Abstract
Background:
Increasingly, as in our institution, operating rooms are located in hospitals and the pathology suite is located at a distant location because of off-site consolidation of pathology services. Telepathology is a technology which bridges the gap between pathologists and offers a means to obtain a consultation remotely. We aimed to evaluate the utility of telepathology as a means to assist the pathologist at the time of intraoperative consultation of lung nodules when a subspecialty pathologist is not available to directly review the slide.
Methods:
Cases of lung nodules suspicious for a neoplasm were included. Frozen sections were prepared in the usual manner. The pathologists on the intraoperative consultation service at two of our system hospitals notified the thoracic pathologist of each case after rendering a preliminary diagnosis. The consultation was performed utilizing a Nikon™ Digital Sight camera and web-based Remote Medical Technologies™ software with live video streaming directed by the host pathologist. The thoracic pathologist rendered a diagnosis without knowledge of the preliminary interpretation then discussed the interpretation with the frozen section pathologist. The interpretations were compared with the final diagnosis rendered after sign-out.
Results:
One hundred and three consecutive cases were included. The frozen section pathologist and a thoracic pathologist had concordant diagnoses in 93 cases (90.2%), discordant diagnoses in nine cases (8.7%), and one case in which both deferred. There was an agreement between the thoracic pathologist's diagnosis and the final diagnosis in 98% of total cases including 8/9 (88.9%) of the total discordant cases. In two cases, if the thoracic pathologist had not been consulted, the patient would have been undertreated.
Conclusions:
We have shown that telepathology is an excellent consultation tool in the frozen section diagnosis of lung nodules.
Keywords: Intraoperative, telepathology, thoracic, virtual
BACKGROUND
The field of telepathology has grown significantly since Ronald Weinstein first coined the term in 1986.[1] Telepathology is a technology which bridges the gap between pathologists and offers a means to obtain a consultation, remotely. It is now used for a wide variety of purposes including quality assurance, intraoperative consultations, remote viewing of gross specimens, and for education of medical students and residents.[2,3,4,5] Telepathology was first used for primary frozen section diagnosis in 1989 in Norway.[6] At our institution, the implementation of telepathology in daily practice grew out of a unique need. In 2010, the pathologists from two nearby hospitals were consolidated at a single off-site location situated 2 miles from the two hospitals. When a pathologist covering intraoperative consultations in one of the hospitals needs a second opinion, often the nearest pathologist is 2 miles away. To circumvent this issue, our institution began implementing inexpensive dynamic (real-time) telepathology as a consultation tool. Our pathologists are trained to use the system and are encouraged to use the technology as needed.
In this study, we focused on lung nodules clinically suspicious for neoplasm. Frozen section interpretation of lung nodules is inherently challenging due to an artifact, benign mimickers of neoplasms, and distinguishing invasive from in situ processes. This is particularly challenging for junior and nonthoracic pathologists who may require assistance from an expert thoracic pathologist. We aimed to evaluate the utility of telepathology as a means to assist the pathologist at the time of intraoperative consultation of lung nodules when an expert pathologist was not immediately available to look directly at the slide.
METHODS
Approval from the Institutional Review Board was obtained. The pathologists on the intraoperative consultation service at two of our system hospitals, Long Island Jewish Medical Center and North Shore University Hospital, were instructed to call the thoracic pathologist (MJ Esposito or TA Bhuiya) for each case in which a lung nodule was clinically suspicious for a neoplasm. The thoracic pathologists were located at the off-site pathology suite. Specimens received for intraoperative consultation included wedge resections, lobectomies, pleural biopsies, and endobronchial biopsies. Frozen sections were prepared in the usual manner, cut at a thickness of 4 μm and prepared using optimal cutting temperature media, a −20°C cryostat, and hematoxylin and eosin staining. Touch preparations or smears, if desired, were prepared with immediate alcohol fixation followed by hematoxylin and eosin staining.
