Abstract
Human Brucella canis infection incidence is unknown. Most identified cases are associated with pet dogs. Contact with pathogenic Brucella spp. can lead to laboratory-acquired infections. We identified a pediatric B. canis case, the source, and other exposed persons. A three-year-old New York City child with fever and dyspnea was hospitalized for 48 hours for bronchiolitis. After her admission blood culture grew B. canis, she was prescribed antimicrobials and recovered. B. canis was isolated from blood of the child's pet dog. Isolates from the child and the dog were genetically similar. The dog originated from an Iowa breeding facility which was quarantined after identification of the puppy's infection. Thirty-one laboratory workers were exposed and subsequently monitored for symptoms; 15 completed post-exposure prophylaxis. This first report strongly suggesting B. canis transmission from a canine to a child in the United States highlights the need for coordinated control policies to minimize human illness.
Keywords: Brucella canis, Pet dogs, Zoonosis
Brucella canis is one of several species of Brucella, most of which cause zoonotic infections. Infection in dogs was first described in 1966 as a cause of reproductive failure and abortion in beagles; however, any canine species can become infected with B. canis when bacteria are shed via oral or venereal routes (1). Surviving offspring born from infected females frequently have asymptomatic infections (2-4). Studies published in the 1970s found 1.5% to 9.4% of tested dogs to be seropositive for B. canis (4-6). The prevalence of disease in dogs in the United States is currently not known, and the seroprevalence might be increasing (7-9).
Human B. canis infections are thought to be rare; 52 cases have been reported in the English literature1. Although the majority of these cases had mild symptoms, human B. canis infections have occasionally resulted in severe illness and complications including mycotic aneurysms, aortic valve vegetations, miscarriage, and osteomyelitis (11-13, 14-16). Pediatric cases have been reported in Argentina and the United States (15,17,18). Most of these children likely acquired infection from exposure to their family dog, although transmission was not confirmed through genetic comparison of human and canine isolates.
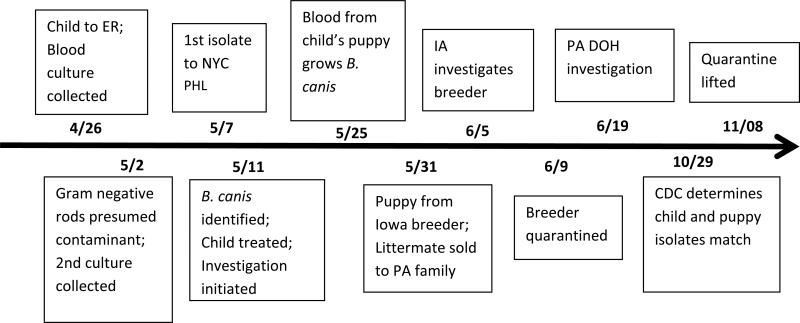
In May 2012, a three-year-old child from New York City (NYC) was diagnosed with B. canis infection. This report describes the clinical course, human and animal investigations, laboratory confirmation, and public health response, and discusses the implications of human B. canis infection. (Figure 1, timeline)
Figure 1.
Timeline of events for B. canis illness and subsequent investigation, New York City, April– October 2012.
Human Case Investigation
On April 26, 2012 (day 0), a three-year-old African-American female with an unremarkable medical history presented to a NYC emergency department (ED) after two days of dry cough, nasal congestion, clear nasal discharge, and one day of dyspnea and temperature of 38.3°C. The child, whose initial physical examination revealed respiratory distress (40 breaths per minute) without wheezing or rhonchi, a heart rate of 154-174 beats per minute and an oxygen saturation of 93% on room air, was admitted to Hospital A. Laboratory examinations revealed a hemoglobin concentration of 11.7 gm/dL, and a white blood cell count of 9,200 cells/mm3 with 62% neutrophils and 24% lymphocytes. Influenza and respiratory syncytial virus rapid tests were negative. Blood and respiratory specimens were collected for culture. A chest x-ray showed a right middle lobe infiltrate and mild peribronchial thickening suggesting either focal atelectasis or consolidation. The child received two doses of nebulized albuterol and one dose of parenteral ceftriaxone in the ED followed by nebulized normal saline every four hours. By the morning of day two, her symptoms had completely resolved, and she was discharged home without antimicrobials. She remained afebrile at home.
