked to defective L-R patterning and polycystic kidney disease (Otto et al., 2003; Sun et al., 2004; Kramer-Zucker et al., 2005; Obara et al., 2006).
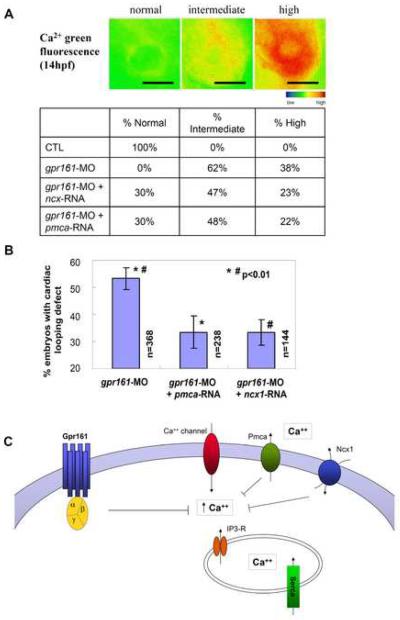
Consistent with a possible role in the Kupffer's vesicle, zebrafish gpr161 is expressed in the posterior mesoderm surrounding this structure during early somitogenesis (14 hpf) (Fig. 1B). Moreover, comparative analysis of zebrafish gpr161 knockdown and the ciliary dynein knockdown (Essner et al, 2005) phenotypes reveals some similarities, including absent or altered lefty1/2 expression. To investigate if gpr161 function is required for either the formation of the Kupffer's vesicle or expression of cilia, we used confocal imaging to visualize tubulin staining of the cilia on cells lining the Kupffer's vesicle. As compared to the control embryos, gpr161 knockdown embryos showed no significant difference in the number or morphology of cilia (Supplementary Fig. 4B). Moreover, in contrast to dynein knockdown embryos, cystic kidneys were not observed in gpr161 knockdown embryos, suggesting no apparent defect in the motility of cilia. Thus, despite some similarities, there are obvious differences between gpr161 and dynein knockdown phenotypes, suggesting they may affect different steps in this process. In this regard, we found that gpr161 may be affecting the generation of the Ca2+ signal within cells lining the Kupffer's vesicle. To monitor the Ca2+ signal in vivo, we injected Ca2+ green indicator into 1-cell stage zebrafish embryos to allow even distribution to all embryonic cells, as described previously (Reinhard et al., 1995; Cox et al., 1996; O'Malley et al., 1996; Nicolson et al., 1998; Fuss et al., 2001; Takahashi et al., 2002; Liu et al., 2003; Webb et al., 2003; Sarmah et al., 2005). Using fluorescence microscopy, we detected elevated Ca2+ levels in cells surrounding the Kupffer's vesicle in gpr161 knockdown embryos at 14 hpf (3- to 6- somite stage) (Fig. 6A; Supplementary Fig. 5). Combining histogram analysis of the Ca2+ imaging (Supplementary Fig. 6) and mathematical modeling (Supplementary Fig. 7), we quantitated the fraction of embryos showing normal, intermediate, and high Ca2+ signals in each group. Compared to control embryos, virtually 100% of the gpr161 knockdown embryos showed intermediate to high Ca2+ levels (Fig. 6A). To determine if removal of excess Ca2+ can rescue the L-R defect that produced the cardiac looping abnormality in gpr161 knockdown embryos, we over-expressed the membrane Ca2+ pump pmca, or the Na+/Ca2+ exchanger ncx1 (Ebert et al, 2005; Langenbacher et al, 2005). Again, combining histogram analysis of the Ca2+ imaging (Supplementary Fig. 6) and mathematical modeling (Supplementary Fig. 7), we showed that injection of either ncx1 or pmca RNA can normalize Ca2+ levels in ~30% of gpr161 knockdown embryos (Fig. 6A), with correlation coefficients of 0.997 in control, 0.995 in gpr161 knockdown, 0.993 in ncx1-RNA rescue and 0.979 in pmca-RNA rescue experiments, respectively. Notably, the percentage of gpr161 knockdown embryos whose Ca2+ levels can be normalized by RNA injection (Fig. 6A) was similar to the percentage of gpr161 knockdown embryos whose cardiac looping defects can be rescued by RNA injection (Fig. 6B; Supplementary Table 6). Collectively, these findings demonstrate that zebrafish gpr161 signaling is essential for the regulation of Ca2+ levels that are necessary for establishment of L-R asymmetry at an early somite stage. In support of our findings, knockdown of the zebrafish Na+/Ca2+ exchanger ncx4a was shown to disrupt Ca2+ homeostasis and perturbed L-R patterning via Ca2+/calmodulin-dependent protein kinase (Shu et al, 2007).
