Abstract
Context:
Androgenetic alopecia (AGA) is a frequent disorder characterized by progressive hair miniaturization in a very similar pattern among all affected men. The pathogenesis is related to androgen-inducible overexpression of transforming growth factor β-1 from balding dermal papilla cells, which is involved in epithelial inhibition and perifollicular fibrosis. Recent research shows that hair follicle androgen sensitivity is regulated by Hic-5, an androgen receptor co-activator which may be activated by the mechanical stimulation. Moreover, the dermis of scalp susceptible to be affected by AGA is firmly bounded to the galea aponeurotica, so the physical force exerted by the occipitofrontalis muscle is transmitted to the scalp skin.
Aims:
To know whether mechanical stress supported by hair follicles is involved in AGA phenomenon.
Materials and Methods:
It is performed with a finite element analysis of a galea model and a schematic representation of AGA progression according to Hamilton–Norwood scale in order to establish the correlation between elastic deformation in scalp and clinical progression of male pattern baldness.
Results:
The result was a highly significant correlation (r: −0.885, P < 0.001) that clearly identifies a mechanical factor in AGA development.
Conclusions:
All these data suggest that mechanical stress determines AGA patterning and a stretch-induced and androgen-mediated mechanotransduction in dermal papilla cells could be the primary mechanism in AGA pathogenesis.
Keywords: Androgenetic alopecia, galea aponeurotica, mechanical stress, mechanosensitivity
INTRODUCTION
Male androgenetic alopecia (AGA), also called male pattern baldness, is a frequent disorder characterized by progressive hair and follicular miniaturization in a very similar pattern among affected men that is classified according to the Hamilton–Norwood scale.[1,2] Genetic predisposition, androgens, and aging are closely related to AGA, but pathogenesis has not been clarified yet.
Dermal papilla is considered a key element in AGA development[3] and thickening and hyperplasia of the dermal sheath is the only universally accepted histopathological evidence in AGA.[4] Both dermal papilla and dermal sheath are considered as a functional unit[5] which constitute the dermal component of the hair follicle, and its metabolism is bidirectional in the anagen-catagen transition.[6] The alteration of this tissue remodeling may cause an excessive collagen network that would not be fully digested later, resulting in physical blocking of the hair canal by a fibrotic process called perifollicular fibrosis.[7,8,9]
Transforming growth factor β-1 (TGFβ-1) is related to fibrosis by upregulating extracellular matrix (ECM) synthesis in many tissues[10] and it causes epithelial inhibition in hair follicle,[11] so it seems to play a fundamental role in AGA pathogenesis. Moreover, TGFβ-1 overexpression is induced by androgens in balding dermal papilla due to enhanced androgen sensitivity.[12] Inui et al. have shown that this androgen sensitivity of dermal papilla cells is regulated by androgen receptor (AR) co-activator Hic-5/ARA55, a focal adhesion associated protein belonging to the family of paxillin.[13] Although Hic-5 is located predominantly in the focal adhesion, it can also be found in the nucleus[14] where it could directly affect TGFβ-1 gene expression in association with AR and dihydrotestosterone. Interestingly, a triggering stimulus that can alter the inactive standby status of Hic-5 is the deformation of the cytoskeleton by physical forces;[15,16] therefore, mechanical stimulation can promote overexpression of molecular signals implicated in AGA pathogenesis.
This fact takes sense if it is considered that scalp skin susceptible to be affected by AGA presents unique anatomical and biomechanical features. Regardless the pattern or degree of severity, AGA is always limited to the skin overlying the galea aponeurotica. This is a thin and relatively inelastic tendon-like tissue sheet that communicates the frontal and occipital bellies of occipitofrontalis muscle.[17] Balding scalp skin is firmly bounded to galea by fibrous rigid subcutaneous layer, so elastic deformation affecting the galea is shared by the three upper layers as a structural unit[18] [Figure 1], whereas the remaining scalp skin freely slides over deeper layer, with low strain transmission to hair follicles and unaffected by AGA.
Figure 1.
