Supplemental Digital Content is available in the text.
Keywords: exercise, heart failure, heart rate
Background—
Heart failure with preserved ejection fraction (HFpEF) is associated with significant morbidity and mortality but is currently refractory to therapy. Despite limited evidence, heart rate reduction has been advocated, on the basis of physiological considerations, as a therapeutic strategy in HFpEF. We tested the hypothesis that heart rate reduction improves exercise capacity in HFpEF.
Methods and Results—
We conducted a randomized, crossover study comparing selective heart rate reduction with the If blocker ivabradine at 7.5 mg twice daily versus placebo for 2 weeks each in 22 symptomatic patients with HFpEF who had objective evidence of exercise limitation (peak oxygen consumption at maximal exercise [Vo2 peak] <80% predicted for age and sex). The result was compared with 22 similarly treated matched asymptomatic hypertensive volunteers. The primary end point was the change in Vo2 peak. Secondary outcomes included tissue Doppler–derived E/e′ at echocardiography, plasma brain natriuretic peptide, and quality-of-life scores. Ivabradine significantly reduced peak heart rate compared with placebo in the HFpEF (107 versus 129 bpm; P<0.0001) and hypertensive (127 versus 145 bpm; P=0.003) cohorts. Ivabradine compared with placebo significantly worsened the change in Vo2 peak in the HFpEF cohort (-2.1 versus 0.9 mL·kg−1·min−1; P=0.003) and significantly reduced submaximal exercise capacity, as determined by the oxygen uptake efficiency slope. No significant effects on the secondary end points were discernable.
Conclusion—
Our observations bring into question the value of heart rate reduction with ivabradine for improving symptoms in a HFpEF population characterized by exercise limitation.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02354573.
Up to half of all patients with the clinical features of heart failure have preserved left ventricular ejection fraction (HFpEF), defined as an EF ≥50%.1–3 Mortality rates in patients with HFpEF are similar to those in patients with reduced EF (HFrEF)1,2,4 and are due largely to cardiovascular death.4,5 In contrast to HFrEF, there are no proven therapies for HFpEF despite the increasing prevalence and hospitalization rate.2,3 The failure of multiple investigational therapies to influence survival or to affect symptoms in HFpEF likely reflects heterogeneous case inclusion (including geographic variation in trial recruitment), suboptimal drug administration with regard to dose, stage or endophenotype of disease, or an incomplete conception of disease pathophysiology.6–9
Editorial see p 1687
Clinical Perspective on p 1725
HFpEF has been conceptualized, in part, as a disorder of diastolic function, reflecting impairments in active relaxation and intrinsic myocardial compliance.10 More broadly, these patients have impairments in ventricular-arterial coupling and of contractile function, albeit insufficient to reduce global left ventricular EF, and abnormally low skeletal muscle O2 extraction.11,12 Given the critical contribution of diastole to ventricular filling and coronary perfusion, reduction of heart rate, with a view to prolonging diastole, especially in atrial fibrillation, has been advocated as a therapeutic strategy to mitigate symptoms in HFpEF13 and has been endorsed by guidelines.14 However, increased heart rate is the major physiological contributor to the rise in cardiac output necessary to meet the metabolic demands of exercise,15 the capacity for which is substantially reduced in both HFpEF and HFrEF.16 Mechanistic studies of patients with HFpEF subject to exercise stress have implicated chronotropic incompetence as a potential contributor to impaired cardiac output reserve and thereby likely to contribute to the exertional dyspnea and effort intolerance characteristic of the syndrome.16–19 Accordingly, we sought to test the hypothesis that heart rate reduction improves exercise tolerance as assessed by peak oxygen consumption (Vo2 peak).
We performed a placebo-controlled, crossover, clinical study to evaluate the effects of short-term selective heart rate reduction with ivabradine (2 weeks), an inhibitor of the sinoatrial pacemaker funny current (If) considered devoid of effects on cardiac contractility,20 on the exercise performance of a homogeneous group of subjects with exercise-limited HFpEF. To substantiate the generalizability of the results and to inform our understanding of mechanisms responsible for exercise limitation, we performed a parallel study in a matched asymptomatic hypertensive group representing less advanced pathophysiology.
Methods
Study Design
We undertook a prospective, double-blind, placebo-controlled, randomized, crossover trial at 2 UK academic hospitals: the John Radcliffe Hospital (Oxford) and the Aberdeen Royal Infirmary. The study was designed to assess the effect of short-term administration of ivabradine on Vo2 peak and other parameters of exercise performance in a well-defined cohort of patients with HFpEF and a comparator asymptomatic hypertensive group. The study was approved by the Ethics Service committees in Aberdeen and Oxford (South Central). All participants provided written informed consent to study. Detailed methods are included in the online-only Data Supplement.
Study Patients
Consensus has not been reached on the optimal method(s) with which to define HFpEF patients; however, there is broad agreement that these dynamic disturbances during exercise cannot be predicted from resting measures of diastolic function.21 For these reasons, our inclusion criteria corresponded to those previously used,22 with rigorous cardiopulmonary exercise testing criteria to establish that the patients were objectively limited compared with age- and sex-predicted normal values.