Fig. 6.
gpr161 knockdown disrupts Ca2+ handling which is essential for establishment of L-R asymmetry. (A) Defective Ca2+ handling resulting in elevated intracellular Ca2+ in gpr161 knockdown embryos surrounding the Kupffer's vesicle. Pseudocolour scale for the intracellular Ca2+ signal as normal, intermediate and high intensity. The percentage of embryos exhibited such level of Ca2+ signal was listed in control, gpr161 knockdown, ncx1- and pmca-RNA rescue of the gpr161 knockdown embryos. (B) Removal of excessive Ca2+ by over-expression of 1pg of Ca2+ pump pmca or Na+/Ca2+ exchanger ncx1 can rescue gpr161 knockdown phenotype. Cardiac looping defect in gpr161-MO (MO#24, 54.1±4.0%; n=368), rescued by pmca-RNA (32.0±6.0%; n=238), rescued by ncx1-RNA (34.0±4.8%; n=144) and results were from 4 injection experiments. n was number of total embryos. Error bars were ± SEM. t-test * and # indicated statistical significance, p<0.01. All scale bars were 100μm. (C) A model of gpr161 involved in Ca2+ handling and essential for L-R asymmetry for cardiac morphogenesis and visceral asymmetry in developing zebrafish embryos. Blocking gpr161 function led to defect in Ca2+ handling and resulted in elevated free cytosolic Ca2+ and loss of cardiac looping and abnormal chamber morphogenesis. The gpr161 knockdown can be rescued by removing excessive cytosolic free Ca2+ using over-expression of Ca2+ pump pmca and Na+/Ca2+ exchanger ncx1.
Discussion
The analysis of the human genome has revealed ~150 family members that are currently classified as orphan GPCRs due to the lack of information regarding their physiological ligands and functions (Vassilatis et al, 2003; Wise et al, 2004). Because orphan GPCRs represent a potential resource for future drug development, various approaches, including transgenic and gene knockout approaches in mice, have been used to decipher their biological roles (Ma and Zemmel 2002; Marchese et al, 1999; Rohrer and Kobilka 1998). Nevertheless, progress on identifying biological functions has been slow due to the time consuming and expensive nature of these approaches (Katugampola and Davenport 2003). Here, we show the utility of combining a RNA knockdown strategy with the rapid developmental program of zebrafish to identify a critical role for the orphan GPCR, gpr161, in vertebrate development. These results add to the growing list of GPCRs that have recently been shown to play critical roles in developmental processes (Kupperman et al, 2000; Leung et al, 2006; Yi et al, 2006).
A highly conserved feature of vertebrate development is the establishment of the L-R axis that determines asymmetric placement of the brain, heart, and visceral organs with respect to the embryonic midline. Although not universally accepted (Levin and Palmer 2007), one model suggests this process is controlled by motile cilia of the mouse embryonic node or the zebrafish Kupffer's vesicle that create a leftward flow of fluid (Hirokawa et al, 2006; Oishi et al, 2006). Disrupting the formation/function of these cilia blocks fluid flow and causes L-R patterning defects (McGrath et al., 2003; Oishi et al., 2006; Speder et al., 2007; Tabin and Vogan, 2003). Although the underlying mechanism is still emerging, this process seems to involve asymmetric Ca2+ flux in the cells surrounding the node/Kupffer's vesicle (Raya et al., 2004; Sarmah et al., 2005; Shimeld et al, 2004; Speder et al., 2007). This asymmetric Ca2+ signal appears to result from activation of polycystin cation channels in the sensory cilia in response to leftward fluid flow produced by the motile cilia (Delmas 2004; Nauli and Zhou 2004; Tabin and Vogan 2003). Perturbing this asymmetric Ca2+ signal disrupts left-sided expression of genes such as spaw and lefty2 in the lateral plate mesoderm (Essner et al, 2005; McGrath et al, 2003; Sarmah et al, 2005) and produces L-R patterning defects.