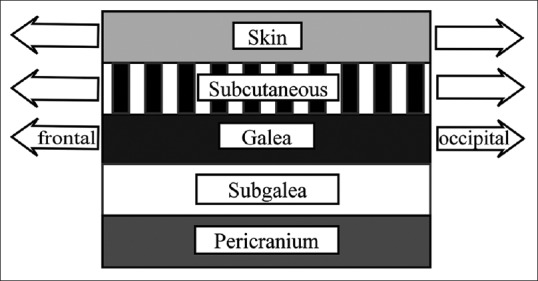
Layers of the scalp. Mechanical strain is transmitted from galea to skin
Thus, the aim of the present study was to analyze the correlation between stress distribution in the galea and clinical progression of male pattern baldness in order to know whether mechanical stress is involved in AGA phenomenon.
MATERIALS AND METHODS
Finite element analysis of stress distribution in the galea
A model for finite element analysis (FEA) was constructed by using the program LISA FEA (version 8.0.0 2013; LISA-Finite element technologies. Missisauga, Canada). The following methodology was applied to develop and resolve the model.
A geometric mesh was developed and adjusted to the galea aponeurotica anatomy. Model dimensions were: 210 mm length, 120 mm width, and 1 mm thick. Total surface was 268 cm2. Length and width dimensions were approximately the average of measurement taken in 10 adult men. The mesh had 252 elements and the total number of nodes was 1075.
The front limit corresponds to the galea insertion with the frontal bellies, which are considered as force effectors. This assumption was based on the study by Kushima et al. about the occipitofrontalis muscle, which classifies frontal bellies into two types: First, the laterally developed type, with no muscle fibers in the metopic portion and second, the generally developed type with muscle fibers also in the metopic portion.[19] These two different morphologies determine the hairline and the direction of the muscle fibers. The author selected the laterally developed type because it is the most frequent type according to this report. Hence, two force vectors were set in a parallel direction to the longitudinal axis of the galea and slightly to the center line (10° to the center line relative to the longitudinal axis). In addition, the front limit of the galea model was deformed by increasing 2 cm of the length in the central metopic portion according to muscle type and hairline shape. As no references were found of the force generated by the frontal bellies, The author applied 1 N to each vector. Nonetheless, this quantitative variable is irrelevant for the purpose of this model because it does not affect symmetric stress distribution.
The rear boundary corresponds to the galea insertion with the occipital bellies, and it was configured as fixation because occipitalis muscles isometric contraction only anchors the galea.[18]
The lateral limits and the galea surface are configured as free because the aponeurotic fibers do not gain a direct insertion onto the bone and the temporal and periauricular muscles are not related to the galea because they are inserted directly into the skull.
The galea was assumed to be an isotropic material due to its nature of regular tendon-like tissue sheet. Classically accepted physical properties for human tendons are attributed: Young's modulus 600 × 106 N/m2 and Poisson's ratio 0.5.[20]
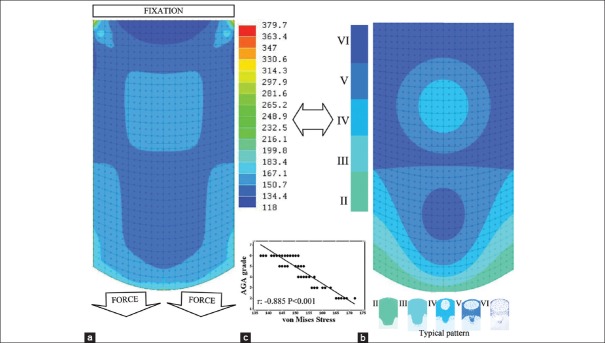
The model was solved as a two-dimensional static stress problem by selecting von Mises stress because it is a proportional scalar to the elastic strain energy generated by external forces at each point of the galea [Figure 2a].
Figure 2.
(a) Von Misses stress in the galea. Hairline and force vectors are configured as laterally developed frontal bellies. (b) Schematic sequence of androgenetic alopecia transition zones according to Hamilton–Norwood scale (typical pattern) (c) Regression line and Pearson product-moment correlation coefficient between A and B
Schematic distribution of androgenetic alopecia progression
The same mesh adjusted to the galea anatomy was applied to represent the clinical course of AGA by transference of the Hamilton–Norwood scale model. The author assigned a numerical value to each hair line transition zone between Grades II and VI of AGA. The overlap between stress distribution and AGA evolution (as well as between the galea and overlaying skin) allowed establishing their correlation in each exact point. The typical pattern of AGA was selected because it is the most frequent.[21] Moreover, this representation is applicable to both typical and vertex types because they are very similar patterns [Figure 2b].
