Abstract
Context:
Premature canities is a common, yet unexplored disorder. Oxidative stress levels have been evaluated within the greying hair follicle but not in the sera of patients with premature canities.
Aims:
To evaluate the oxidative stress parameters in the sera of patients with premature canities.
Settings and Design:
A pilot case-controlled study, conducted in a tertiary care setup in Delhi during November 2011 to December 2012.
Materials and Methods:
Fifty-two self-reporting cases of premature canities (age of onset <20 years) and 30 healthy controls were recruited from outpatient Department of Dermatology. Oxidative stress parameters (serum malonaldehyde (MDA), whole blood reduced glutathione (rGSH) and serum ferric reducing antioxidant potential [FRAP]) were assessed in cases and controls. Mann–Whitney test was used to compare the oxidative stress parameters between the two groups (SPSS version 17.0, SPSS Inc, Chicago, USA; P < 0.05 considered as significant).
Results:
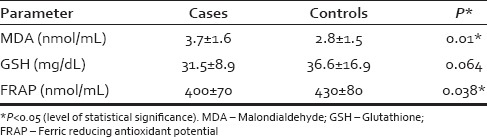
The age and sex distribution of cases and controls was comparable. The mean serum levels of MDA were higher in cases than controls (3.7 ± 1.6 nmol/ml vs. 2.8 ± 1.5 nmol/ml; P = 0.01). The GSH levels were lower in the cases than controls (31.5 ± 8.9 mg/dl vs. 36.6 ± 16.9 mg/dl; P = 0.064). Similarly, the mean FRAP levels were lower in the cases than controls (400 ± 70 nmol/ml vs. 430 ± 80 nmol/ml; P = 0.038).
Conclusions:
Patients with premature canities had a higher level of pro-oxidants and lower levels of antioxidants than controls. This is the first humble attempt to document the oxidative stress parameters in sera of patients with premature canities, further studies with larger sample size are required to reach a definite conclusion.
Keywords: Greying of hair, oxidative stress, premature canities
INTRODUCTION
Premature canities is the development of grey hair over scalp earlier than the expected age. Melanocyte apoptosis and increased oxidative stress has been documented in the Pigmentary Unit of greying hair follicles,[1] but, to the best of our knowledge, assessment of oxidative stress in the sera of patient with premature canities has not been estimated so far. The present study was undertaken to study the oxidative stress parameters including lipid peroxidation and the enzymatic and nonenzymatic antioxidant potential in the sera of patients with premature canities and to compare with healthy controls.
MATERIALS AND METHODS
Fifty-two, apparently healthy, self-reporting individuals with onset of premature greying of scalp hair before the age of 20 years and 30 matched healthy controls, were recruited from the outpatient Department of Dermatology. Institutional Ethical Committee approval was obtained before commencement of the study. Written informed consent was taken from all recruits. Patients with greying of hair as a part of other conditions like vitiligo, cutaneous disease involving the scalp, history of topical application or systemic drug/supplement intake, and use of hair dye in the past 6 months were excluded. Smokers, pregnant or lactating women, and subjects with a history of febrile illness or severe systemic disease within 3 months of onset of greying of hair were also excluded. All patients underwent hematological and biochemical evaluation including complete hemogram, erythrocyte sedimentation rate (ESR), blood sugar (fasting and postprandial), and liver and kidney function tests.
Following oxidative stress parameters were assessed:
Lipid peroxidation (malonaldehyde; MDA) estimation using the colorimetric method as described by Satoh[2]
Whole blood reduced glutathione (rGSH) content in erythrocytes by the method of Beutler et al[3]
Ferric reducing antioxidant potential (FRAP) estimation using the method of Benzie et al.[4]
To compare the various oxidative stress parameters in cases and controls, Mann–Whitney test was used. The statistical software SPSS version 17.0 (SPSS Inc., Chicago, USA) was used and P < 0.05 was considered as significant.
RESULTS
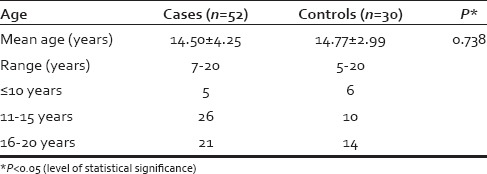
The age and sex distribution of cases and controls were comparable [Table 1]. ESR was found to be raised in cases (mean = 14.49 ± 13.46 mm in 1st h) as compared to controls (mean = 8.33 ± 4.91 mm in 1st h; P = 0.004). Other hematological parameters, fasting and postprandial blood sugars, serum bilirubin, serum glutamic pyruvic transaminase, alkaline phosphatase, calcium, phosphate, total proteins, and albumin were comparable in both the groups.
