Abstract
Keratoconus as the most common cause of ectasia is one of the leading cause of corneal transplants worldwide. The current available therapies do not modify the underlying pathogenesis of the disease, and none of the available approaches but corneal transplant hinder the ongoing ectasia. Several studies document Crosslink defect between collagen fibrils in the pathogenesis of keratoconus. Collagen cross link is a relatively new approach that with the application of the riboflavin and ultraviolet A, new covalent bands reform. Subjective and objective results following this method seem to be promising. Endothelial damage besides other deep structural injury, which is the major concern of this technique have not yet been reported, when applying the standard method.
Keywords: Corneal cross linking, keratoconus, ultraviolet A
Introduction
Keratoconus is a progressive noninflammatory disease of the cornea, affects both eyes once in its progression. It usually begins sometime in puberty and its advance is predictably arrests in the third or fourth decade.[1]
Estimated incidence in a different society ranges from 5 to 20 in 10,000 persons, and a prevalence of 54 in 100,000 is reported in different studies.[2,3] With this prevalence, keratoconus is regarded as the most common corneal ectasia.[4] This thanks to advances in diagnosing modalities, the reported incidence in recent studies seems to be even higher. Females seem to be more affected. And no ethnicity is spare from this disease.[4,5,6]
Method of Literature Review
We searched the National Library of Medicine's PubMed database and Elsevier Scopus database with a subsequent review of the accompanying references. The major search words and word combinations included: Corneal cross linking treatment of keratoconus; corneal cross linking, treatment options of keratoconus, perspective of keratoconus, cross linking and ultraviolet A (UVA), complications of corneal cross linking, outcome of corneal cross linking, methods and techniques of corneal cross linking, corneal cross linking risks.
In addition, the citations from the above searches were also included. Cases from the nonEnglish literature were not included. All identified documents were examined, and those that were relevant were retrieved for inclusion in the review.
Pathogenesis
The exact underlying pathogenesis of the keratoconus despite recent researches remains unknown. Nevertheless, different hypotheses have been proposed. It seems that offending assaults such as trauma, ultraviolet (UV) or atopy fire an inflammatory cascade in susceptible patients that leads to degeneration of the corneal stroma.[7] It has been found that interleukin 1 receptors are presented 4 times more on the surface of keratinocytes of keratoconic patient. This cytokine is postulated to induce apoptosis in keratinocytes and regulates keratinocyte proliferation and differentiation.[8,9] Moreover, a role in modulating the expression of metalloproteinase has been suggested for this cytokine.[9]
Imbalances between other proinflammatory agents and anti-inflammatory agents have also been found. Cellular adhesion molecule-1, vascular cell adhesion molecule-1 and interleukin 6 are even expressed as much as 40 times, whereas expressions of anti-inflammatory agents like interleukin 10 are markedly reduced.[10]
Disparities between proteinase and proteinase inhibitor activity have also been postulated.[11] The discovered disproportion can lead to the corneal stromal degradation; as the core pathological finding in keratoconus. Sawaguchi et al. found that α2-macroglobulin and α1-antiprotease levels as a proteinase inhibitors are significantly less in keratotic eyes than normal eyes[12] and studies demonstrate a higher activity for lysosomal and catabolic enzymes like the proteinase.[13]
Collagen alignment and components are also of significant importance. More than two third of the dry weight of the cornea is composed of collagen.[14] To build up the transparency of the cornea the axis and three-dimensional structure of collagen fibrils should be arranged elaborately. Researchers found differences in certain collagen types between normal and keratoconic eyes and retard in the wound healing process could be attributed to these differences.[15]
There are also reports that demonstrate higher prevalence of keratoconus in patients with a known connective tissue disease such as osteogenesis imperfecta and Ehlers–Danlos.[16,17] Researchers also found that severe mitral valve prolapse pending finally to surgery occurs more commonly in these people.[18] Findings that make a genetic abnormality in connective tissue, especially collagen genes are even more possible.
Collagen cross linking is defective in type VI Ehlers–Danlos due to lysyl hydroxylase deficiency. This type is the most common reported form that has a correlation with keratoconus[19,20] and paucity of these cross links seems to be one of the basis of corneal laxity at least in some patients.
