Abstract
The elderly population is a large and the fastest-growing portion of the population worldwide. The elderly make up the lion's share of patients for certain health conditions including cancer, cardiovascular disease, arthritis, and Parkinson's disease, among others in most parts of the world. Furthermore, elderly make up the majority of patients for many medications treating chronic conditions. Typically, clinical trials conducted in adult population include patients between the ages of 18 and 64 years. However, drugs should be studied in all age groups and trial participants should be representative of the patient population receiving the therapy in daily medical practice. Elderly patients are poorly represented in clinical trials. Hence, there is inadequate evidence and knowledge about responses of geriatric patients to medications. Regulatory authorities in developed countries urge to avoid arbitrary upper age limits and advise researchers and industry not to exclude elderly people from clinical trials without a valid reason. Since last few years Indian regulatory authority has been stipulating upper age limit for studies conducted in India. The Central Drugs Standard Control Organization (CDSCO) will be doing a great contribution to the researchers if it changes its view on stipulating upper age restrictions in clinical studies. This article describes the need for including elderly patients in the clinical trials in order to garner data from geriatric patients who form major medication users in most of the chronic diseases.
Keywords: Clinical trial, drug regulations, elderly, India
INTRODUCTION
Major medical breakthroughs could not happen without the generosity of clinical trial participants young and old. It is important for clinical trials to have participants of different age, sex, race, and ethnicity. When research involves a group of people who are similar, the findings may not apply to or benefit everyone. When clinical trials include diverse participants, the study results may have a much wider applicability.
Some patient groups in the general population may need special studies because they have distinctive risk-benefit considerations that need to be considered during drug development. The elderly are the majority users of many medicines. Although persons aged ≥65 years represent only about 13% of the population, they consume nearly one-third of all medications.[1] Hence, the evaluation of new drugs in the elderly is a major issue. Some clinical efficacy and safety data in elderly will be required by regulators for registration unless a medication is unlikely to be used in elderly.
Geriatric patients can respond differently from younger patients to drug therapy. Age-related physiological changes can affect pharmacokinetics and pharmacodynamics of the drug. Geriatric patients are more prone to adverse effects due to comorbidities and concomitant drugs. The adverse effects can be severe, or less tolerated, and have serious consequences than the younger population. Not all pharmacotherapeutic outcomes that can occur in the geriatric population can be predicted from nongeriatric populations. Therefore, to assess the benefits and risks of a drug that will be used in elderly, these patients should be appropriately represented in clinical trials. Regulatory authorities have recommended the determination of pharmacokinetics in the elderly from a larger group of the representative target population in the efficacy and safety clinical trials.[2] If elderly patients are included in efficacy-safety studies, the population approach can be used to explore the pharmacokinetic variability and associated altered clinical outcome, if any.
Drugs should be studied in all age groups. Poor representation of elderly in clinical trials leads to inadequate evidence and knowledge regarding drug therapy in elderly. Since last few years Indian regulatory authority has been stipulating upper age limit for studies conducted in India. This article describes the need for including elderly patients in the clinical trials in order to garner data from geriatric patients who form major medication users in most of the chronic diseases.