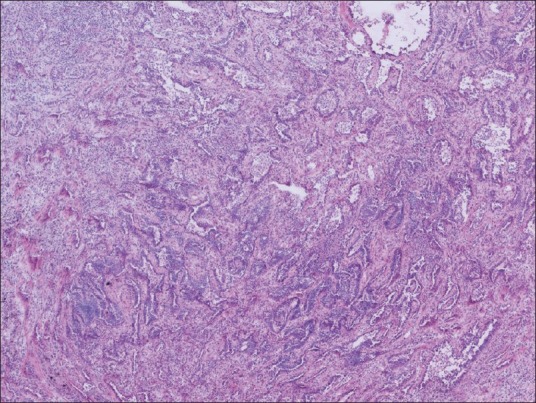
The frozen section pathologist consulted the thoracic pathologist by telepathology utilizing a standard microscope with attached Nikon™ Digital Sight camera and web-based Remote Medical Technologies™ software with live video streaming directed by the frozen section pathologist. Figure 1 shows the image as it appears on the desktop of the consultant pathologist. Relevant clinical information was provided, including patient age, sex, history, and the procedure performed. After the frozen section, pathologist rendered a preliminary diagnosis; he or she notified the thoracic pathologist of the case. The thoracic pathologist rendered his diagnosis without knowledge of the host's interpretation and subsequently discussed the case findings with the host. The interpretation was relayed to the surgeon only after both the host and consultant discussed the case. Concordance of host and consultant diagnosis was noted. The frozen section pathologist was responsible for showing the areas of interest, and the thoracic pathologist was allowed to see as many fields at any magnification as needed before making the diagnosis. The quality of the image transmitted was noted by the consultant (excellent, good, or poor).
Figure 1.
Remote consultation. The host pathologist is responsible for showing the areas of interest to the consultant, at multiple magnifications. The quality of the transmitted image depends on multiple factors, starting with the preparation of the slide. This screenshot shows the transmitted image as it appears on the desktop of the consultant pathologist
Final diagnoses of permanent sections were made in the usual fashion by one of three thoracic pathologists at our institution, including the two participating in the study as consultants. The interpretations made at the time of intraoperative consultation by the host and consultant pathologist were compared with the final diagnosis for concordance. Data on turnaround times was not collected as part of the study. Discrepancies were divided into minor and major based on therapeutic implications. We considered adenocarcinoma versus adenocarcinoma in situ (AIS) a minor discrepancy since the current standard of care is completion lobectomy for both. Deferred cases are not considered major or minor errors, but rather simply as deferred.
RESULTS
Two thoracic pathologists and 17 generalist/nonthoracic subspecialty pathologists participated in the study. One hundred and three consecutive cases were obtained over a period from February 2013 to March 2015. The diagnoses ranged from chronic inflammation to primary and metastatic carcinomas, lymphoma, sarcoma, and carcinoid. The frozen section pathologist and a thoracic pathologist had concordant diagnoses in 93/103 cases (90.2%), discordant diagnoses in 9/103 cases (8.7%), and one case in which both deferred. There was an agreement between the thoracic pathologist diagnosis and the final diagnosis in 98% of total cases including 8/9 (88.9%) of the total discordant cases. There was one case deferred by the host, six minor discrepancies, and two major discrepancies. In two cases, if the thoracic pathologist had not been consulted, the patient would have been undertreated. One of these cases was interpreted as a mucinous neoplasm by the host and was deferred. Interestingly, the host was one of the consultants in the study. In the other case, the host was unsure if the process was inflammatory or malignant.
Frozen section diagnoses were compared with permanent section diagnoses. Of the discordant cases between host and consultant pathologists, the frozen section pathologist was correct in one case and in the remaining eight cases the thoracic pathologists was correct. Discordant cases are shown in Table 1. The most common cause of discordance at the time of frozen section was adenocarcinoma versus AIS [Figure 2]. In three cases, the image was noted to be of lesser quality (darker), however, this did not negatively impact the consultant's diagnosis. In one case noted to have an image of lesser quality, the consultant rendered a diagnosis which was concordant with the final interpretation while the host pathologist's diagnosis was discordant with the final interpretation.
Table 1.