Histopathological Findings
Based on the severity of the disease, microscopic changes happen in each layer of the cornea. The epithelium of the basement membrane disrupts and breaks appear in Bowman's membrane. Posterior proliferation of epithelium to Bowman's membrane creates Z shape deformities that become filled with periodic acid–Schiff positive materials. As the disease progresses, Iron deposits appear within and between epithelial cells and degenerating fibroblasts and keratinocytes appear in the stroma. Arrangement of stromal fibrils gets distorted as well.
Descemet's membrane and endothelium usually do not undergo noteworthy changes. Nevertheless, breaks in Descemet's membrane and increased cellular pleomorphism in endothelial cells have been reported.[21]
Clinical Manifestation
Clinical findings depend on the stage of the disease and are a spectrum from being completely asymptomatic to severely distorted vision. Fortunately, keratoconus barley ends to complete blindness.
At initial steps, there are no symptoms and the disease goes unnoticed by the patient and physician, unless a diagnostic procedure like keratography is done. Gradually the disease distorts the vision so severely that the refractive errors cannot be compensated with spectacles.
Amsler classified the subclinical form as fruste form and defined it as four or less degree downward deflection from the horizontal axis with handheld keratosope.[22,23]
On clinical examination with a slit lamp following findings might be visible
After dilating the pupil, scissoring of the retinoscopic is visible. Conical protrusion, as it comes from the name of the disease, is a frequent finding in the incipient phase. Stromal thinning is very often found commonly inferiorly or inferotemporally. Vogt's striae are fine upright lines paralleling the axis of the cone deep in stroma and Descemet's membrane. Fleischer's ring defined as iron deposition around the cornea. Munson's sign; V shape conformation of the lower lid when gazing downward and Rizzuti's sign; well demarcated beam of light just around the nasal limbus, exhibited by lateral illumination of the cornea. These two latter signs are useful external signs especially in advanced keratoconus. Other additional signs like stromal scars and visible corneal nerves might be found too.[21,22,23]
Classification
Based on disease evolution, Amsler categorized the disease from fruster or subclinical to severe [Table 1].[4,24,25]
Table 1.
The Amsler–Krumeich classification of keratoconus severity[24]
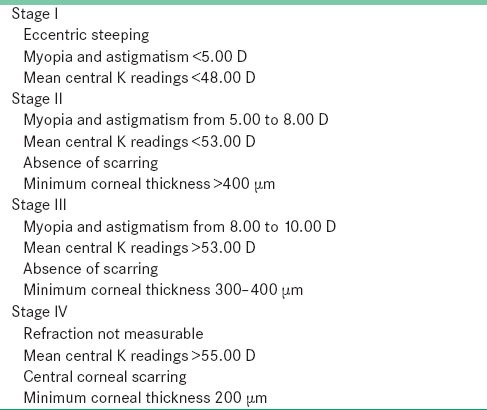
Morphologically the disease is classified as: Nipple with conical diameter <5 mm and round in morphology. This type is easily corrected with contact lenses.
Oval
The cone has a diameter more than 5 mm commonly in the inferotemporal corneal quadrant and is peripherally located. This form is not easily corrected with contact lenses. In keratoglobus, more than 75% of the corneal surface is cone like. Contact lens in visual correction has been almost always disappointing.[26]
Management
Contact lenses are the first choice of therapy for more than 90% of patients. In the early phase of the disease, soft lenses seem to be adequate but with further progression rigid gas permeable lenses are more functional. In very early phase spectacles can also be applied. However, the abnormal shape of the cornea does not let for proper matching with spectacles. In patients with severe keratoconus and those who do not tolerate the contact lens, surgery is the next step. There are different methods of surgeries while penetrating keratoplasty (PKP), in which the full thickness of the cornea is removed enjoys from the most popularity. According deep lamellar keratoplasty, in which the surgeon leaves the descemet's membrane and endothelium intact, less rate of graft rejection is reported. Nevertheless, PKP seems to bestow a better visual acuity. Photorefractive keratectomy, Intra-corneal ring segment and implantation of intraocular lens are other surgical maneuvers that are applied solely or in combination with other techniques.[23,27,28]
Corneal Cross Linking
Although cross linking was a common method in different industries including chemistry to build up polymers, medical engineering for heart prostheses and dentistry for certain material,[29] entrance of this method to ophthalmology comes back to 1997, when Spörl et al. induced cross link in corneal collagens.[30] Collagen cross link created a hopeful prospective for corneal ectasia of all kinds and especially keratoconus. Before that there was no treatment to modify the underlying pathophysiology and arrest further ectasia.