Table 1.
Age distribution of study subjects
Oxidative stress parameters [Table 2] - The mean serum levels of MDA in cases were significantly higher (3.7 ± 1.6 nmol/ml) than in controls (2.8 ± 1.5 nmol/ml; P = 0.01). The glutathione (GSH) levels were lower in the cases (31.5 ± 8.9 mg/dl) as compared to controls (36.6 ± 16.9 mg/dl; P = 0.064), but this difference was insignificant. The mean FRAP levels were lower in the cases than in controls (400 ± 70 nmol/ml vs. 430 ± 80 nmol/ml; P = 0.038). None of these oxidative stress parameters were found to correlate with the duration and severity of greying.
Table 2.
Oxidative stress parameters in cases and controls
DISCUSSION
Canities is the scientific term for the phenomena of physiological greying of hair that occurs with aging and shows variation with race and ethnicity. The normal occurrence of greying of hair, in white races, is at the age of 34.2 ± 9.6 years, and it is said that by the age of 50 years, 50% of the population have at least 50% grey hair, known as the 50/50/50 rule of thumb.[5] This rule of thumb has been revaluated in a large world sample of human subjects from different ethnic and geographic origins. It was concluded that around 6–23% of world population is expected to have 50% grey hair by the age of 50 years, a range far below that predicted by 50/50/50 rule of thumb.[6]
Onset of greying of hair before the age of 20 years in caucasians and before 30 years in Africans is considered as premature canities.[7,8] Unfortunately, there are no studies published so far that define premature greying in Asian population. Though, a cut-off at the age of 25 years is considered by some authorities in India.[9] For the purpose of our study, we defined premature greying if it occurred before the age of 20 years. Usually, the process is progressive and permanent, though there are occasional reports of re-pigmentation of previously grey hair.[10,11]
Melanin, the pigment primarily responsible for hair color, is synthesized in unique membrane bound organelle “melanosomes” present within the melanocytes. Melanins are indole derivatives of 3,4dihydroxyphenylalanine, and they are formed through a series of oxidative steps.[12] Melanogenic activity in the hair follicle is closely linked to the hair cycle. Tyrosinase activity becomes apparent in anagen 3, and pigment transfer to cortical epithelium begins in the anagen 4 stage of development.[13] In pigmented hair follicles, intense melanogenesis continues throughout the remainder of anagen (stage 5 and 6), and then ceases with the onset of catagen.
Oxidative stress seems to play a key role in many pathways within the hair follicle.[14] The process of melanin synthesis in hair generates oxidative stress by a generation of hydrogen peroxide (H2O2) and other free radicals.[15,16,17] As compared to keratinocytes, melanocytes are more vulnerable to oxidative stress, because they are engaged in the production of large quantities of melanin throughout the anagen phase of hair cycle.[1] Besides this, oxidative stress generated outside hair follicle by UV-light exposure, psycho-emotional, or inflammatory stress may add to this endogenous oxidative stress, and may overwhelm the hair follicle melanocyte anti-oxidant capacity, thereby speeding up the terminal damage in the aging hair follicle.[18,19,20,21]
Oxidative stress leads to damage via the generation of free radicals or reactive oxygen species (ROS) namely superoxide (O2), H2O2, and hydroxyl free radical. They are highly unstable and capable of damaging cell membranes (lipid peroxidation of membranes), DNA (produces single-stranded breaks in DNA), and proteins (protein fragmentation and degradation of critical enzymes), due to their extreme reactivity. MDA, a lipid peroxidation product generated by free radical injury in tissues, subsequently appears in serum and acts as a sensitive indicator of lipid peroxidation and oxidative stress.[22] Thus, the raised levels of MDA as in the present study points toward a higher degree of oxidative stress in patients with premature canities. As lipids are an integral component of all cellular membranes, a higher degree of lipid peroxidation reflects a higher grade of cell membrane injury.
As generation of ROS is an integral part of normal metabolic processes, the body has devised antioxidant systems to protect itself from this constant hazard. These include multiple antioxidants and free radical scavenging enzymes like superoxide dismutase, catalase, and GSH peroxidase.[22] GSH peroxidase, a selenium-containing enzyme which catalyzes the reduction of H2O2 and lipid hydro-peroxidases using rGSH.

The activity of enzyme is dependent on the constant availability of rGSH, levels of which are maintained by the enzyme glutathione reductase (GR)[23]

The decrease in GSH levels in human blood is an indicator of reduced antioxidant potential. In the present study, the lower value of GSH observed in cases suggests a reduced ability to fight oxidative stress in cases as compared to the controls. Furthermore, since the GSH redox system represents the aqueous phase of oxidative stress, therefore, its alteration depicts damage and malfunctioning of the hydrophilic cellular structures as well.