Discordant cases among host and consultant pathologists
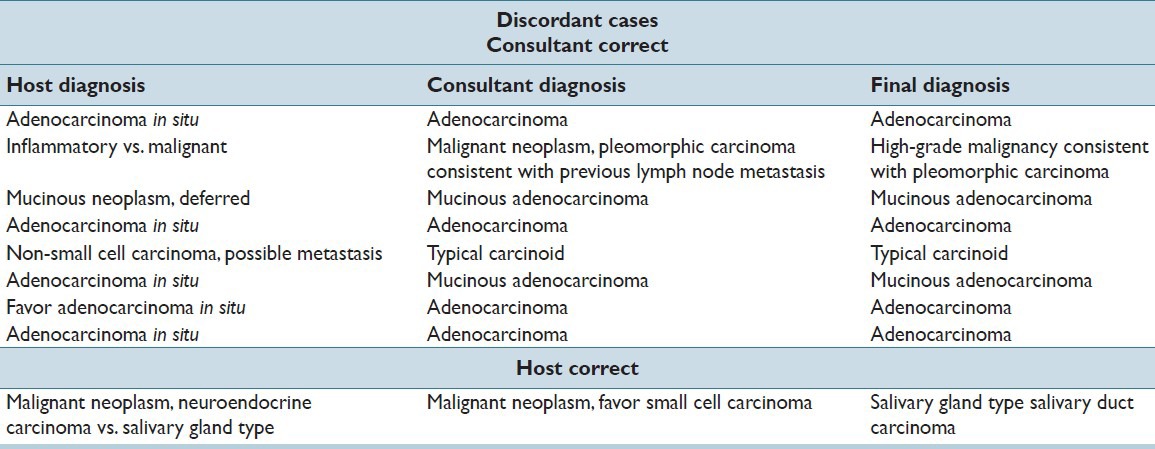
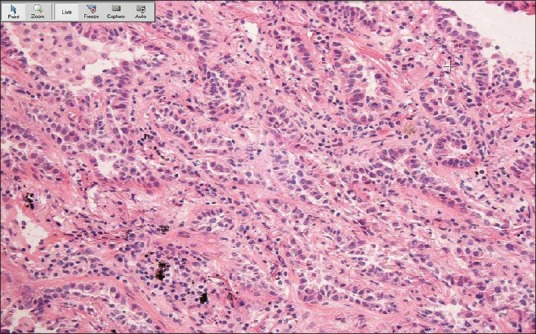
Figure 2.
Adenocarcinoma. The distinction between adenocarcinoma and adenocarcinoma in situ was particularly challenging for the host pathologists in this study. This frozen section slide of adenocarcinoma was correctly interpreted as adenocarcinoma by the consultant pathologist and as AIS by the host pathologist
CONCLUSIONS
Telepathology has grown tremendously over the last nearly 30 years, and the technology has continued to improve to a level in which we use it in daily clinical practice. The centralization of pathology groups away from the hospital setting has created not just an interest, but a need for a remote means of diagnosis.[7] In some institutions, a pathologist is not present in the cutting room, let alone the hospital, and the slides are prepared by a pathology assistant.[8] The urgent nature of the intraoperative consultation requires an efficient approach to obtaining a second opinion if it is needed. Thus, telepathology is an attractive option.[9]
The clinical expertise of the thoracic pathologist, as well as familiarity with the telepathology technology, contributed to the outcomes in this study. One large study showed a concordance of clinically important diagnoses between telepathology and light microscopy in 99.7% of cases (2138/2144).[10] A meta-analysis of more than 1290 cases showed a diagnostic accuracy of 0.91 of the telepathological frozen section diagnosis versus 0.98 for the conventional frozen section.[11] Misdiagnoses on telepathology can be attributed to sampling error, selection of microscopic fields not representative of the lesion, misinterpretation due to poor image quality, or misinterpretation not attributable to image quality.[11]
There are many variables to consider when using telepathology. The preparation of the slide is one of the most important. Frozen section slides are more difficult to interpret than slides prepared from formalin-fixed, paraffin embedded sections.[9] The slides are inherently laden with artifacts including air drying, shattering effect, rolling of the tissue, air bubbles, and thick sections, among others. Aspects of the telepathology technology must be considered. Telepathology can be static, dynamic, or a combination or both. When using static or dynamic telepathology without robotic assistance, the referring pathologist has the responsibility to show the most representative fields of the slide, at various magnifications.[12,13]
Slide scanning technologies include robotic microscopy (RM) and virtual slide (VS) system. The RM system is slower in terms of digitizing the image compared to the newer VS system which can rapidly digitize histologic sections on glass slides.[7] In a study by Evans et al., diagnostic accuracy was reported as 98% with both modalities, and none of their discrepant diagnoses had a clinical impact. They recorded the length of slide review and found that pathologists required an average of 9.65 min to review a slide by RM and only 2.25 min for VS.[7] Wellnitz et al. noted that the time spent when using telepathology as a tool for primary diagnosis is greater than for the second opinion when using a remotely operated RM. This was largely attributed to the remote pathologist having to review nearly the entire slide.[14] We have not implemented slide scanning systems at our institution.
