Abstract
Background:
To evaluate and compare corneal biomechanical indices and their specificity among keratoconus (KC), keratoconus suspect (KCS), and normal eyes (NL) before and after controlling potential confounders.
Materials and Methods:
A total of 160 eyes in three groups were included prospectively: NL, KC, and KCS groups based on clinical examination and topography. Corneal hysteresis (CH) and corneal resistance factor (CRF) were measured by the ocular response analyzer. CH and CRF were compared between the three groups by analysis of variances test.
Results:
The three groups consisted of 80 NL, 48 KC, and 32 KCS eyes. The mean CH measured was 10.4 ± 1.25, 7.83 ± 1.28 and 10.17 ± 1.80 mm Hg in NL, KC and KCS eyes, respectively. The mean CRF was 10.23 ± 1.75, 6.5 ± 1.63 and 9.98 ± 2.00 mm Hg in NL, KC and KCS eyes, respectively. Mean CH and CRF were significantly different between the NL and KC (P < 0.05); however after controlling for central corneal thickness and sex; there was no significant difference between NL and KCS (P > 0.05).
Conclusion:
CH and CRF can be helpful in differentiating KC from NL eyes; however, they are not valuable for detecting KCS that is the main concern for refractive surgery. Future studies focusing on more accurate tests for identifying KCS, using a consistent grading scale for defining KC and KCS are still warranted.
Keywords: Corneal biomechanics, corneal hysteresis, corneal resistance factor, forme fruste keratoconus, keratoconus, ocular response analyzer
Introduction
Keratoconus (KC) is a progressive ectatic disorder of the cornea characterized by corneal thinning that leads to corneal conical shape, irregular astigmatism and decreased vision.[1]
Currently, moderate and severe forms of KC are diagnosed without difficulty in the clinics using corneal topography combined with biomicroscopic, retinoscopic and pachymetric assessment.[2,3] However, the difficulty arises for detecting early stages of the corneal ectatic disorder known as keratoconus suspect (KCS) or forme fruste keratoconus (FFKC). Detecting KCS in clinics is of critical importance because KCS remains as the main cause of corneal ectasia post-refractive surgery, especially laser in situ keratomileusis (LASIK).[4,5]
One of the assumed predisposing factors in developing ectasia is changes in corneal biomechanics.[6] Ocular response analyzer (ORA) (Reichert Ophthalmic Instruments, Buffalo, NY, USA) is a device that measures corneal hysteresis (CH) and corneal resistance factor (CRF), which are associated with corneal biomechanics and strength.
This study was conducted to compare the corneal biomechanical parameters between KC, KCS and normal (NL) eyes and to determine the diagnostic accuracy of CH and CRF for detecting KC from NL ones after controlling for central corneal thickness (CCT), and finally clarify the role of CCT on CH and CRF measured by ORA.
Materials and Methods
A prospective, analytical cross-sectional study was conducted. The study was in concordance with the tenets of the Declaration of Helsinki, and the ethics committee of the Tehran University of Medical Sciences approved it. Consequent patients from June 2010 to January 2012 were included. All patients signed informed consent before entering the study.
The patients were divided into three groups: NL, KC, KCS. KC patients were diagnosed based on clinical evaluation and topographic and tomographic analysis using the Pentacam Rotating Scheimpflug camera (Oculus, Wetzlar, Germany). KCS patients were defined as no clinical sign of KC and area of inferior or superior steepening; minor topographic asymmetry, steep keratometric curvature greater than 47.0 diopters and oblique cylinder greater than 1.5 D.[7] Exclusion criteria were patients aged under18 years old, history of previous ocular disease or surgery, corneal scarring, or any corneal pathology other than KC. NL Eyes were selected from a database of patients who were referred for refractive surgery to the Farabi Eye Hospital and had passed the LASIK pre-operative screening process including a thorough clinical examination and topographic and tomographic analysis.