ELDERLY POPULATION AND CLASSIFICATION
The elderly population is the fastest-growing portion of the population in advanced nations. Globally, the elderly population (aged ≥60 years) is expected to increase to more than 2 billion in 2050.[3] The present population aged ≥65 years in India accounts to 5.3% of the total population. By the year 2025, it is expected that 7.2% of Indian population will contain people aged ≥65 years.[4]
Gerontologists have recognized very different conditions that people experience as they grow older. In developed countries, most people in their 60s and early 70s are still fit, active, and able to care for themselves. However, after 75, they will become increasingly frail, a condition marked by serious mental and physical debilitation. Gerontologists have defined elderly sub-groups as young-old (65–74 years), middle-old (75–84 years), and very old (≥85 years).[5]
The cut-off age for elderly in India is 60 years. Low life expectancy of the Indians compared to developed countries, the normal age for retirement, age boundary for senior citizenship, and literature reports support 60 years as the cut-off age for the elderly in India.[6] However, increasing the life expectancy of Indian population may point toward upsurge in the cut-off age for the elderly in India in the future.[4]
AGING AND PHARMACOLOGY
Aging has a significant effect on the responses to pharmacological interventions. Age-related physiological and pathological changes play a major role in altering pharmacological actions of drugs. Age-related changes in hepatic and renal functions significantly affect the absorption, distribution, metabolism, and excretion of the drugs. Age-related changes in bioavailability may be secondary to changes in absorption or gut wall and hepatic metabolism. Gastric acid secretion decreases with aging.[7] Aging is associated with slowing of gastric emptying, decreased peristalsis, and slowing of colonic transit which influence the Tmax and Cmax than the area under the curve. The active transport of some nutrients is impaired with aging. Gastrointestinal blood flow is probably diminished in the elderly and affects the absorption of drugs.[7] Decrease in albumin with aging increases the unbound concentrations of many drugs.[8] Due to aging-related possible increase in α1-acid glycoprotein, concentration of some unbound drugs like lignocaine decreases.[9] Age-related changes such as an increase in body fat and a decrease in body water may influence volumes of distribution of drugs. Changes in body composition lead to an increased concentration of water-soluble drugs and a prolonged elimination of lipid soluble drugs.[7]
Both Phase I and Phase II of drug metabolism in liver convert drugs into more water soluble molecules to ease the elimination. Phase I metabolism in the liver is affected in the elderly. Hence, decreased the clearance of drugs that undergo Phase I metabolism is expected. Metabolism could also be impaired due to a reduction in liver size and blood flow with aging. Glomerular filtration rate declines with age. Hence, age-dependent decrease in total clearance is expected for drugs that are eliminated by kidneys. The use of standard doses of these drugs may result in increased plasma concentration and increased risk of adverse drug reactions in elderly. The efficacy of renally eliminated drugs is usually increased in states of reduced renal functions.
The relationship between aging and pharmacodynamic effects of drugs are less well-established. Age-related up-regulation and down-regulation of pharmacological receptors are probable. Pharmacodynamic changes can vary among drugs, including the drugs from the same class. In elderly, the central nervous system shows an increased sensitivity to antipsychotic drugs due to the age-related increase in monoamine oxidase activity. Aging brain loses a significant number of active cells and some degree of brain atrophy is common. There is also a reduction in cerebral blood flow and a selective decline in some nerve pathways. The elderly patients are more sensitive to drugs that have anticholinergic effects due to age-related loss of cholinergic neurons and exacerbation of cholinergic deficit by these drugs. The elderly patients’ exhibit increased sensitivity to coumarin anticoagulants due to age-related decline in the hepatic synthesis of vitamin K-dependent clotting factors.
ELDERLY AS CLINICAL STUDY SUBJECTS
Typically, clinical trials include patients aged between 18 and 65 years. However, the study population should ideally reflect the population that will be treated in the real world, particularly if it's studying a drug that will be used by elderly patients. It is just and apt that elderly patients are adequately represented in clinical trials for treatments for the above-listed conditions. There are several benefits of elderly patients’ participation in clinical trials [Table 1].
Table 1.