Little is known about the potential role of GPCR signaling pathways in regulating the asymmetric Ca2+ signal. Here, we present four lines of evidence supporting the hypothesis that gpr161 is essential for Ca2+ signaling in the Kupffer's vesicle and for determination of the L-R axis. Firstly, gpr161 is expressed in the posterior and lateral plate mesoderm in close proximity to the developing Kupffer's vesicle - consistent with its role in this process. Secondly, knockdown of gpr161 results in L-R patterning defects, including abnormalities in cardiac looping and positioning of visceral organs. Thirdly, knockdown of gpr161 disrupts left-sided expression of laterality genes, including lefty2 and spaw in the lateral plate mesoderm and bmp4 in the developing heart tube. Lastly, knockdown of gpr161 produces elevated Ca2+ levels in the posterior mesoderm surrounding the Kupffer's vesicle. Collectively, these results support a model in which gpr161 regulates L-R asymmetry by modulating Ca2+ levels in cells surrounding the Kupffer's vesicle. Since fluorescent measurement of Ca2+ levels can be affected by pH, we cannot rule out an additional involvement of gpr161 in regulating pH levels. However, the finding that over-expression of the membrane Ca2+ pump pmca, or the Na+/Ca2+ exchanger ncx1 normalizes the fluorescent signals and rescues cardiac looping defects in a similar percentage of gpr161 knockdown embryos strongly suggests a primary role in regulating Ca2+ homeostasis.
The signaling pathway(s) acting downstream of gpr161 has not been identified. However, a computational program (Proteome Alliance: http://tp12.pzr.unirostock.de/~moeller/7tmhmm/submission.php) based on structural characteristics of known GPCRs (Möller et al, 2001; Sgourakis et al, 2005) predicts that zebrafish Gpr161 is coupled to a member of the G protein αq family. Members of the Gαq family have been shown to activate phospholipase C-β, which produces diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). Upon acute activation, IP3 increases the cytosolic Ca2+ level by mediating its release from intracellular stores (Hubbard and Hepler 2006). Subsequently, the intracellular stores are replenished by increasing entry of extracellular Ca2+ across the plasma membrane. However, upon chronic stimulation, DAG acting via protein kinase C is thought to prevent Ca2+ overload by inhibiting the entry of extracellular Ca2+ (Fan et al, 2005; Lu et al, 2005; Sternfeld et al 2007). We envision that the zebrafish Gpr161 may act in a similar fashion. Accordingly, we propose a model in which the zebrafish orphan receptor Gpr161 reduces Ca2+ entry through the plasma membrane by inhibiting a Ca2+ channel, Ca2+ pump, or Ca2+ exchanger (Fig. 6C). Further supporting a role for inositol phosphate signaling in L-R axis determination, knockdown of an inositol phosphate kinase (ipk1) has been shown to interfere with asymmetric Ca2+ flux and produce L-R patterning defects (Sarmah et al., 2005). Since gpr161 is expressed in the posterior mesoderm, this signaling pathway may function in the cells surrounding the Kupffer's vesicle. Alternatively, this signaling pathway may function in the dorsal forerunner cells giving rise to this structure (Schneider et al., 2008). Although future studies will be needed to clarify the signaling pathway and identify the earliest site of its action, our results show that the orphan GPCR, gpr161, is critical for the establishment of L-R asymmetry through direct or indirect regulation of Ca2+ homeostasis in zebrafish.
Although a previous report has identified a requirement for a G protein in the initiation of L-R patterning in invertebrates (Bergmann et al., 2003), this is the first report of a GPCR acting in the elaboration and maintenance of L-R patterning in vertebrates. Considering the conservation of molecular components in L-R asymmetry and cardiac morphogenesis, it will be of interest to investigate if the gpr161 signaling pathway is similarly involved in establishment of the L-R axis in mammals. While this paper was under revision, a study appeared linking a probable gain-of-function mutation of mouse Gpr161 gene to abnormal neural fold closure and lens development in mice (Matteson et al., 2008). Taken together, the finding that knockdown phenotypes result from loss-of-function of gpr161 in zebrafish as well as gain-of-function in mice supports an evolutionarily conserved role in vertebrate development.
Materials and methods
Zebrafish, Danio rerio
Florida wildtype strain ('That Fish Place', Lancaster, Pennsylvania). Longfin strain (Scientific Hatcheries, Huntington Beach, CA). Transgenic zebrafish line (TG[cmlc:GFP]) (Huang et al, 2003).