Both the referring and remote pathologists practicing telepathology should be trained in telepathology.[9] The technology must be user-friendly and be able to receive and transmit the image with high speed and quality (i.e., color and resolution) with the ultimate goal of showing the remote pathologist an image which is interpretable, and ideally as true to the physical slide as possible.[15] Image quality and the ability to receive images depend on the computer hardware used and bandwidth of the telecommunications link.[16,17] Standards for these operations have not been set as they have been in teleradiology, partly because of the increased complexities for standardization compared with teleradiology.[18]
Distinguishing AIS versus invasive adenocarcinoma posed the greatest challenge for the host pathologists. In five out of nine discordant cases, the host pathologist diagnosed the tumor as AIS while the consultant correctly identified invasive adenocarcinoma. While completion lobectomy is the current standard of care for AIS, evidence shows that some patients may be treated with limited resection (wedge or segmentectomy).[19,20] As guidelines evolve, it may become imperative for pathologists to be able to accurately distinguish the two entities.
While our institution has embraced telepathology, there is still a perception among some pathologists that the technology is inferior to the live experience in terms of quality and ease of use.[7] Other barriers to implementing the technology include cost and time constraints. In this study, we have shown that telepathology is an excellent consultative tool in the frozen section diagnosis of challenging lung nodules. Learning to use this modality is imperative to the modern pathologist.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2015/6/1/55/168515
REFERENCES
- 1.Weinstein RS, Bloom KJ, Rozek LS. Telepathology and the networking of pathology diagnostic services. Arch Pathol Lab Med. 1987;111:646–52. [PubMed] [Google Scholar]
- 2.Graham AR, Bhattacharyya AK, Scott KM, Lian F, Grasso LL, Richter LC, et al. Virtual slide telepathology for an academic teaching hospital surgical pathology quality assurance program. Hum Pathol. 2009;40:1129–36. doi: 10.1016/j.humpath.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Dee FR, Meyerholz DK. Teaching medical pathology in the twenty-first century: Virtual microscopy applications. J Vet Med Educ. 2007;34:431–6. doi: 10.3138/jvme.34.4.431. [DOI] [PubMed] [Google Scholar]
- 4.Ford JC, Pinder KE, Ovalle WK, Li CH. Pathology education in a multisite urban/rural distributed curriculum. Hum Pathol. 2008;39:811–6. doi: 10.1016/j.humpath.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Helin H, Lundin M, Lundin J, Martikainen P, Tammela T, Helin H, et al. Web-based virtual microscopy in teaching and standardizing Gleason grading. Hum Pathol. 2005;36:381–6. doi: 10.1016/j.humpath.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Nordrum I, Engum B, Rinde E, Finseth A, Ericsson H, Kearney M, et al. Remote frozen section service: Atelepathology project in northern Norway. Hum Pathol. 1991;22:514–8. doi: 10.1016/0046-8177(91)90226-f. [DOI] [PubMed] [Google Scholar]
