Abstract
Stastny, P, Lehnert, M, Zaatar, AMZ, Svoboda, Z, and Xaverova, Z. Does the dumbbell-carrying position change the muscle activity in split squats and walking lunges? J Strength Cond Res 29(11): 3177–3187, 2015—The forward walking lunge (WL) and split squat (SSq) are similar exercises that have differences in the eccentric phase, and both can be performed in the ipsilateral or contralateral carrying conditions. This study aimed to determine the effects of dumbbell-carrying position on the kinematics and electromyographic (EMG) amplitudes of the gluteus medius (Gmed), vastus medialis (VM), vastus lateralis (VL), and biceps femoris during WLs and SSqs. The resistance-trained (RT) and the non–resistance-trained (NT) groups (both n = 14) performed ipsilateral WLs, contralateral WLs, ipsilateral SSqs, and contralateral SSqs in a randomized order in a simulated training session. The EMG amplitude, expressed as a percentage of the maximal voluntary isometric contraction (%MVIC), and the kinematics, expressed as the range of motion (ROM) of the hip and knee, were measured during 5 repetition maximum for both legs. The repeated measure analyses of variance showed significant differences between the RT and NT groups. The NT group showed a smaller knee flexion ROM (p < 0.001, η2 = 0.36) during both types of WLs, whereas the RT group showed a higher eccentric Gmed amplitude (p < 0.001, η2 = 0.46) during all exercises and a higher eccentric VL amplitude (p < 0.001, η2 = 0.63) during contralateral WLs. Further differences were found between contralateral and ipsilateral WLs in both the RT (p < 0.001, η2 = 0.69) and NT groups (p < 0.001, η2 = 0.80), and contralateral WLs resulted in higher eccentric Gmed amplitudes. Contralateral WLs highly activated the Gmed (90% MVIC); therefore, this exercise can increase the Gmed maximal strength. The ipsilateral loading condition did not increase the Gmed or VM activity in the RT or NT group.
Key Words: electromyography, strength training, gluteus medius, ipsilateral loading, contralateral loading, vastus lateralis
Introduction
Exercise selection is a key point when creating a resistance training program. For example, side lunges and forward lunges have been shown to activate the gluteus medius (Gmed) (16,18), which is an important muscle for controlling the frontal plane motion of the pelvic hip complex. The Gmed stabilizes the hip during unilateral stance to prevent the pelvis from dropping on the unsupported side and has also been proven to be critical for controlling internal rotation of the femur during closed kinetic chain activities (24). Weakness of the Gmed has been associated with lower back pain (41), patellofemoral pain syndrome (10,22,30), iliotibial bend syndrome (23), increased injury risks in athletes (35), and decreased sport performance (37). Not only do some exercises target certain muscle groups over others but also the activation of a specific muscle group can be altered by performing variations on the same exercise. For example, specific types of squats and lunges result in different activations of the vastus medialis (VM) compared with the vastus lateralis (VL) (21,31,32). Specifically, squats performed with greater hip adduction not only activate the VM more than the VL but also activate the Gmed more than conventional squats do (21). Additionally, muscle activation differs between unilateral and bilateral squats. For example, the Gmed and hamstrings are more active than the quadriceps during unilateral squats, whereas during bilateral squats, the quadriceps are more active than the Gmed and hamstrings (39).
The VM and VL are 2 of the key muscles that control the frontal plane kinematics of the knee (49), which may also influence the activation of other muscles. An imbalance between VM and VL has been associated with anterior cruciate ligament injury and patellofemoral pain syndrome (19,31). However, knee stability is also dependent on hamstring function, such as the activity of the biceps femoris (BF) (11,47). With the previously mentioned facts in mind, performing variations of squats and lunges may increase the activation of the VM, BF, and Gmed, possibly resulting in changes in strength that may be important for injury prevention and rehabilitation. However, if VL activation exceeds VM activation by a ratio greater than 1:1, the risk of injury may be increased, especially in non-trained individuals and during the rehabilitation process (31). However, high VL activity may be important for resistance-trained (RT) individuals if it is beneficial for performance in specific sports (34,38). Therefore, it is important to determine an individual's specific needs and to select the appropriate exercises to target these needs.
The Gmed, VM, VL, and BF play key roles in knee stability and pathology, suggesting that complex training programs should include a focus on strengthening the specific muscle groups appropriate to the athlete's needs. Different variations of lunges and squats are commonly used in both professional and recreational resistance training. One may consider performing different squat and lunge variations if the aim of the exercises is to activate certain muscles in a closed kinetic chain exercise. Walking lunges (WLs) have been shown to activate the quadriceps more than the hamstrings, whereas jumping lunges with different eccentric characteristics produce even greater electromyographic (EMG) activity of the quadriceps than WLs do (33). WLs and single-leg squats are considered traditional rehabilitation exercises (16,24,45) that are effective in rehabilitation programs (1) and should be included in injury prevention training programs.
Recent studies (19,24,45) have reported the EMG values for a variety of weight-bearing exercises. However, when prescribing an exercise as part of a resistance training program, one should consider that muscle activation can be varied by altering the exercise intensity (32), changing the kinematics (17), changing the way the eccentric actions are performed (33), training experience (12,26,36), and asymmetrical loading (42,43). Forward WLs have kinematics similar to those of stationary split squats (SSqs); the major difference between the 2 is that the dynamic nature of WLs results in impact forces during landing, whereas the SSqs do not because both feet are constantly fixed to the ground, with one foot in front of the other. Therefore, SSqs are included in resistance training programs by practitioners before WLs. In addition, both these exercises allow for a large range of motion (ROM), such as peak knee and hip flexion over 90° angle, even in non–resistance-trained (NT) individuals (14,20,40), and they can be externally loaded using dumbbells in an ipsilateral or contralateral fashion.
To our knowledge, the combined effect of dumbbell-carrying position and the characteristics of the eccentric phase and joint kinematics on muscle activity has not yet been explored. Therefore, the purpose of this study was to determine the effect of dumbbell-carrying position on the EMG amplitudes of Gmed, VM, VL, and BF during WLs and SSqs in RT and NT men. The hypothetical presumption was that the ipsilateral loading condition would yield a greater level of Gmed activity during WLs and SSqs because of the increased need for lateral stabilization, which may also change the level of VL and VM activities and hip and knee kinematics. Another hypothesis was that RT men would exhibit greater EMG amplitude during WLs and SSqs compared with NT men. Specifically, this study has 4 objectives: to determine whether the EMG amplitudes of the selected muscles is associated with changes in knee and hip joint kinematics during SSqs and WLs, to determine whether the EMG amplitude differs between RT and NT men, to determine whether hip and knee joint kinematics are different during WLs and SSqs in RT and NT men, and to determine whether there are differences in muscle activity between ipsilateral and contralateral loading conditions during WL and SSq exercises. The results of this study may provide insight to guide the selection of exercises to include in resistance training programs, specifically in terms of training status, dumbbell-carrying position, and the choice of unilateral exercise.
