Abstract
Background:
The primary cicatricial alopecias (PCAs) are a rare group of diseases where hair follicle is the primary target of destruction. There are a few studies on histopathological and trichoscopic features of PCA.
Aims:
To study the clinical, trichoscopic, and histopathological characteristics of PCAs of the scalp and to find out the concordance between trichoscopic and histopathological diagnosis.
Materials and Methods:
We retrospectively analyzed the clinical, trichoscopic, and histopathological features of 24 PCA patients. Fisher's Chi-square exact test was done to find the significant trichoscopic and histopathological features. Cohen's kappa coefficient was used to determine the agreement between histopathological and trichoscopic diagnosis.
Results:
A total of 24 patients of PCA were seen with a male: female ratio of 2:1. There were 10 (41.7%) patients of discoid lupus erythematosus (DLE), 5 (20.8%) of lichen planopilaris (LPP), 3 (12.5%) of dissecting cellulitis of scalp, and 2 (8.3%) each of pseudopelade of brocq, folliculitis decalvans, and frontal fibrosing alopecia. The important histopathological findings of DLE were follicular plugging, vacuolar changes in the basal layer, necrotic keratinocytes, and superficial and deep perifollicular and perivascular lymphocytic infiltrate. Histopathology of LPP showed vacuolar changes in the basal layer and lichenoid infiltrate involving the infundibulum and isthmus. Trichoscopy of DLE showed follicular plugging, yellow dots, and thick arborizing blood vessels. The peripilar cast was important finding in LPP. The characteristic yellow dot with three-dimensional structure was noted in dissecting cellulitis of the scalp. The Cohen's kappa agreement was 0.89 between histopathological and trichoscopic diagnosis.
Conclusion:
The diagnosis of PCA is challenging because of overlapping features clinically and histopathologically. Trichoscopy may provide quick and reliable diagnosis and obviate the necessity of scalp biopsy in busy clinics.
Keywords: Histopathology, primary cicatricial alopecia, trichoscopy
INTRODUCTION
Primary cicatricial alopecia (PCA) is an uncommon and clinically diverse set of disorders in which the hair follicle is irreversibly destroyed leading to permanent alopecia. In 2001, North American Hair Research Society, issued a consensus opinion on classification of the PCA based on principal inflammatory cell type in a representative biopsy sample from the scalp.[1]
The diagnosis of PCA is challenging because of overlapping features both clinically and histopathologically. Trichoscopy is a noninvasive procedure which shows characteristic features and aids to the diagnosis. The aim of the present study was to retrospectively analyze the clinical, trichoscopic, and histopathological characteristics of PCA of the scalp and to find out the concordance between trichoscopic and histopathological diagnosis in PCA.
MATERIALS AND METHODS
All the patients of PCA who attended Dermatology Outpatient Department from April 2013 to September 2014 were included in the present study. We retrospectively analyzed the clinical data, trichoscopic, and histopathological features of 24 patients of PCA. The diagnosis was confirmed on the basis of clinical, histopathological, and trichoscopic features. The age, sex, duration, clinical findings, concomitant diseases, and other relevant history were recorded. The blood tests like complete blood count, blood sugar, liver and kidney function, and antinuclear antibody were performed in relevant cases. The trichoscopy was performed with a digital dermoscope and the images saved. Punch biopsy of 4 mm was taken from the active margin of alopecia patches, and in most cases vertical sectioning was done. The slides were stained with eosin and hematoxylin and studied under microscope. Pus for culture and sensitivity was done in relevant patients. The study was approved by Institutional Ethics Committee.
Statistical analysis
The collected data were analyzed using SPSS version 22 (IBM SPSS Statistics, Version 22.0. Armonk, NY: IBM Corp.). Fisher's Chi-square exact test was performed for intergroup comparisons. A P < 0.05 were considered statistically significant. The agreement between histopathological diagnosis and trichoscopic diagnosis was calculated using Cohen's kappa coefficient.