Collagen is one of the core structural proteins in the body. Its three-dimensional structure gives especial features to this protein. With minor changes in the structure of this super molecule, the elasticity, tensile rigidity, and resistance capacity differs significantly.
Each molecule of collagen is made of three left-handed α-helix chains. Each chain is a repeat of three amino acids: Pro-X-Gly or Hypro-X-Gly. Glycine is a small amino acid that its presence is of paramount importance of forming strong bonds between α-helices. Substitution of this aa with another amino acids lead to severe connective tissue disorders. Collagen fibrils are linked to gather in a network pattern. Lysyl hydroxylase is the key enzyme for creating covalent bonds between collagen fibrils.[14] And as it was already mentioned this enzyme is defective in certain types of Ehlers–Danlos syndrome and keratoconus is more prevalent in this group. Other researchers have also shown that less covalent cross linkage is associated with decreased mechanical strength.[31] Nevertheless, Cannon and Foster declared that natural paucity in cross linkages does not substantially lead to keratoconus.[32]
As the age increases, more covalent bonds form between collagen fibrils, also called the tropocollagen, in all parts of the body including the cornea.[33,34] This cross linkage increases Young's modulus.[35] Finding that might explain the arrest in the progression of keratoconus after the fourth decade.[36]
Three-dimensional images of normal corneal stromal collagen, which were taken using femtosecond high energy laser to induce signals in a process named second harmonic generation, demonstrated highly interwoven network in the anterior part of the cornea; “sutural lamella” that insert to Bowman layer. This highly organized “sutural lamella” does not appear in keratoconic eyes.[37]
Mechanism
Increase in collagen cross links to arrest the conical progression has been a reasonably appealing strategy.
In 1998, Spoerl applied Glutaraldehyde and Karnovsky solution to induce collagen cross links in vivo. This technique was efficient. Yet the toxicity, deep penetration of the substance and consequent scar formation makes the in vivo application restricted.[30]
Wollensak et al. were the first group who innovated riboflavin and UV for creating collagen crosslinks.[35]
The collagen cross-link is a photo-induced reaction. Riboflavin (Vitamin B2) functions as a photosynthesizer that becomes excited with UVA at 370 microns and goes to higher levels of energy. The resultant is oxidizing substance and free radicals cause formation of new covalent bonds between collagen fibrils.[38,39]
Technique
Despite trivial differences in detail, most researchers use the instruction first described by Dresden: After mechanical debridement of 7–9 mm of corneal epithelium under local anesthesia chemical debridement with alcohol similar to debridement technique used in LASIK laser in situ keratomileusis can also be applied. Riboflavin 0.1% solution is used every 2–3 min for 30 min. After this time fluorescence appears while examining the eye with a slit lamp. Then UVA radiation starts. Before radiation, ultrasonic pachymetry is necessary to confirm that thinnest part of the stroma is not <400 microns. This least diameter has been shown in the literature to keep the posterior structures protected from UVA radiation. The wavelength of UVA is arranged at 370 nm. UVA is mostly absorbed by riboflavin in this wavelength. Using the procedure, the stroma is always saturated with riboflavin. After the surgery antibiotic and corticosteroids, both topically are used, and a bandage contact lens is inserted. This contact lens will be removed in the 3rd day.[40]
Postsurgical Outcomes
Biomechanical changes
After the cross linking, cornea shows more resistance against the enzymatic degeneration and its stress-strain measurement increase.[35,41] Diameter of collagen fibrils is also reported to be increased.[42]
Clinical studies demonstrate significant improvement and all published publications reported arrest in corneal ectasia.[43] In the first case series that was done by Wollensak et al. on 23 patients, a significant decrease in the topographic index (K max) by 2.01 D and spherical equivalent (SE) by 1.14 D was observed. Best corrected visual acuity (BCVA) was improved by 1.26 as well.[44] Further studies in other countries showed similar findings in K max, BCVA, and spherical equivalent.[45,46,47,48,49,50,51] Improvement in visual acuity seems to be due to astigmatism correction,[45,46,47] which is not necessarily accompanied by better keratometric indices. Modification in corneal anterior surface deformity and astigmatism correction lead to improvement in higher order aberrations especially coma aberration. With all very promising results, reverse in the presence ectatic cornea is subtle.