HFpEF was defined according to the presence of both symptoms and signs of HF and EF ≥50%, a nondilated left ventricle and relevant structural heart disease in the form of left ventricular hypertrophy, left atrial enlargement, or evidence of diastolic dysfunction on echocardiography (mitral inflow E/A ratio, e′ measured at the mitral annulus, and E/e′ ratio).23 Eligible patients with HFpEF were at least 60 years of age with subjective exercise limitation owing to breathlessness or fatigue and objective evidence of exercise limitation as a measured Vo2 peak on cardiopulmonary exercise testing of <80% predicted for age and sex, with an appropriate pattern of gas exchange.24,25
Screening and Intervention
Eligible participants underwent screening assessment by history taking and physical examination, quality-of-life assessment measured by the Minnesota Living With Heart Failure Questionnaire,26 biochemical blood analysis, 12-lead ECG, transthoracic echocardiography, spirometry, and cardiopulmonary exercise testing.
We screened 65 patients for the HFpEF group and selected 34 matched asymptomatic hypertensive patients from a hypertension database over a 2-year period from December 2011 through January 2014 (Figure 1). Of these, 30 patients were found to be eligible to enter the HFpEF group, and all 34 patients were eligible for the asymptomatic hypertension group. The first 24 consecutive patients took part in the HFpEF group, of which 2 participants were excluded in the final analysis: 1 patient did not complete the study and dropped out during the second visit, and the other was excluded because of suboptimal exercise testing based on a respiratory exchange ratio of 0.81 during the second visit. Twenty-two asymptomatic hypertensive patients consented and participated in the study, and all were included in the final analysis. In line with existing trial protocols of ivabradine in HFrEF aiming for a heart rate target of 50 to 60 bpm,27,28 eligible participants were randomly assigned through block randomization to receive either ivabradine 7.5 mg twice daily or matching placebo tablets for 2 weeks (period 1). At the end of period 1, all screening assessments were repeated; in addition, cardiovascular magnetic resonance imaging was performed in the HFpEF cohort. After a 2-week washout period, subjects were then assigned to the alternative treatment arm (placebo or ivabradine) for a further 2 weeks (period 2). At the end of this, all period 1 assessments were repeated. Participants, investigators, and outcome assessors were all blinded to treatment allocation.
Figure 1.
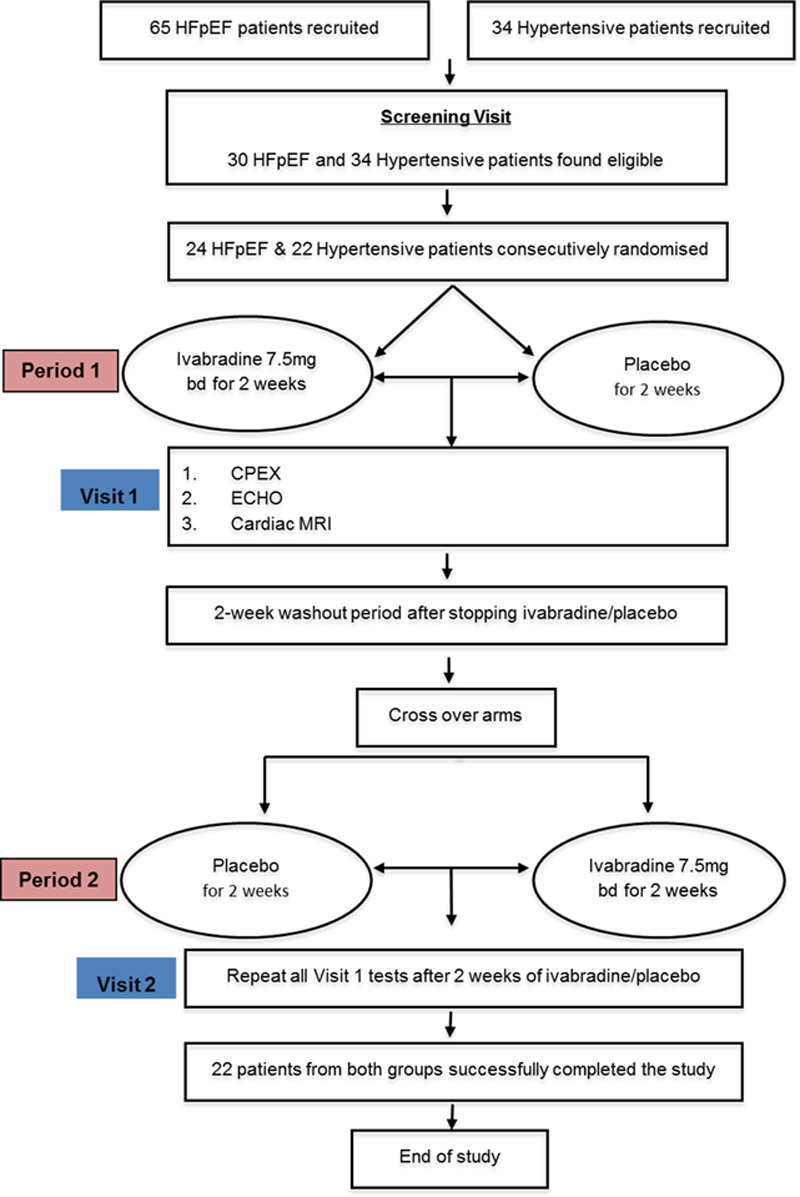
Flow diagram of the study. CPEX indicates cardiopulmonary exercise testing; ECHO, echocardiography; HFpEF, heart failure with preserved ejection fraction; and MRI, magnetic resonance imaging.