Statistical analysis
Data were coded, entered, and analyzed using Minitab 17 software for statistical science (version 17.1.0.0 20013; Minitab Inc. State College, PA, USA). Statistical analysis included mean value, student's t-test, standard error, and Pearson product-moment correlation coefficient (r) that were expressed in terms of P value. P ≤ 0.05 was considered statistically significant. Sample size was n = 228 and it corresponds to the central point of each mesh element with a surface of approximately 1 cm2. Regression line of correlation between von Mises stress in the galea and quantitative grade of AGA in each point is also performed. Elements close to occipital fixing are eliminated because the occipital skin is not firmly bounded to the galea at this level [Figure 2c].[19]
RESULTS
In the present study, the mean von Mises stress was 153.5 ± 16.5 and of AGA transition zones was 4 ± 2. Stress distribution in the galea showed a highly significant correlation with AGA patterning (r = −0.885, P < 0.001). The negative value is due to the inverse relationship between strain and terminal-vellus hair ratio.
The main limitation of this model was to place it in a two-dimensional mesh, and not in a three-dimensional geometry that corresponds to skull anatomy. Nonetheless, von Mises stress values only vary slightly due to the assumption of static stress model. Moreover, the curves of the subjacent cranial vault are little pronounced at this level, so even if a certain degree of error in this schematic model is assumed, the stress distribution obtained in this analysis would be very approximate to real conditions.
In Grade VII of AGA, the hair line advances beyond the limits of the galea. In this case, the mechanical strain could be transmitted to the follicles from the scalp linked to the galea itself. Logically, stress would be lower at this zone and would affect only to most severe and advanced cases. Needless to say, this correlation is only relevant to male pattern baldness. Despite female pattern hair loss has been classically considered an AGA analogous disorder; recent studies have demonstrated important differences in the pathological process.[22]
CONCLUSIONS
The result of this analysis indicates a constant linear dependence between elastic deformation of scalp and AGA patterning, which clearly identifies mechanical stress as an active factor in AGA.
The involuntary tonic contraction of occipitofrontalis muscle is related to psychological stress conditions,[23] facial expression,[24] the maintenance of visual field,[19] and an aponeurotic tension model of human craniofacial growth,[25] so the galea aponeurotica supports a continuous stress which is transmitted to ECM and cells of each tissue, dermal papilla, and dermal sheath cells included. The deformation energy does not cause apparent damage to scalp skin, but its interplay with androgens could be fatal in organ remodeling of hair follicles. This androgen-mediated molecular response to mechanical stimulation can play the anabolic role instead of biological virilization role, as it has been extensively studied in tissues whose function is closely linked to the physical force support.[26] Furthermore, it has been reported that TGFβ-1 increases the expression of Hic-5 in hypertrophic scars fibroblasts[27] and it potentiates AR transactivity in balding dermal papilla cells[28] by autocrine loop [Figure 3]. Hence, the long-lasting cyclic strain would cause a slow, chronic, and progressive environmental adaptation process in balding hair follicles since puberty.
Figure 3.
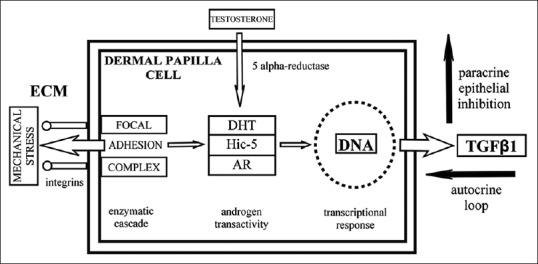
Mechanotransduction of mechanical stimuli to nucleus through Hic-5 shuttling
All these data suggest that stress distribution in the scalp determines AGA patterning and a stretch-induced and androgen-mediated mechanotransduction process in dermal papilla cells could be the primary mechanism in AGA pathogenesis.