Possible Complications and Risk
Corneal edema happens very often post surgically and is usually a self-limited consequence.[52]
Corneal persistent haziness is also observed in the anterior two third of the cornea that is the result of a lacunar honey comb-like hydration pattern, which is visible under confocal microscope and is a positive indication of sufficient cross linking. This phenomenon does not seem to distort the vision significantly.[53,54]
Epithelial damage, corticosteroid use and contact lens bandage after the procedure render the cornea to infection.[55]
Escherichia coli, Staphylococcus epidermis, poly microbial infections with Streptococcus salivarius, Streptococcus oralis, and coagulase negative staphylococcus specimens have been the offending agents in different studies.[56,57,58]
Severe keratitis caused by Acanthamoeba and Pseudomonas have been reported.[59,60] Primary herpetic keratitides with geographical ulcer and iritis,[61] as well as recurrence of herpetic keratitis, is also reported.[61,62]
Coskunseven et al. found a subtle increase in intraocular pressure by 2 mmHg.[43,51,52]
Ensuing stiffness of the cornea after cross linking is the suggesting hypothesis. Nevertheless, these findings were not repeated in other studies.[62]
Endothelial damage
The major concern in applying UVA for corneal cross linkage was endothelial damage. UVA is potentially dangerous for endothelial cells and these cells are incapable of regeneration and offence at any level is irreversible.
Ultraviolet A induces apoptosis in keratinocytes. This process continues even after the surgery and peaks 24 hours then after. In corneal diameter <400 microns, apoptotic changes were seen even in the endothelial layer. The depth of the injury depends on corneal diameter and UVA density of energy. With the current protocol, crosslink changes occur maximum 350 micron from the surface and the max energy density is 0.18 mW/cm2, which is far less than the threshold for endothelial damage (0.35 mW/cm2).[50] All researchers who demonstrated adherence to the standard protocol reported no injury to the endothelium.
Keratinocytes start regeneration 2–3 weeks postoperative and become compete in 6 months.[63]
Conclusion
Keratoconus is one of the leading causes of cornea transplant. Therapies based on the spectacles and contact lenses do not hinder the progression of the disease. Surgeries and corneal transplantation though preserved as the first choice for the severe variants of the disease do have risks like rejection of the transplanted cornea. Moreover surgical results are not always satisfying.
Corneal crosslink is a noble strategy based on the underlying pathology of the disease. Experimental and clinical researchers have demonstrated the efficacy of this approach. Side effects like endothelial damage that were the major concerns have not been reported under the standard method.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 2.Hofstetter HW. A keratoscopic survey of 13,395 eyes. Am J Optom Arch Am Acad Optom. 1959;36:3–11. doi: 10.1097/00006324-195901000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101:267–73. doi: 10.1016/0002-9394(86)90817-2. [DOI] [PubMed] [Google Scholar]
- 4.Romero-Jiménez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: A review. Cont Lens Anterior Eye. 2010;33:157–66. doi: 10.1016/j.clae.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 5.McMonnies CW. Abnormal rubbing and keratectasia. Eye Contact Lens. 2007;33:265–71. doi: 10.1097/ICL.0b013e31814fb64b. [DOI] [PubMed] [Google Scholar]
- 6.Weed KH, MacEwen CJ, Giles T, Low J, McGhee CN. The Dundee University Scottish Keratoconus study: Demographics, corneal signs, associated diseases, and eye rubbing. Eye (Lond) 2008;22:534–41. doi: 10.1038/sj.eye.6702692. [DOI] [PubMed] [Google Scholar]
- 7.Cristina Kenney M, Brown DJ. The cascade hypothesis of keratoconus. Cont Lens Anterior Eye. 2003;26:139–46. doi: 10.1016/S1367-0484(03)00022-5. [DOI] [PubMed] [Google Scholar]
