Abstract
Lung allografts are prone to rejection, even though recipients undergo aggressive immunosuppressive therapy. Lymphatic vessels serve as conduits for immune cell trafficking and have been implicated in the mediation of allograft rejection. In this issue of the JCI, Cui et al. provide compelling evidence that lymphatic vessel formation improves lung allograft survival in a murine transplant model. Moreover, their data suggest a potential mechanism for the beneficial effects of lymphatics that does not involve immune cell or antigen transport. Together, the results of this study provide new insight into the role of lymphatic vessels in transplant tolerance.
Lymphatic vessels: protective or detrimental for transplants?
Lymphatic vessels play a central role in adaptive immune responses by providing a highway for immune cells and foreign antigens to move from tissues to secondary lymphoid organs, where primary immune responses are initiated (1). In this classic model, lymphatics are positive immune regulators required for initiation of a primary immune response. Thus, the predicted role of lymphatic vessels in the transplanted organ would be to facilitate immune rejection. Indeed, this prediction is supported by studies of corneal transplants, which show that inhibition of lymphangiogenesis improves graft survival (2). In this issue, Cui et al. test the role of lymphatic vessels in the transplanted mouse lung and find the opposite to be true: lymphatic vessels protect the transplanted lung from rejection (3). This provocative study by Cui and colleagues should stimulate a reevaluation of the role of lymphatics in the immune rejection of transplanted organs as well as the mechanism underlying this role.
Cui et al. used an elegant, and technically formidable, mouse model to examine the role of lymphatics during allogeneic lung transplantation (3). Murine lung transplantation was performed in a manner similar to that used in humans, such that the transplanted lung was attached by connecting the main airways and blood vessels but not the draining lymphatics. Prior studies in animal models have suggested that at least a week is required to reestablish lymphatic drainage of the transplanted lung; however, the mechanism and vascular architecture of these donor-recipient lymphatic connections are unknown (4). Cui et al. compared lung transplants between isogeneic (genetically matched) and allogeneic (genetically mismatched) mice and revealed a reduction in lymphatic vessel density in the rejected allografts compared with the healthy isografts 30 days after transplantation. The authors systemically administered VEGF-C, a potent lymphangiogenic factor, to test whether increasing lymphatics could reduce allograft rejection. VEGF-C treatment not only successfully increased lymphatic vessel density, but also reduced inflammation and improved lung function in the allografted lung. Why would increased lymphatic function slow rather than accelerate lung rejection? Cui and colleagues propose a novel mechanism that is unconnected to the classic role of lymphatics in the cellular immune response. Lymphatic endothelial cells (LECs) express high levels of LYVE-1, a receptor for hyaluronic acid (HA). Previous studies have linked high levels of HA to severe, chronic forms of lung rejection such as bronchiolitis obliterans (5), but whether HA is a cause or merely a marker of worsening lung inflammation has not been clear. Cui et al. demonstrated that blocking the interaction of LYVE-1 and HA with anti-LYVE–specific antibodies reversed the VEGF-C–mediated improvement in allograft rejection, despite an increase in the density of lymphatic vessels (Figure 1). Furthermore, increased HA levels correlated with human lung transplant rejection, suggesting that these may be translatable findings.
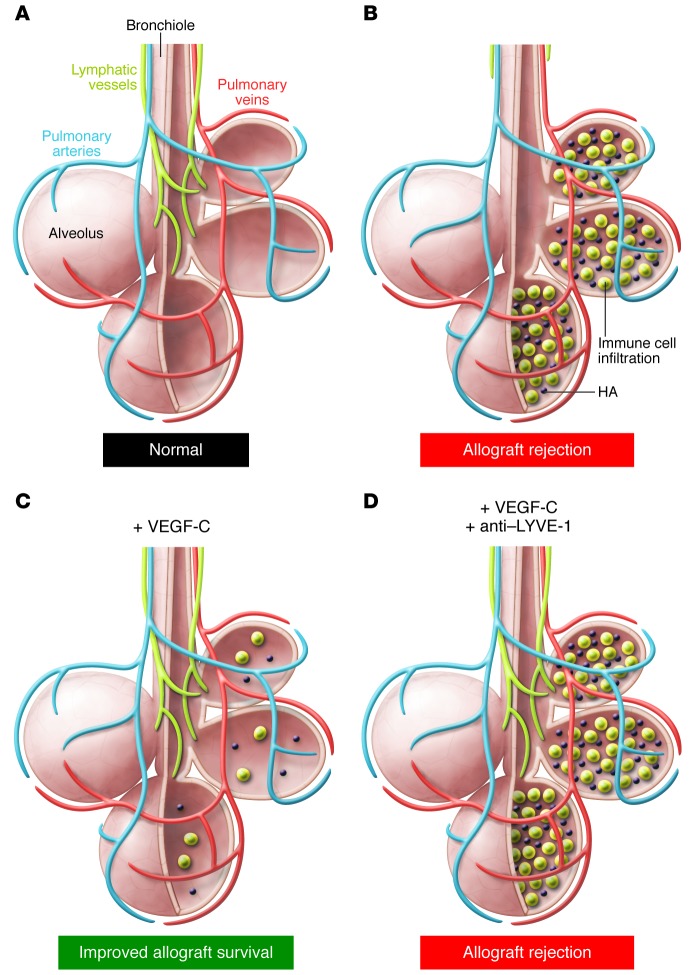
Figure 1. Lymphatic vessels improve lung allograft survival and function.