RESULTS
Clinical features
There were 24 patients of PCA, with a male: female ratio of 2:1. The age group ranged from 3 to 76 years. The study comprised of 10 cases of discoid lupus erythematosus (DLE), five cases of lichen planopilaris (LPP), three cases of dissecting cellulitis of scalp, two cases each of pseudopelade of brocq, folliculitis decalvans, and frontal fibrosing alopecia (FFA) [Figures 1–6]. The various clinical features are summarized in Table 1.
Figure 1.
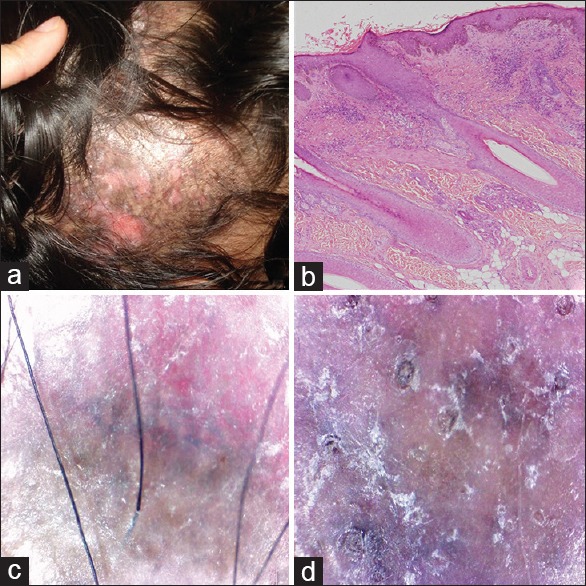
Discoid lupus erythematosus (a) clinical (b) histopathology H and E, ×40 (c and d) dermoscopic findings
Figure 6.
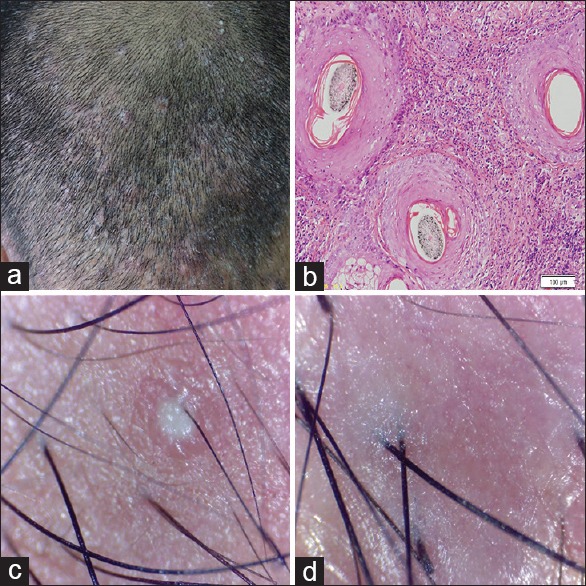
Folliculitis decalvans (a) clinical (b) histopathology H and E, ×100 (c and d) dermoscopic findings
Table 1.
Summary of clinical features of primary cicatricial alopecia
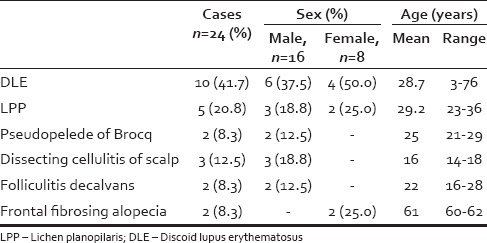
Figure 2.
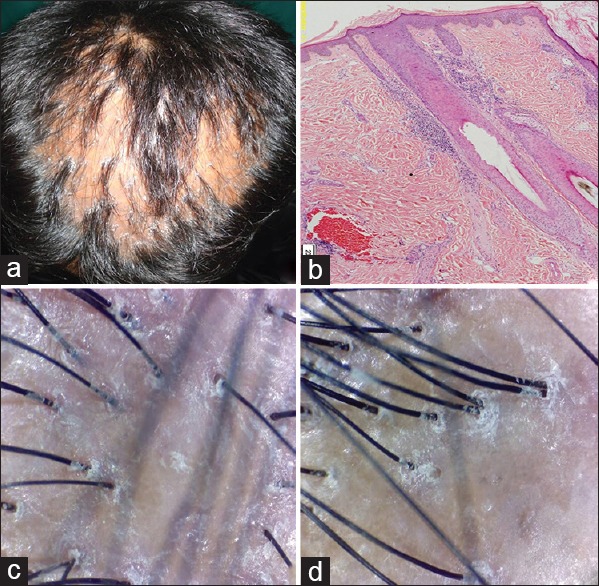
Lichen planopilaris (a) clinical (b) histopathology H and E, ×40 (c and d) dermoscopic findings
Figure 3.
