Abstract
Objectives:
Spontaneous reporting of adverse drug reaction (ADR) is the backbone of pharmacovigilance program. Under reporting by prescribers is still exist. This study was done to assess the knowledge, attitude, and practice (KAP) of undergraduate students about pharmacovigilance.
Materials and Methods:
It was a questionnaire-based cross-sectional study. Study tool was a validated questionnaire containing 21 questions to evaluate KAP of pharmacovigilance among undergraduate medical students in a Tertiary Care Teaching Hospital of South India.
Results:
All data were analyzed by using Microsoft Excel sheet, Chi-square, and ANOVA. The mean score of final, prefinal, and 2nd year students is respectively (4.76, 5.63, and 4.73) for knowledge, (4.26, 4.95, and 4.53) for attitude and (1.66, 1.55, and 1.28) for the practice. There is a significant difference in mean score between three groups for knowledge and attitude, but not for practice. They have a better attitude, but poor in knowledge and practice regarding pharmacovigilance.
Conclusion:
Students lack adequate knowledge and skill of reporting ADR, but they have a positive attitude toward pharmacovigilance program. The integration of pharmacovigilance with undergraduate curriculum may help in improving ADR monitoring and reporting.
Keywords: Adverse drug reporting, pharmacovigilance, undergraduate
INTRODUCTION
Drug therapy is an integral part of the medical management. It has many beneficial effects, but side-effects and adverse drug reactions (ADRs) are some of its major disadvantages. ADR is defined by World Health Organization (WHO) as “a response to a drug that is noxious and unintended, and which occurs at doses normally used in man for prophylaxis, diagnosis or therapy of disease or for the modification of physiological function”.[1] ADR is responsible for significant morbidity and mortality; it is fourth to sixth leading cause of death in USA.[2] Studies suggested that ADR is responsible for 0.2-24% of hospital admission.[3,4] ADR also has a significant impact on health care cost.[5] Pharmacovigilance is defined by WHO as “the science and activities relating to the detection, understanding, and prevention of adverse effects or any other drug-related problems”.[6] To promote drug safety WHO started Program for International Drug Monitoring in 1961 and subsequent to that it promoted pharmacovigilance program at country level in collaboration with Center for International Drug Monitoring, Uppsala.[7]
To detect and spontaneously report ADR and to ensure drug safety, National Pharmacovigilance Program was initiated in India in the year 2004.[8] It is now renamed as Pharmacovigilance Program of India and operational since July 2010 under the aegis of Central Drug Standard Control Organization.[9]
The Uppsala Monitoring Centre (UMC), Sweden maintains the international database of ADR report received from different countries. India is an active participant in this program and its contribution to UMC database has rose from 0.5% in 2012 to 2% in 2013 making it seventh largest contributor of UMC drug safety database.[10] Although it has shown some improvement, but still lot is required to be done to increase the spontaneous reporting. Spontaneous reporting of ADR by health care professionals is backbone of pharmacovigilance program, but under reporting of ADR is still prevalent and is the cause of concern. Study showed that only 6-10% of all ADR cases are reported. Health care professional has a major role in pharmacovigilance program.[11] ADR reporting does not currently appear to be considered part of routine professional practice by health care professionals. This is essentially due to the absence of a vibrant and active ADR monitoring system and also lack of a reporting culture among health care professionals.[12,13,14] Medical students could play a major role and bring a paradigm shift in successful implementation of pharmacovigilance program if adequate knowledge and skill are imparted to them during undergraduate training career, but at present they don’t have any significant role which is due to inadequate training to them regarding ADR reporting.[15,16] Very few studies are there to assess the knowledge, attitude, and practice (KAP) of pharmacovigilance among undergraduate medical students. Hence, this study has been done to assess of KAP among medical students about same and to compare the result among different groups according to year of study.