- 7.Evans AJ, Chetty R, Clarke BA, Croul S, Ghazarian DM, Kiehl TR, et al. Primary frozen section diagnosis by robotic microscopy and virtual slide telepathology: The university health network experience. Hum Pathol. 2009;40:1070–81. doi: 10.1016/j.humpath.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Dunn BE, Choi H, Recla DL, Kerr SE, Wagenman BL. Robotic surgical telepathology between the Iron Mountain and Milwaukee Department of Veterans Affairs Medical Centers: A twelve year experience. Hum Pathol. 2009;40:1092–9. doi: 10.1016/j.humpath.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan KJ, Burgess JR, Sandberg GD, Myers CP, Bigott TR, Greenspan RB. Use of robotic telepathology for frozen-section diagnosis: A retrospective trial of a telepathology system for intraoperative consultation. Mod Pathol. 2002;15:1197–204. doi: 10.1097/01.MP.0000033928.11585.42. [DOI] [PubMed] [Google Scholar]
- 10.Dunn BE, Choi H, Almagro UA, Recla DL, Krupinski EA, Weinstein RS. Routine surgical telepathology in the department of veterans affairs: Experience-related improvements in pathologist performance in 2200 cases. Telemed J. 1999;5:323–37. doi: 10.1089/107830299311899. [DOI] [PubMed] [Google Scholar]
- 11.Wellnitz U, Binder B, Fritz P, Friedel G, Schwarzmann P. Reliability of telepathology for frozen section service. Anal Cell Pathol. 2000;21:213–22. doi: 10.1155/2000/904578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halliday BE, Bhattacharyya AK, Graham AR, Davis JR, Leavitt SA, Nagle RB, et al. Diagnostic accuracy of an international static-imaging telepathology consultation service. Hum Pathol. 1997;28:17–21. doi: 10.1016/s0046-8177(97)90273-2. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein LJ, Epstein JI, Edlow D, Westra WH. Static image analysis of skin specimens: The application of telepathology to frozen section evaluation. Hum Pathol. 1997;28:30–5. doi: 10.1016/s0046-8177(97)90275-6. [DOI] [PubMed] [Google Scholar]
- 14.Wellnitz U, Fritz P, Voudouri V, Linder A, Toomes H, Schmid J, et al. The validity of telepathological frozen section diagnosis with ISDN-mediated remote microscopy. Virchows Arch. 2000;437:52–7. doi: 10.1007/s004280000187. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein RS, Bloom KJ, Rozek LS. Telepathology. Long-distance diagnosis. Am J Clin Pathol. 1989;914(Suppl 1):S39–42. [PubMed] [Google Scholar]
- 16.Weinberg DS, Allaert FA, Dusserre P, Drouot F, Retailliau B, Welch WR, et al. Telepathology diagnosis by means of digital still images: An international validation study. Hum Pathol. 1996;27:111–8. doi: 10.1016/s0046-8177(96)90363-9. [DOI] [PubMed] [Google Scholar]
- 17.Krupinski EA. Virtual slide telepathology workstation-of-the-future: Lessons learned from teleradiology. Semin Diagn Pathol. 2009;26:194–205. doi: 10.1053/j.semdp.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein RS, Graham AR, Richter LC, Barker GP, Krupinski EA, Lopez AM, et al. Overview of telepathology, virtual microscopy, and whole slide imaging: Prospects for the future. Hum Pathol. 2009;40:1057–69. doi: 10.1016/j.humpath.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 19.El-Sherif A, Gooding WE, Santos R, Pettiford B, Ferson PF, Fernando HC, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: A 13-year analysis. Ann Thorac Surg. 2006;82:408–15. doi: 10.1016/j.athoracsur.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura H, Kawasaki N, Taguchi M, Kabasawa K. Survival following lobectomy vs limited resection for stage I lung cancer: A meta-analysis. Br J Cancer. 2005;92:1033–7. doi: 10.1038/sj.bjc.6602414. [DOI] [PMC free article] [PubMed] [Google Scholar]