DISCUSSION
The result of this study can only be understood if it assumes that the hair follicle is a mechanosensitive organ. However, is it possible that quantitative differences in mechanical stress may cause AGA development? Although many genetic factors could affect stress in scalp hair follicles, probably the mechanical variable is not too different among all men, like androgen levels are similar between those AGA affected and those who are not.[29] The increase of occipitofrontalis muscle activity due to any circumstance could be considered a hair loss accelerator, but it is unlikely that the mechanical variable can trigger the early development of baldness itself. Undoubtedly, the primary cause of AGA is intrinsic to hereditary predisposition, and a possible genetic factor could be the degree of response to mechanical stimuli in each individual. The pathology that presents greater association with AGA is benign prostatic hyperplasia (BPH)[30] and recent research links BPH pathogenesis with prostatic pressure.[31] Thus, the genes that regulate mechanosensitivity in androgen target tissues could be critically involved in AGA etiology.
The involvement of mechanical stress in AGA implies that hair follicles do not have genetically preprogramed androgen sensitivity. It is imperative at this point to mention the ingenious experiment by Nordstrom, who transplanted hair follicles from both balding and occipital scalp to the forearm. The result was the loss of hair from the balding scalp whereas the occipital hair continued growing.[32] This study is considered a proof of genetic follicle preprograming, but according to the approach of the present paper, it would be necessary to know the strain supported by the forearm skin and to realize that the hair follicles close to receding hairline have already started a countdown toward the miniaturization, but not the occipital follicles. In hair transplantation, the grafted follicles start a new “balding clock,” but hair growth would be guaranteed for many years even without preventive pharmacotherapy.
It is common to see that some patients (men as well as women) present a transient hypertrichosis localized in a hurt extremity after immobilization, so elimination of stretch in the skin seems to promote the anagen phase in body hair. In this sense, the treatment of scalp muscles with botulinum toxin Type A is effective in slowing hair loss.[33] This therapy dramatically reduces occipitofrontalis muscle tone, which temporarily interrupts AGA evolution. The function of mechanosensitivity in dermal papilla could play a pivotal role in hair cycle control due to information received from the hair itself (e.g., hair length or traction).
Hence, to search for therapeutic agents that target the mechanotransduction signaling pathway in any level is a promising research field. Minoxidil, an internationally accepted drug against hair loss whose mechanism of action is not yet clarified could affect the first step of stretch-induced response by alteration of K+ mechanosensitive ion channels.[34] Unfortunately, the effectiveness of these treatments would be limited to hair loss prevention and regrowth of follicles without advanced perifollicular fibrosis. The AGA pathological process ends by the complete destruction of some affected follicles,[4] but most of them remain as vellus-like hair, so a large recovery is possible in theory. However, these therapies would face one of the biggest challenges of medicine today: Reversing a fibrotic process.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The author thank Dr. Maria del Mar Tellez for her lessons in molecular biology and Mario Escoriza, Marisol Cruz, Pablo Mellado, Joe Canton, Maico Viciana and Manuel Canton for their invaluable help in literature search, traslation and text review.