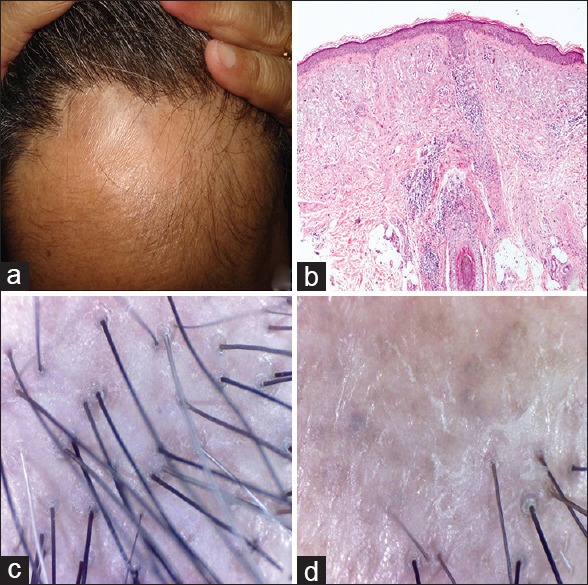
Frontal fibrosing alopecia (a) clinical (b) histopathology H and E, ×40 (c and d) dermoscopic findings
Figure 4.
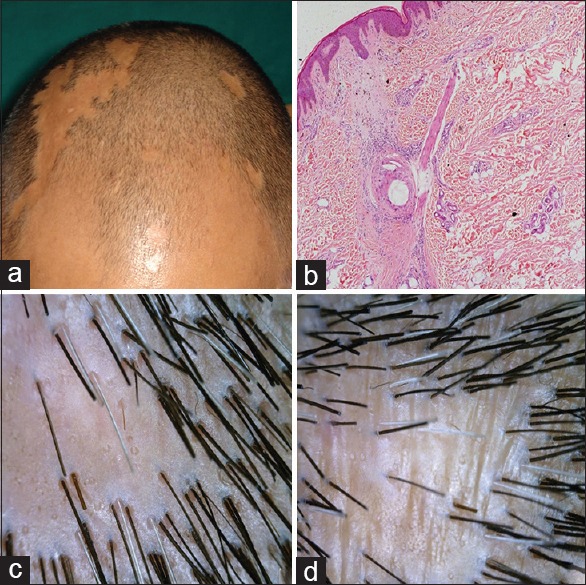
Pseudopelade of brocq (a) clinical (b) histopathology H and E, ×40 (c and d) dermoscopic findings
Figure 5.
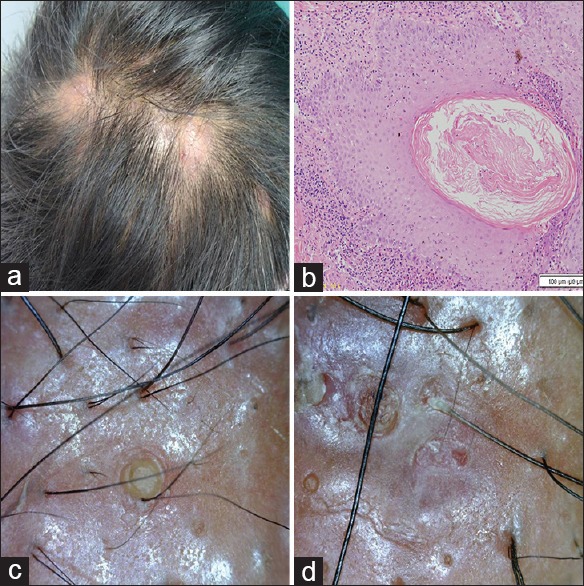
Dissecting cellulitis of scalp (a) clinical (b) histopathology H and E, ×100 (c and d) dermoscopic findings
Discoid lupus erythematosus
The patients include six males and four females. The most common site was occipital scalp in females and frontoparietal scalp in males. There were multiple, erythematous, scaly, atrophic, hypopigmented patches with peripheral hyperpigmentation. In 4 (40%) patients, the lesions were also present on other body areas. In 3 (30%) cases, scalp DLE was associated with systemic lupus erythematosus.