The child's respiratory viral culture was negative, however, her ED blood culture grew Gram-negative rods by May 2 (day six), and she was called back to the ED for evaluation. Her clinical exam was unremarkable, the ED blood culture was thought to be contaminated, and a second set of blood cultures was drawn. No treatment was prescribed.
On May 11 (day 15), the first blood specimen for culture (collected in the ED on day 0) was identified as B. canis by the NYC Public Health Laboratory (PHL) and the child was again called back for evaluation and treatment. Additional laboratory analysis of the child's blood revealed that her liver enzymes were elevated (alkaline phosphatase, 339 IU/L; aspartate aminotransferase [AST], 52 IU/L; alanine aminotransferase [ALT], 81 IU/L; total bilirubin 0.2 mg/dL). The second blood culture, collected on day six, also grew similar colonies of Gram-negative rods. A third blood specimen for culture was collected after which trimethoprim (TMP)/sulfamethoxazole (SMX) and rifampin were prescribed for six weeks.
Follow-up visits were conducted during the three months after hospital discharge, and the child remained asymptomatic. Blood was obtained one and six weeks after antimicrobial treatment. Cultures were negative, and AST and ALT concentrations had returned to normal levels after one week of treatment.
Epidemiologic investigation
On day 15, the NYC Department of Health and Mental Hygiene (DOHMH) interviewed the child's mother regarding potential exposures for the child's infection. In March 2012, the family had purchased an eight-week-old male Yorkshire Terrier from a NYC pet store. The mother reported that the child and puppy had frequent contact. The puppy remained in the apartment except for brief periods and did not have contact with other children. Aside from a second dog, a spayed female Lhasa Apso, who had been with the family for seven years, the puppy had no contact with other dogs. The child did not attend daycare, nor did she have frequent contact with other dogs.
The child lived in the household with her parents, neither of whom reported symptoms consistent with brucellosis. Their physical examinations were normal, and they declined to submit clinical specimens for analysis. Visitors to the household included the child's aunt and grandmother, neither of whom reported illness.
The child's mother stated that the puppy appeared healthy since arriving in the home. On day 16, a private veterinarian determined that the Yorkshire Terrier and Lhasa Apso appeared healthy on physical exam. Serologic specimens from both dogs were sent to a private laboratory for B. canis antibody screening with commercially-available tests. The adult dog's sera was B. canis positive on an immunofluorescent antibody screen and was submitted to the Animal Health Diagnostic Center (AHDC), New York State Veterinary Diagnostic Laboratory, Cornell University College of Veterinary Medicine, a designated canine-brucellosis serological reference laboratory, for screening by microscopic slide agglutination antibody test and confirmation with B. canis cytoplasmic protein agar gel immunodiffusion (AGID2). The screening test was positive; however, the AGID2 was negative. Interpretations of these results include: (1) the dog was not infected with B. canis (the screening was false-positive) or (2) the dog was acutely infected, as the AGID2 requires a specimen obtained 8-12 weeks post exposure for seroconversion to a positive test status. The puppy's serologic specimen was not submitted to AHDC since the commercial laboratory's screening test was negative. Subsequently, whole blood from both the puppy and the adult dog was submitted to the AHDC for culturing on day 27.
The puppy's blood culture grew B. canis; the adult dog's did not. Standard protocols were followed for phenotypic and molecular identification of suspect Brucella colonies (19). The USDA AMOS protocol was followed to identify colonies using DNA primers specific for B. canis (20). These results confirmed the blood culture isolate from the puppy as B. canis, and the isolate was submitted to the Centers for Disease Control and Prevention (CDC) for comparison with the case-patient's isolate.
