Abstract
The oocyte is the sole source of the female genetic material that will be fertilized by sperm to form an embryo. Many extrinsic and intrinsic factors are critical for oocyte development and survival; however, these mediators are incompletely understood. In this issue of the JCI, Weinberg-Shukron et al. uncover a novel recessive missense mutation in the gene encoding nucleoporin-107 (NUP107) that results in abnormal ovarian development. Recapitulation of the human mutation in the Drosophila NUP107 ortholog resulted in poor follicular development and demonstrated an evolutionarily conserved and ovary-specific role of NUP107. While NUP107 is required for nuclear pore complex function in somatic cells of flies and women, this specific amino acid change appears only to be disruptive in the ovary. All together, these findings imply that missense mutations in other genes could be specifically disruptive of ovarian or testicular function, while leaving extragonadal function intact.
Oocyte development and function
Although there are morphological differences between the ovaries of invertebrates and vertebrates, in all egg-producing animals, the ovary is critical for propagation of the species and is responsible for nourishing and maturing the female genetic material contained within the egg (or oocyte). In Drosophila, the oocyte shares the cytoplasm with 15 nurse cells due to incomplete cytokinesis (1). Together, these cells form an interconnected 16-cell cluster that is surrounded by a layer of somatic follicle cells. Both nurse cells and follicle cells serve as supporting cells for the oocyte and produce key nutrients and factors for oocyte development (Figure 1). In women, cognate supporting cells, called granulosa cells, provide the nutrient system for the mammalian gamete (Figure 1). Whereas communication between the supporting cells and the fly oocyte is mostly unidirectional (2), communication between the mammalian oocyte and granulosa cells involves bidirectional paracrine signaling pathways that control oocyte destiny (3–6). These pathways include KIT ligand signaling from the granulosa cells to the oocyte (7) and secretion of growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) from the oocyte, which engage a receptor system on the surrounding granulosa cells (8). The effects of disrupting these pathways have been explored in detail in mouse models and flies; however, few mutations that cause intrinsic defects in the ovary have been uncovered in women with infertility.
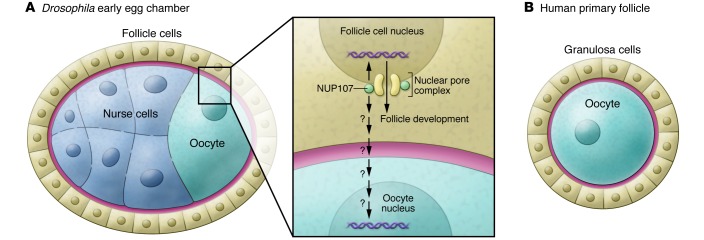
Figure 1. Schematic drawing of a Drosophila early egg chamber and a human primary follicle at equivalent stages of oogenesis.
(A) A Drosophila early egg chamber. The inset illustrates that NUP107 plays a critical cell-autonomous role in regulating the Drosophila follicle cell development. (B) A human primary follicle. NUP107 might play a role in follicle-cell communication with the oocyte as well as a cell-autonomous role in oocyte development. Cell types are indicated in the figure.
In this issue, Weinberg-Shukron and colleagues (9) identify and characterize four related patients with XX female gonadal dysgenesis. All of these women had the normal complement of X chromosomes but displayed small or streak ovaries. This ovarian phenotype resulted in reduced levels of estrogen and a consequential loss of negative feedback on the synthesis of pituitary gonadotropins, such as follicle-stimulating hormone and luteinizing hormone. Women with hypergonadotropic hypogonadism fail to have menstrual cycles or initiate puberty and have other defects in secondary sex characteristics (10, 11). Excitingly, Weinberg-Shukron et al. (9) identified a recessive mutation in nucleoporin-107 (NUP107) as the underling cause of XX gonadal dysgenesis in all four of the female family members they described. This lone point mutation (c.1339G>A) results in a single amino acid change in an evolutionarily conserved amino acid (p.D447N) of NUP107. Based on structural models, this single mutation was predicted to be detrimental, and Weinberg-Shukron et al. speculated that this D447N change would impair NUP107 interactions in the nuclear pore only in the ovary.