Study End Points
The predefined primary end point was the change in Vo2 peak. Secondary end points were changes in Doppler-derived E/e′, brain natriuretic peptide levels, and quality of life assessed by the Minnesota Living With Heart Failure Questionnaire.
Statistical Analysis
For a crossover study design, power calculation indicated that to detect a mean absolute difference in Vo2 peak of 2.5 mL·kg−1·min−1 (SD, 2.5 mL·kg−1·min−1), 22 patients could provide 90% power at an overall 2-sided α level of 0.05. Continuous variables are reported as mean±SD. Data sets were evaluated for normality by the Kolmogorov-Smirnov test. Comparisons between HFpEF and the asymptomatic hypertensive group were assessed by a 2-tailed Student t test. The comparisons between ivabradine and placebo treatments within patient groups were assessed by a 2-tailed paired Student t test. All statistical analyses were performed with the use of SPSS Statistics, version 19 (IBM). A value of P≤0.05 with a 2-tailed test was considered statistically significant.
Results
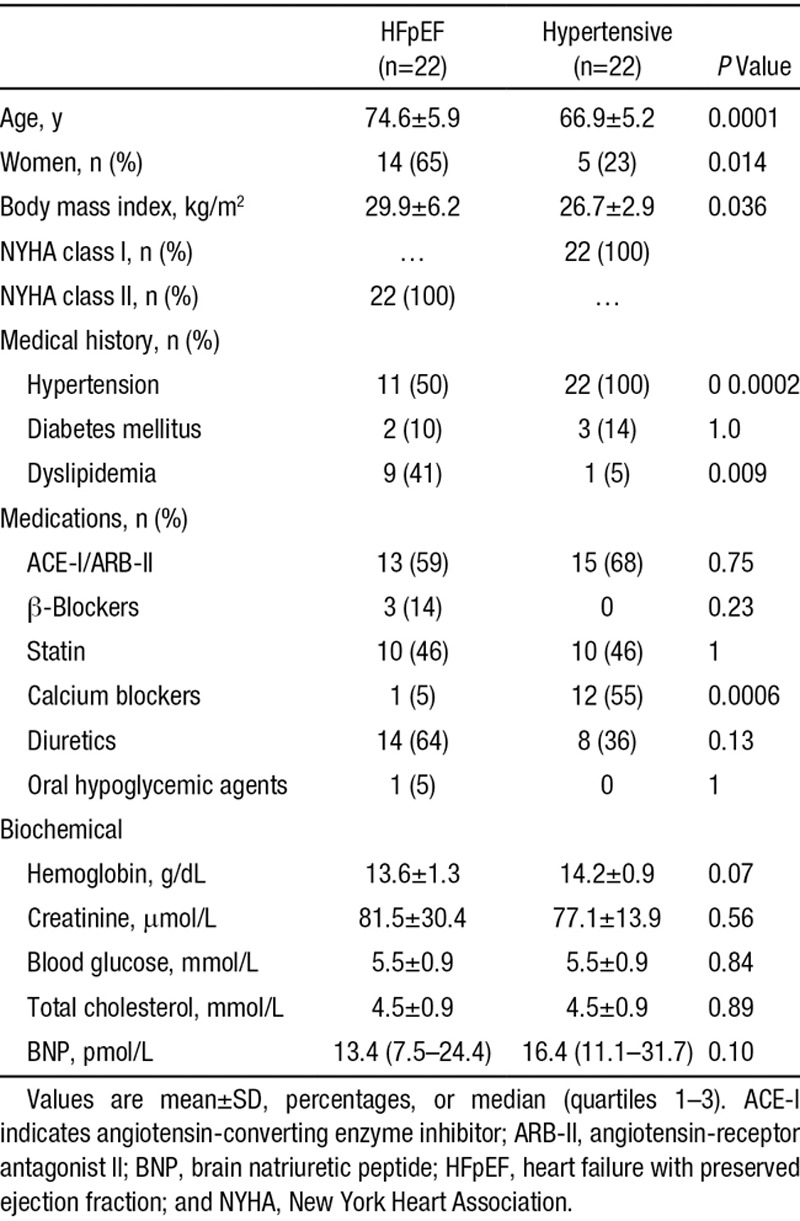
Baseline Clinical Characteristics
Baseline clinical characteristics of the patients are shown in Table 1. Compared with the hypertensive group, the patients with HFpEF were older (74.6 versus 66.9 years; P=0.0001), were more likely to be female (65% versus 23%; P=0.014), and had a lower proportion of hypertension (50% versus 100%; P=0.0002). Cardiovascular drug therapy was similar between groups, but there was a significantly greater use of calcium channel blockers in the hypertensive cohort (55% versus 5% in the HFpEF cohort; P=0.0006). Three HFpEF patients but no hypertensive individuals were taking β-blockers (P=0.23). All HFpEF patients but none in the hypertension group scored significantly on the Minnesota Living With Heart Failure Questionnaire at baseline. There were no differences in resting plasma brain natriuretic peptide. The baseline echocardiographic characteristics of HFpEF patients are shown in Table I in the online-only Data Supplement.