The ORA device (Reichert Ophthalmic Instruments) was used to obtain a biomechanical waveform from which the CH and CRF be obtained.[6] The ORA and corneal thickness measurements were taken during the same visit. ORA measurements were obtained four times sequentially without topical anesthesia and averaged for statistical analysis. All the measurements were performed between 1 pm and 5 pm. Pentacam was used for measuring the CCT. For the potential effect of CCT, age sex, these were also evaluated in all groups.
Data were analyzed using statistical package for social sciences (PASW, version 18, Chicago, Inc., USA). The mean values of the study groups were compared by analysis of variances test. Univariate linear regression was used to evaluate the linear best-fit relationship between CH, CRF and CCT. Logistic regression was used to control for CCT and sex as possible confounders in association of CH and CRF with KC. The results were considered statistically significant at P < 0.05. Two receiver operating characteristic curves (ROC) were used to identify the best CH and CRF cut-off points for having the maximum sensitivity and specificity in differentiating KC from NL corneas.
Results
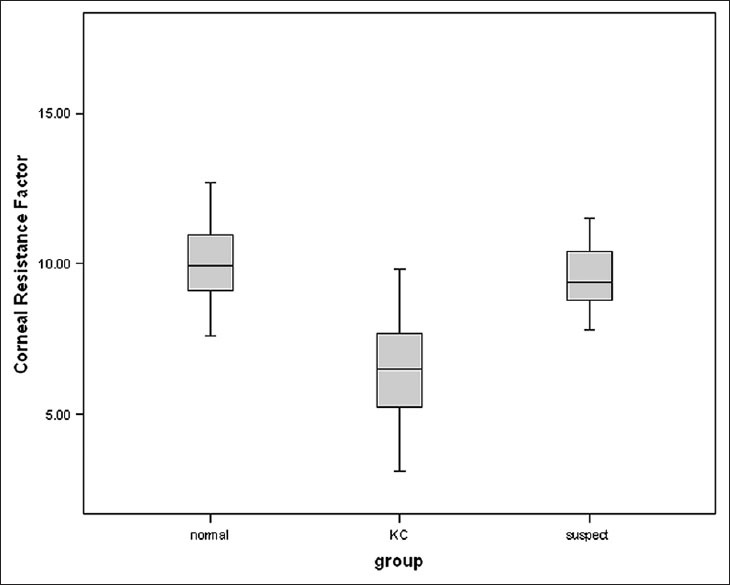
Table 1 summarizes the demographic data, ORA parameters and anterior segment properties obtained from each of the groups. There were no significant differences between the groups for all parameters except CH, CRF, and CCT. Box and whisker plots comparing CH and CRF between the three groups are shown in Figures 1 and 2.
Table 1.
Summary data for normal, KC and KCS
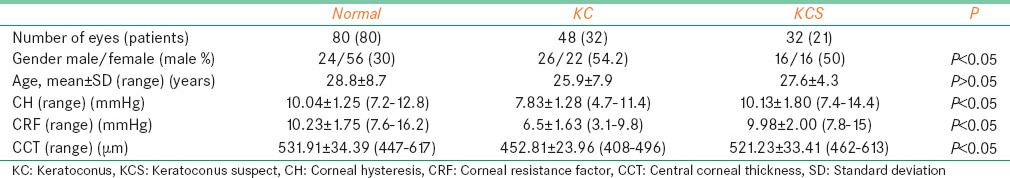
Figure 1.