RNA Over-expression
Zebrafish gpr161 over-expression construct was generated by PCR using primers (listed below) and then subcloned into pCS2+ vector at BamH1/Xho1 and Cla1/Xho1 sites, respectively. Capped sense RNA was synthesized using SP6 RNA polymerase and the mMESSAGE mMACHINE system (Ambion, Austin, TX). Canine ncx1 was kindly provided by Dr. Ken Philipson and zebrafish pmca was kindly provided by Dr. Jau-Nian Chen. For microinjection of gpr161, ncx1 and pmca mRNA or morpholino antisense oligos, zebrafish embryos were injected at 1 cell stage with injection volume of about 0.5 nl, subsequently incubated in 0.3x Danieau's medium at 28.5°C. Embryos were maintained in the above condition until they reached different developmental stages for total RNA preparation and whole mount in situ hybridization. PCR primers for the gpr161-RNA construct were:
gpr161FL-F1: 5'-AATGAACGGCTCTAAGAATGGGACG;
gpr161FL-R1: 5'-CTGTACCTTCAAAAGCTGCAGATTAAA
Antisense morpholino oligos
gpr161-MO (translational blocking antisense morpholinos) synthesized by GeneTools, LLC, Philomath, Oregon:
(MO#24, targeting at ATG start codon): 5'-CGTCCCATTCTTAGAGCCGTTCATT; (MO#36, targeting at 5'UTR): 5'-GAGGCTTCTGGTTGCCAATGACTTG.
5-mismatch-MO control (MO#28): 5'-CCTCCGATTGTTAGAGCCCTTGATT (mismatch were underlined).
For gpr161 RNA rescue experiment, MO#36 targeting the 5'UTR was used. All other experiments, MO#24 targeting the ATG start codon was used. Morpholinos were injected into zebrafish embryos at 1 cell stage with injection volume of about 0.5 nl at 100μM and 300uM for MO#24 and MO#36, respectively. Zebrafish gpr161 acc. no. (EU090912).
Whole mount in situ hybridization
Details of the whole mount in situ hybridization protocol and probes used in this study are given in Supplementary Methods.
RT-PCR
Details of the RT-PCR protocol and primers for gpr161 and actin are provided in the Supplementary Methods.
Detection of MO blocking function by in vitro system
An in vitro transcription and translation coupled system to assay the efficacy and specificity of the MO is described in the Supplementary Methods.
Detection of intracellular Ca2+ in zebrafish embryos
Ca2+ green-dextran 10,000 MW (Molecular probe/Invitrogen, Carlsbad, CA) was used at 0.05% (w/v) for microinjection into zebrafish embryos at 1 cell stage of injection volume of about 0.5 nl. Embryos were incubated in 0.3x Danieau's medium at 28.5°C. Fluorescent image at 500ms exposure of the Kupffer's vesicle (KV) at 3 to 6 somite stage was documented using a MZFL3 stereomicroscope with GFP Plus fluorescence filter set of excitation filter 480/40 nm and barrier filter 510 nm (Leica Microsystems, Wetzlar, Germany) and images were converted equally to an intensity scale using ImagePro+ software (Media Cybernetics, Silver Spring, MD; red indicating high intensity; yellow, moderate; green, low).
Supplementary Material
Acknowledgements
We are grateful to Drs. Deborah Yelon, Jau-Nian Chen, Ken Philipson, Michael Rebagliati, Matthais Hammerschmidt and Wolfgang Driever for sharing reagents; Dr. William Schwindinger for mathematical modeling of histogram analysis and Dr. Carl Hansen for valuable suggestion on the analysis; and Cynthia Rhone, Gail Gregory, Shannon Wescott and Steven Krasucki for animal care. We thank Drs. Jau-Nian Chen and William Schwindinger, and Soniya Sinha for critical review of the manuscript. We are very thankful to our editorial reviewers that gave us the critical comments and suggestions in our manuscript. This study is funded by NIH grants GM58191 and GM39867 awarded to JDR. While this paper was being prepared for submission, an online version of a paper describing a hypomorphic allele of mouse Gpr161 gene with congenital cataracts and neural tube defects was published (Matteson et al., 2008).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at doi:
References