Estimation of “FRAP” measures the combined antioxidant effect of nonenzymatic defenses in biological fluids. It is based on the measurement of total antioxidant status of blood by assessing the extent of reduction of TPTZ-Fe3+ to TPTZ-Fe2+ (TPTZ-tripyridyltriazine). The lower levels of FRAP in the cases (mean = 400 ± 70 nmol/ml) compared to controls (430 ± 80 nmol/ml; P = 0.038) is again suggestive of a low total antioxidant status of blood. None of these oxidative stress parameters were found to correlate with the duration and severity of greying.
In a study by Arck et al.,[14] a higher frequency of oxidative stress associated mitochondrial DNA damage was observed in greying hair follicles. This was accompanied by an absence of oxidative stress-protectors, such as Bcl-2, and melanocyte growth factors, such as c-Kit.
Role of H2O2 mediated oxidative stress has been documented in senile hair greying by Wood et al.[24] While another study on premature canities documented an intrinsic deficiency of catalase in grey hair compared to pigmented hair, leading to decreased ability to reduce H2O2, and higher concentration of the same in the greying hair follicles.[25] Kauser et al. demonstrated lower levels of catalase in hair follicles melanocytes derived from aged hair as compared to those from younger donors.[26] Low H2O2 concentrations increase tyrosinase activity, while high concentrations irreversibly deactivate the enzyme.[27,28] Abundant evidence indicates that many proteins and peptides, including the H2O2-reducing enzyme catalase are structurally damaged and functionally altered by H2O2-mediated oxidation.[24,29] This acts as a vicious cycle in perpetuating the ROS-induced damage to the hair follicle melanocytes.
All these studies indicate the role of ROS at the level of hair follicles without elaborating on the systemic state of oxidative stress in a patient. To the best of our knowledge, there are no studies in English literature which document the oxidative stress parameters in the sera of patients with premature greying, despite their documented role in its causation.
CONCLUSION
All the three tests of oxidative stress, MDA, GSH, and FRAP point in the same direction of raised levels of pro-oxidants and reduced ability of body to fight the same, that is, low levels of antioxidants, both enzymatic and nonenzymatic. Hence, the role of antioxidants in the treatment of premature canities can be postulated emphatically. However, in view of the small sample size of the present study and possible confounding influence of multiple sociocultural and psychological factors on oxidative stress of an individual, further studies with larger sample size are recommended.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Aknowledgments
We are immensely thankful to Dr. Neeraj Kumar Singh, PhD. Medical biochemistry, Institute of Human Behaviour and Allied Sciences and Ms. Kumari Asha, Department of Biochemistry, University College of Medical Sciences, Delhi for their technical assistance in conducting the tests of oxidative stress parameters.
REFERENCES
- 1.Arck PC, Overall R, Spatz K, Liezman C, Handjiski B, Klapp BF, et al. Towards a “free radical theory of graying”: Melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006;20:1567–9. doi: 10.1096/fj.05-4039fje. [DOI] [PubMed] [Google Scholar]
- 2.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 3.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 4.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 5.Keogh EV, Walsh RJ. Rate of greying of human hair. Nature. 1965;207:877–8. doi: 10.1038/207877a0. [DOI] [PubMed] [Google Scholar]
- 6.Panhard S, Lozano I, Loussouarn G. Greying of the human hair: A worldwide survey, revisiting the ‘50’ rule of thumb. Br J Dermatol. 2012;167:865–73. doi: 10.1111/j.1365-2133.2012.11095.x. [DOI] [PubMed] [Google Scholar]
- 7.Trüeb RM. Pharmacologic interventions in aging hair. Clin Interv Aging. 2006;1:121–9. doi: 10.2147/ciia.2006.1.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odom RB, James WD, Berger TG. Diseases of the skin appendages. In: James WD, Berger TG, Odom RB, editors. Andrew's Diseases of the Skin Clinical Dermatology. 9th ed. Philadelphia: WS Saunders; 2000. p. 955. [Google Scholar]
- 9.Pasricha JS, Verma K. Treatment of Skin Diseases. 5th ed. New Delhi: Mehta Publishers; 2008. Diseases of the appendages; p. 289. [Google Scholar]