Benefits of elderly patients’ participation in clinical studies
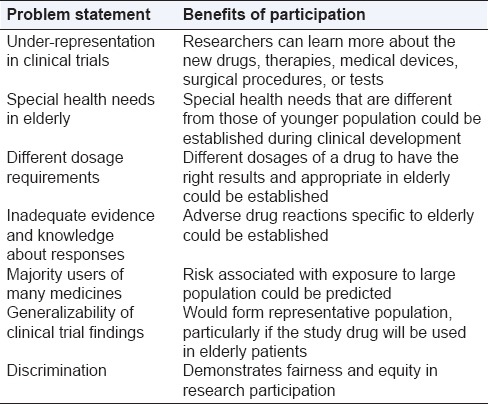
There is inadequate evidence and knowledge about responses of geriatric patients to medications. The older patient population is poorly represented in clinical trials, with up to 35% of published trials excluding older people. In a recent commentary, it was contended that the crisis of aging must be addressed by the development of broad expertise and research into geriatric pharmacology. It has been found that the elderly are underrepresented in cancer clinical trials, more pronounced in trials for early-stage cancers than in trials for late-stage cancers.[10]
In USA, though the elderly aged ≥65 years account for 61% of all new cancer cases and 70% of all cancer deaths, in the clinical trials active between 1993 and 1996, the elderly comprised only 25% of oncology trial participants.[11] A study audited 226 clinical research proposals recording exclusion of patients based on an arbitrary upper age limit and found that significant proportion (13.7%) of clinical trials excluded patients based arbitrarily on an upper age limit.[12] However, none (9.8%) of the trials submitted by geriatricians excluded patients based solely on age. The mean upper age limit used over all trials as a cut-off was 69.2 years. Over 50% trials submitted by neurology/psychiatry excluded patients based on an upper age limit.[12]
Although elderly patients represent the majority of the heart failure (HF) population, and have a worse prognosis compared to younger cohort commonly included in trials, targeted treatment strategies have been insufficiently developed for them. The elderly HF phenotype is characterized by an increased prevalence of HFpEF, with a greater burden of cardiac and noncardiac co-morbidities. The present knowledge is limited by the enrollment of patients with HF with reduced EF in most trials, with the exclusion of those with increased frailty.[13]
People are living longer and expect that treatments will cure and improve their quality of life. Hence, there is a clear need for evidence-based pharmacotherapy of elderly patients. To deny them this opportunity runs counter to the precepts of medical practice and could even be considered unethical. In a survey of nine EU countries, the majority (87%) of the medical professionals believed that excluding people on age grounds alone was unjustified and over 70% agreed that not having enough older people in clinical trials resulted in difficulties for older patients.[14]
REGULATORY VIEW ON ELDERLY IN CLINICAL STUDIES
The scientific, ethical, and regulatory principles that determine the conduct of clinical trials in younger individuals apply equally to older people. In addition, the development of drugs to be used in elderly requires an awareness of physiological, pathophysiological, and sociological considerations. This has inevitably meant that the drug development process must increasingly recognize the importance of identifying and developing therapeutic targets relevant to elderly. However, there are several reasons why elderly are underrepresented in clinical trials [Table 2].
Table 2.
Reasons for under-representation of elderly patients in clinical studies

The International Conference on Harmonization (ICH) of technical requirements for registration of pharmaceuticals for human use (ICH) called for including elderly in clinical trials for all therapies intended for adults. “Studies in Support of Special Populations: Geriatrics,” issued by ICH in 1994 urged clinical trial protocols to avoid arbitrary upper age limits. It also recommended not excluding elderly from trials even if they have other ailments, since doing so prevents uncovering interactions of multiple drugs or diseases.[15]
Federal laws require that cancer trials enroll representative samples of women and members of a minority group. No such law for representative samples of elderly exists today. A 7 years review of elderly patients’ enrollment in cancer drug registrations by U.S. Food and Drug Administration (USFDA) found statistically significant under-representation of the elderly.[16] The USFDA guidance advises researchers not to exclude trial participants solely on the basis of age, and also urges inclusion of an appropriate representation of elderly patients in clinical trials to measure safety and effectiveness in older patients, and to allow comparisons with their nonelderly patients.[17]
European medicines agency has described a strategy for geriatric medicines. Five of the 10 new medicinal product dossiers reviewed by Committee for Human Medicinal Products included specific studies for the elderly and all of them analyzed the results for patients aged ≥65 years.[18] Governmental officials, industry, health professionals, and other stakeholders from across Europe are working to improve the translation of research into practice by specifically addressing the lack of clear, accessible evidence about new technology, including medications for elderly people.[19]
Canadian government's panel on research ethics suggests that researchers should not exclude elderly people from research unless there is a valid reason for doing so. Furthermore, exclusion of the elderly shall not be based on easily remediable issues that are not germane to the research question. The Canadian government also recommends that when considering the inclusion of elderly people in research, researchers, and REBs shall consider their physical and social needs to ensure adequate protections such as reasonable accommodation for mobility, transportation support, and other types of assistance to facilitate their participation in research.[20]
In India, the schedule Y of drugs and cosmetic rules stipulates that geriatric patients should be included in Phase III trials and in Phase II trials at the sponsor's option in meaningful numbers in four situations viz., if the disease intended to be treated is characteristically a disease of aging or the population to be treated is known to include substantial number of geriatric patients or when there is specific reason to expect that conditions common in the elderly are likely to be encountered or when the new drug is likely to alter the geriatric patient's response (with regard to safety or efficacy) compared with that of the nongeriatric patient.[21]
DISCUSSION
The Indian elderly are more likely to suffer from chronic than acute illness. There is a rise in Non Communicable Diseases (NCD), particularly cardiovascular, metabolic, and degenerative disorders, as well as communicable diseases. NCD-related disability is estimated to increase and contribute to a higher proportion of overall national disability. Studies that estimate the disease burden in Indian elderly are limited. Disease conditions in elderly usually include Alzheimer’s, dementia, Parkinson's disease, urinary incontinence, heart diseases, arthritis, vision and eye diseases, diabetes, sleep disorders, depression, hearing loss, osteoporosis, and lung diseases. The elderly also make up the lion's share of patients for certain health conditions including cancer, cardiovascular disease, arthritis, and Parkinson's disease, among others in most parts of the world. Cardiovascular disease is the leading cause of death among the elderly. The prevalence of morbidity among the elderly due to infectious diseases is quite high. Considering the increasing disease burden in elderly, it is essential to establish the information associated with medications used in elderly.