Table 1.
Clinical and Demographic Characteristics of the HFpEF and Asymptomatic Hypertensive Cohorts at Baseline
Response to Exercise
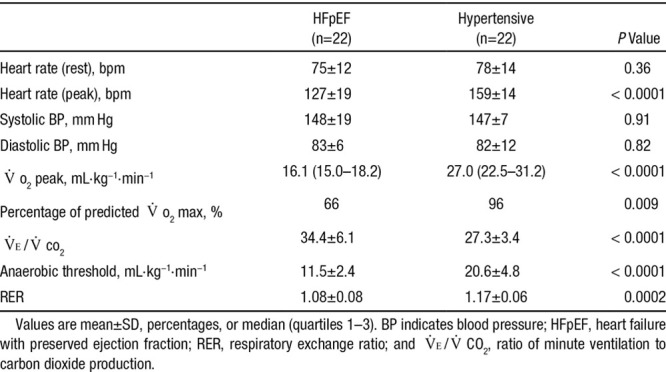
Cardiopulmonary exercise testing of HFpEF patients at baseline revealed a significantly lower Vo2 peak (16.1 versus 27.0 mL·kg−1·min−1 [P<0.0001] despite satisfactory effort indicated by a respiratory exchange ratio >1.0), anaerobic threshold (11.5 versus 20.6 mL·kg−1·min−1; P<0.0001), and maximal workload achieved (4.5 versus 7.7 metabolic equivalents; P<0.0001) compared with the hypertensive group but an increased ventilatory response to exercise, as indicated by a higher ratio of minute ventilation to carbon dioxide production (VE/Vco2; Table 2). Despite being asymptomatic, the hypertensive cohort had Vo2 peak values that were below mean age- and sex-predicted normal values (28.0 mL·kg−1·min−1). HFpEF patients had marked chronotropic dysfunction with lower peak exercise heart rates (129 versus 145 bpm; P<0.0001).
Table 2.
Baseline Hemodynamics and Cardiopulmonary Exercise Testing Characteristics of HFpEF and Asymptomatic Hypertensive Cohort
Selective Heart Rate Lowering With Ivabradine in the HFpEF Cohort
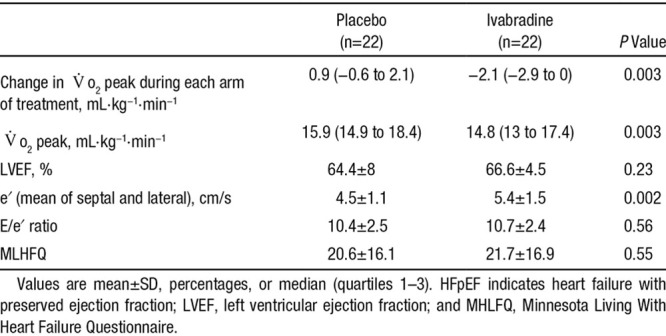
Table 3 and Table II in the online-only Data Supplement show the comparison of the effects of ivabradine versus placebo on resting hemodynamic, cardiac imaging, and exercise parameters in the HFpEF cohort. Ivabradine reduced the mean resting heart rate by 20 bpm (77 to 57 bpm; P<0.0001) without any effect on blood pressure or left ventricular EF. Similarly, ivabradine treatment reduced the chronotropic response to exercise (peak heart rate, 129 versus 107 bpm; P<0.0001). The heart rate reduction was accompanied by reduced peak oxygen consumption in the majority of HFpEF patients (19 patients had a reduction in the Vo2 peak), with a diminution in the Vo2 peak from 15.9 to 14.8 mL·kg−1·min−1 (P=0.003), without significantly affecting VE/Vco2 slope or the anaerobic threshold. Moreover, a paired comparison of the changes in Vo2 peak resulting from the 2-week intervention blocks demonstrated a consonant lowering in the ivabradine group (−2.1 versus 0.9 mL·kg−1·min−1; P=0.003; Figure 2). Compared with placebo, ivabradine treatment induced small but significant increases in the transmitral E/A ratio (0.6 versus 0.65; P=0.026) and mean e′ velocity (4.5 versus 5.4 cm/s; P=0.002), with no effect on the E/e′ ratio, ratio of myocardial phosphocreatine to adenosine triphosphate, or symptomatic status (Minnesota Living With Heart Failure Questionnaire; Table 3).