- 8.Wilson SE, He YG, Weng J, Li Q, McDowall AW, Vital M, et al. Epithelial injury induces keratocyte apoptosis: Hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996;62:325–7. doi: 10.1006/exer.1996.0038. [DOI] [PubMed] [Google Scholar]
- 9.Bureau J, Fabre EJ, Hecquet C, Pouliquen Y, Lorans G. Modification of prostaglandin E2 and collagen synthesis in keratoconus fibroblasts, associated with an increase of interleukin 1 alpha receptor number. C R Acad Sci III. 1993;316:425–30. [PubMed] [Google Scholar]
- 10.Lema I, Durán JA, Ruiz C, Díez-Feijoo E, Acera A, Merayo J. Inflammatory response to contact lenses in patients with keratoconus compared with myopic subjects. Cornea. 2008;27:758–63. doi: 10.1097/ICO.0b013e31816a3591. [DOI] [PubMed] [Google Scholar]
- 11.Sawaguchi S, Yue BY, Sugar J, Gilboy JE. Lysosomal enzyme abnormalities in keratoconus. Arch Ophthalmol. 1989;107:1507–10. doi: 10.1001/archopht.1989.01070020581044. [DOI] [PubMed] [Google Scholar]
- 12.Sawaguchi S, Twining SS, Yue BY, Chang SH, Zhou X, Loushin G, et al. Alpha 2-macroglobulin levels in normal human and keratoconus corneas. Invest Ophthalmol Vis Sci. 1994;35:4008–14. [PubMed] [Google Scholar]
- 13.Godel V, Blumenthal M, Iaina A. Congenital leber amaurosis, keratoconus, and mental retardation in familial juvenile nephronophtisis. J Pediatr Ophthalmol Strabismus. 1978;15:89–91. doi: 10.3928/0191-3913-19780301-09. [DOI] [PubMed] [Google Scholar]
- 14.Smolin G, Theft R. 1st ed. Boston: Little Brown; 1983. The Cornea. Scientific Foundations and Clinical Practice; pp. 21–5. [Google Scholar]
- 15.Zimmermann DR, Fischer RW, Winterhalter KH, Witmer R, Vaughan L. Comparative studies of collagens in normal and keratoconus corneas. Exp Eye Res. 1988;46:431–42. doi: 10.1016/s0014-4835(88)80031-9. [DOI] [PubMed] [Google Scholar]
- 16.Kuming BS, Joffe L. Ehlers-Danlos syndrome associated with keratoconus. A case report. S Afr Med J. 1977;52:403–5. [PubMed] [Google Scholar]
- 17.Beckh U, Schönherr U, Naumann GO. Autosomal dominant keratoconus as the chief ocular symptom in Lobstein osteogenesis imperfecta tarda. Klin Monbl Augenheilkd. 1995;206:268–72. doi: 10.1055/s-2008-1035438. [DOI] [PubMed] [Google Scholar]
- 18.Sharif KW, Casey TA, Coltart J. Prevalence of mitral valve prolapse in keratoconus patients. J R Soc Med. 1992;85:446–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Maumenee IH. The cornea in connective tissue diseases. Ophthalmology. 1978;85:1014–7. doi: 10.1016/s0161-6420(78)35591-3. [DOI] [PubMed] [Google Scholar]
- 20.Maumenee IH. Hereditary connective tissue diseases involving the eye. Trans Ophthalmol Soc U K. 1974;94:753–63. [PubMed] [Google Scholar]
- 21.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 22.Maguire LJ, Meyer RF. Ectatic corneal degenerations. In: Kaufman HE, editor. The cornea. New York: Churchill Livingstone; 1988. pp. 485–510. [Google Scholar]
- 23.Gordon MO, Steger-May K, Szczotka-Flynn L, Riley C, Joslin CE, Weissman BA, et al. Baseline factors predictive of incident penetrating keratoplasty in keratoconus. Am J Ophthalmol. 2006;142:923–30. doi: 10.1016/j.ajo.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 24.Amsler M. Classic keratoconus and forme fruste keratoconus, unitary arguments. Ophthalmologica. 1946;111:96–101. doi: 10.1159/000300309. [DOI] [PubMed] [Google Scholar]
- 25.Alió JL, Shabayek MH. Corneal higher order aberrations: A method to grade keratoconus. J Refract Surg. 2006;22:539–45. doi: 10.3928/1081-597X-20060601-05. [DOI] [PubMed] [Google Scholar]
- 26.Perry HD, Buxton JN, Fine BS. Round and oval cones in keratoconus. Ophthalmology. 1980;87:905–9. doi: 10.1016/s0161-6420(80)35145-2. [DOI] [PubMed] [Google Scholar]