Box and whisker plots of corneal hysteresis in normal, keratoconus suspect, and keratoconus eyes
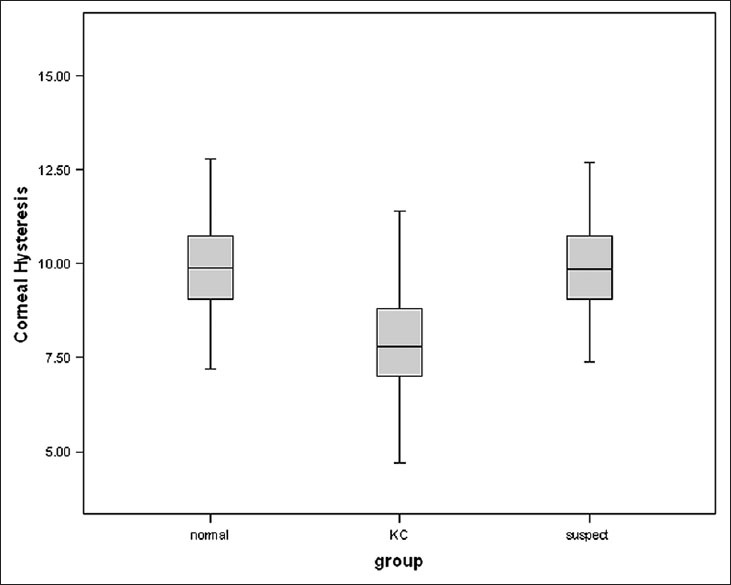
Figure 2.
Box and whisker plots of corneal resistance factor in normal, keratoconus suspect, and keratoconus eyes
Mean CCT was 531.91 ± 34.39 μm (range: 447-617 μm) in the NL group, 452.81 ± 23.96 μm (range: 408-496 μm) in the KC group and 521.23 ± 33.41 μm (range: 462-613 μm) in the KCS group.
Corneal hysteresis and CRF were correlated with CCT (P < 0.0001 and P < 0.0001, respectively) but not with sex (P = 0.285 and P = 0.925, respectively) and age (P = 0.708 and 0.540, respectively) in the NL group. In the KC group, CH and CRF were not significantly correlated with age, sex and CCT (P > 0.05). In the KCS group, CH and CRF were correlated with sex (P = 0.003 for both) and CCT (P < 0.0001 for both) and CRF was correlated with age (P = 0.02), but CH was not (P = 0.057).
Corneal hysteresis was significantly lower in KC compared with NL (P < 0.001) but no significant difference was seen between NL and KCS eyes (P = 0.948). CRF was significantly lower in KC as compared with NL eyes (P < 0.001), but no significant difference was seen between NL and KCS eyes (P > 0.05) [Figure 2]. CCT was significantly lower in KC eyes as compared with NL (P < 0.001) [Table 1].
The linear regression analysis showed a positive correlation of CH, CRF and CCT respectively [Figures 3 and 4]. After adjustments for Sex and CCT, the CH and CRF differences remained significant between NL and KC eyes but it was not significant between NL and KCS eyes. The linear regression line provided in the NL group was CH = (0.017 × CCT) + 0.84, as calculated from this line each 10 μm increase in CCT lead to 0.17 increases in CH. As the CH was not significantly correlated to CCT in KC, we calculated a corrected CH based on the regression line of the NL group for NL, KC and KCS, the calculated CH was then compared between the three groups, the mean corrected CH was 9.88 ± 0.58 (9.73-10.03) in the NL, 8.54 ± 0.41 (8.41-8.67) in KC and 9.7 ± 0.57 (9.48-9.93) in KCS (P < 0.05). The mean corrected CH was significantly lower in KC compared with NL, but there was no significant difference between NL and KCS.
Figure 3.