NYC pet store investigation
On day 35, the New York State Department of Agriculture and Markets (NYS DAM) contacted the pet store that sold the implicated puppy. The store owner stated that the puppy and its littermate, purchased from an Iowa breeder, arrived at the store in March and sold quickly. The pet store, licensed by NYS DAM, had been inspected on April 30, 2012 as part of the annual license renewal process, and was found to be in compliance with all applicable laws and regulations. At the time of the April inspection, there were 24 puppies on the premises. The two puppies met all import requirements to enter NYS (testing for B. canis is not required). The pet store provided the name of the man who purchased the puppy's littermate, a Pennsylvania (PA) resident.
Trace forward; Pennsylvania Department of Health investigation
On June 19, the PA Department of Health (PADOH) was notified that one of their residents purchased the affected puppy's littermate. After investigation, PADOH reported that this female puppy had serum tested at AHDC; both the screening and confirmatory tests were positive. No blood culture was performed. Among those exposed to the second puppy were a child and a pregnant woman. Due to the difficulty of successfully treating B. canis infection in dogs and the potential complication risk if infection occurred in the pregnant woman, the PADOH, in consultation with the private veterinarian, recommended euthanizing the puppy. The owner was reluctant to euthanize, and it was agreed that the private veterinarian would treat the puppy with antimicrobials, perform an ovariohysterectomy, and re-test the puppy (21). Follow-up serologic testing by AHDC revealed that the puppy remained positive for B. canis; the owner did not plan to euthanize their pet.
The PADOH advised the child and the pregnant woman to limit contact with the puppy until it could be re-tested. They also recommended that the owners consult with their primary medical care provider. The PADOH offered to facilitate testing exposed persons but testing was refused.
Iowa breeding facility inspection
On day 40, NYS DAM notified the Iowa State Veterinarian that a puppy with canine brucellosis sold to a NYC pet store, and its littermate, had originated from an Iowa breeder. The Iowa Department of Agriculture and Land Stewardship investigated the authorized commercial breeding facility and collected blood specimens from the dam and sire of the two puppies. Both (parent) dogs were positive on a serologic canine brucellosis tube test at a Missouri laboratory and were euthanized. The breeding facility was issued an Order of Quarantine on June 19. Per state protocol for the release of the quarantine, all sexually intact dogs over six months of age were required to be serologically negative for canine brucellosis on two consecutive tests conducted 30 days apart. On July 9, blood samples from 70 eligible dogs were tested by the United States Department of Agriculture (USDA) National Veterinary Services Laboratory (NVSL) in Ames, Iowa using an in-house 2-Mercaptoethanol (ME) Tube Agglutination Test; 13 dogs that were positive by this test were euthanized. On August 20, 56 dogs were tested, including the remaining eligible dogs plus those that had attained six months of age); five positive dogs were euthanized. On September 25, 53 of the remaining test-eligible dogs were negative for canine brucellosis. On October 29, 66 dogs six weeks of age and older were serologically negative. The quarantine was released on November 8, 2012. Information regarding the disposition of prior litters from the parent dogs of the puppy that exposed the index case was not available, nor was information regarding the offspring of other positive dogs.
Laboratory investigation
Hospital A
On day 0, Hospital A laboratory (Laboratory A) set up cultures from the child's blood specimen. On day five, colonies of gram-negative rods from a subculture of the blood culture bottle were growing on blood and chocolate agar plates. On day nine, the isolate was forwarded to the NYC PHL for identification and further analysis.