Methods
Experimental Approach to the Problem
The present investigation was a cross-sectional study that was performed in the biomechanics laboratory at Palacky University during the power-lifting preseason (May and June 2014). The hypothetical presumption was that the ipsilateral and contralateral loading conditions (independent variables) would result in reciprocal differences in EMG and kinematics as dependent variables. The testing procedure was performed in the same form as a training session (described below). This procedure tested the hypotheses that the ipsilateral loading condition would result in a greater level of Gmed, VL, or VM EMG amplitude during WLs and SSqs and that hip and knee joint kinematics are associated with the EMG amplitudes of selected muscles. The differences between RT and NT men were tested by examining dependent variables in these research groups.
Subjects
The participants included 28 men between the age of 24 and 35 years divided into a RT group and a NT, as described in Table 1, along with isometric performance. At the time of data collection, none of the subjects had reported having recently implemented ipsilateral or contralateral loading of SSq or WL in their training programs. The RT group included 14 competitive power lifters competing in the Czech championships during the 2014 season (deep back squat 1 repetition maximum [1RM], 149 ± 37 kg; strength training age, 12 ± 6 years) with at least 5 years of strength training experience in a self-reported structured training program, which included at least 3 resistance training sessions per week for the lower limbs. The NT group included 14 recreational sportsmen recruited from Palacky University who performed fewer than 2 lower-limb resistance training sessions per week. All the participants were older than 18 years of age and lacked any pathologies or injuries. Written informed consent was provided by all participants, and the testing protocol with informed consent was approved by the local ethics committee at Palacky University in Olomouc, in accordance with the ethical standards of the Helsinki Declaration of 1983. All the participants were informed of and shown the testing protocols and all aspects of the investigation when they signed the written informed consent form for the study. Additionally, written informed consent was obtained from the subject pictured in Figure 1 for the publication of his image.
Table 1.
Subject and group characteristics with isometric measurement results.*
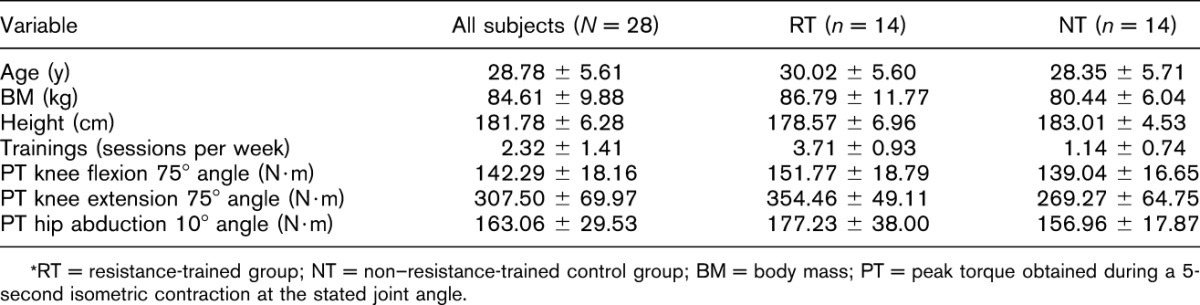
Figure 1.
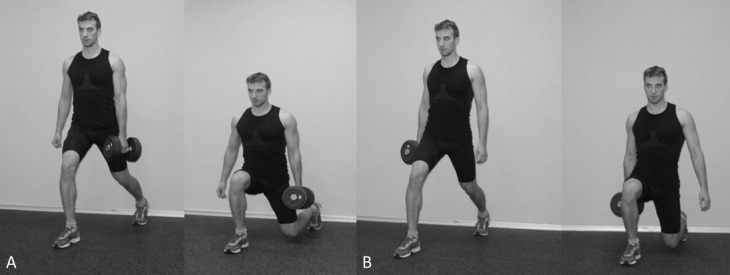
Dumbbell position during ipsilateral vs. contralateral split squats. A) Contralateral split squat. B) Ipsilateral split squat.
Procedures
Initial anthropometric measurements were taken to record the participants' height, body mass, leg length, knee width, ankle width, and greater trochanter-to-anterior-superior iliac spine distance. The warm-up procedure consisted of 5 minutes of stationary cycling and one set of 25 bodyweight squats using different foot positions. After the warm-up, EMG electrodes were secured to the skin over the belly of the VM, the VL, the BF, and the Gmed and were kept in place throughout the entire measurement period. The participants performed a 5-second maximal voluntary isometric contraction (MVIC) on an isokinetic dynamometer for knee extension, knee flexion, and hip abduction to establish the EMG signal during maximum effort. Three-dimensional (3D) reflective markers were taped bilaterally onto each subject before the WL and SSq exercises. Four exercises (WLs and SSqs with the dumbbell on the ipsilateral and contralateral sides) were performed in a random order. Each exercise was performed first with bodyweight for 5 repetitions with one leg as the stance leg (i.e., the leg that was in front during the lunge or squat) followed by 60 seconds of rest and then 5 bodyweight repetitions with the other leg as the stance leg. After a 1- to 3-minute rest period, the first dumbbell load of 12.5 kg was used for the next set of 5 repetitions for each leg with 60 seconds of rest between legs followed by another 1–3 minutes of rest period and another weight increase of approximately 12.5 kg for 5 repetitions with 60 seconds of rest between legs. This process was repeated until the dumbbell mass exceeded the subject's ability to perform 5 repetitions (5RM). The recommendations of the American Society of Exercise Physiology were followed for this task (6): at least 60 seconds and a maximum of 3 minutes of rest were included between subsequent sets of each exercise (i.e., each increase in dumbbell load) but only the 5RM sets were included in the statistical analyses. Once the 5RM was determined for the first exercise, the same protocol was used for the remaining 3 exercises, which were performed in random order.
Instrumentation
Maximal voluntary isometric contraction was determined using an isokinetic dynamometer IsoMed 2000 (D&R Ferstl GmbH, Hemau, Germany), which has been reported to have high reproducibility for peak torque measurement (15). The EMG data were collected with a Noraxon 1400A device (Noraxon, Scottsdale, AZ, USA). Kinematic data were collected using a 6-camera Vicon-612 infrared motion analysis system (Oxford Metrics, Oxford, United Kingdom) with established validity (48), which was completed with 2 force plates (Kistler Instrumente, Winterthur, Switzerland). The Vicon motion analysis system, EMG, and force plate outputs were connected to and fully synchronized via analogue signal with the Vicon Nexus software (Oxford Metrics). These procedures are further explained below.