Figure 2.
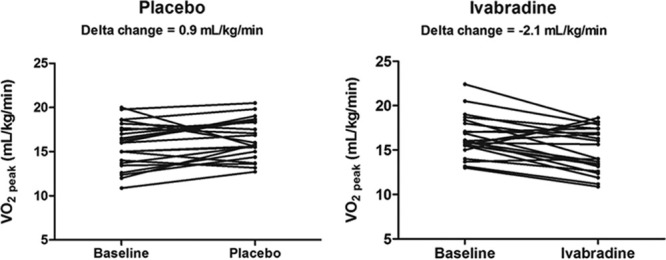
Effect of ivabradine on Vo2 peak in the heart failure with preserved ejection fraction (HFpEF) cohort. Depicts the change in Vo2 peak (mL·kg−1·min−1) with placebo (left) and ivabradine (right; ivabradine vs placebo, P=0.003) in the HFpEF cohort.
Table 3.
Effect of Ivabradine Versus Placebo on Cardiac Imaging and Exercise Parameters in the HFpEF Cohort
To assess the influence of ivabradine on submaximal exercise performance in patients with HFpEF, an analysis of the relationship between oxygen consumption and ventilation, defined as the oxygen uptake efficiency slope (OUES), was also undertaken (Table II and Figure I in the online-only Data Supplement). OUES is a submaximal measure of cardiorespiratory reserve less sensitive to exercise duration and has strong prognostic value in HF.29 Compared with placebo, an assessment of the OUES at 75% of the duration of exercise identified a significant reduction with ivabradine [1834 versus 1621 (mL/min O2)/(L/min VE); (P=0.04)].
Selective Heart Rate Lowering With Ivabradine in the Asymptomatic Hypertensive Cohort
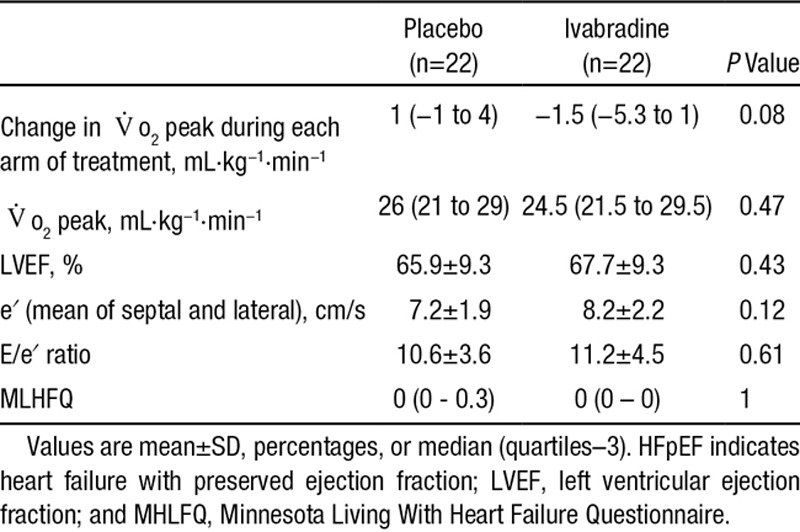
As with the HFpEF group, administration of ivabradine at 7.5 mg twice daily significantly reduced resting heart rate compared with placebo (from 74 to 61 bpm; P=0.001). Peak exercise heart rate was blunted by ivabradine use (145 versus 127 bpm; P=0.003). Ivabradine use was associated with a statistically nonsignificant reduction in the primary end point, Vo2 peak (26 versus 24.5 mL·kg−1·min−1; P=0.47; Table 4 and Table III in the online-only Data Supplement). Compared with placebo, ivabradine treatment was associated with a small but significant increase in the VE/Vco2 ratio (27.4 versus 29.2; P=0.004) but did not affect the anaerobic threshold or peak workload.
Table 4.
Effect of Ivabradine Versus Placebo on Cardiac Imaging and Exercise Parameters in the Asymptomatic Hypertensive Cohort
Discussion
We undertook a short term, placebo-controlled, randomized, crossover study examining the effect of selective heart rate lowering with the If inhibitor ivabradine on exercise capacity in a well-defined cohort of patients with symptomatic HFpEF. With individuals acting as their own controls, we found that 2 weeks of heart rate reduction with ivabradine at a dose of 7.5 mg twice daily in patients with HFpEF almost uniformly exacerbated already abnormal exercise physiology, resulting in a significant reduction in the primary end point, Vo2peak.