- 27.Watson SL, Ramsay A, Dart JK, Bunce C, Craig E. Comparison of deep lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus. Ophthalmology. 2004;111:1676–82. doi: 10.1016/j.ophtha.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Sray WA, Cohen EJ, Rapuano CJ, Laibson PR. Factors associated with the need for penetrating keratoplasty in keratoconus. Cornea. 2002;21:784–6. doi: 10.1097/00003226-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Snibson GR. Collagen cross-linking: A new treatment paradigm in corneal disease-A review. Clin Experiment Ophthalmol. 2010;38:141–53. doi: 10.1111/j.1442-9071.2010.02228.x. [DOI] [PubMed] [Google Scholar]
- 30.Spörl E, Huhle M, Kasper M, Seiler T. Increased rigidity of the cornea caused by intrastromal cross-linking. Ophthalmologe. 1997;94:902–6. doi: 10.1007/s003470050219. [DOI] [PubMed] [Google Scholar]
- 31.Andreassen TT, Simonsen AH, Oxlund H. Biomechanical properties of keratoconus and normal corneas. Exp Eye Res. 1980;31:435–41. doi: 10.1016/s0014-4835(80)80027-3. [DOI] [PubMed] [Google Scholar]
- 32.Cannon DJ, Foster CS. Collagen crosslinking in keratoconus. Invest Ophthalmol Vis Sci. 1978;17:63–5. [PubMed] [Google Scholar]
- 33.Malik NS, Moss SJ, Ahmed N, Furth AJ, Wall RS, Meek KM. Ageing of the human corneal stroma: Structural and biochemical changes. Biochim Biophys Acta. 1992;1138:222–8. doi: 10.1016/0925-4439(92)90041-k. [DOI] [PubMed] [Google Scholar]
- 34.Daxer A, Misof K, Grabner B, Ettl A, Fratzl P. Collagen fibrils in the human corneal stroma: Structure and aging. Invest Ophthalmol Vis Sci. 1998;39:644–8. [PubMed] [Google Scholar]
- 35.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29:1780–5. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 36.Tuft SJ, Moodaley LC, Gregory WM, Davison CR, Buckley RJ. Prognostic factors for the progression of keratoconus. Ophthalmology. 1994;101:439–47. doi: 10.1016/s0161-6420(94)31313-3. [DOI] [PubMed] [Google Scholar]
- 37.Morishige N, Nishida T, Jester JV. Second harmonic generation for visualizing 3-dimensional structure of corneal collagen lamellae. Cornea. 2009;28:S46–53. [Google Scholar]
- 38.Wollensak G. Crosslinking treatment of progressive keratoconus: New hope. Curr Opin Ophthalmol. 2006;17:356–60. doi: 10.1097/01.icu.0000233954.86723.25. [DOI] [PubMed] [Google Scholar]
- 39.McCall AS, Kraft S, Edelhauser HF, Kidder GW, Lundquist RR, Bradshaw HE, et al. Mechanisms of corneal tissue cross-linking in response to treatment with topical riboflavin and long-wavelength ultraviolet radiation (UVA) Invest Ophthalmol Vis Sci. 2010;51:129–38. doi: 10.1167/iovs.09-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26:385–9. doi: 10.1097/ICO.0b013e3180334f78. [DOI] [PubMed] [Google Scholar]
- 41.Spoerl E, Wollensak G, Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004;29:35–40. doi: 10.1080/02713680490513182. [DOI] [PubMed] [Google Scholar]
- 42.Wollensak G, Wilsch M, Spoerl E, Seiler T. Collagen fiber diameter in the rabbit cornea after collagen crosslinking by riboflavin/UVA. Cornea. 2004;23:503–7. doi: 10.1097/01.ico.0000105827.85025.7f. [DOI] [PubMed] [Google Scholar]
- 43.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: Long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 44.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–7. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 45.Caporossi A, Baiocchi S, Mazzotta C, Traversi C, Caporossi T. Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A rays induced cross-linking of corneal collagen: Preliminary refractive results in an Italian study. J Cataract Refract Surg. 2006;32:837–45. doi: 10.1016/j.jcrs.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 46.Agrawal VB. Corneal collagen cross-linking with riboflavin and ultraviolet-A light for keratoconus: Results in Indian eyes. Indian J Ophthalmol. 2009;57:111–4. doi: 10.4103/0301-4738.44515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vinciguerra P, Albè E, Trazza S, Rosetta P, Vinciguerra R, Seiler T, et al. Refractive, topographic, tomographic, and aberrometric analysis of keratoconic eyes undergoing corneal cross-linking. Ophthalmology. 2009;116:369–78. doi: 10.1016/j.ophtha.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 48.Wittig-Silva C, Whiting M, Lamoureux E, Lindsay RG, Sullivan LJ, Snibson GR. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: Preliminary results. J Refract Surg. 2008;24:S720–5. doi: 10.3928/1081597X-20080901-15. [DOI] [PubMed] [Google Scholar]