The second blood culture obtained from the child on day six was also growing gram-negative rods by day nine. All manipulation, including making and staining smears, subculturing, vortexing and panel/kit inoculation, was done on an open bench in Laboratory A. After identifying the isolate as a Brucella spp., Laboratory A workers were evaluated for exposure risk. Because the laboratory was small and the testing panels required manipulation of liquid suspensions, all 17 laboratorians were considered to have had a high-risk exposure, and post-exposure prophylaxis (PEP) was recommended (22). Two laboratorians opted not to complete PEP. No workers developed symptoms during the six months post-exposure follow up.
NYC Public Health Laboratory
On day 11, the NYC PHL received the first isolate from the child for identification. A suspected organism was not indicated on the requisition. Per protocol, a Gram stain was performed, and the isolate was sub-cultured onto multiple agars (Becton Dickinson Diagnostic Systems, Franklin Lakes, NJ). The Gram stain revealed small, gram-negative coccobacilli. Sufficient growth was detected on both the blood and chocolate agars for inoculation of API NE strip, which yielded a low probability identification of Brucella spp. and Oligella ureolyticus. Based on the Gram stain morphology and the positive urea obtained from the API NE, a MIDI gas liquid chromatography (GLC) analysis was performed (Sherlock® Microbial Identification System, Newark, DE)(16, 23). The GLC yielded an identification of Brucella melitensis (the only Brucella spp. in its IBA1 version 1.10 database). The organism was immediately transferred to the BioThreat Response Laboratory where Laboratory Response Network (LRN) procedures for identification of Brucella spp. (both PCR and conventional testing) were set up. Brucella spp. markers were detected by the LRN PCR, and the submitting laboratory was notified. On day 15, conventional tests identified the isolate as B. canis. The isolate was sent to the CDC for confirmation and molecular typing.
At the NYC PHL, initial testing was performed on an open bench. Fourteen laboratorians, three of whom were classified as high-risk, were potentially exposed. The high-risk workers were offered PEP; all declined. All laboratorians were monitored for fever during the six months following this exposure. None developed symptoms.
CDC Bacterial Special Pathogens Branch
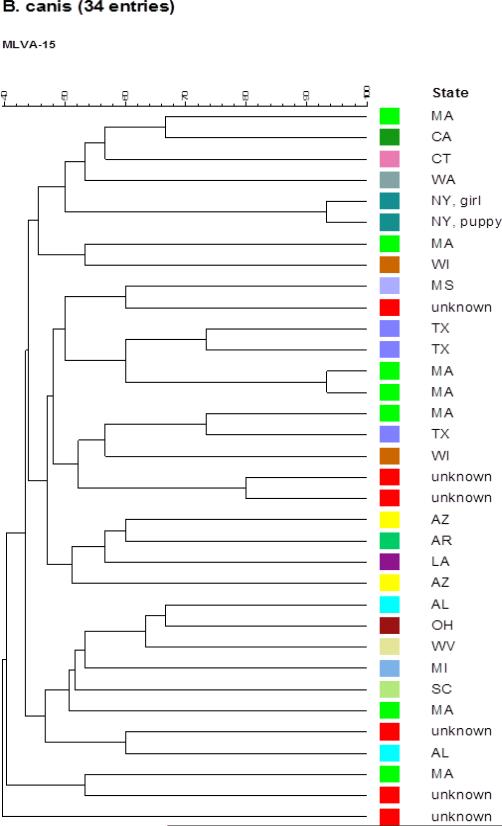
CDC typed the isolates from the child and the puppy based on multiple-locus variable-number tandem-repeat (VNTR) analysis (MLVA 15) as described (24). Purified genomic DNA preparations of both Brucella isolates were used to generate the 15 VNTR amplicons, the PCR products were then analyzed on an ABI Prism 3130 automated fluorescent capillary DNA sequencer (Applied Biosystems, CA). Detailed analysis of 15 allele designations was performed using GeneMapper (version 3.7) software package (Applied Biosystems, CA), and a comparative phylogenetic tree of these isolates was generated along with recent U.S. B. canis isolates from CDC as described (24). Both B. canis isolates carried the same alleles at 12 VNTRs, and only differed at three alleles. (Figure 2) The phylogenetic data show the close genetic similarity of these two B. canis isolates, and thus strongly suggest the puppy was the source of the child's infection.
