Abstract
Introduction:
An increased number of screen failure patients in a clinical trial increases time and cost required for the recruitment. Assessment of reasons for screen failure can help reduce screen failure rates and improve recruitment.
Materials and Methods:
We collected retrospective data of human epidermal growth factor receptor (HER2) positive Indian breast cancer patients, who failed screening for phase 3 clinical trials and ascertained their reasons for screen failure from screening logs. Statistical comparison was done to ascertain if there are any differences between private and public sites.
Results:
Of 727 patients screened at 14 sites, 408 (56.1%) failed screening. The data on the specific reasons for screen failures was not available at one of the public sites (38 screen failures out of 83 screened patients). Hence, after excluding that site, further analysis is based on 644 patients, of which 370 failed screening. Of these, 296 (80%) screen failure patients did not meet selection criteria. The majority -266 were HER2 negative. Among logistical issues, 39 patients had inadequate breast tissue sample. Sixteen patients withdrew their consent at private sites as compared to six at public sites. The difference between private and public sites for the above three reasons was statistically significant.
Conclusion:
Use of prescreening logs to reduce the number of patients not meeting selection criteria and protocol logistics, and patient counseling to reduce consent withdrawals could be used to reduce screen failure rate.
Keywords: Breast cancer, consent withdrawal, patient enrolment, screen failure, selection criteria
INTRODUCTION
Enrolment in a clinical trial is considered a way to gain access to new drugs, the potential of getting the better therapeutic benefit, an increase in survival rate and improved quality of life for cancer patients.[1] However, only 5–10% of cancer patients are included in the clinical trials.[1] For breast cancer trials with a human epidermal growth factor receptor (HER2) targeted therapy only 4.5%, eligible patients are included in the trials.[2]
The barriers to enrolment in a clinical trial, especially oncology trials are varied-uncertainty about treatment allocation, potential side-effects, fear, perceived harm, commitment of time, direct and indirect costs of participation, family considerations, study design, especially restrictive inclusion/exclusion criteria, and complex procedures.[3,4] In addition, the current Indian regulatory challenges - audio-visual (AV) recording of the consent process, restriction of 3 trials per investigator, Ethics Committee registration-make patient recruitment quite difficult and demanding.[5]
Despite these barriers, patients who choose to consent and participate may fail screening due to multiple reasons and hence, are unable to get potential benefits of participating in a trial. High screen failure rates also lead to increased time, resources and costs during the conduct of a trial. It is, therefore, imperative to assess the reasons for screen failure.
American cancer studies have reported poor performance, inadequate organ functions or laboratory parameters, estimated short life expectancy, lack of a specific biomarker or lack of archived tumor tissues, low education, being a minority, and longer screening delays as reasons for screen failure.[6,7] However, no such studies have been conducted in India. Hence, we analyzed data of breast cancer patients who failed screening for participation in a clinical trial and tried to ascertain reasons for screen failure.
MATERIALS AND METHODS
We analyzed retrospective data from screening logs of breast cancer phase 3 trials for HER2 positive Indian female patients. A clinical trial participant was considered a screen failure when a potential clinical trial participant did not get enrolled into the trial after undergoing the screening process.[6,8] The data analyzed were: Total number of patients screened, the number of patients who failed screening, and reasons for screen failure.
Following reasons for screen failure were analyzed:
Patient not meeting selection criteria
Logistical issues – inadequate breast tissue sample, screening duration exceeded beyond protocol requirement
Consent withdrawal by the patient
Investigator discretion
Patient noncompliance-screened patient who did not return for the enrolment visit
No reason provided.
In addition, we also compared these data between public and private clinical trial sites, all being hospitals.
We compared the difference in the number of screen failure and enrolled patients in private and public sites using Chi-square test. The reasons why patients failed screening were also compared between private and public sites. The chi-square test was used for categories: Patient not meeting selection criteria, consent withdrawal, and logistical issues. Fisher's exact test was used for categories: Investigator discretion, patient noncompliance, and no reason provided.