- 49.Hoyer A, Raiskup-Wolf F, Spörl E, Pillunat LE. Collagen cross-linking with riboflavin and UVA light in keratoconus. Results from Dresden. Ophthalmologe. 2009;106:133–40. doi: 10.1007/s00347-008-1783-2. [DOI] [PubMed] [Google Scholar]
- 50.Fournié P, Galiacy S, Arné JL, Malecaze F. Corneal collagen cross-linking with ultraviolet-A light and riboflavin for the treatment of progressive keratoconus. Fr Ophtalmol. 2009;32:1–7. doi: 10.1016/j.jfo.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Grewal DS, Brar GS, Jain R, Sood V, Singla M, Grewal SP. Corneal collagen crosslinking using riboflavin and ultraviolet-A light for keratoconus: One-year analysis using Scheimpflug imaging. J Cataract Refract Surg. 2009;35:425–32. doi: 10.1016/j.jcrs.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 52.Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009;35:1358–62. doi: 10.1016/j.jcrs.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 53.Mazzotta C, Balestrazzi A, Baiocchi S, Traversi C, Caporossi A. Stromal haze after combined riboflavin-UVA corneal collagen cross-linking in keratoconus: In vivo confocal microscopic evaluation. Clin Experiment Ophthalmol. 2007;35:580–2. doi: 10.1111/j.1442-9071.2007.01536.x. [DOI] [PubMed] [Google Scholar]
- 54.Wollensak G, Herbst H. Significance of the lacunar hydration pattern after corneal cross linking. Cornea. 2010;29:899–903. doi: 10.1097/ICO.0b013e3181ca3293. [DOI] [PubMed] [Google Scholar]
- 55.Dhawan S, Rao K, Natrajan S. Complications of corneal collagen cross-linking. J Ophthalmol 2011. 2011 doi: 10.1155/2011/869015. 869015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pérez-Santonja JJ, Artola A, Javaloy J, Alió JL, Abad JL. Microbial keratitis after corneal collagen crosslinking. J Cataract Refract Surg. 2009;35:1138–40. doi: 10.1016/j.jcrs.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 57.Pollhammer M, Cursiefen C. Bacterial keratitis early after corneal crosslinking with riboflavin and ultraviolet-A. J Cataract Refract Surg. 2009;35:588–9. doi: 10.1016/j.jcrs.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 58.Zamora KV, Males JJ. Polymicrobial keratitis after a collagen cross-linking procedure with postoperative use of a contact lens: A case report. Cornea. 2009;28:474–6. doi: 10.1097/ICO.0b013e31818d381a. [DOI] [PubMed] [Google Scholar]
- 59.Rama P, Di Matteo F, Matuska S, Paganoni G, Spinelli A. Acanthamoeba keratitis with perforation after corneal crosslinking and bandage contact lens use. J Cataract Refract Surg. 2009;35:788–91. doi: 10.1016/j.jcrs.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 60.Sharma N, Maharana P, Singh G, Titiyal JS. Pseudomonas keratitis after collagen crosslinking for keratoconus: Case report and review of literature. J Cataract Refract Surg. 2010;36:517–20. doi: 10.1016/j.jcrs.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 61.Kymionis GD, Portaliou DM, Bouzoukis DI, Suh LH, Pallikaris AI, Markomanolakis M, et al. Herpetic keratitis with iritis after corneal crosslinking with riboflavin and ultraviolet A for keratoconus. J Cataract Refract Surg. 2007;33:1982–4. doi: 10.1016/j.jcrs.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 62.Coskunseven E, Jankov MR, 2nd, Hafezi F. Contralateral eye study of corneal collagen cross-linking with riboflavin and UVA irradiation in patients with keratoconus. J Refract Surg. 2009;25:371–6. doi: 10.3928/1081597X-20090401-02. [DOI] [PubMed] [Google Scholar]
- 63.Vinciguerra P, Albè E, Mahmoud AM, Trazza S, Hafezi F, Roberts CJ. Intra-and postoperative variation in ocular response analyzer parameters in keratoconic eyes after corneal cross-linking. J Refract Surg. 2010;26:669–76. doi: 10.3928/1081597X-20100331-01. [DOI] [PubMed] [Google Scholar]