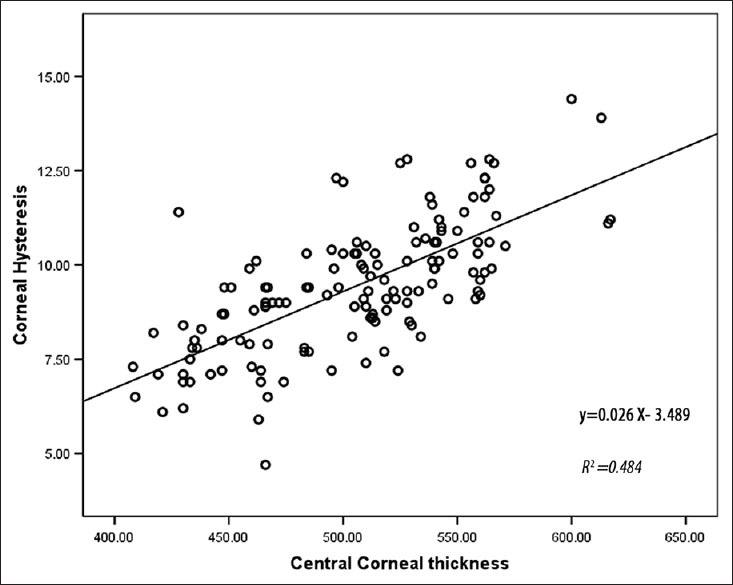
Correlation between corneal hysteresis and central corneal thickness in the three groups
Figure 4.
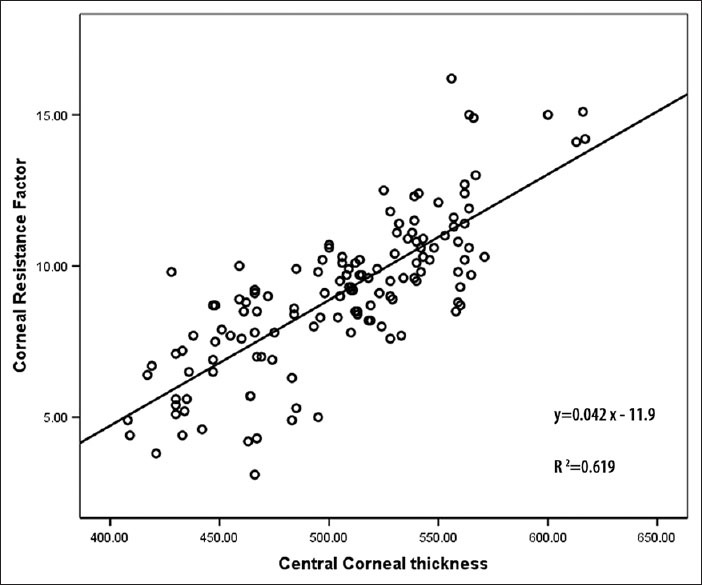
Correlation between corneal resistance factor and central corneal thickness in the three groups
The ROC curve analysis [Figure 5] showed overall predictive accuracy of 84% for CH. The optimal cut-off point was 8.75 with 75% sensitivity and 89% specificity. The area under the curve was 0.895 (P = 0.000 with the null hypothesis true area = 0.05). The ROC curve analysis [Figure 6] showed overall predictive accuracy of 91.4% for CRF. The optimal cutoff point was 8.45 with 90% sensitivity and 93% specificity. The area under the curve was 0.966 (P = 0.000 with the null hypothesis true area = 0.05).
Figure 5.
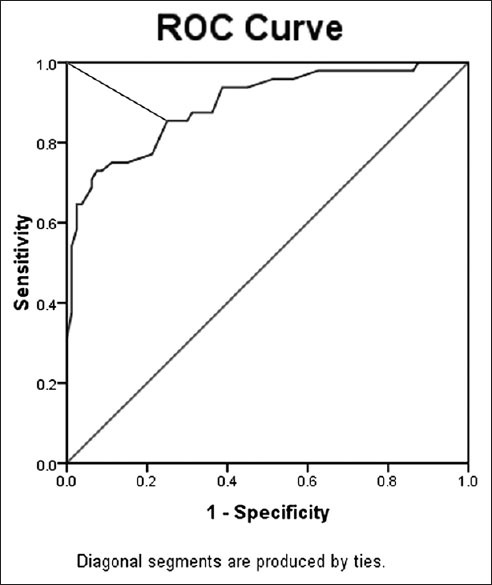
Receiver operating characteristic curve for corneal hysteresis data. The cutoff was 8.75 with 75% sensitivity and 89% specificity (test accuracy, 84%)
Figure 6.