(A) Normal lung parenchyma showing air-filled alveoli, blood vessels, and lymphatic vessels that reach the alveoli along the airway. (B) During lung transplantation, the major airways and blood vessels are preserved, but lymphatic vessel density is reduced. In this issue, Cui et al. (3) demonstrate that in rejecting murine lung allografts, inflammatory cells infiltrate the alveoli and HA levels increase. (C) Animals given systemic VEGF-C, which promotes lymphangiogenesis, exhibit increased lymphatic vessel density, reduced HA accumulation, and improved allograft survival. (D) In VEGF-C–treated animals, inhibition of the interaction between HA and LYVE-1 reverses the beneficial effects of VEGF-C treatment, despite the increase in lymphatic vessel density.
As formation of lymphatic vessels has been thought to facilitate graft rejection, the finding that lymphatic function may tip the balance toward increased protection and away from acute rejection and damage after lung transplantation is unexpected. Perhaps it is not surprising that lymphatic vascularization of an avascular organ such as the cornea would increase immune responsiveness; however, testing the role of lymphatic function in larger organ transplantation has been more challenging. Almost a half century ago, classic studies addressed this question using a guinea pig skin transplant model in which the recipient skin was physically separated from the body except for a vascular pedicle that maintained blood but not lymphatic flow (6). In contrast to the present report by Cui et al., the studies in the skin transplant model strongly demonstrated that lymphatics contribute to the establishment of tissue rejection. Why these different sets of transplantation experiments have yielded such distinct results is not yet clear, but timing may be a critical factor in the discrepancy between studies. Cui et al. examined events after 20 days, a time point at which lymphatic drainage is likely reestablished and chronic inflammatory events drive rejection. It is conceivable that disconnecting the draining lymphatics during lung transplantation initially protects the transplanted organ from early immune responses that initiate graft rejection. Alternatively, it is possible that the protective effect observed at this later time point could be even more powerful if lymphatic drainage was established earlier, such as through microsurgical techniques at the time of transplantation to reconnect collecting lymphatic vessels. A second explanation may be that acute rejection of transplanted lungs, unlike skin or hearts, can be initiated in situ and not require coordination by secondary lymphoid organs (7). Future studies in both animal models and human transplantation are needed to better understand the early and late roles of lymphatic function in organ rejection in general, and in the lung in particular.
Prevention of rejection through an unanticipated lymphatic function
A second unexpected finding in the study by Cui et al. is that the mechanism of protection of the transplanted lung by lymphatics may have nothing to do with the transport of antigens and immune cells, but instead may be tied to HA removal (3). The contribution of HA in organ rejection and inflammation is murky. HA is an abundant matrix glycosaminoglycan of very high molecular weight that contributes to physical properties, such as the sponginess of joints, and is important for tissue morphogenesis (8). HA synthesis and breakdown are prominent in inflammatory states, and HA has been proposed to stimulate innate immune receptors, such as the TLRs (9). Cui and colleagues propose a model in which lymphatics protect the transplanted lung by using LYVE-1 receptors to take up and remove proinflammatory HA; however, additional genetic and mechanistic insights are needed to test this provocative hypothesis. What are the HA target cells and receptors in the transplanted lung? There are other inflammatory mediators that might be removed by lymphatic drainage and alternative receptors, such as CD44, that may transport HA. In addition, LYVE-1–deficient mice are healthy and fertile (10), suggesting that lymphatic LYVE-1 would have to serve to transport HA exclusively in nonphysiologic inflammatory states. Challenging LYVE-1–deficient animals, either with pulmonary inflammation or transplantation of LYVE-1–deficient donor lungs, along with complementary studies to reduce HA synthesis or proteolysis, is needed to more thoroughly evaluate the contribution of LYVE-1–dependent HA transport in protecting lung allografts from acute rejection.