Lichen planopilaris
The involvement in all patients was on the frontoparietal scalp. Violaceous patches of scarring alopecia with perifollicular scaling were seen. Itching was an important symptom in all the patients. None had lesions on the mouth or other body sites.
Frontal fibrosing alopecia
Two postmenopausal females presented with band like progressive scarring alopecia of the frontal scalp. The hairs on the active margin showed perifollicular scaling. There was no involvement of eyebrows. However, lichen planus pigmentosus lesion was present on other body areas in one of the patients.
Pseudopelade of brocq
There were multiple skin colored atrophic patches of scarring alopecia of variable sizes. There was no history of any inflammation on the affected area. One patient had associated scalp psoriasis.
Dissecting cellulitis of scalp
All patients had multiple, painful, pustules, nodules, and abscesses with areas of scarring alopecia on the scalp. Pus for culture and sensitivity was sterile. All had associated moderate acne vulgaris.
Folliculitis decalvans
Two patients had recurrent follicular pustules on the scalp with areas of scarring alopecia on the previously affected site. Follicular tufting was not seen in our patients.
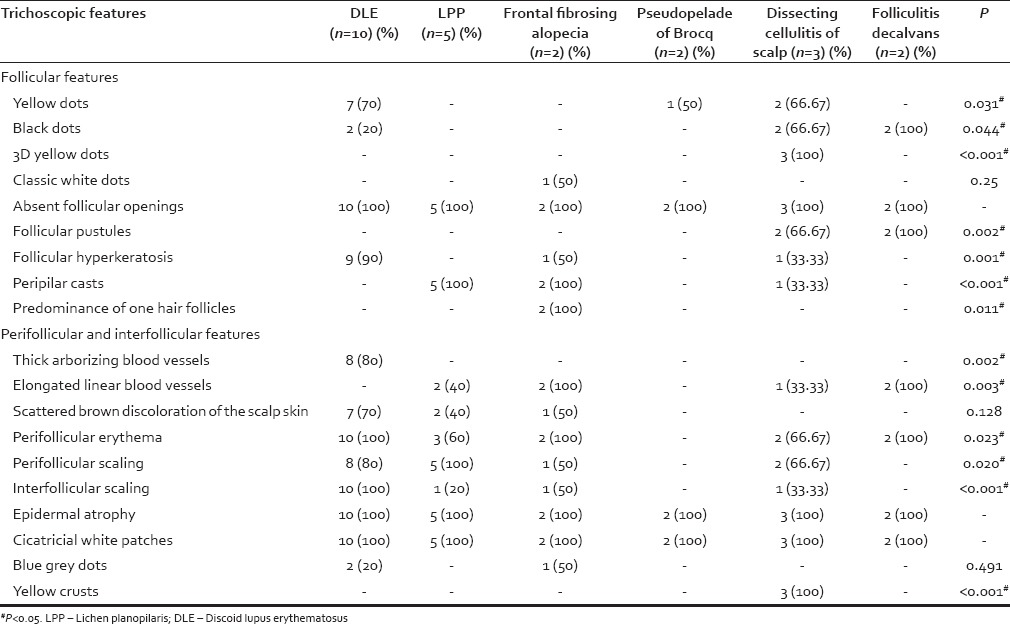
Trichoscopic features
Trichoscopy showed absent follicular opening and epidermal atrophy in all the patients. Scattered brownish discoloration was seen in 70% DLE patients while thick arborizing blood vessels were seen in 80% cases of DLE. The peripilar cast was seen in 100% patients of LPP. Scattered single hair follicles were noted in FFA. Follicular pustules were seen in folliculitis decalvans. A three-dimensional (3D) yellowish structure was specifically seen in all patients of dissecting cellulitis of the scalp. The various trichoscopic features are shown in Table 2.