- Amsterdam A, Burgess S, Golling G, Chen W, Sun Z, Townsend K, Farrington S, Haldi M, Hopkins N. A large-scale insertional mutagenesis screen in zebrafish. Genes Dev. 1999;13:2713–2724. doi: 10.1101/gad.13.20.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann DC, Lee M, Robertson B, Tsou MF, Rose LS, Wood WB. Embryonic handedness choice in C. elegans involves the Galpha protein GPA-16. Development. 2003;130:5731–5740. doi: 10.1242/dev.00839. [DOI] [PubMed] [Google Scholar]
- Bisgrove BW, Essner JJ, Yost HJ. Multiple pathways in the midline regulate concordant brain, heart and gut left-right asymmetry. Development. 2000;127:3567–3579. doi: 10.1242/dev.127.16.3567. [DOI] [PubMed] [Google Scholar]
- Chen JN, Fishman MC. Zebrafish tinman homolog demarcates the heart field and initiates myocardial differentiation. Development. 1996;122:3809–3816. doi: 10.1242/dev.122.12.3809. [DOI] [PubMed] [Google Scholar]
- Chen JN, van Eeden FJ, Warren KS, Chin A, Nusslein-Volhard C, Haffter P, Fishman MC. Left-right pattern of cardiac BMP4 may drive asymmetry of the heart in zebrafish. Development. 1997;124:4373–4382. doi: 10.1242/dev.124.21.4373. [DOI] [PubMed] [Google Scholar]
- Chocron S, Verhoeven MC, Rentzsch F, Hammerschmidt M, Bakkers J. Zebrafish Bmp4 regulates left-right asymmetry at two distinct developmental time points. Dev. Biol. 2007;305:577–588. doi: 10.1016/j.ydbio.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Cox KJ, Fetcho JR. Labeling blastomeres with a calcium indicator: a non-invasive method of visualizing neuronal activity in zebrafish. J. Neurosci. Methods. 1996;68:185–191. doi: 10.1016/0165-0270(96)00067-2. [DOI] [PubMed] [Google Scholar]
- Delmas P. Polycystins: from mechanosensation to gene regulation. Cell. 2004;118:145–148. doi: 10.1016/j.cell.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Malicki J, Stemple DL, Stainier DY, Zwartkruis F, Abdelilah S, Rangini Z, Belak J, Boggs C. A genetic screen for mutations affecting embryogenesis in zebrafish. Development. 1996;123:37–46. doi: 10.1242/dev.123.1.37. [DOI] [PubMed] [Google Scholar]
- Ebert AM, Hume GL, Warren KS, Cook NP, Burns CG, Mohideen MA, Siegal G, Yelon D, Fishman MC, Garrity DM. Calcium extrusion is critical for cardiac morphogenesis and rhythm in embryonic zebrafish hearts. Proc. Nal. Acad. Sci. U. S. A. 2005;102:17705–17710. doi: 10.1073/pnas.0502683102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ. Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development. 2005;132:1247–1260. doi: 10.1242/dev.01663. [DOI] [PubMed] [Google Scholar]
- Fan G, Jiang YP, Lu Z, Martin DW, Kelly DJ, Zuckerman JM, Ballou LM, Cohen IS, Lin RZ. A transgenic mouse model of heart failure using inducible Galpha q. J. Biol. Chem. 2005;280:40337–40346. doi: 10.1074/jbc.M506810200. [DOI] [PubMed] [Google Scholar]
- Fraser LR, Adeoya-Osiguwa SA, Baxendale RW. First messenger regulation of capacitation via G protein-coupled receptors. Mol. Hum. Reprod. 2003;9:739–748. doi: 10.1093/molehr/gag097. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Schioth HB. The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol. Pharmacol. 2005;67:1414–1425. doi: 10.1124/mol.104.009001. [DOI] [PubMed] [Google Scholar]
- Fuss SH, Korsching SI. Odorant feature detection: activity mapping of structure response relationships in the zebrafish olfactory bulb. J. Neurosci. 2001;21:8396–8407. doi: 10.1523/JNEUROSCI.21-21-08396.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler R, Rauch GJ, Geiger-Rudolph S, Albrecht A, van Bebber F, Berger A, Busch-Nentwich E, Dahm R, Dekens MP, Dooley C, Elli AF, Gehring I, Geiger H, Geisler M, Glaser S, Holley S, Huber M, Kerr A, Kirn A, Knirsch M, Konantz M, Küchler AM, Maderspacher F, Neuhauss SC, Nicolson T, Ober EA, Praeg E, Ray R, Rentzsch B, Rick JM, Rief E, Schauerte HE, Schepp CP, Schönberger U, Schonthaler HB, Seiler C, Sidi S, Söllner C, Wehner A, Weiler C, Nüsslein-Volhard C. Large-scale mapping of mutations affecting zebrafish development. BMC Genomics. 2007;8:11. doi: 10.1186/1471-2164-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin CT, Srinivasan Y, Zheng YW, Huang W, Coughlin SR. A role for thrombin receptor signaling in endothelial cells during embryonic development. Science. 2001;293:1666–1670. doi: 10.1126/science.1061259. [DOI] [PubMed] [Google Scholar]
- Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, van Eeden FJ, Jiang YJ, Heisenberg CP, Kelsh RN, Furutani-Seiki M, Vogelsang E, Beuchle D, Schach U, Fabian C, Nüsslein-Volhard C. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left-right asymmetry. Cell. 2006;125:33–45. doi: 10.1016/j.cell.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac S, Rebagliati M, Stainier DY. A cellular framework for gut-looping morphogenesis in zebrafish. Science. 2003;302:662–665. doi: 10.1126/science.1085397. [DOI] [PubMed] [Google Scholar]
- Huang CJ, Tu CT, Hsiao CD, Hsieh FJ, Tsai HJ. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev. Dyn. 2003;228:30–40. doi: 10.1002/dvdy.10356. [DOI] [PubMed] [Google Scholar]
- Hubbard KB, Hepler JR. Cell signaling diversity of the Gqalpha family of heterotrimeric G proteins. Cell. Signal. 2006;18:135–150. doi: 10.1016/j.cellsig.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Katugampola S, Davenport A. Emerging roles for orphan G-protein-coupled receptors in the cardiovascular system. Trends Pharmacol. Sci. 2003;24:30–35. doi: 10.1016/s0165-6147(02)00007-x. [DOI] [PubMed] [Google Scholar]
- Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Kupperman E, An S, Osborne N, Waldron S, Stainier DY. A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature. 2000;406:192–195. doi: 10.1038/35018092. [DOI] [PubMed] [Google Scholar]
- Langenbacher AD, Dong Y, Shu X, Choi J, Nicoll DA, Goldhaber JI, Philipson KD, Chen JN. Mutation in sodium-calcium exchanger 1 (NCX1) causes cardiac fibrillation in zebrafish. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17699–17704. doi: 10.1073/pnas.0502679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung T, Chen H, Stauffer AM, Giger KE, Sinha S, Horstick EJ, Humbert JE, Hansen CA, Robishaw JD. Zebrafish G protein gamma2 is required for VEGF signaling during angiogenesis. Blood. 2006;108:160–166. doi: 10.1182/blood-2005-09-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Palmer AR. Left-right patterning from the inside out: widespread evidence for intracellular control. Bioessays. 2007;29:271–287. doi: 10.1002/bies.20545. [DOI] [PubMed] [Google Scholar]
- Lin F, Sepich DS, Chen S, Topczewski J, Yin C, Solnica-Krezel L, Hamm H. Essential roles of Gα12/13 signaling in distinct cell behaviors driving zebrafish convergence and extension gastrulation movements. J. Cell Biol. 2005;169:777–787. doi: 10.1083/jcb.200501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KS, Gray M, Otto SJ, Fetcho JR, Beattie CE. Mutations in deadly seven/notch1a reveal developmental plasticity in the escape response circuit. J. Neurosci. 2003;23:8159–8166. doi: 10.1523/JNEUROSCI.23-22-08159.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S, Ahmad N, Rebagliati M. The zebrafish nodal-related gene southpaw is required for visceral and diencephalic left-right asymmetry. Development. 2003;130:2303–2316. doi: 10.1242/dev.00436. [DOI] [PubMed] [Google Scholar]
- Lu Z, Jiang YP, Ballou LM, Cohen IS, Lin RZ. Galpha q inhibits cardiac L-type Ca2+ channels through phosphatidylinositol 3-kinase. J. Biol. Chem. 2005;280:40347–40354. doi: 10.1074/jbc.M508441200. [DOI] [PubMed] [Google Scholar]
- Ma P, Zemmel R. Value of novelty? Nat. Rev. Drug Discov. 2002;1:571–572. doi: 10.1038/nrd884. [DOI] [PubMed] [Google Scholar]
- Marchese A, George SR, Kolakowski LF, Jr, Lynch KR, O'Dowd BF. Novel GPCRs and their endogenous ligands: expanding the boundaries of physiology and pharmacology. Trends Pharmacol. Sci. 1999;20:370–375. doi: 10.1016/s0165-6147(99)01366-8. [DOI] [PubMed] [Google Scholar]