NUP107 conserved between fly and human oogenesis
Weinberg-Shukron et al. (9) used Drosophila as a model to predict the potential function of NUP107 in human female gonadal dysgenesis. RNAi-mediated knockdown of NUP107 in Drosophila somatic gonadal cells led to female sterility due to defective oogenesis, including decreased egg production. While NUP107 knockdown resulted in a dramatic female phenotype, NUP107-deficient male flies continued to be fertile. Introduction of an RFP-NUP107 transgene in NUP107-null flies rescued the lethal phenotype and fertility of female flies: however, introduction of an RFP-NUP107D364N transgene, which recapitulates the mutation identified in the patients with XX female gonadal dysgenesis, rescued the developmental phenotype, but these flies had reduced female fertility, abnormal egg production, decreased egg quality, and increased apoptosis. Despite the vast evolutionary distance and morphological difference between Drosophila and humans, some of the most fundamental aspects of gonadogenesis and gametogenesis are well conserved between the two. For example, meiosis is conserved, including arrest at prophase I during oogenesis and resumption during oocyte maturation. Moreover, the codevelopment of the oocyte and the somatic follicle lineage that eventually form a multicellular complex called an egg chamber in Drosophila and the follicle in humans is also preserved. This multicellular complex of supporting somatic cells, known as follicle cells in Drosophila and granulosa cells in humans, surrounds each oocyte and plays an important role in nourishing the oocyte and regulating its development. As expected, the conservation of these oogenic processes likely reflects common underlying molecular mechanisms that are shared between Drosophila and humans. For example, the Drosophila diaphanous (dia) gene is involved in cytokinesis and other morphogenetic processes that are mediated by actin during gametogenesis, and mutations in a human homolog of dia correlate to premature ovarian failure (12).
The study by Weinberg-Shukron et al. (9) provides yet another clear example of an oogenic mechanism that is conserved between humans and Drosophila and supports a role for the NUP107 mutation in the development of human XX gonadal dysgenesis. In particular, these results illustrate the importance of NUP107 function in Drosophila follicle cells during oogenesis, a finding that predicts a possible function of NUP107 in human granulosa cells that is related to the XX gonadal dysgenesis. In flies expressing the orthologous XX gonadal digenesis–associated NUP107 variant, the organization and morphology of follicle cells in the Drosophila egg chamber as well as their derivative structure in the mature egg, the eggshell, are both severely defective. In addition, the Drosophila experiments predict an additional possible function of NUP107 in human granulosa cells in controlling oocyte development, as specific knockdown of NUP107 expression in Drosophila follicle cells causes defects in nurse cells and oocytes, both of which are germline cells.
Outstanding questions and future directions
While the work of Weinberg-Shukron et al. shows a remarkable phenotype when NUP107 is disrupted in the follicle cells, it will be important for future experiments to assess whether NUP107 has cell-autonomous functions in the germline. Such analysis could be easily achieved by specific knockdown of Nup107 in the germline with a germline-specific driver, such as nanos-Gal4, to induce expression of a UAS-nup107-RNAi transgene. An even more informative experiment would monitor the development of a germline-specific Nup107 knockout and assess its affect on oogenesis. Complete, germline-specific deletion can be readily achieved by applying FRT-FLP–mediated recombination techniques to generate the null nup107EB allele in germ cells. While systematic comparison between the global knockout phenotype and the soma-specific knockdown phenotype might have allowed indirect inference on the germline-autonomous function of NUP107, Weinberg-Shukron and colleagues did not make this comparison, likely due to the complex and variable defects of the nup107EB mutant and the somatic nup107-RNAi–treated flies, compounded with the incomplete knockout of the NUP107 protein in follicle cells. Despite the lack of an analysis of germline autonomy, the conserved function of Drosophila and human NUP107 during oogenesis provides an exciting opportunity for exploring mechanisms that relate to the nuclear pore complexes and are responsible for oogenesis in humans. In particular, the specific defects in oogenesis associated with NUP107 mutants in the female reproductive system in both humans and Drosophila is quite surprising, given that this protein and the nuclear pore complex are generally known for their housekeeping function in every nucleated cell.