RESULTS
A total of 727 patients suffering from breast cancer were screened at 14 sites (6 private and 8 public) for recruitment. The pooled data [Table 1] of public and private sites showed that 408 (56.1%) patients failed screening. The number of patients screened and enrolled was more at public sites as compared with private sites. The difference in a number of screen failure and enrolled patients between private and public sites was statistically significant.
Table 1.
Screen failure rates in breast cancer trials
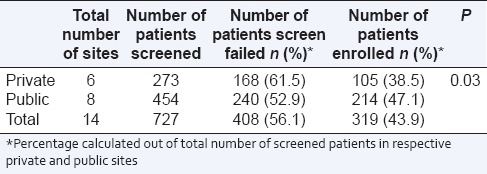
At one of the public sites, there were 38 screen failures out of 83 screened patients. However, no data on the specific reasons for screen failures was available. Hence, further analysis is based on 644 patients, of which 370 failed screening [Table 2]. Among the 6 reasons of screen failure, not meeting selection criteria accounted for 296 (80%) screen failure patients. Of these, 266 patients (89.9%) were HER2 negative. This means 378 (58.7% out of total 644 patients were HER2 positive). The difference between the number of screen failure patients from public sites and private sites due to the reason of selection criteria not met was statistically significant (P = 0.013).
Table 2.
Reasons for screen failure in breast cancer trials
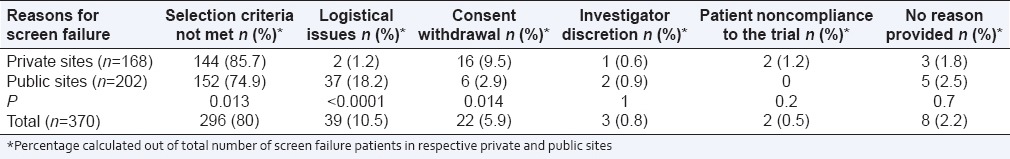
Screen failure due to logistical issues was reported in 39 patients (10.5%). Of these, 36 patients (92.3%) had inadequate breast tissue sample. Total screen failures due to HER2 negative status and inadequate breast tissue sample were 302 (266 + 36). In the remaining three patients, screening period extended beyond the protocol requirement. The difference between a number of logistical issues at private and public sites (2 and 37 respectively) was statistically significant (P < 0.0001).
A total number of patients who withdrew consent prior to enrolment was 22 (5.9%). Sixteen patients withdrew their consent at private sites as compared to six at public sites, and this difference was statistically significant (P = 0.014).
There was no significant difference between private and public sites for the remaining reasons for screen failure.
DISCUSSION
The overall screen failure rate in our study of patients suffering from breast cancer was 56.1%. Of all the reasons for screen failure, patients not meeting selection criteria especially HER2 status formed 80% of screen failure patients. Of 644 screened patients, there were 378 (58.7%) patients with HER2 positive status eligible for enrolment. Data from seven published studies from India have shown a wide range of variability in the prevalence of HER2 positive patients, ranging from 22% to 46%.[2,8,9] Since the rate of HER2 positive patients could be low and variable, an approach to prescreening of patients for HER2 status would have saved time, cost and resources spent on other screening procedures.
An inadequate tissue sample from 36 patients (5.7% out of total 644 screened patients), all from public sites formed the major logistical issue in our study. In a study from a tertiary care public hospital, Ghosh et al. reported that HER2 data were missing in 79 (3.9%), due to poor preservation of the histological sample or insufficient material.[2] As the patients in a tertiary care public hospital are often referred from other towns, there is no control over the quality of biopsy specimen. The institutions engaged in a clinical trial can reduce chances of screen failure by revisiting the selection criteria and the protocol logistics, prior to screening the clinical trial participants.