MATERIALS AND METHODS
It was a cross-sectional questionnaire-based study carried out in a Tertiary Care Teaching Hospital and Medical College in Puducherry. One hundred and eighty undergraduate MBBS students, 60 from each batch attending clinical posting and willing to participate and gave written informed consent were included in the study. A KAP questionnaire was designed by following preceding studies.[17,18] Questionnaire was pretested in a small group of students by doing a pilot study. Modified questionnaire was given to participants. The questionnaire contains 21 questions, 10 to test knowledge, seven to test the attitude, four to test practice. Study was initiated after obtaining clearance from the Institutional Ethics Committee. The study involved 2nd year, prefinal year, and final year undergraduate medical students. The questionnaire was handed to the students after explaining the purpose of the study. Any doubts regarding questionnaire were clarified by investigator. 25 min was given for filling the questionnaire. A score of 1 was allocated for each correct answer or positive response and score 0 was allocated for wrong, unattempted answer, or negative response. Maximum possible score was 10, 7 and 4 for KAP, respectively. Mean Score of <50%, 50-69%, and 70% or above of maximum possible score were considered as poor, average, and good performance, respectively. Data were compiled, entered in Microsoft Excel sheet by using SPSS version 19 and analyzed by descriptive statistics, Chi-square and ANOVA test.
RESULTS
There were 10 knowledge-based questions. Among the respondents 61% of final year, 80% of prefinal year and 61.67% of 2nd year student responded correctly to the definition of ADR (P < 0.05). 41. 67% of final year, 55% of prefinal year, and 56.67% of 2nd year students were aware about locality of National Pharmacovigilance Center. Thirty-eight percent, 44% and 40% of final, prefinal and 2nd year students knew who can report ADR. Thirty percent of final, 41% of prefinal and 22% of 2nd year student know the definition of pharmacovigilance (P < 0.05). The details regarding the responses of the medical students for knowledge based questions are listed in Table 1. Mean knowledge score of prefinal year students is more than final and 2nd year student. Difference in knowledge score among three groups is statistically significant as shown in Table 4 and Figure 1.
Table 1.
Response of students to knowledge-based questions
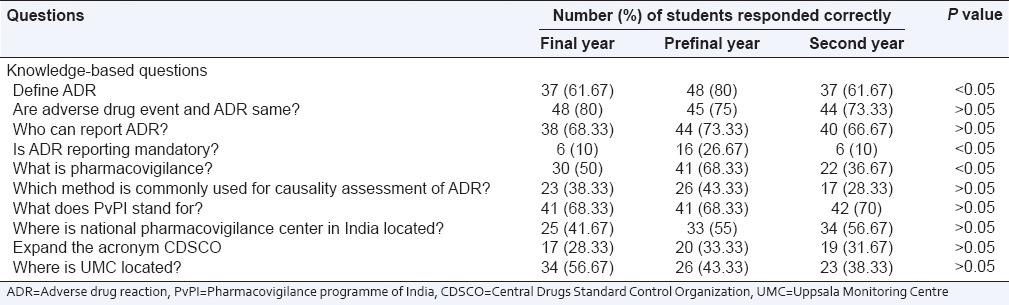
Table 4.
Comparison of mean score
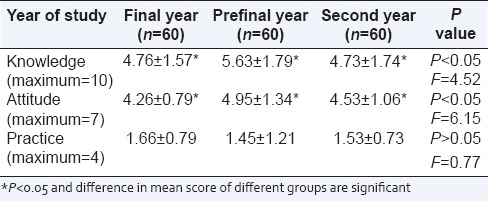
Figure 1.
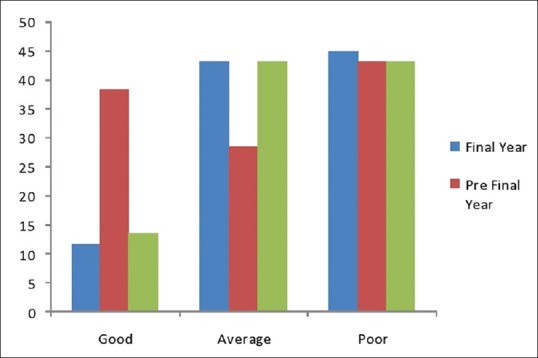
Knowledge score
Total number of questions to test the attitude was seven. Ninety-five percent, 88%, 91% final, prefinal, and 2nd year student, respectively felt ADR reporting is necessary. Students thought it relevant to have a discussion on pharmacovigilance in clinical posting. The details regarding the responses of the medical students for knowledge-based questions are listed in Table 2. Mean score of attitude between three groups is respectively, and difference in score among them is statistically significant as depicted in Table 4 and Figure 2.
Table 2.
Response of students to attitude based questions

Figure 2.