Indeed, the nuclear pore complex has been implicated in other tissue-specific functions. For example, human NUP155 mutations correlated with familial atrial fibrillation and early sudden cardiac death (13). A human NUP62 mutation that causes infantile striatonigral necrosis has also been identified (14). In Drosophila, a structural nucleoporin of the NUP107-160 complex, SEH1, is specifically required in germ cells during oogenesis but is dispensable for the development of somatic tissues (15). Despite the unexpected findings of Weinberg-Shukron et al., the molecular mechanisms responsible for the tissue-specific functions of the nuclear pore complex remain elusive. The 3D homology modeling of the human NUP107 with the yeast ortholog NUP84 by Weinberg-Shukron et al. (9) suggests that the D447N mutation likely disrupts the overall 3D structure of NUP107 and thus its interaction with other components of nuclear pore complexes. The oogenesis-specific function of NUP107 could reflect a more stringent dose requirement of a housekeeping gene during gametogenesis, as often seen among Drosophila female sterile mutations. Alternatively, these results could reflect an oocyte-specific function of NUP107, such as an interaction with oogenesis-specific proteins, as suggested by Weinberg-Shukron and colleagues (9). In any case, the oogenic defects of the D447N-equivalent mutation in the Drosophila NUP107 homolog provide an effective entry point for using this powerful genetic model to systematically investigate the molecular mechanisms mediated by NUP107 and other components of the nuclear pore complex that are accountable for oogenesis-specific functions. The new mechanisms and principles that have emerged from the Drosophila studies by Weinberg-Shukron and colleagues can further be efficiently tested and validated in mammalian models. Such results might also shed light on how NUP107 and the nuclear pore complex achieve specific functions in other tissues.
Acknowledgments
Reproductive biology research in our laboratories has been supported by NIH grants DP1-CA174418 and R37HD42012 (to H. Lin) and HD032067, HD033438, HD007495, and HD076508 (to M.M. Matzuk).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2015;125(11):4005–4007. doi:10.1172/JCI84692.
See the related article beginning on page 4295.
References
- 1.Deng W, Lin H. Asymmetric germ cell division and oocyte determination during Drosophila oogenesis. Int Rev Cytol. 2001;203:93–138. doi: 10.1016/s0074-7696(01)03005-4. [DOI] [PubMed] [Google Scholar]
- 2. Spradling AC. Developmental genetis of oogenesis. In: Bate M, Martinez-Arias A, eds. The development of Drosophila melanogaster. Cold Spring Harbor, New York, USA: Cold Spring Harbor Press; 1993:1–70. [Google Scholar]
- 3.Matzuk MM, Burns K, Viveiros MM, Eppig J. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296(5576):2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- 4.Eppig JJ, Wigglesworth K, Pendola FL. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci U S A. 2002;99(5):2890–2894. doi: 10.1073/pnas.052658699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wigglesworth K, Lee KB, O’Brien MJ, Peng J, Matzuk MM, Eppig JJ. Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes. Proc Natl Acad Sci U S A. 2013;110(39):E3723–E3729. doi: 10.1073/pnas.1314829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peng J, Eppig JJ, Matzuk MM. Bi-directional communication between the oocyte and surrounding somatic cells is required for successful follicular development and oocyte maturation. In: Ten critical topics in reproductive medicine. Washington, DC, USA: Science/AAAS Custom Publishing; 2013:13–15. [Google Scholar]
- 7.Zhang H, et al. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol. 2014;24(21):2501–2508. doi: 10.1016/j.cub.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Peng J, et al. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci U S A. 2013;110(8):E776–E785. doi: 10.1073/pnas.1218020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinberg-Shukron A, et al. A mutation in the nucleoporin-107 gene causes XX gonadal dysgenesis. J Clin Invest. 2015;125(11):4295–4304. doi: 10.1172/JCI83553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy A, Matzuk MM. Reproductive tract function and dysfunction in women. Nat Rev Endocrinol. 2011;7(9):517–525. doi: 10.1038/nrendo.2011.79. [DOI] [PubMed] [Google Scholar]
- 11.Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30(6):624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bione S, et al. A human homologue of the Drosophila melanogaster diaphanous gene is disrupted in a patient with premature ovarian failure: evidence for conserved function in oogenesis and implications for human sterility. Am J Hum Genet. 1998;62(3):533–541. doi: 10.1086/301761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, et al. Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell. 2008;135(6):1017–1027. doi: 10.1016/j.cell.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Basel-Vanagaite L, et al. Mutated nup62 causes autosomal recessive infantile bilateral striatal necrosis. Ann Neurol. 2006;60(2):214–222. doi: 10.1002/ana.20902. [DOI] [PubMed] [Google Scholar]
- 15.Senger S, Csokmay J, Akbar T, Jones TI, Sengupta P, Lilly MA. The nucleoporin Seh1 forms a complex with Mio and serves an essential tissue-specific function in Drosophila oogenesis. Development. 2011;138(10):2133–2142. doi: 10.1242/dev.057372. [DOI] [PMC free article] [PubMed] [Google Scholar]