Table 2.
Trichoscopic features of various types of primary scarring alopecias
Histopathological features
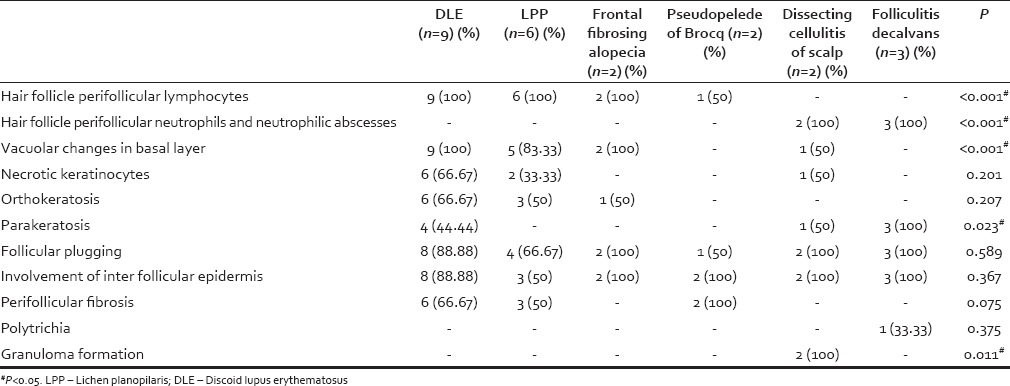
Hair follicles were replaced by fibrous connective tissue with a variable degree in all the patients. The sebaceous glands were either decreased or absent. Interface changes were noted in both LPP (100%) and DLE (90%). In LPP, the perifollicular lymphocytic infiltrate was mostly seen in the superficial dermis. However, in DLE, the infiltrate was both perifollicular and perivascular, and located in both superficial and deep dermis. Mucin deposition was noted only in DLE. Neutrophilic abscesses were seen in both folliculitis decalvans and dissecting cellulitis of the scalp. Granuloma with foreign body giant cells was seen in both cases of dissecting cellulitis of the scalp. Minor inflammation was evident in pseudopelade of brocq. The salient histopathological features observed are summarized in Table 3.
Table 3.
Histopathological features of various types of primary cicatricial alopecias
The diagnosis of PCA based on trichoscopic feature and histopathological features was compared. There was a perfect agreement (kappa coefficient = 0.89) between histological and trichoscopic diagnosis.
DISCUSSION
In PCA, the inflammatory process is folliculocentric with the hair follicle as the main target.[2] The destruction of epithelial stem cells in the bulge of the outer root sheath prevents the regeneration of hair.[3,4] In a retrospective study by Whiting, cicatricial alopecia was diagnosed in 7.3% (n = 427) of all patients who underwent an evaluation over a 10-year period.[5] In two 5-year retrospective studies by Tan et al. and Qi et al., PCA was reported in 3.2% (n = 112) and 2.1% (n = 59) of the patients, respectively.[2,6] Whiting observed pseudopelade predominantly (40.6%), followed by LPP (12.6%) and folliculitis decalvans (11.2%).[5] Tan et al. reported DLE as the most common (33.9%), followed by pseudopelade (24.1%) and LPP (22.3%).[2] Trachsler and Trueb studied 136 biopsy specimens of scarring alopecia and found that the most frequent diagnosis was LPP (26%), followed by DLE (21%), folliculitis decalvans (20%), and pseudopelade of Brocq (10%).[7] In these studies, most of the cases were lymphocytic but one study from China reported folliculitis decalvans as the most common (40%).[6] The different clinical presentations could be because of ethnic and demographic variations.