- 10.Ortonne JP. Vitiligo and other disorders of hypopigmentation. In: Jorizzo JL, Rapini R, Bolognia JL, editors. Dermatology. 2nd ed. Missouri: Mosby Elsevier; 2008. p. 936. [Google Scholar]
- 11.Lorincz AL. Disturbances of melanin pigmentation. In: Hurley HJ, Moschella SL, editors. Dermatology Moschella and Hurley. 2nd ed. Philadelphia: WS Saunders; 1985. p. 1297. [Google Scholar]
- 12.Sarangarajan R, Apte SP. The polymerization of melanin: A poorly understood phenomenon with egregious biological implications. Melanoma Res. 2006;16:3–10. doi: 10.1097/01.cmr.0000195699.35143.df. [DOI] [PubMed] [Google Scholar]
- 13.Slominski A, Paus R. Melanogenesis is coupled to murine anagen: Toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101:90S–7S. doi: 10.1111/1523-1747.ep12362991. [DOI] [PubMed] [Google Scholar]
- 14.Van Neste D, Tobin DJ. Hair cycle and hair pigmentation: Dynamic interactions and changes associated with aging. Micron. 2004;35:193–200. doi: 10.1016/j.micron.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Nappi AJ, Vass E. Hydrogen peroxide generation associated with the oxidations of the eumelanin precursors 5,6-dihydroxyindole and 5,6-dihydroxyindole-2-carboxylic acid. Melanoma Res. 1996;6:341–9. doi: 10.1097/00008390-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Jiménez-Cervantes C, Martínez-Esparza M, Pérez C, Daum N, Solano F, García-Borrón JC. Inhibition of melanogenesis in response to oxidative stress: Transient downregulation of melanocyte differentiation markers and possible involvement of microphthalmia transcription factor. J Cell Sci. 2001;114:2335–44. doi: 10.1242/jcs.114.12.2335. [DOI] [PubMed] [Google Scholar]
- 17.Pavel S. Dynamics of melanogenesis intermediates. J Invest Dermatol. 1993;100:162S–5S. [PubMed] [Google Scholar]
- 18.Kadekaro AL, Kavanagh RJ, Wakamatsu K, Ito S, Pipitone MA, Abdel-Malek ZA. Cutaneous photobiology. The melanocyte vs. the sun: Who will win the final round? Pigment Cell Res. 2003;16:434–47. doi: 10.1034/j.1600-0749.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 19.Emerit I, Filipe P, Freitas J, Vassy J. Protective effect of superoxide dismutase against hair graying in a mouse model. Photochem Photobiol. 2004;80:579–82. doi: 10.1562/0031-8655(2004)080<0579:PEOSDA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Irie M, Asami S, Nagata S, Miyata M, Kasai H. Relationships between perceived workload, stress and oxidative DNA damage. Int Arch Occup Environ Health. 2001;74:153–7. doi: 10.1007/s004200000209. [DOI] [PubMed] [Google Scholar]
- 21.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latha B, Babu M. The involvement of free radicals in burn injury: A review. Burns. 2001;27:309–17. doi: 10.1016/s0305-4179(00)00127-3. [DOI] [PubMed] [Google Scholar]
- 23.Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol. 2001;54:176–86. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood JM, Decker H, Hartmann H, Chavan B, Rokos H, Spencer JD, et al. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. FASEB J. 2009;23:2065–75. doi: 10.1096/fj.08-125435. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y, Luo LF, Liu XM, Zhou Q, Xu SZ, Lei TC. Premature graying as a consequence of compromised antioxidant activity in hair bulb melanocytes and their precursors. PLoS One. 2014;9:e93589. doi: 10.1371/journal.pone.0093589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood JM, Schallreuter KU. Studies on the reactions between human tyrosinase, superoxide anion, hydrogen peroxide and thiols. Biochim Biophys Acta. 1991;1074:378–85. doi: 10.1016/0304-4165(91)90088-x. [DOI] [PubMed] [Google Scholar]
- 27.Kauser S, Westgate GE, Green MR, Tobin DJ. Human hair follicle and epidermal melanocytes exhibit striking differences in their aging profile which involves catalase. J Invest Dermatol. 2011;131:979–82. doi: 10.1038/jid.2010.397. [DOI] [PubMed] [Google Scholar]
- 28.Wood JM, Chavan B, Hafeez I, Schallreuter KU. Regulation of tyrosinase by tetrahydropteridines and H2O2. Biochem Biophys Res Commun. 2004;325:1412–7. doi: 10.1016/j.bbrc.2004.10.185. [DOI] [PubMed] [Google Scholar]
- 29.Tobin DJ. Aging of the hair follicle pigmentation system. Int J Trichology. 2009;1:83–93. doi: 10.4103/0974-7753.58550. [DOI] [PMC free article] [PubMed] [Google Scholar]