Conclusions and future directions
Do these studies identify new approaches for treating human lung transplant patients? It is noteworthy that Cui and colleagues were able to functionally evaluate the effect of increasing lymphatic density by systemically injecting murine transplant recipients with VEGF-C, an approach that might not have been predicted to impact distant lymphatic function in the lung. These experiments show that VEGF-C functions, at least in part, by preventing LEC apoptosis, a mechanism that explains the ability of this lymphangiogenic factor to affect distant lymphatics following systemic delivery. Previous studies aimed at modeling VEGF-C therapies have been primarily directed at the growth of new lymphatics, such as after lymph node resection that is performed for the treatment of breast cancer (11). In this context, VEGF-C treatment has not been wildly successful, most likely because lymphatic vessel growth is regulated by complex molecular mechanisms that control VEGF-C localization and activity as well as by other molecular pathways required to build new lymphatic vessels (12). The use of systemic VEGF-C therapy to simply prevent LEC apoptosis may be a more practical therapeutic strategy for maintaining lymphatic function. Nevertheless, differences between the mouse model used by Cui et al. and human lung transplants need to be more fully addressed to assess the potential translational impact of these studies. Human lung allografts with acute rejection exhibit an increased, not decreased, density of lymphatic vessels at 14 days (13). Additionally, an increase in lymphoid tissue has been seen in bronchiolitis obliterans syndrome, the chronic form of lung rejection (14). Indeed, Cui and colleagues did not observe a difference in lymphatic vessel density in human lung allografts treated for acute rejection, despite observing a decrease in the amount of HA. Further studies that test whether loss of lymphatics is as prominent in human transplantation as in the mouse model are needed to assess the potential therapeutic role for VEGF-C. Together, the studies presented by Cui et al. provide a new perspective on this important clinical problem and may yield unprecedented ideas regarding the pathogenesis and treatment of rejection in the transplanted lung.
Acknowledgments
J.S. Maltzman is supported by NIH grants R01-AI085160 and R56-AI108786. M.L. Kahn is supported by NIH grants HL111553 and HL120872.
Footnotes
Conflict of interest: Jonathan S. Maltzman has a family member who is employed by and has an equity interest in Gilead.
Reference information:J Clin Invest. 2015;125(11):3999–4001. doi:10.1172/JCI84549.
See the related article beginning on page 4255.
References
- 1.Card CM, Yu SS, Swartz MA. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J Clin Invest. 2014;124(3):943–952. doi: 10.1172/JCI73316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dietrich T, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010;184(2):535–539. doi: 10.4049/jimmunol.0903180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui Y, et al. Therapeutic lymphangiogenesis ameliorates established acute lung allograft rejection. J Clin Invest. 2015;125(11):4255–4268. doi: 10.1172/JCI79693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruggiero R, et al. Detection of canine allograft lung rejection by pulmonary lymphoscintigraphy. J Thorac Cardiovasc Surg. 1994;108(2):253–258. [PubMed] [Google Scholar]
- 5.Todd JL, et al. Hyaluronan contributes to bronchiolitis obliterans syndrome and stimulates lung allograft rejection through activation of innate immunity. Am J Respir Crit Care Med. 2014;189(5):556–566. doi: 10.1164/rccm.201308-1481OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker CF, Billingham RE. The role of afferent lymphatics in the rejection of skin homografts. J Exp Med. 1968;128(1):197–221. doi: 10.1084/jem.128.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelman AE, et al. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol. 2009;182(7):3969–3973. doi: 10.4049/jimmunol.0803514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toole BP. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001;12(2):79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 9.Jiang D, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11(11):1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 10.Gale NW, et al. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol. 2007;27(2):595–604. doi: 10.1128/MCB.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tammela T, et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med. 2007;13(12):1458–1466. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- 12.Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest. 2014;124(3):878–887. doi: 10.1172/JCI71603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dashkevich A, et al. Lymph angiogenesis after lung transplantation and relation to acute organ rejection in humans. Ann Thorac Surg. 2010;90(2):406–411. doi: 10.1016/j.athoracsur.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Sato M, et al. The role of intrapulmonary de novo lymphoid tissue in obliterative bronchiolitis after lung transplantation. J Immunol. 2009;182(11):7307–7316. doi: 10.4049/jimmunol.0803606. [DOI] [PubMed] [Google Scholar]