There are very few studies on cicatricial alopecia from India,[8,9] and to our knowledge, this is the first report of trichoscopic as well as the histopathological study of PCA from India. In both the Indian studies, DLE was the most common cause of PCA reported in 79.16% and 49% cases, respectively. In our study also, DLE was the most common, seen in 41.7% cases. Dissecting cellulitis of the scalp (12.5%) and FFA (8.3%) were not observed in previously reported Indian studies. Traditionally, the criteria for diagnosis of DLE in histopathology are epidermal atrophy, interface dermatitis, superficial and deep dermal lymphocytic inflammation, periadnexal inflammation, papillary dermal fibrosis, dermal mucin, and thickened periodic acid-Schiff-positive epidermal and follicular basement membrane.[10] These criteria were observed in most of our cases. The classic histological features described in LPP are the presence of a lichenoid interface dermatitis of the upper portions of the follicle.[10,11] The inflammation in LPP is mostly lymphocytic with preferential involvement of the peri-infundibular and isthmic regions. The superficial and deep perifollicular and perivascular lymphocytic infiltrate, the presence of abundant dermal melanophages in DLE helped to differentiate with LPP in our cases.
Trichoscopy (dermoscopic examination of scalp and hair) is a very useful technique for the diagnosis and follow-up of hair and scalp disorders.[12,13] Trichoscopy of primary scarring alopecia is characterized by decreased hair density and loss of follicular openings. The most characteristic trichoscopic features of DLE of the scalp are thick arborizing vessels and large yellow dots.[14] Scattered brown discoloration may be seen in some patients.[14] In our patients, thick arborizing vessels were seen only in 80% cases. Red dots are considered a good prognostic factor of hair regrowth.[15] It was reported in 25% and 38% DLE cases in previous studies.[6,15] The red dots were not seen in our patients. This may be due to brown skin color of our patients. The trichoscopic and pathologic features of FFA and LPP are identical and include absence of follicular openings, cicatricial white patches, peripilar casts, blue-gray dots, and perifollicular erythema.[16]
In FFA, the hair follicles are of single hair follicular units. The most characteristic trichoscopic feature of classic LPP is white perifollicular scaling and scales entangling hair shafts up to 2–3 mm above scalp surface.[14] Peripilar casts were also seen in all cases of LPP and FFA. Folliculitis decalvans is characterized by the presence of multiple hairs in one follicular unit.[17] Tufted hairs were not seen in our patients. It was seen in 12.5% cases in a recent study.[6] The finding of tufted hair may be related to the severity of folliculitis decalvans.[18] A specific finding in dissecting cellulitis of the scalp is yellow dots with 3D structure imposed over a thick, black, hair shaft residue.[19] We noted this finding in all cases of dissecting cellulitis of the scalp. Trichoscopic features of classic pseudopelade of Brocq are nonspecific. Thus, pseudopelade of Brocq is a diagnosis of exclusion both clinically and trichoscopically.[20,21]
There was 89% concordance between trichoscopic and histopathological diagnosis in our study. A limitation of this study is retrospective design and a small number of patients in each type of PCA. As there is too much overlap in the histopathological features of folliculitis decalvans and dissecting cellulitis of the scalp, clinicopathological correlation and trichoscopic evaluation of neutrophilic variants of PCA help in proper diagnosis. We suggest that for proper diagnosis of PCA, both histopathology and trichoscopy are essential and for follow-up trichoscopy is very helpful. However, trichoscopy may provide quick and reliable diagnosis and obviate the necessity of scalp biopsy in a busy clinic.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Olsen EA, Bergfeld WF, Cotsarelis G, Price VH, Shapiro J, Sinclair R, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103–10. doi: 10.1067/mjd.2003.68. [DOI] [PubMed] [Google Scholar]