Even though the proportionate participation of the elderly in trials may not be desirable or feasible, it is important to determine the factors that influence elderly participation in trials and to ensure that the elderly have access to trials. It may be necessary to design more trials that focus exclusively on elderly subjects or that address the treatment of elderly patients with specific comorbidities. Similarly, it may be important that certain trials enroll elderly patients in sufficient numbers to allow for statistically meaningful subgroup analyses. More information is needed on the differential response to and tolerance of chemotherapies by age and in individuals with and without comorbidities.
If the subject groups involved in Phase II and III trials reflect the patient populations that will use the drugs, the safety of marketed drugs could be significantly improved. By excluding representatives of the target population, many clear safety questions go unidentified. Sponsors and investigators have at times in the past followed the path of inclusion criterion of 18–64 years for most clinical trials. With the increasing life span and increasing number of elderly population world over, the sponsors recently have moved away from stipulating upper age limit as exclusion criteria and have stipulated only the lower age limit of 18 years. This is a bold move and would result in consolidating the data on elderly patients. Regulators world over have always been making efforts to promote the inclusion of elderly in clinical trials. The authors are of the opinion that the regulatory authorities in countries like India have several concerns to include patients aged ≥65 years in clinical trials [Table 3].
Table 3.
Authors’ viewpoint: Indian regulatory authority concerns to include patients aged >65 years in clinical trials

Increasingly, medical researchers are recommending that clinical trial sponsors raise age limits for trial participants (say, from 65 to 75 years) or eliminate them entirely, and that any age limits in trial protocols be medically justified. They also urge building into trial designs one or more subgroups of elderly patients, based on their other ailments and medications, to better study interactions. They also want contract research organizations to train investigators better in dealing with elderly patients’ special difficulties and to pay more attention to accommodating them. Several measures can be taken to protect elderly patients volunteering to participate in clinical trials [Table 4].
Table 4.
Measures to protect elderly in clinical trials

When the world over consensus is building up for including more elderly in the studies, Indian regulatory authority viz., CDSCO have decided arbitrarily to fix upper age limit for studies conducted in India. This would have negative impact on two counts: Viz., data on elderly in India would not be available at the end of the study and the sponsors of global studies may be unwilling to make this change on age restriction only for India with the fear that this could jeopardize the study in some way.
With the introduction of suitable guidelines and legislations for compensation related to clinical trial injury or death,[22] the internal guideline of the CDSCO which stipulates arbitrary age restrictions on patient recruitment should be made redundant and done away with upper age limits for patients participating in the trials. The CDSCO should allow researchers to pursue research in the elderly so that drug later approved by them may be used without restriction in the elderly patients. With the changing demographics, it is time for regulators to move the standard upper age limit for clinical trials from 65 years to no restrictions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Avorn J. Medication use and the elderly: Current status and opportunities. Health Aff (Millwood) 1995;14:276–86. doi: 10.1377/hlthaff.14.1.276. [DOI] [PubMed] [Google Scholar]
- 2.Gad SC. Evaluation of human tolerance and safety in clinical studies: Phase I and beyond. In: Gad SC, editor. Drug Safety Evaluation. New York: John Wiley and Sons, Inc; 2002. pp. 764–830. [Google Scholar]
- 3.United Nations. World Population Prospects: The 2010 Revision. [Last accessed on 2015 Apr 27]. Available from: http://www.esa.un.org/unpd/wpp .