Consistent with previous reports,16–19 our cohort of HFpEF patients had poor exercise tolerance, a significantly impaired peak oxygen uptake, low Vo2 at the anaerobic threshold, increased ventilatory response, and a reduction of the chronotropic response to exercise. Because of the broader pathogenesis of HFpEF, including prominent defects in skeletal muscle metabolism,11,12 our patients with HFpEF were not diagnosed on the basis of resting diastolic dysfunction but rather on the basis of subjective exercise limitation with normal left ventricular EF and the absence of significant valvular disease, together with objective exercise limitation. In the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) study, of 935 patients with HFpEF, diastolic function was normal in approximately one third of gradable participants.30 We also have observed a poor agreement between exercise E/E′, cardiopulmonary exercise testing categorization, and current criteria based on resting diastolic function.31 We and others have found that HFpEF is characterized by dynamic disturbances of left ventricular active relaxation during exercise.32–34 Furthermore, plasma brain natriuretic peptide is often relatively normal at rest in HFpEF, especially in those with a high body mass index in whom brain natriuretic peptide appears to be suppressed but rises dramatically on exercise (unpublished data).31,34,35
The choice of Vo2 peak as the primary end point in this study is supported by its objective measurement of cardiac reserve, its robust correlation with survival,36 and the difficulties in obtaining a true maximal oxygen uptake (Vo2max), which relies on exercise to absolute exhaustion with plateauing of oxygen uptake despite continued exercise.29 In common with Vo2max, Vo2 peak is effort dependent and does not provide insight into potential differences in submaximal exercise capacity that may be more reflective of the levels of exertion that result in symptoms in HF patients. To address this possibility, we also evaluated a measure of submaximal cardiopulmonary reserve, the OUES, the value of which has, unlike Vo2, been shown to be relatively independent of exercise duration and an even more powerful predictor of prognosis than conventional measures of exercise performance.29 We found that ivabradine treatment also significantly reduced submaximal cardiorespiratory reserve in HFpEF. Although there was a significant change in certain parameters of exercise capacity, ivabradine treatment did not discernibly alter the cardiac energetic status (ratio of phosphocreatine to adenosine triphosphate) of the HFpEF or hypertensive patients.
In contrast to the Class Ia evidence in HFrEF, limited evidence-based treatment options exist for the management of HFpEF. Current therapy includes heart rate reduction, a strategy based on physiological observations dating to the late 19th century that, primarily at higher heart rates, shortening of the diastolic filling period impairs cardiac filling and results in lower stroke volumes.37 The findings from the present study question this widely held approach, indicating that even short-term selective heart rate lowering in the presently defined population of HFpEF patients acts to impair exercise capacity, not least as the relationship between decreasing heart rate and increasing stroke volume is asymmetrical. Moreover, the observation that despite ameliorating some measures of cardiac filling (eg, e′), heart rate reduction almost uniformly adversely affects exercise tolerance highlights the need for a broader conceptualization of HFpEF as a chronic, complex disorder of integrated cardiovascular reserve rather than a purely diastolic disease.11,12,16,38
Kosmala et al13 reported increased exercise capacity in patients with HFpEF after short-term treatment with ivabradine. The reasons underlying the discrepancy with the present study are unclear but may reflect the younger population studied (mean age, 67 years), atypical of the clinical population seen with HFpEF, shorter duration, and lower dose of ivabradine (7 days of 2.5–5 mg twice daily, resulting in a reduction in resting heart rate of 10 bpm), which did not appear to affect peak heart rate response to exercise and perhaps study design (not crossover). Our patients, being older and at an age more typical of the HFpEF population, with advanced chronotropic incompetence and diminished stroke volume reserve (a largely fixed stroke volume) were more sensitive to heart rate reduction.
The present proof-of-concept study was not designed to address whether selective heart rate slowing had longer-term effects on survival or hospitalization. However, the significant and consistent reduction in multiple metabolic stress testing parameters linked to mortality in HF, including reduced Vo2 peak, increased VE/Vco2,39 low chronotropic response,39 and reduced OUES,29 suggests the need for caution with indiscriminate heart rate reduction in this population of patients. Harmful off-target effects beyond the inhibition of sinoatrial If are possible. Indeed, subgroup analyses of the Effects Of Ivabradine in Patients With Stable Coronary Artery Disease Without Heart Failure (SIGNIFY) trial using ivabradine suggested a signal for cardiovascular harm in stable coronary artery disease.40 However, we speculate that we are observing mechanism-related drug effects and that reducing heart rate with other agents (eg, β-adrenergic blockade) may confer similar or more profound adverse effects in HFpEF as a result of their impact on heart rate and exercise-dependent ventricular lusitropy.41
The following represent potential limitations of our study. First, the sample size studied was small, but the treatment was not found to be beneficial in either group. Importantly, in this crossover study, we observed no evidence of a carryover or period effect; the washout period of 2 weeks (336 hours) exceeds the biological half-life of ivabradine (2 hours). Nevertheless, the study needs to be replicated in a larger clinical trial examining a well-defined homogeneous cohort powered to look at mortality and morbidity end points that may support this and point to alternative strategies to improve exercise intolerance. Second, the heart rate reduction of 20 bpm in our study group was greater than previously studied in trials using ivabradine. A beneficial effect resulting from a lesser heart rate reduction cannot be excluded.