Another important reason for screen failure seen was consent withdrawal. The online survey of 5701 respondents by The Center for Information and Study on Clinical Research Participation (CISCRP) in 2013 showed that 69% participants from Asia-Pacific region found informed consent form (ICF) difficult to understand as compared to 12% from North America.[10] An Indian study of a mock trial to assess consenting decision showed that only about 30% of patients consented and had poor understanding of ICF. Common reasons for withholding consent were a reluctance to give blood or take a new drug.[11] The CISCRP study showed that participants, who dropped out early from the study had lower levels of self-confidence, difficulty in understanding the ICF and considered site visits stressful.[10] The other factors affecting the consent of a patient could be – fear of trial, lack of understanding, travel distance to and from hospital, availability of accompanying caregiver, expectations versus experience of trial procedures and conduct; behavior and attitude of the site staff, etc. The patient's family, friends and the primary physician may be unaware about clinical trials and their bias, and negative perception could affect the patient's decision about participating in the trial.[12] Hence, the investigator and his/her team need to have constant and reassuring communication with the patient throughout the study to reduce chances of consent withdrawal and to encourage the patient to comply with the requirements of clinical trial protocol, procedures and follow-up visits.
Most Western studies suggested that higher educational level, income and socioeconomic status of patients increased the probability of consent and participation in clinical trials.[7,13,14] However, in our study, the proportion of patients withdrawing consent was lower at public sites compared to private sites. It is likely that the patients at public sites, who may have low literacy levels and low socioeconomic status, may have less access to alternative costly cancer treatments and hence, are unlikely to withdraw consent. The latter group, with higher socioeconomic status, may consider and be able to afford alternative treatments; if they are uncertain about benefits of participating in a clinical trial or are experiencing any discomfort in compliance to protocol procedures.
It is also important to consider the impact of failing to enroll in a clinical trial on the participants. For Indian patients, health benefits, the cure of current disease, free medication, lack of alternative therapy and detailed knowledge are reported motivating factors for participating in a clinical tral.[15,16] However, when they are unable to participate because of failing the screening process, they may feel disappointed as their chance to get a potentially better drug is lost. Their financial burden increases as many of the tests and procedures which would have been provided free in a trial are no longer free.[15] There is also the loss of time, effort and opportunity of alternative treatment as patients go through the screening process. The CISCRP 2013 study showed that around 35% of patients, who were ineligible for a trial, did not know the reasons for screen failure and two-thirds of them chose not to participate in future trials.[10] Disappointment of patients and lack of understanding as to why they were unable to get enrolled may prevent them from participating in the future trials.
There is also an impact of high screen failure rates on the sponsor of the trial. A considerable amount of time, effort and resources are required to carry out the screening procedures, thereby having a financial impact. In such cases, low probability of recruitment along with high cost of screening will increase the financial burden on the sponsor. High screen failure rate means extended recruitment period, which will also increase the cost and could also delay the eventual availability of the drug in the market. Trial investigators are also impacted – the time and efforts spent in obtaining consent along with AV recording takes a long time out of their busy clinical practice. It would be worthwhile to consider any strategies to reduce screen failure rate so that not only the time and effort is reduced but also the investigators continue to remain motivated for conducting the trial. The time and effort saved can instead be used for better patient management. Thus, strategies developed at reducing screen failure rate will benefit the patients, the investigators, and the industry.