- 4.Mari Bhat PN. Demographic Scenario, 2025. Planning Commission, Government of India. [Last accessed on 2015 Apr 27]. Available from: http://www.planningcommission.nic.in/reports/sereport/ser/vision2025/demogra.pdf .
- 5.Zizza CA, Ellison KJ, Wernette CM. Total water intakes of community-living middle-old and oldest-old adults. J Gerontol A Biol Sci Med Sci. 2009;64:481–6. doi: 10.1093/gerona/gln045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harugeri A, Joseph J, Parthasarathi G, Ramesh M, Guido S. Potentially inappropriate medication use in elderly patients: A study of prevalence and predictors in two teaching hospitals. J Postgrad Med. 2010;56:186–91. doi: 10.4103/0022-3859.68642. [DOI] [PubMed] [Google Scholar]
- 7.McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev. 2004;56:163–84. doi: 10.1124/pr.56.2.4. [DOI] [PubMed] [Google Scholar]
- 8.Davis D, Grossman SH, Kitchell BB, Shand DG, Routledge PA. The effects of age and smoking on the plasma protein binding of lignocaine and diazepam. Br J Clin Pharmacol. 1985;19:261–5. doi: 10.1111/j.1365-2125.1985.tb02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grandison MK, Boudinot FD. Age-related changes in protein binding of drugs: Implications for therapy. Clin Pharmacokinet. 2000;38:271–90. doi: 10.2165/00003088-200038030-00005. [DOI] [PubMed] [Google Scholar]
- 10.Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–9. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–7. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 12.Briggs R, Robinson S, O’Neill D. Ageism and clinical research. Ir Med J. 2012;105:311–2. [PubMed] [Google Scholar]
- 13.Lazzarini V, Mentz RJ, Fiuzat M, Metra M, O’Connor CM. Heart failure in elderly patients: Distinctive features and unresolved issues. Eur J Heart Fail. 2013;15:717–23. doi: 10.1093/eurjhf/hft028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartlam B, Lally F, Crome P. The PREDICT study: Increasing the Participation of the Elderly in Clinical Trials. The Opinions of Patients and Carers. Lay Report. Keele University. 2010 [Google Scholar]
- 15.U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for Industry. E7 Studies in Support of Special Populations: Geriatrics. Questions and Answers. 2012. [Last accessed on 2015 Apr 27]. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm189544.pdf .
- 16.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–31. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 17.Yancik R, Ries LA. Aging and cancer in America. Demographic and epidemiologic perspectives. Hematol Oncol Clin North Am. 2000;14:17–23. doi: 10.1016/s0889-8588(05)70275-6. [DOI] [PubMed] [Google Scholar]
- 18.Committee for Human Medicinal Products (CHMP). Adequacy of Guidance on the Elderly Regarding Medicinal Products for Human Use. European Medicines Agency. 2007. [Last accessed on 2015 Apr 20]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500049541.pdf .
- 19.Fahy N, McKee M, Busse R, Grundy E. How to meet the challenge of ageing populations. BMJ. 2011;342:d3815. doi: 10.1136/bmj.d3815. [DOI] [PubMed] [Google Scholar]
- 20.Tri-Council Policy Statement. Fairness and Equity in Research Participation. [Last accessed on 2015 Apr 27]. Available from: http://www.pre.ethics.gc.ca .
- 21.Ministry of Health and Family Welfare. Government of India. Drugs and Cosmetic Rules. 1945. [Last accessed on 2015 Apr 27]. Available from: http://www.cdsco.nic.in/writereaddata/Drugs&CosmeticAct.pdf .
- 22.Gupta YK, Pradhan AK, Goyal A, Mohan P. Compensation for clinical trial-related injury and death in India: Challenges and the way forward. Drug Saf. 2014;37:995–1002. doi: 10.1007/s40264-014-0230-3. [DOI] [PubMed] [Google Scholar]