Conclusion
The results of the present study do not support a general strategy of heart rate reduction in HFpEF and question its role in improving symptoms in these patients.
Sources of Funding
The study was funded by a project grant from the Chest Heart and Stroke Society. Drs Mahmod, Neubauer, and Ashrafian are supported by the National Institute for Health Research Oxford Biomedical Research Center based at The Oxford University Hospitals Trust at the University of Oxford. Dr Yavari is supported by the UK Department of Health’s National Institute for Health Research.
Acknowledgments
Dr Ashrafian acknowledges support from the BHF Center of Research Excellence, Oxford, UK. The research was also supported by the National Institute for Health Research Oxford Biomedical Research Center Program and by the National Institute for Health Research Rare Diseases Translational Research Collaboration (NIHR RD-TRC).
Disclosures
None.
Supplementary Material
Footnotes
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
Drs Frenneaux and Ashrafian contributed equally.
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.115.017119/-/DC1.
CLINICAL PERSPECTIVE
Heart failure with preserved ejection fraction (HFpEF) accounts for nearly 50% of all the heart failure, with mortality figures now comparable to those in patients with heart failure with reduced ejection fraction. Despite this unmet clinical need, our limited understanding of the broad systemic pathophysiology has translated into a lack of proven therapies for HFpEF. Predicated on the hypothesis that heart rate reduction promotes diastolic filling with corresponding benefits in diastolic function in HFpEF, we investigated the influence of heart rate reduction with ivabradine in these patients in a double-blind crossover trial. Ivabradine is a particularly useful agent in this context because it acts on the If channels in the sinoatrial node and lowers the heart rate without substantially affecting cardiac contractility. The diagnosis of HFpEF was made with conventional echocardiographic criteria and with cardiopulmonary exercise testing to capture the dynamic impairments of physiology characteristic of HFpEF. We found heart rate reduction with ivabradine to have a largely consistently detrimental effect on peak oxygen uptake and on submaximal exercise capacity. Although a larger clinical end-point study is required, we believe that this report has implications for the widespread practice of heart rate reduction in this context.
References
- 1.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC Get With the Guidelines Scientific Advisory Committee and Investigators. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 4.Tribouilloy C, Rusinaru D, Mahjoub H, Soulière V, Lévy F, Peltier M, Slama M, Massy Z. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J. 2008;29:339–347. doi: 10.1093/eurheartj/ehm554. doi: 10.1093/eurheartj/ehm554. [DOI] [PubMed] [Google Scholar]
- 5.Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, Lopez-Sendon J, Teerlink JR, White M, McMurray JJ, Komajda M, McKelvie R, Ptaszynska A, Hetzel SJ, Massie BM, Carson PE I-Preserve Investigators. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 2010;121:1393–1405. doi: 10.1161/CIRCULATIONAHA.109.909614. doi: 10.1161/CIRCULATIONAHA.109.909614. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 7.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 8.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 9.Kristensen SL, Køber L, Jhund PS, Solomon SD, Kjekshus J, McKelvie RS, Zile MR, Granger CB, Wikstrand J, Komajda M, Carson PE, Pfeffer MA, Swedberg K, Wedel H, Yusuf S, McMurray JJ. International geographic variation in event rates in trials of heart failure with preserved and reduced ejection fraction. Circulation. 2015;131:43–53. doi: 10.1161/CIRCULATIONAHA.114.012284. doi: 10.1161/CIRCULATIONAHA.114.012284. [DOI] [PubMed] [Google Scholar]
- 10.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure: abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 11.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS, Lewis GD. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8:286–294. doi: 10.1161/CIRCHEARTFAILURE.114.001825. doi: 10.1161/CIRCHEARTFAILURE.114.001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosmala W, Holland DJ, Rojek A, Wright L, Przewlocka-Kosmala M, Marwick TH. Effect of If-channel inhibition on hemodynamic status and exercise tolerance in heart failure with preserved ejection fraction: a randomized trial. J Am Coll Cardiol. 2013;62:1330–1338. doi: 10.1016/j.jacc.2013.06.043. doi: 10.1016/j.jacc.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 14.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 15.Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–291. doi: 10.1161/01.res.58.2.281. doi: 10.1161/01.RES.58.2.281. [DOI] [PubMed] [Google Scholar]
- 16.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 17.Brubaker PH, Joo KC, Stewart KP, Fray B, Moore B, Kitzman DW. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil. 2006;26:86–89. doi: 10.1097/00008483-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Phan TT, Shivu GN, Abozguia K, Davies C, Nassimizadeh M, Jimenez D, Weaver R, Ahmed I, Frenneaux M. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:29–34. doi: 10.1161/CIRCHEARTFAILURE.109.877720. doi: 10.1161/CIRCHEARTFAILURE.109.877720. [DOI] [PubMed] [Google Scholar]