Figure 2.
Phylogenetic tree, 34 B. canis isolates, US, 2002- 2013
Although no validated human serological test is available to detect B. canis antibodies, baseline serology was performed by CDC on 31 exposed Laboratory A and NYC PHL staff using the Brucella Microagglutination Test (BMAT) to rule out antibody response to Brucella spp. other than canis; all baseline titers were negative.
Discussion
Epidemiologic information, clinical course and genetic analysis of bacterial isolates from the child and the puppy suggest this is the first confirmed zoonotic transmission of B. canis in the U.S. This human case of B. canis infection resulted in a multistate, multi-agency investigation to determine the source of the child's infection, origin of the puppy, location of its littermate, additional human exposures, and an investigation of the breeding facility.
Brucella spp. have a low infectious dose and can be aerosolized during laboratory manipulations resulting in occupationally-acquired infections, thus prophylaxis of exposed laboratorians is recommended (22, 26). Biosafety level three (BSL-3) practices are recommended for handling Brucella cultures. Unknown isolates, however, are not always handled using these precautions. In Laboratory A, the child's blood culture was handled on an open bench resulting in exposure to 17 workers who required evaluation and PEP. An in-service review regarding work with unknown isolates was conducted for all microbiology staff members. A revised protocol was established with an emphasis on safety and early recognition of Brucella spp. or any unusual organisms. The importance of Laboratory A's role in identification of unusual organisms was emphasized, and workers were reminded to forward all suspicious isolates to the NYC PHL for further identification.
The NYC PHL regularly receives unknown isolates from sentinel clinical laboratories. The isolates may come with little or no information regarding suspected identification, phenotypic information, or patient clinical information. In this instance, 14 PHL laboratorians were exposed to Brucella since all of the initial testing was performed outside of the biological safety cabinet. As a result of this exposure, a dedicated BSL2+ lab area was designated for all testing of unknown isolates, including staining, media inoculation, and preliminary identification testing. All work will be performed under BSL2+ conditions until Bacillus anthracis, Francisella tularensis, Brucella spp., Burkholderia mallei/pseudomallei, Yersinia pestis and Neisseria meningitidis have been ruled-out. Room access has been limited to reduce the risk of exposure. In total, the isolates for this one case resulted in post-exposure evaluation of 31 laboratory workers, 15 of whom received a full course of PEP. Serial serological monitoring, as recommended by CDC following exposure to Brucella spp. (25), was not possible because of the lack of a validated serological assay.
Of the 52 human cases of B. canis reported during 1968-2010, 38 occurred in the United States (10). Most of these have been associated with close contact with dogs, but cases have also been reported in laboratory workers (14,17,26,27,28). Although human B. canis infection appears to be rare, the true burden of disease is unknown. Human B. canis infections might be under-recognized and reported, because validated serologic tests for human infection with B. canis are unavailable in the United States, laboratories handling human specimens typically have limited experience culturing and identifying this organism, bacteremia can be intermittent, and diagnostic suspicion might be low.
As was the case with this child, the clinical presentation of human B. canis infection includes non-specific symptoms (22, 29). Human B. canis infection is typically milder than infections with other Brucella species; however, serious infections have been described and there is interest in determining whether some culture-negative cases of endocarditis, osteomyelitis and septic arthritis may be caused by B. canis (12, 30). Because the child did not have any of the typical risk factors for Brucella infection, including travel to an endemic country, work in kennels or abattoirs, or consumption of unpasteurized dairy products, Brucella was not initially suspected. In many cases, patients presenting with similar symptoms to those of this patient might not have blood cultures collected. Even when specimens are obtained, the organism might not be readily identified due to limited experience with this organism in many human clinical laboratories.