Exercises
All 4 exercises were performed with both the dominant and nondominant legs in a randomized order. A 60-second rest period was included between the exercise set with the first limb as the stance leg and the set with the opposite limb as the stance leg.
The ipsilateral WLs started with the subjects standing with their feet together on one force platform and their hands parallel to their trunks. The dumbbell was carried in one hand, and the lunge step was initiated by the ipsilateral leg stepping on the second force platform. The end of the exercise was defined as the end of foot contact with the second force platform, when the stance (loaded) leg was returned to the starting position. The full range of the lunge was performed while the trunk was kept in an upright position, and the participants were instructed to “lunge down as far as possible” (17). The step distance was equal to the leg length, which was determined by measuring from the anterior-superior iliac spine to the medial malleolus of the tibia (5,17).
The contralateral WLs started and finished in the same manner as the ipsilateral WLs, and the subjects were given the same verbal instruction. The only difference was that the leg opposite the hand holding the dumbbell was the leg that performed the lunge.
For the ipsilateral SSqs, the participant started by standing in the lunge position (described above) with one foot on each force plate, with the supported (rear) leg standing on the toes and the stance leg flat on the force plate. The dumbbell was carried in the hand that was ipsilateral to the stance leg (Figure 1B). The full range of the SSq was performed with the trunk kept in an upright position. The step distance was equal to the leg length, as determined by measuring from the anterior-superior iliac spine to the medial malleolus of the tibia (5,17), and the participants were instructed to “squat down as far as possible.”
The contralateral SSqs (Figure 1A) started and finished in the same manner as the ipsilateral SSqs, and the subjects were given the same verbal instruction. The only difference was that the leg opposite the hand holding the dumbbell was the leg that performed the squat.
Isometric Strength Measurement
To obtain the maximal value of the EMG signal, the subjects performed a 5-second MVIC on the dynamometer for unilateral knee flexion and extension and hip abduction for both legs. Each participant performed 2 consecutive measurements of each muscle group with 45 seconds of rest intervals. A full passive ROM and 2 submaximal isometric trials against resistance were performed on the dynamometer before each MVIC attempt to avoid injury. The greatest EMG value was used for the statistical analyses, and the peak torque of that trial was used to describe maximal isometric strength, as shown in Table 1.
First, the knee extensors (VM and VL) and knee flexors (BF) were tested for each leg. Maximal voluntary isometric contractions were measured in the standard sitting position with 75° angle knee flexion. The backrest of the dynamometer seat was set to an angle of 75° angle, and the angle of the hip joint was 100° angle. The arm of the dynamometer lever was fixed to the distal part of the shin, and the lower edge of the shin pad was placed 2.5 cm over the medial apex malleolus. The subjects were secured with belts in the pelvic region and the thigh region of the tested lower limbs that did not interfere with the electrodes placed on the VM and VL. Adjustable straps and pads were placed on the shoulders, and the participants held handgrips along the seats. The mechanical axis of the dynamometer was aligned with the knee axis according to the standard position for knee flexion/extension (15).
Reference Gmed values for MVIC were obtained during side-lying hip abduction (8,35). The subjects were positioned with the tested lower extremity at 10° angle of hip abduction and 10° angle of hip flexion. The arm of the dynamometer lever was fixed to the lateral thigh of the tested limb 1 cm above the patella. To keep the testing position of the tested leg fixed, a strap was used. The axis of rotation of the dynamometer was aligned with the greater trochanter of the femur.
The participants were provided with concurrent visual feedback in the form of an isokinetic strength curve displayed on the dynamometer monitor. Verbal encouragement was also provided.
Electromyographic Measurement
Raw EMG signals were recorded bilaterally by 8 leads and sampled at 1,000 Hz. Two bipolar surface electrodes (adhesive disposable electrode; Kendall, Mansfield, MA, USA) were taped over each muscle with a 10-mm interelectrode distance and were secured with a strap to reduce the possibility of EMG signal artifacts resulting from electrode displacement (during the measurement, there were no other motion artifacts in the signal). The input impedance was greater than 10 MΩ at 100 Hz, with a frequency bandwidth of 16–800 Hz and a common mode rejection ratio of 60 Hz (80 dB).
The electrodes for the VM were placed over the distal third of the muscle belly and were oriented 55° angle to the vertical. The electrode for the VL was placed over the muscle belly in the distal third, and it was oriented 15° angle to the vertical (25). The Gmed was located by palpating the iliac crest and placing electrodes parallel to the muscle fibers at 33% of the distance between the iliac crest and the greater trochanter (3,4), which is similar to the locations used by O'Sullivan et al. (44) for the posterior Gmed. The electrodes for the BF were placed over the distal third of the belly of the long head. The ground electrode was placed over the tibia bone.
Three-Dimensional Kinematics Measurement
Six cameras were placed around the walking track with 2 force plates in the middle, and the kinematic data were recorded at 200 Hz in accordance with the Plug-In Gait model (13). Reflective markers that were 14 mm in diameter were bilaterally attached to the subject's skin over the following landmarks: the anterior-superior iliac spine, posterior-superior iliac spine, lateral thigh, lateral femoral epicondyle, tibia, lateral malleolus, heel, and metatarsal head of the second toe. The force plates were used to detect and standardize the beginning of foot contact during the ipsilateral and contralateral WLs with a contact sensitivity of 20 N.
Data Acquisition
The Vicon Nexus software program was used to compute knee angles in the sagittal plane and hip angles in the sagittal, frontal, and transverse planes; separate the eccentric phase and concentric phase of each exercise repetition; and separate the EMG amplitudes for the eccentric and concentric phases. The knee and hip angles were relative for each subject. Knee angles were defined as the angles between the thigh and the shank, and hip angles were defined as the angles between the pelvis and the thigh. Each segment was determined as a body-fixed and rigid coordinate system detected by at least 3 nonlinear markers, in accordance with the Plug-In Gait model (13), which also includes the anthropometry of the lower limb. The pelvis coordinate system was constructed from the 3D location vectors of the 3 pelvic markers located near the center of the hip, midway between the anterior-superior iliac spines, and the posterior-superior iliac spine. The knee center location was determined using a thigh-embedded coordinate system located at the lateral knee marker. The ankle center location was determined by a shank-embedded coordinate system located at the lateral ankle marker. The eccentric and concentric phases of the SSqs and WLs were separated at the peak knee flexion of the stance (loaded) leg. The kinematic values, expressed in degrees, were the peak angles and total ROM of the knee and hip throughout the exercise movement. These included peak hip adduction, peak hip external rotation, peak hip flexion, peak knee flexion, hip abduction/adduction ROM, hip external/internal rotation ROM, hip flexion/extension ROM, and knee flexion/extension ROM. ROM was calculated as an absolute difference in both directions of the selected movement. For example, knee flexion was measured from minimum to maximum flexion angle, whereas hip external rotation may not have started at a neutral position, meaning that both internal and external rotation needed to be considered when calculating the total hip rotation ROM. All these variables were obtained for both legs but were only evaluated for the stance leg during the SSq and WL exercises.