- 19.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–785. doi: 10.1093/eurjhf/hft026. doi: 10.1093/eurjhf/hft026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiFrancesco D. The role of the funny current in pacemaker activity. Circ Res. 2010;106:434–446. doi: 10.1161/CIRCRESAHA.109.208041. doi: 10.1161/CIRCRESAHA.109.208041. [DOI] [PubMed] [Google Scholar]
- 21.Senni M, Paulus WJ, Gavazzi A, Fraser AG, Díez J, Solomon SD, Smiseth OA, Guazzi M, Lam CS, Maggioni AP, Tschöpe C, Metra M, Hummel SL, Edelmann F, Ambrosio G, Stewart Coats AJ, Filippatos GS, Gheorghiade M, Anker SD, Levy D, Pfeffer MA, Stough WG, Pieske BM. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J. 2014;35:2797–2815. doi: 10.1093/eurheartj/ehu204. doi: 10.1093/eurheartj/ehu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Löffler M, Düngen HD, Tschöpe C, Herrmann-Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309:781–791. doi: 10.1001/jama.2013.905. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 23.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology: developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 24.ATS/ACCP statement on cardiopulmonary exercise testing. Am J Resp Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 25.Edelmann F, Wachter R, Pieske B. Aldosterone inhibition in patients with heart failure with preserved ejection fraction: reply. JAMA. 2013;310:205–207. doi: 10.1001/jama.2013.7976. doi: 10.1001/jama.2013.7976. [DOI] [PubMed] [Google Scholar]
- 26.Garin O, Ferrer M, Pont A, Rué M, Kotzeva A, Wiklund I, Van Ganse E, Alonso J. Disease-specific health-related quality of life questionnaires for heart failure: a systematic review with meta-analyses. Qual Life Res. 2009;18:71–85. doi: 10.1007/s11136-008-9416-4. doi: 10.1007/s11136-008-9416-4. [DOI] [PubMed] [Google Scholar]
- 27.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61259-7. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 28.Fox K, Ford I, Steg PG, Tendera M, Ferrari R BEAUTIFUL Investigators. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807–816. doi: 10.1016/S0140-6736(08)61170-8. doi: 10.1016/S0140-6736(08)61170-8. [DOI] [PubMed] [Google Scholar]
- 29.Baba R, Nagashima M, Goto M, Nagano Y, Yokota M, Tauchi N, Nishibata K. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol. 1996;28:1567–1572. doi: 10.1016/s0735-1097(96)00412-3. doi: 10.1016/S0735-1097(96)00412-3. [DOI] [PubMed] [Google Scholar]
- 30.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O’Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014;7:740–751. doi: 10.1161/CIRCHEARTFAILURE.114.001583. doi: 10.1161/CIRCHEARTFAILURE.114.001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahadevan G, Dwivedi G, Jiminez D, Williams L, Frenneaux MP. How valid are the European Society of Cardiology echocardiographic diastolic criteria in diagnosing heart failure with normal ejection fraction? Eur Heart J. 2011;32:778–778. [Google Scholar]
- 32.Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A, Frenneaux M. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009;54:402–409. doi: 10.1016/j.jacc.2009.05.012. doi: 10.1016/j.jacc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97:964–969. doi: 10.1136/hrt.2010.212787. doi: 10.1136/hrt.2010.212787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, Shah SJ. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:870–876. doi: 10.1016/j.amjcard.2012.05.014. doi: 10.1016/j.amjcard.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.cir.83.3.778. doi: 10.1161/01.CIR.83.3.778. [DOI] [PubMed] [Google Scholar]
- 37.Nagayama T, Takimoto E, Sadayappan S, Mudd JO, Seidman JG, Robbins J, Kass DA. Control of in vivo left ventricular [correction] contraction/relaxation kinetics by myosin binding protein C: protein kinase A phosphorylation dependent and independent regulation. Circulation. 2007;116:2399–2408. doi: 10.1161/CIRCULATIONAHA.107.706523. doi: 10.1161/CIRCULATIONAHA.107.706523. [DOI] [PubMed] [Google Scholar]
- 38.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–515. doi: 10.1038/nrcardio.2014.83. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 39.Robbins M, Francis G, Pashkow FJ, Snader CE, Hoercher K, Young JB, Lauer MS. Ventilatory and heart rate responses to exercise: better predictors of heart failure mortality than peak oxygen consumption. Circulation. 1999;100:2411–2417. doi: 10.1161/01.cir.100.24.2411. doi: 10.1161/01.CIR.100.24.2411. [DOI] [PubMed] [Google Scholar]
- 40.Fox K, Ford I, Steg PG, Tardif JC, Tendera M, Ferrari R SIGNIFY Investigators. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. 2014;371:1091–1099. doi: 10.1056/NEJMoa1406430. doi: 10.1056/NEJMoa1406430. [DOI] [PubMed] [Google Scholar]
- 41.Cheng CP, Igarashi Y, Little WC. Mechanism of augmented rate of left ventricular filling during exercise. Circ Res. 1992;70:9–19. doi: 10.1161/01.res.70.1.9. doi: 10.1161/01.RES.70.1.9. [DOI] [PubMed] [Google Scholar]