- Matteson PG, Desai J, Korstanje R, Lazar G, Borsuk TE, Rollins J, Kadambi S, Joseph J, Rahman T, Wink J, Benayed R, Paigen B, Millonig JH. The orphan G protein-coupled receptor, Gpr161, encodes the vacuolated lens locus and controls neurulation and lens development. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2088–2093. doi: 10.1073/pnas.0705657105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- Möller S, Vilo J, Croning MD. Prediction of the coupling specificity of G protein coupled receptors to their G proteins. Bioinformatics. 2001;17:S174–S181. doi: 10.1093/bioinformatics/17.suppl_1.s174. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Zhou J. Polycystins and mechanosensation in renal and nodal cilia. Bioessays. 2004;26:844–856. doi: 10.1002/bies.20069. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene 'knockdown' in zebrafish. Nat. Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Nicolson T, Rüsch A, Friedrich RW, Granato M, Ruppersberg JP, Nüsslein-Volhard C. Genetic Analysis of Vertebrate Sensory Hair Cell Mechanosensation: the Zebrafish Circler Mutants. Neuron. 1998;20:271–283. doi: 10.1016/s0896-6273(00)80455-9. [DOI] [PubMed] [Google Scholar]
- Obara T, Mangos S, Liu Y, Zhao J, Wiessner S, Kramer-Zucker AG, Olale F, Schier AF, Drummond IA. Polycystin-2 immunolocalization and function in zebrafish. J. Am. Soc. Nephrol. 2006;17:2706–2718. doi: 10.1681/ASN.2006040412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenthal J, Nusslein-Volhard C. Fork head domain genes in zebrafish. Dev. Genes Evol. 1998;208:245–258. doi: 10.1007/s004270050179. [DOI] [PubMed] [Google Scholar]
- Offermanns S, Mancino V, Revel JP, Simon MI. Vascular system defects and impaired cell chemokinesis as a result of Gα13 deficiency. Science. 1997;275:533–536. doi: 10.1126/science.275.5299.533. [DOI] [PubMed] [Google Scholar]
- Oishi I, Kawakami Y, Raya A, Callol-Massot C, Izpisua Belmonte JC. Regulation of primary cilia formation and left-right patterning in zebrafish by a noncanonical Wnt signaling mediator, duboraya. Nat. Genet. 2006;38:1316–1322. doi: 10.1038/ng1892. [DOI] [PubMed] [Google Scholar]
- O'Malley DM, Kao Y-H, Fetcho JR. Imaging the Functional Organization of Zebrafish Hindbrain Segments during Escape Behaviors. Neuron. 1996;17:1145–1155. doi: 10.1016/s0896-6273(00)80246-9. [DOI] [PubMed] [Google Scholar]
- Otto EA, Schermer B, Obara T, O'Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, Foreman JW, Goodship JA, Strachan T, Kispert A, Wolf MT, Gagnadoux MF, Nivet H, Antignac C, Walz G, Drummond IA, Benzing T, Hildebrandt F. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat. Genet. 2003;34:413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RT, Link BA, Dowling JE, Schreiber SL. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc. Natl. Acad. Sci. U. S. A. 2000;97:12965–12969. doi: 10.1073/pnas.97.24.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raya A, Kawakami Y, Rodríguez-Esteban C, Ibañes M, Rasskin-Gutman D, Rodríguez-León J, Büscher D, Feijó JA, Izpisúa-Belmonte JC. Notch activity acts as a sensor for extracellular calcium during vertebrate left-right determination. Nature. 2004;427:121–128. doi: 10.1038/nature02190. [DOI] [PubMed] [Google Scholar]
- Reinhard E, Yokoe H, Niebling KR, Allbritton NL, Kuhn MA, Meyer T. Localized calcium signals in early zebrafish development. Dev. Biol. 1995;170:50–61. doi: 10.1006/dbio.1995.1194. [DOI] [PubMed] [Google Scholar]
- Rohrer DK, Kobilka BK. G protein-coupled receptors: functional and mechanistic insights through altered gene expression. Physiol. Rev. 1998;78:35–52. doi: 10.1152/physrev.1998.78.1.35. [DOI] [PubMed] [Google Scholar]
- Romo X, Pastén P, Martínez S, Soto X, Lara P, de Arellano AR, Torrejón M, Montecino M, Hinrichs MV, Olate J. xRic-8 is a GEF for Gsalpha and participates in maintaining meiotic arrest in Xenopus laevis oocytes. J. Cell. Physiol. 2008;214:673–680. doi: 10.1002/jcp.21257. [DOI] [PubMed] [Google Scholar]
- Ruppel KM, Willison D, Kataoka H, Wang A, Zheng YW, Cornelissen I, Yin L, Xu SM, Coughlin SR. Essential role for Gα13 in endothelial cells during embryonic development. Proc. Natl. Acad. Sci. U. S. A. 2005;102:8281–8286. doi: 10.1073/pnas.0503326102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmah B, Latimer AJ, Appel B, Wente SR. Inositol polyphosphates regulate zebrafish left-right asymmetry. Dev. Cell. 2005;9:133–145. doi: 10.1016/j.devcel.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Concordet JP, Ingham PW. Regulation of left-right asymmetries in the zebrafish by Shh and BMP4. Dev. Biol. 1999;210:277–287. doi: 10.1006/dbio.1999.9214. [DOI] [PubMed] [Google Scholar]