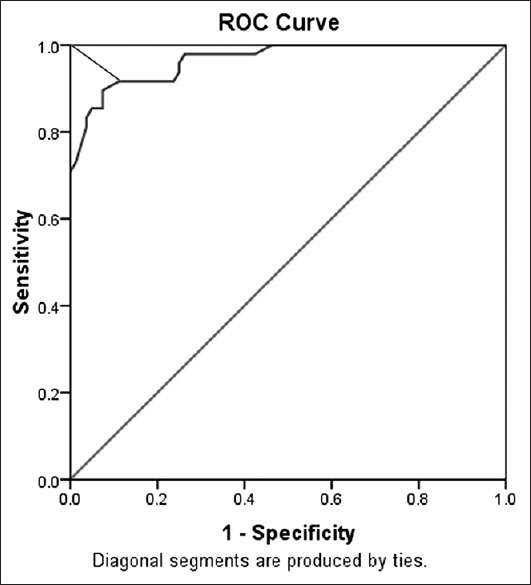
Receiver operating characteristic curve for corneal resistance factor data. The cut-off was 8.45 with 90% sensitivity and 93% specificity (test accuracy, 91.4%)
Discussion
Diagnosing mild to severe forms of KC is not difficult, however diagnosing FFKC or KCS eyes remains challenging. Despite the improvements in detecting KCS eyes using topographic and tomographic tools, there is no specific accepted consensus for categorizing an eye as KCS[8] and new cases of corneal ectasia after refractive surgery are still reported.[4,5,9,10,11] Therefore, a test that could detect KCS eyes in doubtful cases, with high accuracy is mandatory.
Since Luce[6] developed ORA for measuring corneal biomechanics in vivo; various studies have evaluated the ORA parameters, CH and CRF accuracy for detecting KC and KCS from NL eyes.[12,13,14,15,16] These studies have determined that CH and CRF are significantly lower in KC eyes than in NL eyes and reported CH and CRF as poor properties for discriminating mild KC from NL eyes.[15,16,17] Despite the various studies performed to evaluate the ORA accuracy for detecting KC and KCS from NL eyes, the diagnostic performance of the CH and CRF remains of limited value and the role of CCT as a confounding factor is not yet clearly defined.[12,17]
Our study assessed the diagnostic accuracy of ORA parameters for detecting clinical and subclinical forms of KC from NL eyes. Our results showed that mean CH and CRF were both significantly lower in KC eyes compared to NL ones, but no significant difference was seen in CCT, CH and CRF parameters of KCS eyes compared with NL eyes. After adjustment for CCT and sex CH and CRF remained significant in KC eyes compared with NL eyes.
Various studies have assessed the CH and CRF between NL and KC eyes with similar results to those of our study.[17,18] Fontes et al.[17] found significantly lower CH and CRF in KC in comparison to NL eyes. Also, Galletti et al.[18] reported significantly lower CH and CRF in KC eyes compared to NL eyes after controlling for CCT.
Our results showed the mean CH, CRF and CCT in KCS did not differ from NL eyes. Kirwan et al.[13] using the principle Orbscan criterion to identify KCS that was a difference of 1.5 diopters or greater between superior and inferior corneal curvature, did not find any significant difference between groups. Saad et al.[7] using a computer-based calculation from Nidek OPD scan videokeratographer, found a significant difference between NL and KCS first, which failed to remain significant after controlling for CCT.
A possible explanation for this finding might be the confounding role of corneal thickness on corneal biomechanics, especially CH and CRF. CH and CRF are known to be highly correlated to corneal thickness.[19,20,21] As corneal thickness decreases significantly in keratoconic eyes[1] and usually is within NL limits in KCS and NL eyes, any changes in CH and CRF could be related to the changes in CCT. After controlling for the CCT, the CH and CRF differences between NL and KC remained significant. The CCT between NL and KCS were not significantly different, therefore, could not play a confounding role.