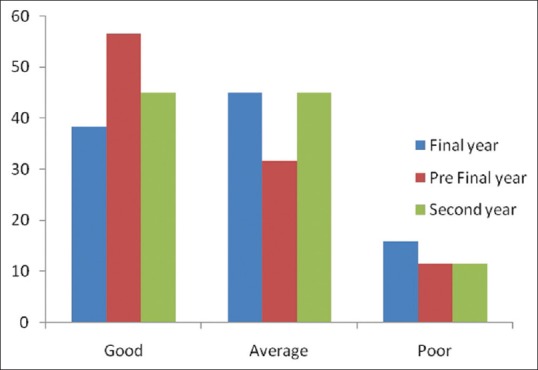
Attitude Score
There were four practice related questions. It was seen that only 18.13% students of final, 12.5% of prefinal and 5% students of 2nd year ever played any role in reporting ADR. About 80%, 63.3% and 85% of final, prefinal and 2nd year students, respectively updating their knowledge about new drug regularly (P < 0.05). The details regarding the responses of the healthcare professionals for these questions are listed in Table 3. Difference in mean practice score among three groups is not statistically significant as shown in Table 4 and Figure 3.
Table 3.
Response of students to practice based questions
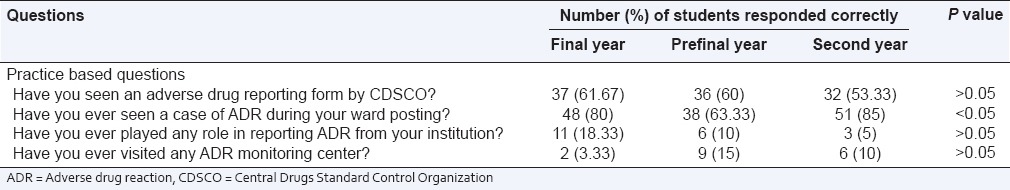
Figure 3.
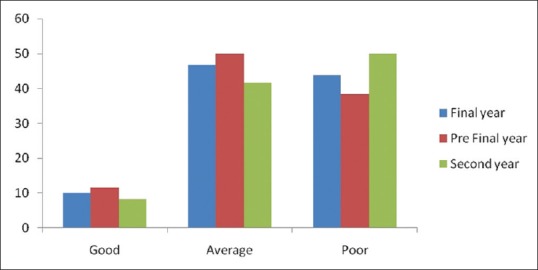
Practice Score
DISCUSSION
Pharmacovigilance is an integral part of holistic health care. It helps in detection and prevention of ADR of medicinal products. Spontaneous reporting of ADR is vital for the success of pharmacovigialnce program. There are innumerable studies to evaluate the KAP of health care providers toward pharmacovigilance program, but a very few study have been done among the budding doctors to capture their knowledge about same.[13,14,17] This study is one of the few studies done among undergraduate medical students regarding KAP of pharmacovigilance.
In this study, most students have an average or poor score in KAP. Among them, prefinal year students have a relatively better score than final and 2nd year students. From this study, it was clear that students have inadequate knowledge about pharmacovigilance, which corroborates with the finding of Vora et al.[16] The aim of pharmacovigilance is to ensure patient safety and rational use of medicines. It has played a major role in detection of ADRs, but previous studies suggests that under-reporting of ADRs is one of the major problems associated with pharmacovigilance program.[19] Major reason for under reporting is lack of knowledge and skill about pharmacovigilance program, which was reflected in our study, and it corroborates with the finding of studies done previously.[20,21] It can be overcome by educational intervention program like incorporation of it in undergraduate practical, continuous medical education (CME), and workshop on pharmacovigilance.[22] Students showed better attitude, but limited knowledge and poor practice toward pharmacovigilance. The findings of the study suggest a huge scope for improving the awareness and knowledge about pharmacovigilance among the students who will be the backbone of health care delivery in future. For this, there is a need for continuous educational initiatives like CME, and it should also be included in their curriculum as part of their study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.International drug monitoring: The role of national centres. Report of a WHO meeting. World Health Organ Tech Rep Ser. 1972;498:1–25. [PubMed] [Google Scholar]
- 2.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 3.Einarson TR. Drug-related hospital admissions. Ann Pharmacother. 1993;27:832–40. doi: 10.1177/106002809302700702. [DOI] [PubMed] [Google Scholar]
- 4.Ramesh M, Pandit J, Parthasarathi G. Adverse drug reactions in a south Indian hospital – their severity and cost involved. Pharmacoepidemiol Drug Saf. 2003;12:687–92. doi: 10.1002/pds.871. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Monguió R, Otero MJ, Rovira J. Assessing the economic impact of adverse drug effects. Pharmacoeconomics. 2003;21:623–50. doi: 10.2165/00019053-200321090-00002. [DOI] [PubMed] [Google Scholar]
- 6.Geneva: World Health Organization; 2002. The Importance of Pharmacovigilance. Safety Monitoring of Medicinal Products. World Health Organization Collaborating Centre for International Drug Monitoring. [Google Scholar]
- 7.Geneva: World Health Organization; 2002. World Health Organization. Safety of Medicine. A Guide for Detecting and Reporting Adverse Drug Reaction. [Google Scholar]
- 8.Adithan C. National pharmacovigilance programme. Indian J Pharmacol. 2005;37:34. [Google Scholar]
- 9.Pharmacovigilance programme of India 2010. CDSCO, Ministry of Health and Family Welfare, Government of India; 2010, Nov. [Last accessed on 2013 Dec 20]. Available from: http://www.cdsco.nic.in/pharmacovigilance .