REFERENCES
- 1.Hamilton JB. Patterned loss of hair in man; types and incidence. Ann N Y Acad Sci. 1951;53:708–28. doi: 10.1111/j.1749-6632.1951.tb31971.x. [DOI] [PubMed] [Google Scholar]
- 2.Norwood OT. Male pattern baldness: Classification and incidence. South Med J. 1975;68:1359–65. doi: 10.1097/00007611-197511000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Inui S, Itami S. Molecular basis of androgenetic alopecia: From androgen to paracrine mediators through dermal papilla. J Dermatol Sci. 2011;61:1–6. doi: 10.1016/j.jdermsci.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 4.El-Domyati M, Attia S, Saleh F, Abdel-Wahab H. Androgenetic alopecia in males: A histopathological and ultrastructural study. J Cosmet Dermatol. 2009;8:83–91. doi: 10.1111/j.1473-2165.2009.00439.x. [DOI] [PubMed] [Google Scholar]
- 5.Iguchi M, Hara M, Manome H, Kobayasi H, Tagami H, Aiba S. Communication network in the follicular papilla and connective tissue sheath through gap junctions in human hair follicles. Exp Dermatol. 2003;12:283–8. doi: 10.1034/j.1600-0625.2003.120308.x. [DOI] [PubMed] [Google Scholar]
- 6.Commo S, Bernard BA. Immunohistochemical analysis of tissue remodelling during the anagen-catagen transition of the human hair follicle. Br J Dermatol. 1997;137:31–8. [PubMed] [Google Scholar]
- 7.Jaworsky C, Kligman AM, Murphy GF. Characterization of inflammatory infiltrates in male pattern alopecia: Implications for pathogenesis. Br J Dermatol. 1992;127:239–46. doi: 10.1111/j.1365-2133.1992.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 8.Mahé YF, Michelet JF, Billoni N, Jarrousse F, Buan B, Commo S, et al. Androgenetic alopecia and microinflammation. Int J Dermatol. 2000;39:576–84. doi: 10.1046/j.1365-4362.2000.00612.x. [DOI] [PubMed] [Google Scholar]
- 9.Yoo HG, Kim JS, Lee SR, Pyo HK, Moon HI, Lee JH, et al. Perifollicular fibrosis: Pathogenetic role in androgenetic alopecia. Biol Pharm Bull. 2006;29:1246–50. doi: 10.1248/bpb.29.1246. [DOI] [PubMed] [Google Scholar]
- 10.Branton MH, Kopp JB. TGF-beta and fibrosis. Microbes Infect. 1999;1:1349–65. doi: 10.1016/s1286-4579(99)00250-6. [DOI] [PubMed] [Google Scholar]
- 11.Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Identification of androgen-inducible TGF-beta1 derived from dermal papilla cells as a key mediator in androgenetic alopecia. J Investig Dermatol Symp Proc. 2003;8:69–71. doi: 10.1046/j.1523-1747.2003.12174.x. [DOI] [PubMed] [Google Scholar]
- 12.Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Androgen-inducible TGF-beta1 from balding dermal papilla cells inhibits epithelial cell growth: A clue to understand paradoxical effects of androgen on human hair growth. FASEB J. 2002;16:1967–9. doi: 10.1096/fj.02-0043fje. [DOI] [PubMed] [Google Scholar]
- 13.Inui S, Fukuzato Y, Nakajima T, Kurata S, Itami S. Androgen receptor co-activator Hic-5/ARA55 as a molecular regulator of androgen sensitivity in dermal papilla cells of human hair follicles. J Invest Dermatol. 2007;127:2302–6. doi: 10.1038/sj.jid.5700883. [DOI] [PubMed] [Google Scholar]
- 14.Shibanuma M, Mori K, Nose K. HIC-5: A mobile molecular scaffold regulating the anchorage dependence of cell growth. Int J Cell Biol. 2012;2012:426138. doi: 10.1155/2012/426138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim-Kaneyama JR, Suzuki W, Ichikawa K, Ohki T, Kohno Y, Sata M, et al. Uni-axial stretching regulates intracellular localization of Hic-5 expressed in smooth-muscle cells in vivo. J Cell Sci. 2005;118:937–49. doi: 10.1242/jcs.01683. [DOI] [PubMed] [Google Scholar]
- 16.Guignandon A, Boutahar N, Rattner A, Vico L, Lafage-Proust MH. Cyclic strain promotes shuttling of PYK2/Hic-5 complex from focal contacts in osteoblast-like cells. Biochem Biophys Res Commun. 2006;343:407–14. doi: 10.1016/j.bbrc.2006.02.162. [DOI] [PubMed] [Google Scholar]