Doxycycline in combination with another antimicrobial agent, for a minimum of six weeks is recommended to treat brucellosis caused by any species; however, doxycycline is contraindicated in children less than 8 years old (31). In this case, TMP/SMX was substituted for doxycycline and given in combination with rifampin, which has a similar rate of relapse to doxycycline and rifampin (31). Humans infected with B. canis appear to respond quickly to treatment; however, long-term follow up of cases has been inconsistent.
Most dogs infected with B. canis are asymptomatic and can shed the organism for months or years in reproductive fluids (2,3). Clinical signs are typically associated with the reproductive tract, and complications including infertility might occur (28, 32). Confirmatory diagnosis of canine brucellosis is made by isolation of the organism from a clinical specimen, although a negative culture does not rule out brucellosis, because bacteremia is intermittent (3,27). Serologic specimens from young dogs may be negative on commercially available assays due to interference from maternal antibody. Sera from dogs testing positive on commercially available antibody screening tests can be confirmed using the B. canis cytoplasmic protein AGID2 antigen available at a reference center.
Recent reports suggest that the prevalence of B. canis might be increasing in the canine population, particularly in breeding kennels (7,9). There is concern that breeding practices might be facilitating infections in dogs that are subsequently sold as pets throughout the United States (32) . The interstate transfer and sale of breeding and pet dogs, and failure to regularly test these dogs for B. canis infection, has been linked with outbreaks in kennels and infected pet dogs (7, 26, 33). There are no requirements for B. canis testing for dogs traveling within or being imported into the United States for sale.
Due to possible under-recognition of human B. canis infections, and the increasing seroprevalence in dogs, the National Association of State Public Health Veterinarians (NASPHV) developed a position statement in 2012 on human B. canis infections (34). The statement urged for the development of a human diagnostic assay and improved communication and data sharing between state departments of health and departments of agriculture regarding human and canine B. canis infections. In addition, the Council for State and Territorial Epidemiologists has recommended including the infecting Brucella species when notifying CDC of human brucellosis cases (34). In parallel, the American Veterinary Medical Association (AVMA) has developed a B. canis-specific policy, advocating for the continued commitment of state and federal agencies to the eradication of brucellosis in all species (35). The policy encourages development and validation of diagnostic assays, clarification of susceptible populations, and development of a disease management plan. In addition to the efforts by CSTE, NASPHV and the AVMA, there is also a need for a coordinated approach to the regional and national guidelines for the management of B. canis and other canine zoonoses. Currently, each state determines requirements for testing, and conditions for imposing and lifting breeding facility quarantine. Standardization of investigative protocols and quarantine requirements would facilitate investigations, assist in the control of canine brucellosis, and decrease the public health risk to the human population.
The non-specific presentation and innocuous exposure history of this B. canis case might explain the infrequency of reported human B. canis infections. If the case had not been identified, additional B. canis-infected dogs from the Iowa kennel would have been sold, resulting in additional opportunities for zoonotic transmission. Research is needed to describe the burden of human B. canis infection, develop a diagnostic assay for human specimens, and establish effective coordination among human and animal public health agencies to control disease and prevent disease transmission between dogs and humans.
Article Summary Line.
This first confirmed report of Brucella canis transmission from a canine to a child in the United States highlights the need for coordination among animal and human health agencies.
Acknowledgements
This journal article was supported by Epidemiology and Laboratory Capacity (ELC) for Infectious Diseases Cooperative Agreement Number 3U50CI000899-02S3 and the Public Health Emergency Preparedness (PHEP) Grant Number 5U90TP000546 from the Centers for Disease Control and Prevention (CDC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC.
Biography
Catherine M. Dentinger, FNP, MPH, is a CDC Career Epidemiology Field Officer assigned to the Bureau of Communicable Disease at the New York City Department of Health and Mental Hygiene.