- 10.Smith CC, Bennett PM, Pearce HM, Harrison PI, Reynolds DJ, Aronson JK, et al. Adverse drug reactions in a hospital general medical unit meriting notification to the Committee on Safety of Medicines. Br J Clin Pharmacol. 1996;42:423–9. doi: 10.1046/j.1365-2125.1996.04376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirodkar SN. India become seventh largest contributor to WHO-UMC's drug safety data base. [Last accessed on 2014 Apr 13]. Available from: http://www.pharmabiz.com .
- 12.Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: A systematic review. Drug Saf. 2009;32:19–31. doi: 10.2165/00002018-200932010-00002. [DOI] [PubMed] [Google Scholar]
- 13.Gupta P, Udupa A. Adverse drug reporting and pharmacovigilance: Knowledge, attitude and perception among resident doctors. J Pharm Sci Res. 2011;3:1064–6. [Google Scholar]
- 14.Desai CK, Iyer G, Panchal J, Shah S, Dikshit RK. An evaluation of knowledge, attitude, and practice of adverse drug reaction reporting among prescribers at a tertiary care hospital. Perspect Clin Res. 2011;2:129–36. doi: 10.4103/2229-3485.86883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rehan HS, Vasudev K, Tripathi CD. Adverse drug reaction monitoring: Knowledge, attitude and practices of medical students and prescribers. Natl Med J India. 2002;15:24–6. [PubMed] [Google Scholar]
- 16.Vora MB, Paliwal NP, Doshi VG, Barvaliya MJ, Tripathi CB. Knowledge of adverse drug reactions and pharmacovigilance activity among the undergraduate students of Gujarat. Int J Pharm Sci Res. 2012;3:1511–5. [Google Scholar]
- 17.Palaian S, Ibrahim MI, Mishra P. Health professionals’ knowledge, attitude and practices towards pharmacovigilance in Nepal. Pharm Pract (Granada) 2011;9:228–35. doi: 10.4321/s1886-36552011000400008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angamo MT, Testa A, Nabe NT. Knowledge, attitude and practice of Adverse drug reaction reporting among health professionals in south west Ethiopia. TAF Prev Med Bull. 2012;10:397–406. [Google Scholar]
- 19.Chatterjee S, Lyle N, Ghosh S. A survey of the knowledge, attitude and practice of adverse drug reaction reporting by clinicians in eastern India. Drug Saf. 2006;29:641–2. doi: 10.2165/00002018-200629070-00009. [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Zhang SM, Chen HT, Fang SP, Yu X, Liu D, et al. Awareness and attitudes of healthcare professionals in Wuhan, China to the reporting of adverse drug reactions. Chin Med J (Engl) 2004;117:856–61. [PubMed] [Google Scholar]
- 21.Radhakrishnan R, Vidyasagar S, Varma DM. An educational intervention to assess knowledge attitude practice of pharmacovigilance among health care professionals in an Indian Tertiary Care Teaching Hospital. Int J PharmTech Res. 2011;3:678–92. [Google Scholar]
- 22.Subish P, Mahamed Izam MI, Mishra P, Shankar PR, Alam K. Education session for pharmacy students on pharmacovigilance. A preliminary study. J Clin Diagn Res. 2010;4:2427–32. [Google Scholar]