However, our results are not in agreement with a number of previous studies. Schweitzer et al.[14] used the NL fellow eye of a manifest keratoconic with KISA index of <60% for defining KCS. They found a significant difference between NL and KCS eyes which remained significant after controlling for CCT in the thinner groups. Johnson et al.[12] used the KSS score developed by the collaborative longitudinal evaluation of KC study group to define and grade the severity of KCS also found a significant difference between KCS and NL eyes after adjustment for CCT, age and sex. The KCS grading of severity performed in this study has made the comparison of their result with other studies directly impossible. This discrepancy between the studies may be caused by the various definitions of KCS used in different studies as proposed by Johnson et al.[12] therefore the use of a unified grading scale for defining KCS in future studies is highly useful for comparing the results.
As the ROC curve analysis between KC and NL eyes showed, selecting the cutoff points for CH (8.75) and CRF (8.45) provided the predictive values of 84% and 91.4% respectively. Because there was no significant difference between KCS and NL eyes in CH and CRF, the ROC curve analysis was only done for NL and KC group and the KCS were not included in our study. The ROC curve analysis performed in the Fontes study[17] showed a poor overall predictive value of CH (74.83%) and CRF (76.97%) with the cutoff points of 9.64 mmHg and 9.60 mmHg respectively. In comparison to Fontes et al.,[17] our study showed higher overall predictive values for both CH and CRF, especially for CRF.
The ORA parameters provided by ORA can be helpful in differentiating KC eyes from NL ones, but CH and CRF cannot detect KCS from NL eyes; therefore ORA alone would not be a helpful diagnostic tool for detecting KCS eyes. However, as mentioned before, the clinical challenge is to have a test that could detect KCS with high accuracy from NL eyes. Our findings show that ORA is not valuable for detecting KCS eyes and CH and CRF are strongly correlated to the corneal thickness rather than early topographic changes of ectasia. Future studies with focusing on more accurate tests for finding KCS are still warranted. A consistent grading scale for defining KCS and KC is of great value in reporting the data of future studies of being capable of comparing the data and getting into a net conclusion. We suggest using CCT adjusted CH and CRF, to compare the corneal biomechanics without the confounding role of CCT.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 2.Holladay JT. Corneal topography using the Holladay Diagnostic Summary. J Cataract Refract Surg. 1997;23:209–21. doi: 10.1016/s0886-3350(97)80344-6. [DOI] [PubMed] [Google Scholar]
- 3.Kalin NS, Maeda N, Klyce SD, Hargrave S, Wilson SE. Automated topographic screening for keratoconus in refractive surgery candidates. CLAO J. 1996;22:164–7. [PubMed] [Google Scholar]
- 4.Mohammadpour M. Corneal ectasia after LASIK in one eye and uneventful PRK in the fellow eye. J Cataract Refract Surg. 2007;33:1677. doi: 10.1016/j.jcrs.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 5.Mohammadpour M. Risk for ectasia with LASIK. J Cataract Refract Surg. 2008;34:181–2. doi: 10.1016/j.jcrs.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31:156–62. doi: 10.1016/j.jcrs.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 7.Saad A, Lteif Y, Azan E, Gatinel D. Biomechanical properties of keratoconus suspect eyes. Invest Ophthalmol Vis Sci. 2010;51:2912–6. doi: 10.1167/iovs.09-4304. [DOI] [PubMed] [Google Scholar]