References
- 1.Carmichael LE. Abortion in 200 beagles. Journal of the American Veterinary Medical Association. 1966;149:1126. [Google Scholar]
- 2.Wanke MM. Canine brucellosis. Anim Reprod Sci. 2004;82-83:195–207. doi: 10.1016/j.anireprosci.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Hollett RB. Canine brucellosis: outbreaks and compliance. Theriogenology. 2006;66:575–87. doi: 10.1016/j.theriogenology.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Boebel FW, Ehrenford FA, Brown GM, Angus RD, Thoen CO. Agglutinins to Brucella canis in stray dogs from certain counties in Illinois and Wisconsin. Journal of the American Veterinary Medical Association. 1979;175:276–7. [PubMed] [Google Scholar]
- 5.Fredrickson LE, Barton CE. A serologic survey for canine brucellosis in a metropolitan area. Journal of the American Veterinary Medical Association. 1974;165:987–9. [PubMed] [Google Scholar]
- 6.Lovejoy GS, Carver HD, Moseley IK, Hicks M. Serosurvey of dogs for Brucella canis infection in Memphis, Tennessee. American Journal of Public Health. 1976;66:175–6. doi: 10.2105/ajph.66.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brower A, Okwumabua O, Massengill C, et al. Investigation of the spread of Brucella canis via the U.S. interstate dog trade. International Journal of Infectious Diseases. 2007;11:454–8. doi: 10.1016/j.ijid.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Increased prevalence of canine brucellosis in Oklahoma 1998-2003. Oklahoma Veterinary Medical Association; [January 17, 2013]. (online). at http://okvma.affiniscape.com/displaycommon.cfm?an=1&subarticlenbr=748.) [Google Scholar]
- 9.Carter T, Johnson C. Brucella canis: a threat to canine and human health. In: England G, Kutzler M, Comizzoli P, Nizanski W, Rijsselaere T, Concannon P, editors. International Symposium on Canine and Feline Reproduction. Whistler; Canada: 2012. 2012. [Google Scholar]
- 10.Lehman MW. Review of Human Brucella canis cases in the United States. 2013 unpublished. [Google Scholar]
- 11.McKee MA, Ballard JL. Mycotic aneurysms of the tibioperoneal arteries. Annals of vascular surgery. 1999;13:188–90. doi: 10.1007/s100169900240. [DOI] [PubMed] [Google Scholar]
- 12.Ying W, Nguyen MQ, Jahre JA. Brucella canis endocarditis: case report. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1999;29:1593–4. doi: 10.1086/313545. [DOI] [PubMed] [Google Scholar]
- 13.Marzetti S, Carranza C, Roncallo M, Escobar GI, Lucero NE. Recent trends in human Brucella canis infection. Comparative immunology, microbiology and infectious diseases. 2013;36:55–61. doi: 10.1016/j.cimid.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Carmichael LE, Barol SR, Broad RH, Freitag JL. Human infection with the agent of canine abortion. Morbidity and Mortality Weekly. 1968;17:285–86. [Google Scholar]
- 15.Piampiano P, McLeary M, Young LW, Janner D. Brucellosis: unusual presentations in two adolescent boys. Pediatric radiology. 2000;30:355–7. doi: 10.1007/s002470050760. [DOI] [PubMed] [Google Scholar]
- 16. [January 9, 2014];Sherlock Microbial Identification System, Version 6.2, Operating Manual. 2012 ( http://www.midiinc.com/pdf/Sherlock_MIS_Operating_Manual.pdf)
- 17.Lucero NE, Corazza R, Almuzara MN, et al. Human Brucella canis outbreak linked to infection in dogs. Epidemiol Infect. 2010;138:280–5. doi: 10.1017/S0950268809990525. [DOI] [PubMed] [Google Scholar]