The EMG data were band-pass filtered (16–500 Hz) and smoothed using a root mean square algorithm with a sliding window function and a time constant of 25 milliseconds and were normalized to the EMG during MVIC to be expressed as a percentage of MVIC (%MVIC). The EMG mean amplitudes (expressed as %MVIC) were separated for the eccentric and concentric phases as muscle activity values: concentric Gmed, VM, VL, and BF amplitudes and eccentric Gmed, VM, VL, and BF amplitudes.
Statistical Analyses
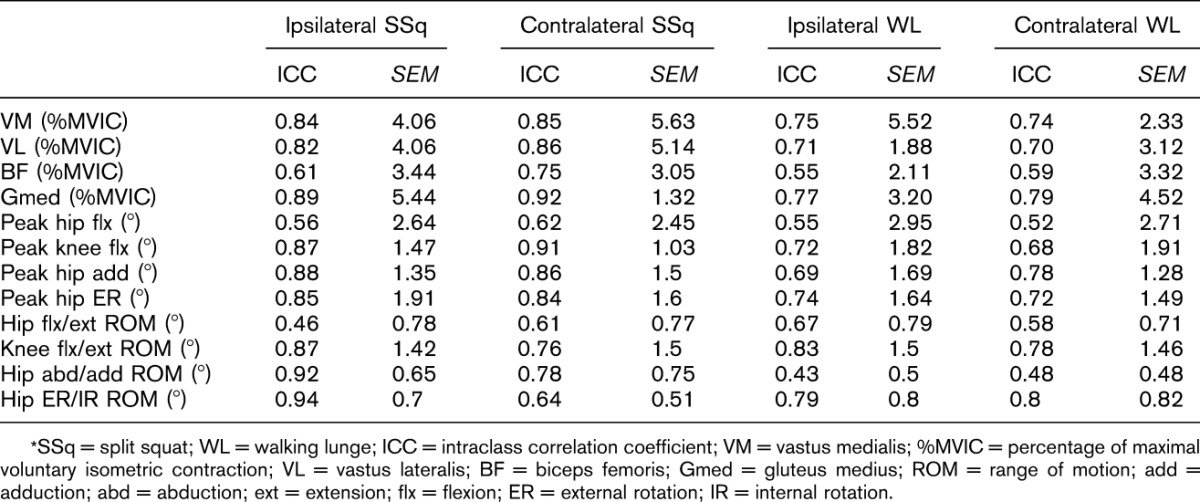
All statistical analyses were performed with STATISTICA version 12 (StatSoft, Inc., Tulsa, OK, USA) with α = 0.05. The first 4 repetitions of each leg during the 5RM trial of each exercise were averaged for further statistical analyses. The intraclass correlation coefficient (ICC) across 4 repetitions for each individual was determined to confirm whether the EMG and 3D measurements were stable within each subject (Table 2). Kendal rank-order correlations (Kendall tau b “τ”) were used to determine the dependence of the EMG amplitudes and the kinematics during all exercises without categorizing them by group (RT or NT). For this test, the kinematic values were regarded as one group of variables (predictors), whereas the associated EMG amplitudes were a second group of variables (predictants). Kendal's τ was used because this coefficient does not require any assumptions of correlation linearity and is not dependent on the number of involved cases (46).
Table 2.
Within-subject reliability.*
To determine whether the EMG amplitudes and kinematics varied between groups, a 2 × 4 (groups × exercises) analysis of variance (ANOVA) for repeated measures was performed on 4 variables (exercises). This analysis was repeated for each EMG and kinematic measurement separately while regarding between-subject (group) factors as a result. Furthermore, the 1-way repeated measure ANOVA was used to compare differences in EMGs and kinematics among all exercises for both groups separately. The dependent variables were all 4 exercises without consideration of the categorical factors. Both ANOVAs were followed by Tukey's post hoc tests. The effect size (partial eta square, η2) of each test was calculated for all analyses and was classified according to Hopkins (29). Statistical significance was set at p ≤ 0.05.
Results
The within-subject reliability analyses across the first 4 repetitions of the 5RM for the individuals resulted in ICC values ranging from 0.43 to 0.94 (Table 2) for both EMG amplitudes and kinematics during all exercises, which indicate a high or very high reliability (9) of measurement.
The EMG and kinematic values exhibited 3 moderate relationships. The peak hip abduction predicted (correlated with) the Gmed amplitude (τ = 0.4, p = 0.042) and the BF amplitude (τ = 0.6, p < 0.002) during eccentric actions; furthermore, hip abduction/adduction ROM predicted Gmed amplitude (τ = 0.36, p < 0.001) during eccentric actions. Other variables exhibited a τ value of under 0.3, which is considered a weak relationship (46).
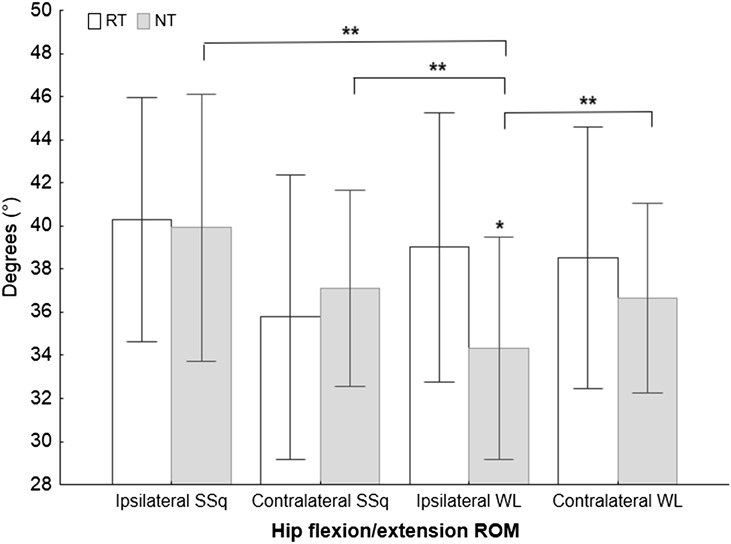
The repeated measure ANOVA results showed significant differences between the RT and NT groups in the total ROM and EMG variables collected from the eccentric phase of the movements. Significant knee flexion ROM differences (F4,51 = 7.12, p < 0.001, η2 = 0.36) were found between the RT and NT groups for both ipsilateral and contralateral WLs (Figure 2, Table 3), and the NT group showed a smaller knee flexion ROM (Table 3). The hip flexion ROM differed significantly between the RT and NT groups (F4,51 = 5.04, p = 0.002, η2 = 0.28) for the ipsilateral WLs (Figure 3, Table 3), and the NT group showed a smaller hip flexion ROM (Table 3). A significant Gmed amplitude difference (F7,91 = 23.10, p < 0.001, η2 = 0.46) was found between the groups during the eccentric phase of both the SSq and WL exercises, and the NT group showed a lower amplitude (Figure 4, Table 3). Furthermore, significant VL amplitude differences (F7,91 = 28, p < 0.001, η2 = 0.63) were found during the eccentric phase of the contralateral WL exercise (Figure 4, Table 3), and the RT group exhibited a higher eccentric VL amplitude. No other differences between groups were found for any other variables.