- Schneider I, Houston DW, Rebagliati MR, Slusarski DC. Calcium fluxes in dorsal forerunner cells antagonize beta-catenin and alter left-right patterning. Development. 2008;135:75–84. doi: 10.1242/dev.004713. [DOI] [PubMed] [Google Scholar]
- Schwartz TW, Frimurer TM, Holst B, Rosenkilde MM, Elling CE. Molecular mechanism of 7TM receptor activation-a global toggle switch model. Annu. Rev. Pharmacol. Toxicol. 2006;46:481–519. doi: 10.1146/annurev.pharmtox.46.120604.141218. [DOI] [PubMed] [Google Scholar]
- Scott IC, Masri B, D'Amico LA, Jin SW, Jungblut B, Wehman AM, Baier H, Audigier Y, Stainier DY. The G protein-coupled receptor agtrl1b regulates early development of myocardial progenitors. Dev. Cell. 2007;12:403–413. doi: 10.1016/j.devcel.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Sgourakis NG, Bagos PG, Hamodrakas SJ. Prediction of the coupling specificity of GPCRs to four families of G-proteins using hidden Markov models and artificial neural networks. Bioinformatics. 2005;21:4101–4106. doi: 10.1093/bioinformatics/bti679. [DOI] [PubMed] [Google Scholar]
- Shimeld SM. Calcium turns sinister in left-right asymmetry. Trends Genet. 2004;20:277–280. doi: 10.1016/j.tig.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Shu X, Huang J, Dong Y, Choi J, Langenbacher A, Chen JN. Na+,K+-ATPase alpha2 and Ncx4a regulate zebrafish left-right patterning. Development. 2007;134:1921–1930. doi: 10.1242/dev.02851. [DOI] [PubMed] [Google Scholar]
- Speder P, Petzoldt A, Suzanne M, Noselli S. Strategies to establish left/right asymmetry in vertebrates and invertebrates. Curr. Opin. Genet. Dev. 2007;17:1–8. doi: 10.1016/j.gde.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Sternfeld L, Duddenhoffer M, Ludes A, Heinze D, Anderie I, Krause E. Activation of muscarinic receptors reduces store-operated Ca2+ entry in HEK293 cells. Cell. Signal. 2007;19:1457–64. doi: 10.1016/j.cellsig.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development. 2004;131:4085–4093. doi: 10.1242/dev.01240. [DOI] [PubMed] [Google Scholar]
- Tabin CJ, Vogan KJ. A two-cilia model for vertebrate left-right axis specification. Genes Dev. 2003;17:1–6. doi: 10.1101/gad.1053803. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Narushima M, Oda Y. In vivo imaging of functional inhibitory networks on the mauthner cell of larval zebrafish. J. Neurosci. 2002;22:3929–3938. doi: 10.1523/JNEUROSCI.22-10-03929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilatis DK, Hohmann JG, Zeng H, Li F, Ranchalis JE, Mortrud MT, Brown A, Rodriguez SS, Weller JR, Wright AC, Bergmann JE, Gaitanaris GA. The G protein-coupled receptor repertoires of human and mouse. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4903–4908. doi: 10.1073/pnas.0230374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelon D, Horne SA, Stainier DY. Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev. Biol. 1999;214:23–37. doi: 10.1006/dbio.1999.9406. [DOI] [PubMed] [Google Scholar]
- Yi P, Han Z, Li X, Olson EN. The mevalonate pathway controls heart formation in Drosophila by isoprenylation of Ggamma1. Science. 2006;313:1301–1303. doi: 10.1126/science.1127704. [DOI] [PubMed] [Google Scholar]
- Webb SE, Miller AL. Imaging intercellular calcium waves during late epiboly in intact zebrafish embryos. Zygote. 2003;11:175–182. doi: 10.1017/s0967199403002211. [DOI] [PubMed] [Google Scholar]
- Wise A, Jupe SC, Rees S. The identification of ligands at orphan G-protein coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2004;44:43–66. doi: 10.1146/annurev.pharmtox.44.101802.121419. [DOI] [PubMed] [Google Scholar]
- Zeng XX, Wilm TP, Sepich DS, Solnica-Krezel L. Apelin and its receptor control heart field formation during zebrafish gastrulation. Dev. Cell. 2007;12:391–402. doi: 10.1016/j.devcel.2007.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.