- 2.Tan E, Martinka M, Ball N, Shapiro J. Primary cicatricial alopecias: Clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25–32. doi: 10.1016/j.jaad.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Harries MJ, Paus R. The pathogenesis of primary cicatricial alopecias. Am J Pathol. 2010;177:2152–62. doi: 10.2353/ajpath.2010.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harries MJ, Trueb RM, Tosti A, Messenger AG, Chaudhry I, Whiting DA, et al. How not to get scar (r) ed: Pointers to the correct diagnosis in patients with suspected primary cicatricial alopecia. Br J Dermatol. 2009;160:482–501. doi: 10.1111/j.1365-2133.2008.09008.x. [DOI] [PubMed] [Google Scholar]
- 5.Whiting DA. Cicatricial alopecia: Clinico-pathological findings and treatment. Clin Dermatol. 2001;19:211–25. doi: 10.1016/s0738-081x(00)00132-2. [DOI] [PubMed] [Google Scholar]
- 6.Qi S, Zhao Y, Zhang X, Li S, Cao H, Zhang X. Clinical features of primary cicatricial alopecia in Chinese patients. Indian J Dermatol Venereol Leprol. 2014;80:306–12. doi: 10.4103/0378-6323.136833. [DOI] [PubMed] [Google Scholar]
- 7.Trachsler S, Trueb RM. Value of direct immunofluorescence for differential diagnosis of cicatricial alopecia. Dermatology. 2005;211:98–102. doi: 10.1159/000086436. [DOI] [PubMed] [Google Scholar]
- 8.Kumar UM, Yelikar BR. The spectrum of histopathological lesions in scarring alopecia: A prospective study. J Clin Diagn Res. 2013;7:1372–6. doi: 10.7860/JCDR/2013/5138.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inchara YK, Tirumalae R, Kavdia R, Antony M. Histopathology of scarring alopecia in Indian patients. Am J Dermatopathol. 2011;33:461–7. doi: 10.1097/DAD.0b013e318201abcd. [DOI] [PubMed] [Google Scholar]
- 10.Sperling LC, Cowper SE. The histopathology of primary cicatricial alopecia. Semin Cutan Med Surg. 2006;25:41–50. doi: 10.1016/j.sder.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Tandon YK, Somani N, Cevasco NC, Bergfeld WF. A histologic review of 27 patients with lichen planopilaris. J Am Acad Dermatol. 2008;59:91–8. doi: 10.1016/j.jaad.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Olszewska M, Rudnicka L, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy. Arch Dermatol. 2008;144:1007. doi: 10.1001/archderm.144.8.1007. [DOI] [PubMed] [Google Scholar]
- 13.Jain N, Doshi B, Khopkar U. Trichoscopy in alopecias: Diagnosis simplified. Int J Trichology. 2013;5:170–8. doi: 10.4103/0974-7753.130385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rakowska A, Slowinska M, Kowalska-Oledzka E, Warszawik O, Czuwara J, Olszewska M, et al. Trichoscopy of cicatricial alopecia. J Drugs Dermatol. 2012;11:753–8. [PubMed] [Google Scholar]
- 15.Tosti A, Torres F, Misciali C, Vincenzi C, Starace M, Miteva M, et al. Follicular red dots: A novel dermoscopic pattern observed in scalp discoid lupus erythematosus. Arch Dermatol. 2009;145:1406–9. doi: 10.1001/archdermatol.2009.277. [DOI] [PubMed] [Google Scholar]
- 16.Duque-Estrada B, Tamler C, Sodré CT, Barcaui CB, Pereira FB. Dermoscopy patterns of cicatricial alopecia resulting from discoid lupus erythematosus and lichen planopilaris. An Bras Dermatol. 2010;85:179–83. doi: 10.1590/s0365-05962010000200008. [DOI] [PubMed] [Google Scholar]
- 17.Otberg N, Kang H, Alzolibani AA, Shapiro J. Folliculitis decalvans. Dermatol Ther. 2008;21:238–44. doi: 10.1111/j.1529-8019.2008.00204.x. [DOI] [PubMed] [Google Scholar]
- 18.Sillani C, Bin Z, Ying Z, Zeming C, Jian Y, Xingqi Z. Effective treatment of folliculitis decalvans using selected antimicrobial agents. Int J Trichology. 2010;2:20–3. doi: 10.4103/0974-7753.66908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudnicka L, Rakowska A, Olszewska M. Trichoscopy: How it may help the clinician. Dermatol Clin. 2013;31:29–41. doi: 10.1016/j.det.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Rudnicka L, Olszewska M, Rakowska A, editors. London: Springer-Verlag; 2012. Trichoscopy: Dermoscopy of hair and scalp. [Google Scholar]
- 21.Alzolibani AA, Kang H, Otberg N, Shapiro J. Pseudopelade of brocq. Dermatol Ther. 2008;21:257–63. doi: 10.1111/j.1529-8019.2008.00207.x. [DOI] [PubMed] [Google Scholar]