Figure 2.
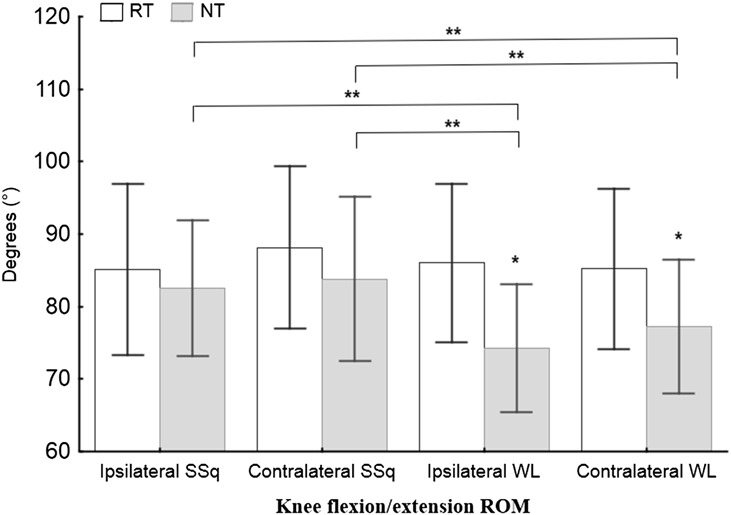
ANOVA results for knee flexion during all 4 exercises. ANOVA = analysis of variance; ROM = range of motion; RT = resistance-trained group; NT = non–resistance-trained group; SSq = split squat; WL = walking lunge. *Significance between the RT and NT groups according to Tukey's post hoc test; **significance between the exercises according to Tukey's post hoc test.
Table 3.
EMG during the eccentric phase and kinematics of all exercises (mean ± SD).*
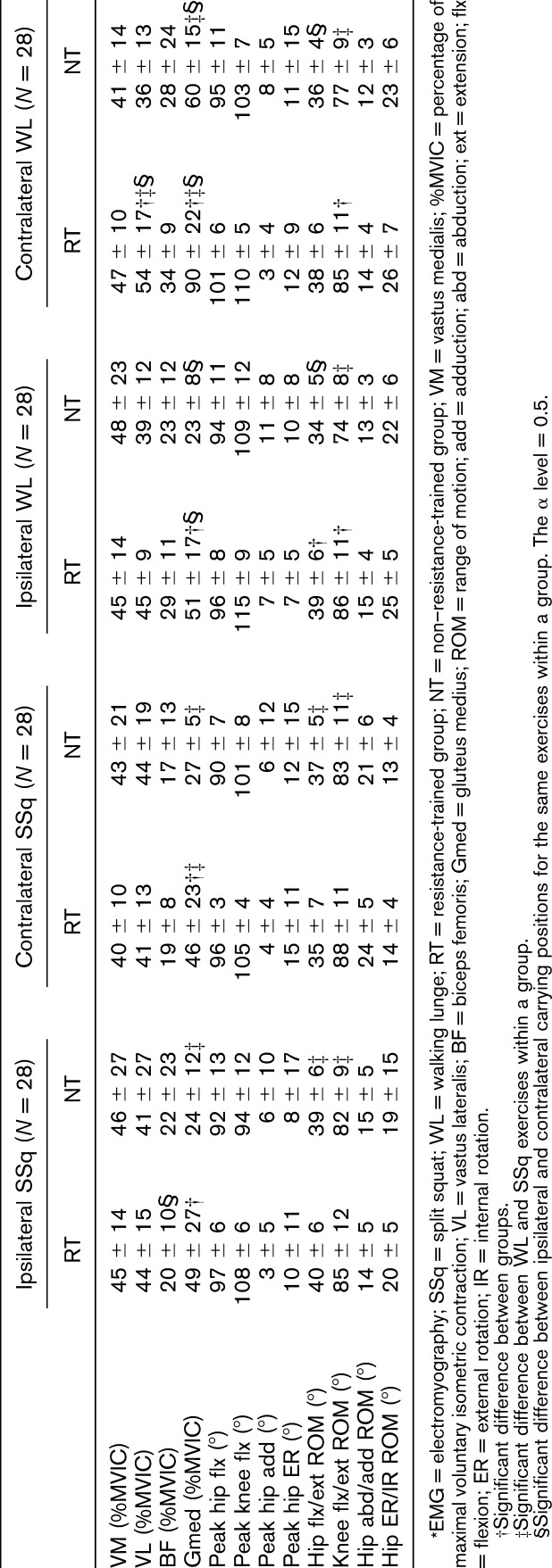
Figure 3.
ANOVA results for hip flexion during all 4 exercises. ANOVA = analysis of variance; ROM = range of motion; RT = resistance-trained group; NT = non–resistance-trained group; SSq = split squat; WL = walking lunge. *Significance between the RT and NT groups according to Tukey's post hoc test; **significance between the exercises according to Tukey's post hoc test.
Figure 4.
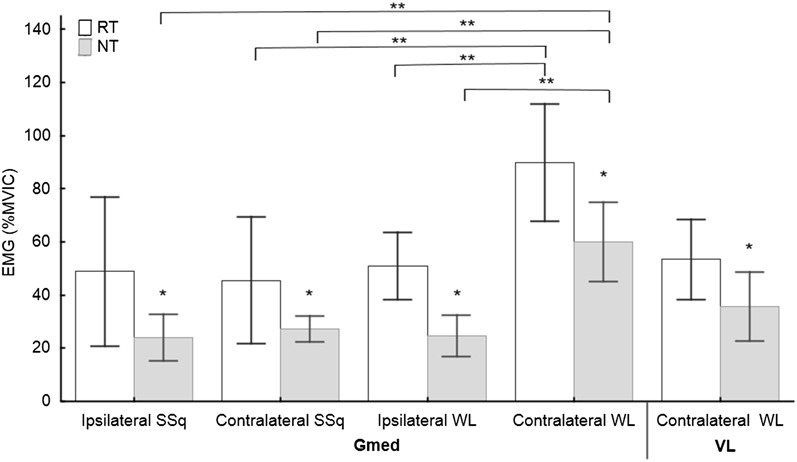
ANOVA results for the Gmed during all 4 exercises and for the VL during contralateral WLs. ANOVA = analysis of variance; EMG = electromyography; %MVIC = percentage of maximum voluntary isometric contraction; Gmed = gluteus medius; VL = vastus lateralis; RT = resistance-trained group; NT = non–resistance-trained group; SSq = split squat; WL = walking lunge. *Significance between the RT and NT groups according to Tukey's post hoc test; **significance between the exercises according to Tukey's post hoc test.