- 17.Sharman AM, Kirmi O, Anslow P. Imaging of the skin, subcutis, and galea aponeurotica. Semin Ultrasound CT MR. 2009;30:452–64. doi: 10.1053/j.sult.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Seery GE. Surgical anatomy of the scalp. Dermatol Surg. 2002;28:581–7. doi: 10.1046/j.1524-4725.2002.12015.x. [DOI] [PubMed] [Google Scholar]
- 19.Kushima H, Matsuo K, Yuzuriha S, Kitazawa T, Moriizumi T. The occipitofrontalis muscle is composed of two physiologically and anatomically different muscles separately affecting the positions of the eyebrow and hairline. Br J Plast Surg. 2005;58:681–7. doi: 10.1016/j.bjps.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Vergari C, Pourcelot P, Holden L, Ravary-Plumioën B, Gerard G, Laugier P, et al. True stress and Poisson's ratio of tendons during loading. J Biomech. 2011;44:719–24. doi: 10.1016/j.jbiomech.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 21.Krupa Shankar D, Chakravarthi M, Shilpakar R. Male androgenetic alopecia: Population-based study in 1,005 subjects. Int J Trichology. 2009;1:131–3. doi: 10.4103/0974-7753.58556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cousen P, Messenger A. Female pattern hair loss in complete androgen insensitivity syndrome. Br J Dermatol. 2010;162:1135–7. doi: 10.1111/j.1365-2133.2010.09661.x. [DOI] [PubMed] [Google Scholar]
- 23.Pritchard DW, Wood MM. EMG levels in the occipitofrontalis muscles under an experimental stress condition. Biofeedback Self Regul. 1983;8:165–75. doi: 10.1007/BF01000546. [DOI] [PubMed] [Google Scholar]
- 24.Bérzin F. Occipitofrontalis muscle: Functional analysis revealed by electromyography. Electromyogr Clin Neurophysiol. 1989;29:355–8. [PubMed] [Google Scholar]
- 25.Standerwick RG, Roberts WE. The aponeurotic tension model of craniofacial growth in man. Open Dent J. 2009;3:100–13. doi: 10.2174/1874210600903010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liegibel UM, Sommer U, Tomakidi P, Hilscher U, Van Den Heuvel L, Pirzer R, et al. Concerted action of androgens and mechanical strain shifts bone metabolism from high turnover into an osteoanabolic mode. J Exp Med. 2002;196:1387–92. doi: 10.1084/jem.20021017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dabiri G, Tumbarello DA, Turner CE, Van de Water L. Hic-5 promotes the hypertrophic scar myofibroblast phenotype by regulating the TGF-beta1 autocrine loop. J Invest Dermatol. 2008;128:2518–25. doi: 10.1038/jid.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inui S, Itami S. Androgen receptor transactivity is potentiated by TGF-ß1 through Smad3 but checked by its coactivator Hic-5/ARA55 in balding dermal papilla cells. J Dermatol Sci. 2011;64:149–51. doi: 10.1016/j.jdermsci.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Faydaci G, Bilal E, Necmettin P, Fatih T, Asuman O, Ugur K. Baldness, benign prostate hyperplasia, prostate cancer and androgen levels. Aging Male. 2008;11:189–92. doi: 10.1080/13685530802400995. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Yang CC, Chen GY, Wu MC, Sheu HM, Tzai TS. Patients with a large prostate show a higher prevalence of androgenetic alopecia. Arch Dermatol Res. 2004;296:245–9. doi: 10.1007/s00403-004-0514-z. [DOI] [PubMed] [Google Scholar]
- 31.Hegarty P, Watson RW, Hegarty NJ, Coffey RN, Fitzpatrick JM. Pressure effects on cellular systems: Is there a link with benign prostatic hyperplasia? Urology. 2004;64:195–200. doi: 10.1016/j.urology.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 32.Nordström RE. Synchronous balding of scalp and hair-bearing grafts of scalp transplanted to the skin of the arm in male pattern baldness. Acta Derm Venereol. 1979;59:266–8. [PubMed] [Google Scholar]
- 33.Freund BJ, Schwartz M. Treatment of male pattern baldness with botulinum toxin: A pilot study. Plast Reconstr Surg. 2010;126:246e–8e. doi: 10.1097/PRS.0b013e3181ef816d. [DOI] [PubMed] [Google Scholar]
- 34.Messenger AG, Rundegren J. Minoxidil: Mechanisms of action on hair growth. Br J Dermatol. 2004;150:186–94. doi: 10.1111/j.1365-2133.2004.05785.x. [DOI] [PubMed] [Google Scholar]