- 18.Tosi MF, Nelson TJ. Brucella canis infection in a 17-month-old child successfully treated with moxalactam. The Journal of pediatrics. 1982;101:725–7. doi: 10.1016/s0022-3476(82)80301-6. [DOI] [PubMed] [Google Scholar]
- 19.Alton GG, Jones LM, Pietz DE. Laboratory techniques in brucellosis. Monograph series World Health Organization. 1975:1–163. [PubMed] [Google Scholar]
- 20.Bricker BJ, Halling SM. Differentiation of Brucella abortus bv. 1, 2, and 4, Brucella melitensis, Brucella ovis, and Brucella suis bv. 1 by PCR. Journal of clinical microbiology. 1994;32:2660–6. doi: 10.1128/jcm.32.11.2660-2666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmichael LE, Greene CE. Canine Brucellosis. In: G CE, editor. Infectious Disease of the Dog and Cat. 2nd edition ed. W.B. Saunders Company; Philadelphia PA: 1990. [Google Scholar]
- 22.CDC Laboratory-acquired brucellosis--Indiana and Minnesota, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:39–42. [PubMed] [Google Scholar]
- 23.Lim DV, Simpson JM, Kearns EA, Kramer MF. Current and developing technologies for monitoring agents of bioterrorism and biowarfare. Clinical microbiology reviews. 2005;18:583–607. doi: 10.1128/CMR.18.4.583-607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiller RV, De BK, Boshra M, et al. Comparison of two multiple-locus variable-number tandem-repeat analysis methods for molecular strain typing of human Brucella melitensis isolates from the Middle East. Journal of clinical microbiology. 2009;47:2226–31. doi: 10.1128/JCM.02362-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Traxler RM, Guerra MA, Morrow MG, et al. Review of Brucellosis Cases from Laboratory Exposures in the United States, 2008-2011, and Improved Strategies for Disease Prevention. J Clin Microbiol. 2013 doi: 10.1128/JCM.00813-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nomura A, Imaoka K, Imanishi H, et al. Human Brucella canis infections diagnosed by blood culture. Emerg Infect Dis. 2010;16:1183–5. doi: 10.3201/eid1607.090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawaczeck E, Toporek J, Cwikla J, Mathison BA. Brucella canis in a HIV-Infected Patient. Zoonoses Public Health. 2010;58:150–2. doi: 10.1111/j.1863-2378.2010.01334.x. [DOI] [PubMed] [Google Scholar]
- 28.Shin S, Carmichael LE. Canine Brucellosis Caused by Brucella Canis. In: Carmichael LE, editor. Recent advances in canine infectious diseases. International Veterinary Information Service; Ithaca, NY: 1999. [Google Scholar]
- 29.Rumley RL, Chapman SW. Brucella canis: an infectious cause of prolonged fever of undetermined origin. Southern medical journal. 1986;79:626–8. doi: 10.1097/00007611-198605000-00027. [DOI] [PubMed] [Google Scholar]
- 30.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. The New England journal of medicine. 2005;352:2325–36. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 31.Al-Tawfiq JA. Therapeutic options for human brucellosis. Expert review of anti-infective therapy. 2008;6:109–20. doi: 10.1586/14787210.6.1.109. [DOI] [PubMed] [Google Scholar]
- 32.Scheftel J. Brucella canis: Potential for Zoonotic Transmission. Compendium and Veterinary Technician. 2003;25:846–53. [Google Scholar]
- 33.Brennan SJ, Ngeleka M, Philibert HM, Forbes LB, Allen AL. Canine brucellosis in a Saskatchewan kennel. Canadian Veterinary Journal. 2008;49:703–8. [PMC free article] [PubMed] [Google Scholar]
- 34.(CSTE) CoSaTE CSTE official list of nationally notifiable conditions. CSTE position statement 12-ID-03. 2012 [Google Scholar]
- 35.Canine brucellosis policy adopted. JAVMA News. 2013 [Google Scholar]