The 1-way repeated measure ANOVA showed significant differences in both EMG and kinematics in the NT group. Significant differences were found for knee flexion ROM (F3,81 = 12.75, p < 0.001, η2 = 0.32) between SSqs and WLs in the NT group, and WLs resulted in less knee flexion (Figure 2, Table 3). Hip flexion ROM was significantly different in the NT group between the ipsilateral SSqs (F3,81 = 6.87, p < 0.001, η2 = 0.20) and the other 3 exercises, and ipsilateral WLs showed a smaller hip ROM compared with the other exercises (Figure 3, Table 3). Significant differences were found in the RT group for the Gmed in the eccentric phase between the contralateral WL (F3,39 = 44.8, p < 0.001, η2 = 0.69) and the 2 other exercises (the contralateral SSq and ipsilateral WL exercises), and the contralateral WL showed a higher amplitude (Figure 4, Table 3). A higher eccentric VL amplitude was also found in the RT group for the contralateral WL (F3,39 = 3, p < 0.041, η2 = 0.18) compared with the other exercises. Furthermore, significant differences were found in the NT group for the Gmed in the eccentric phase between the contralateral WL (F4,54 = 18.9, p < 0.001, η2 = 0.80) and the 3 other exercises, and the contralateral WL yielded a higher amplitude (Figure 4, Table 3). No other differences among exercises were found for any of the other variables.
Discussion
The aim of this study was to determine the effect of dumbbell-carrying position on kinematics and EMG amplitudes in WLs and SSqs. The study identified differences in EMG amplitudes in the Gmed and VL; these differences were associated with both the dumbbell-carrying position and kinematic changes. Hip abduction (Gmed) strength during bilateral and single-leg squats has been previously associated with knee valgus (39), which is similar to the association between hip abduction/adduction ROM and Gmed activity. The subjects involved in this study did not have any hip abduction weakness (Table 1) according to normality data (2,7); therefore, the observed increases in Gmed activity were the result of its higher enhancement in the chosen muscle chain rather than actual weakness of individual muscles. The same results were found for VL activity. However, the Gmed might have been the weakest point in the muscle chain of measured muscles, which may partially explain why its activity increased more than that of the other muscles during 5RM of SSqs or WLs. The association between peak hip abduction and BF amplitude does not resemble the findings of previous research; moreover, BF activity did not differ among exercises. Thus, it is possible that the BF acts in a specific manner to stabilize the knee during SSqs and WLs, regardless of the dumbbell-carrying position.
The finding that the kinematics did not differ between the RT and NT groups during SSqs but did differ during WLs supports the idea of including SSq exercises at the beginning of a training program, before WLs are performed. In other words, some of the non-trained individuals may be unable to perform the full range of WLs, but this limitation is not observed in SSqs. Two crucial differences were found for ipsilateral WLs, which resulted in a decreased range of knee and hip flexion in the NT group. The knee and hip joint kinematics during contralateral WLs were similar to the values found in a previous study (20), where the peak knee (110 ± 6) and hip angles (87 ± 12) during traditional WLs varied according to the trunk position.
The WL is an exercise with an eccentric phase that includes landing; therefore, the greater ICC for the EMG values during the SSq exercises (Table 2) may be attributed to the inclusion of only an ascending phase and the lack of a landing phase in WLs. This result is similar to the previous findings (16) in which forward lunges resulted in lower ICC values than single-leg squats, and exercises that included jumps resulted in an ICC of less than 0.5. However, the presented EMG and kinematic measurements exhibit acceptable reliability. Furthermore, the eccentric phase of movement was the only variable that showed any significant differences in EMG amplitudes.
The kinematic variables exhibited 3 moderate correlations with EMG amplitudes, indicating that the observed EMG changes appeared not only because of the kinematic change but also because of other variables, such as the dumbbell-carrying position, the presence of an impact force during WLs, or training experience. Because the peak angles during WLs in the NT group were similar to or greater than the peak angles reached in a previous study (peak knee flexion = 87.5 ± 11.2, peak hip flexion = 74.2 ± 14.4) (17), we can assume that an appropriate load was applied for the NT. In this regard, a 5RM already represents the threshold load at which NT men can maintain the prescribed exercise technique.
The between-group analysis indicated differences in Gmed activity during the eccentric phase, where the RT group exhibited higher amplitude values than the NT group. This finding is consistent with contemporary knowledge that strength training increases EMG values, such as the root mean square (12) or MVIC results (36), in primal movers, whereas decreasing the training intensity also influences the EMG (27). However, this finding was not reported for the BF in a previous study (28) or in this study. Previously, contradictions were found in the VM and VL; strength training performed twice a week resulted in large increases in the maximal voluntary activation of the VL or VM during both isometric and concentric knee extension actions (28). Our study found that EMG differences based on training experience were only present for the eccentric part of the SSq or WL exercises and not for any concentric action; the minimum difference in training experience between the groups was 2 lower-leg trainings per week. The EMG differences between the RT and NT subjects in our study might be related to the exercise variation or to the fact that the group averages were calculated using the values for both legs of every individual, which may explain the contradictions between our study and studies in which only the dominant leg was measured.
Contralateral WLs resulted in greater Gmed activation during the eccentric phase in both the RT (90% MVIC) and NT (60% MVIC) groups compared with the contralateral SSqs (RT = 46% MVIC, NT = 27% MVIC). This result shows that contralateral WLs have a high activation effect on the Gmed even for NT individuals because a Gmed activation of 60% MVIC is considered a high amount of activity (45). Unfortunately, a reduction in knee flexion was found in the NT group (compared with the RT group), meaning that this exercise might be recommended for beginners only if ROM is not negatively affected. Contralateral WL showed increased VL activity in the RT group (54% MVIC), which was not accompanied by the same increase in VM activity. This finding suggests that contralateral WLs may increase the muscle imbalance between the VM and VL, especially in RT individuals. Alternatively, contralateral WLs appear to be beneficial for Gmed and VL strengthening, if such strengthening is the aim of a training program. The forward bodyweight lunge was estimated for the Gmed activity and was found to exhibit a moderate (15.5–19% MVIC) (5,17) or high (29–42% MVIC) (16,18) level of activity. This degree of muscle activity should be exceeded when the external load is increased from bodyweight to 5RM, which was observed in both groups during contralateral WLs but not during SSqs.