Being a retrospective study we had limited control over data collection. Among the patients who withdrew their consent, we do not know the exact reasons why they withdrew consent. Nevertheless, our data on screen failure patients for breast cancer trials provides us with various factors which may play a role in recruitment and retention of patients. We cannot completely eliminate screen failures, but having a proactive approach to minimize them can definitely be adopted. Reducing the number of screen failure patients goes a long way in controlling both time and cost. It would be desirable to conduct a prospective study in breast cancer patients to identify reasons for screen failure and also test whether simple measures, e.g., prescreening logs, patient counseling can help reduce the screen failure rates amongst clinical trial participants.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tournoux C, Katsahian S, Chevret S, Levy V. Factors influencing inclusion of patients with malignancies in clinical trials. Cancer. 2006;106:258–70. doi: 10.1002/cncr.21613. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh J, Gupta S, Desai S, Shet T, Radhakrishnan S, Suryavanshi P, et al. Estrogen, progesterone and HER2 receptor expression in breast tumors of patients, and their usage of HER2-targeted therapy, in a tertiary care centre in India. Indian J Cancer. 2011;48:391–6. doi: 10.4103/0019-509X.92245. [DOI] [PubMed] [Google Scholar]
- 3.Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer. 2008;112:228–42. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 4.Mills EJ, Seely D, Rachlis B, Griffith L, Wu P, Wilson K, et al. Barriers to participation in clinical trials of cancer: A meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7:141–8. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt A. India's next challenge: Rebooting recruitment. Perspect Clin Res. 2014;5:93–4. doi: 10.4103/2229-3485.134295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Pearce T, Cobani V, Zekaj M, Adams N, Williamson A, et al. Lessons from the other side of clinical trial accrual: Screen failures at the Josephine Ford Cancer Center/Henry Ford Health System in 2010. J Clin Oncol. 2011;29(15) suppl; abstr e16624. [Google Scholar]
- 7.Siddiqi AE, Sikorskii A, Given CW, Given B. Early participant attrition from clinical trials: Role of trial design and logistics. Clin Trials. 2008;5:328–35. doi: 10.1177/1740774508094406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambroise M, Ghosh M, Mallikarjuna VS, Kurian A. Immunohistochemical profile of breast cancer patients at a tertiary care hospital in South India. Asian Pac J Cancer Prev. 2011;12:625–9. [PubMed] [Google Scholar]
- 9.Vaidyanathan K, Kumar P, Reddy CO, Deshmane V, Somasundaram K, Mukherjee G. ErbB-2 expression and its association with other biological parameters of breast cancer among Indian women. Indian J Cancer. 2010;47:8–15. doi: 10.4103/0019-509X.58852. [DOI] [PubMed] [Google Scholar]
- 10.Center for Information and Study on Clinical Research Participation. 2013 Perceptions and Insights Study. Report on Study Participant Experiences. [Last accessed on 2015 Jan 07]. Available from: http://www.ciscrp.org/wp-content/uploads/2014/01/2013-CISCRP-Study-Study-ParticipantExperiences.pdf .
- 11.Gitanjali B, Raveendran R, Pandian DG, Sujindra S. Recruitment of subjects for clinical trials after informed consent: Does gender and educational status make a difference? J Postgrad Med. 2003;49:109–13. [PubMed] [Google Scholar]
- 12.Denson AC, Mahipal A. Participation of the elderly population in clinical trials: Barriers and solutions. Cancer Control. 2014;21:209–14. doi: 10.1177/107327481402100305. [DOI] [PubMed] [Google Scholar]
- 13.Challenges in Clinical Research. Washington (DC): National Academies Press (US); 2010. [Last accessed on 2015 Jan 28]. Institute of Medicine (US) Forum on Drug Discovery, Development, and Translation. Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary; p. 3. Available from: http://www.ncbi.nlm.nih.gov/books/NBK50888/ [PubMed] [Google Scholar]
- 14.Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014;111:1684–7. doi: 10.1038/bjc.2014.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalal J. Factors Influencing Patient Participation in Clinical Trials in India. ACRP Clinical Researcher. 2015;29:50–5. [Google Scholar]
- 16.Shah JY, Phadtare A, Rajgor D, Vaghasia M, Pradhan S, Zelko H, et al. What leads Indians to participate in clinical trials? A meta-analysis of qualitative studies. PLoS One. 2010;5:e10730. doi: 10.1371/journal.pone.0010730. [DOI] [PMC free article] [PubMed] [Google Scholar]