- 8.Schlegel Z, Hoang-Xuan T, Gatinel D. Comparison of and correlation between anterior and posterior corneal elevation maps in normal eyes and keratoconus-suspect eyes. J Cataract Refract Surg. 2008;34:789–95. doi: 10.1016/j.jcrs.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 9.Touboul D, Roberts C, Kérautret J, Garra C, Maurice-Tison S, Saubusse E, et al. Correlations between corneal hysteresis, intraocular pressure, and corneal central pachymetry. J Cataract Refract Surg. 2008;34:616–22. doi: 10.1016/j.jcrs.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 10.Klein SR, Epstein RJ, Randleman JB, Stulting RD. Corneal ectasia after laser in situ keratomileusis in patients without apparent preoperative risk factors. Cornea. 2006;25:388–403. doi: 10.1097/01.ico.0000222479.68242.77. [DOI] [PubMed] [Google Scholar]
- 11.Kymionis GD, Bouzoukis D, Diakonis V, Tsiklis N, Gkenos E, Pallikaris AI, et al. Long-term results of thin corneas after refractive laser surgery. Am J Ophthalmol. 2007;144:181–85. doi: 10.1016/j.ajo.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Johnson RD, Nguyen MT, Lee N, Hamilton DR. Corneal biomechanical properties in normal, forme fruste keratoconus, and manifest keratoconus after statistical correction for potentially confounding factors. Cornea. 2011;30:516–23. doi: 10.1097/ICO.0b013e3181f0579e. [DOI] [PubMed] [Google Scholar]
- 13.Kirwan C, O’Malley D, O’Keefe M. Corneal hysteresis and corneal resistance factor in keratoectasia: Findings using the Reichert ocular response analyzer. Ophthalmologica. 2008;222:334–7. doi: 10.1159/000145333. [DOI] [PubMed] [Google Scholar]
- 14.Schweitzer C, Roberts CJ, Mahmoud AM, Colin J, Maurice-Tison S, Kerautret J. Screening of forme fruste keratoconus with the ocular response analyzer. Invest Ophthalmol Vis Sci. 2010;51:2403–10. doi: 10.1167/iovs.09-3689. [DOI] [PubMed] [Google Scholar]
- 15.Shah S, Laiquzzaman M, Bhojwani R, Mantry S, Cunliffe I. Assessment of the biomechanical properties of the cornea with the ocular response analyzer in normal and keratoconic eyes. Invest Ophthalmol Vis Sci. 2007;48:3026–31. doi: 10.1167/iovs.04-0694. [DOI] [PubMed] [Google Scholar]
- 16.Shah S, Laiquzzaman M, Yeung I, Pan X, Roberts C. The use of the ocular response analyser to determine corneal hysteresis in eyes before and after excimer laser refractive surgery. Cont Lens Anterior Eye. 2009;32:123–8. doi: 10.1016/j.clae.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Fontes BM, Ambrósio R, Jr, Jardim D, Velarde GC, Nosé W. Corneal biomechanical metrics and anterior segment parameters in mild keratoconus. Ophthalmology. 2010;117:673–9. doi: 10.1016/j.ophtha.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 18.Galletti JG, Pförtner T, Bonthoux FF. Improved keratoconus detection by ocular response analyzer testing after consideration of corneal thickness as a confounding factor. J Refract Surg. 2012;28:202–8. doi: 10.3928/1081597X-20120103-03. [DOI] [PubMed] [Google Scholar]
- 19.Fontes BM, Ambrósio R, Jr, Velarde GC, Nosé W. Corneal biomechanical evaluation in healthy thin corneas compared with matched keratoconus cases. Arq Bras Oftalmol. 2011;74:13–6. doi: 10.1590/s0004-27492011000100003. [DOI] [PubMed] [Google Scholar]
- 20.Fontes BM, Ambrósio R, Jr, Alonso RS, Jardim D, Velarde GC, Nosé W. Corneal biomechanical metrics in eyes with refraction of −19.00 to +9 D in healthy Brazilian patients. J Refract Surg. 2008;24:941–5. doi: 10.3928/1081597X-20081101-14. [DOI] [PubMed] [Google Scholar]
- 21.Kamiya K, Hagishima M, Fujimura F, Shimizu K. Factors affecting corneal hysteresis in normal eyes. Graefes Arch Clin Exp Ophthalmol. 2008;246:1491–4. doi: 10.1007/s00417-008-0864-x. [DOI] [PubMed] [Google Scholar]