The ipsilateral and contralateral loading condition EMGs did not differ for the SSqs but did differ for the WLs in both groups. The ipsilateral loading condition for both the WLs and SSqs did not exhibit any advantage over the contralateral condition in terms of muscle activity. Changes in the muscle activities resulting from the dumbbell-carrying position were found for the WLs. However, both types of SSqs exhibited high Gmed activity (>40% MVIC) in the RT group, suggesting that this exercise is appropriate for Gmed strengthening.
Most of the exercises used in this study produced an EMG signal amplitude for the VM and VL of less than 45% MVIC; thus, we would consider these exercises beneficial for moderate activity (18) in this muscle group. The BF did not exhibit any significant differences, and its activity was the lowest for the observed muscles, as previously reported (33).
This study has a limitation regarding the number of selected muscles because the electrodes were placed bilaterally, but unilateral exercises were measured. A bilateral placement was chosen considering the way exercises are performed during a training session, in which both legs have to be trained. Some of the EMG values recorded during the eccentric part of the WL exceeded 100% MVIC in some individuals, which is reasonable because the MVIC was measured in the isometric (not eccentric) condition. It would be more appropriate to measure EMG activity during different RMs, but this would likely produce inaccurate results because the NT group was involved. Another limitation concerns the variations in ROM between groups. Instead of standardizing the ROM, we instructed the participants to “lunge down as far as possible” at a standardized step distance to respect both the individuality of each person's movement and the idea of taking the measurement in a condition similar to that of a training session.
Practical Applications
The results of this study demonstrate that NT individuals perform WLs with less knee flexion ROM, whereas this reduction is not observed during SSqs. Thus, SSqs should be performed during the initial period of a resistance training program (in NT men) before WLs are performed to ensure that participants achieve a full ROM and moderate Gmed activity during this exercise.
The other results demonstrate that contralateral WLs produce greater Gmed and VL activities compared with ipsilateral WLs and both types of SSqs. Thus, the ipsilateral loading condition may not be ideal for increasing the Gmed or VM activity in trained or nontrained men. Contralateral WLs activate the Gmed in both RT and NT individuals but with an additional preference for VL activity in RT individuals. Contralateral WLs (at 5RM) target the Gmed in terms of its maximal activity; thus, this exercise may increase maximal Gmed strength. As hypothesized, changes in muscle activity are dependent on a combination of factors, including different dumbbell-carrying positions, training history, and the selected exercise. Therefore, this study adds to the body of evidence that highlights the importance of prescribing specific exercises to meet each individual's specific needs.
Acknowledgments
This study was funded by project POST-UP II, CZ.1.07/2.3.00/30.0041 and co-funded by the European Social Fund and the Government of the Czech Republic.
References
- 1.Alkjær T, Simonsen EB, Peter Magnusson S, Aagaard H, Dyhre-Poulsen P. Differences in the movement pattern of a forward lunge in two types of anterior cruciate ligament deficient patients: Copers and non-copers. Clin Biomech 17: 586–593, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Bohannon RW. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil 78: 26–32, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Bolgla LA, Uhl TL. Electromyographic analysis of hip rehabilitation exercises in a group of healthy subjects. J Orthop Sports Phys Ther 35: 487–494, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bolgla LA, Uhl TL. Reliability of electromyographic normalization methods for evaluating the hip musculature. J Electromyogr Kinesiol 17: 102–111, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Boudreau SN, Dwyer MK, Mattacola CG, Lattermann C, Uhl TL, McKeon JM. Hip-muscle activation during the lunge, single-leg squat, and step-up-and-over exercises. J Sport Rehabil 18: 91–103, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Brown LE, Weir J. ASEP procedures recommendation I: Accurate assessment of muscular strength and power. J Exerc Physiol Online 4: 1–21, 2001. [Google Scholar]
- 7.Buchanan PA, Vardaxis VG. Lower-extremity strength profiles and gender-based classification of basketball players ages 9-22 years. J Strength Cond Res 23: 406–419, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Burnet EN, Pidcoe PE. Isometric gluteus medius muscle torque and frontal plane pelvic motion during running. J Sports Sci Med 8: 284–288, 2009. [PMC free article] [PubMed] [Google Scholar]
- 9.Chandler TJ, Brown LE. Conditioning for Strength and Human Performance: Philadelphia, PA: Lippincott Williams & Wilkins, 2008. [Google Scholar]
- 10.Cichanowski HR, Schmitt JS, Johnson RJ, Niemuth PE. Hip strength in collegiate female athletes with patellofemoral pain. Med Sci Sports Exerc 39: 1227–1232, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Danneskiold-Samsoe B, Bartels EM, Bulow PM, Lund H, Stockmarr A, Holm CC, Watjen I, Appleyard M, Bliddal H. Isokinetic and isometric muscle strength in a healthy population with special reference to age and gender. Acta Physiol (Oxf) 197(Suppl 673): 1–68, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Dasteridis G, Pilianidis T, Mantzouranis N, Aggelousis N. The effects of athletics training on isometric strength and EMG activity in adolescent athletes. Biol Exerc 8: 37–46, 2012. [Google Scholar]
- 13.Davis RB, Õunpuu S, Tyburski D, Gage JR. A gait analysis data collection and reduction technique. Hum Mov Sci 10: 575–587, 1991. [Google Scholar]
- 14.Desloovere K, Wong P, Swings L, Callewaert B, Vandenneucker H, Leardini A. Range of motion and repeatability of knee kinematics for 11 clinically relevant motor tasks. Gait Posture 32: 597–602, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Dirnberger J, Kösters A, Müller E. Concentric and eccentric isokinetic knee extension: A reproducibility study using the IsoMed 2000-dynamometer. Isokinetics Exerc Sci 20: 31–35, 2012. [Google Scholar]
- 16.Distefano LJ, Blackburn JT, Marshall SW, Padua DA. Gluteal muscle activation during common therapeutic exercises. J Orthop Sports Phys Ther 39: 532–540, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Dwyer MK, Boudreau SN, Mattacola CG, Uhl TL, Lattermann C. Comparison of lower extremity kinematics and hip muscle activation during rehabilitation tasks between sexes. J Athl Train 45: 181–190, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekstrom R, Donatelli R, Carp K. Electromyographic analysis of core trunk, hip, and thigh muscles during 9 rehabilitation exercises. J Orthop Sports Phys Ther 37: 754, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Fagan V, Delahunt E. Patellofemoral pain syndrome: A review on the associated neuromuscular deficits and current treatment options. Br J Sports Med 42: 789–795, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Farrokhi S, Pollard CD, Souza RB, Chen YJ, Reischl S, Powers CM. Trunk position influences the kinematics, kinetics, and muscle activity of the lead lower extremity during the forward lunge exercise. J Orthop Sports Phys Ther 38: 403–409, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Felício LR, Dias LA, Silva APMC, Oliveira AS, Bevilaqua-Grossi D. Muscular activity of patella and hip stabilizers of healthy subjects during squat exercises [in English, Portuguese]. Rev Bras Fisioter 15: 206–211, 2011. [PubMed] [Google Scholar]
- 22.Finnoff JT, Hall MM, Kyle K, Krause DA, Lai J, Smith J. Hip strength and knee pain in high school runners: A prospective study. PM R 3: 792–801, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Fredericson M, Cookingham CL, Chaudhari AM, Dowdell BC, Oestreicher N, Sahrmann SA. Hip Abductor weakness in distance runners with iliotibial band syndrome. Clin J Sport Med 10: 169–175, 2000. [DOI] [PubMed] [Google Scholar]
- 24.French H, Dunleavy M, Cusack T. Activation levels of gluteus medius during therapeutic exercise as measured with electromyography: A structured review. Phys Ther Rev 15: 92–105, 2010. [Google Scholar]
- 25.Gilleard W, McConnell J, Parsons D. The effect of patellar taping on the onset of vastus medialis obliquus and vastus lateralis muscle activity in persons with patellofemoral pain. Phys Ther 78: 25–32, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Häkkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H, Mälkiä E, Kraemer WJ, Newton RU, Alen M. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol (1985) 84: 1341–1349, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Häkkinen K, Komi P, Alén M, Kauhanen H. EMG, muscle fibre and force production characteristics during a 1 year training period in elite weight-lifters. Eur J Appl Physiol Occup Physiol 56: 419–427, 1987. [DOI] [PubMed] [Google Scholar]
- 28.Häkkinen K, Kraemer WJ, Newton RU, Alen M. Changes in electromyographic activity, muscle fibre and force production characteristics during heavy resistance/power strength training in middle-aged and older men and women. Acta Physiol Scand 171: 51–62, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Hopkins WG. Linear models and effect magnitudes for research, clinical and practical applications. Sportscience 14: 49–57, 2010. [Google Scholar]
- 30.Ireland ML, Willson JD, Ballantyne BT, Davis IM. Hip strength in females with and without patellofemoral pain. J Orthop Sports Phys Ther 33: 671–676, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Irish SE, Millward AJ, Wride J, Haas BM, Shum GLK. The effect of closed-kinetic chain exercise and open-kinetic chain exercise on the muscle activity of vastus medialis oblique and vastus lateralis. J Strength Cond Res 24: 1256–1262, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Jang EM, Heo HJ, Kim MH, Yoo WG. Activation of VMO and VL in squat exercises for women with different hip adduction loads. J Phys Ther Sci 25: 257–258, 2013. [Google Scholar]
- 33.Jönhagen S, Halvorsen K, Benoit DL. Muscle activation and length changes during two lunge exercises: Implications for rehabilitation. Scand J Med Sci Sports 19: 561–568, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Kamen G, Knight CA. Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Med Sci Med Sci 59: 1334–1338, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Leetun DT, Ireland ML, Willson JD, Ballantyne BT, Davis IM. Core stability measures as risk factors for lower extremity injury in athletes. Med Sci Sports Exerc 36: 926–934, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Maeo S, Takahashi T, Takai Y, Kanehisa H. Trainability of muscular activity level during maximal voluntary co-contraction: Comparison between bodybuilders and nonathletes. PLoS One 8: e79486, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masuda K, Kikuhara N, Demura S, Katsuta S, Yamanaka K. Relationship between muscle strength in various isokinetic movements and kick performance among soccer players. J Sports Med Phys Fitness 45: 44–52, 2005. [PubMed] [Google Scholar]
- 38.McBride J, Triplett-McBride T, Davie A, Newton R. The effect of heavy-vs. light-load jump squats on the development of strength, power, and speed. J Strength Cond Res 16: 75–82, 2002. [PubMed] [Google Scholar]
- 39.McCurdy K, O'Kelley E, Kutz M, Langford G, Ernest J, Torres M. Comparison of lower extremity EMG between the 2-leg squat and modified single-leg squat in female athletes. J Sport Rehabil 19: 57–70, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Moonot P, Mu S, Railton GT, Field RE, Banks SA. Tibiofemoral kinematic analysis of knee flexion for a medial pivot knee. Knee Surg Sports Traumatol Arthrosc 17: 927–934, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Nadler SF, Malanga GA, DePrince M, Stitik TP, Feinberg JH. The relationship between lower extremity injury, low back pain, and hip muscle strength in male and female collegiate athletes. Clin J Sport Med 10: 89–97, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Neumann DA, Cook TM. Effect of load and carrying position on the electromyographic activity of the gluteus medius muscle during walking. Phys Ther 65: 305–311, 1985. [DOI] [PubMed] [Google Scholar]
- 43.Neumann DA, Cook TM, Sholty RL, Sobush DC. An electromyographic analysis of hip abductor muscle activity when subjects are carrying loads in one or both hands. Phys Ther 72: 207–217, 1992. [DOI] [PubMed] [Google Scholar]
- 44.O'Sullivan K, Smith SM, Sainsbury D. Electromyographic analysis of the three subdivisions of gluteus medius during weight-bearing exercises. Sports Med Arthrosc Rehabil Ther Technol 2: 17, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reiman MP, Bolgla LA, Loudon JK. A literature review of studies evaluating gluteus maximus and gluteus medius activation during rehabilitation exercises. Physiother Theory Pract 28: 257–268, 2012. [DOI] [PubMed] [Google Scholar]
- 46.Sheskin DJ. Handbook of Parametric and Nonparametric Statistical Procedures. London: CRC Press, 2003. [Google Scholar]
- 47.Shields RK, Madhavan S, Gregg E, Leitch J, Petersen B, Salata S, Wallerich S. Neuromuscular control of the knee during a resisted single-limb squat exercise. Am J Sports Med 33: 1520–1526, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Windolf M, Götzen N, Morlock M. Systematic accuracy and precision analysis of video motion capturing systems—Exemplified on the Vicon-460 system. J Biomech 41: 2776–2780, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Wünschel M, Leichtle U, Obloh C, Wülker N, Müller O. The effect of different quadriceps loading patterns on tibiofemoral joint kinematics and patellofemoral contact pressure during simulated partial weight-bearing knee flexion. Knee Surg Sports Traumatol Arthrosc 19: 1099–1106, 2011. [DOI] [PubMed] [Google Scholar]


