Abstract
Background:
Articular cartilage injuries of the knee are among the most debilitating injuries leading to osteoarthritis due to limited regenerative capability of cartilaginous tissue. The use of platelet concentrates containing necessary growth factors for cartilage healing has recently emerged as a new treatment method.
Objectives:
The efficacy of two types of different platelet concentrates were compared in the treatment of acute articular cartilage injuries of the knee in an animal model.
Materials and Methods:
Eighteen adult Iranian mixed breed male dogs were used to conduct this experimental study. Full thickness articular cartilage defects (diameter 6 mm, depth 5 mm) were created in the weight bearing area of femoral condyles of both hind limbs in all dogs (n = 72). Twelve dogs were randomly selected to receive treatment and their right and left hind limb defects were treated by L-PRP and L-PRF implantation respectively, while no treatment was undertaken in six other dogs as controls. The animals were euthanized at 4, 16 and 24 weeks following surgery and the resultant repair tissue was investigated macroscopically and microscopically. At each sampling time, 4 treated dogs and 2 control dogs were euthanized, therefore 8 defects per group were evaluated.
Results:
Mean macroscopic scores of the treated defects were higher than the controls at all sampling times with significant differences (P < 0.05) observed between L-PRF treated and control defects (10.13 vs. 8.37) and L-PRP treated and control defects (10 vs. 8.5) at 4 and 16 weeks, respectively. A similar trend in mean total microscopic scores was observed with a significant difference (P < 0.05) between L-PRP treated and control defects at 4 (9.87 vs. 7.62) and 16 (13.38 vs. 11) weeks. No significant difference was observed between the platelet concentrate treated defects in either mean macroscopic scores or mean total microscopic scores.
Conclusions:
Both L-PRP and L-PRF could be used to effectively promote the healing of articular cartilage defects of the knee.
Keywords: Platelet-Rich Plasma, Cartilage, Knee Joint, Dogs, Articular Cartilage
1. Background
Articular cartilage injuries associated with trauma or overuse are common in the knee joint and if untreated can become symptomatic and progress to osteoarthritis. In a retrospective review of over 31000 knee arthroscopies, chondral lesions were found in 63% of patients with an average of 2.7 lesions per knee, while another study of 993 consecutive knee arthroscopies reported articular cartilage pathology in 66% of patients with localized full thickness lesions observed in 11% of the knees (1, 2). Articular cartilage is devoid of blood vessels and nerves with low presence of chondrocytes with minimum mitotic activity, therefore this particular tissue has little or no potential for healing compared with other tissues. The inability of articular cartilage to regenerate was first reported by Hunter in 1743 (2, 3).
It has been reported that different growth factors namely transforming growth factor β, basic fibroblast growth factor, insulin like growth factor I, vascular endothelial growth factor and platelet derived growth factor positively influence the repair and regeneration of hyaline cartilage (4, 5). Platelets are the natural reservoir of growth factors in the body and the abovementioned growth factors are released from the alpha granules of activated platelets following injury and the process of inflammation and tissue repair is initiated (6, 7). In the recent years, first generation platelet rich products in the form of autologous platelet rich plasma (PRP) have been used as a biological treatment in articular cartilage repair. In vitro studies indicated that PRP stimulates cellular proliferation and production of cartilage matrix by chondrocytes and preclinical animal model studies indicated that PRP positively influences cartilage repair (8-12). Platelet rich fibrin (PRF) is a second generation platelet concentrate with many advantages over the first generation PRP, which is produced by collecting autologous blood in glass tubes without any anticoagulant and immediate centrifugation. The resultant product is a true biomaterial containing fibrin clot, platelets and leukocytes with a high concentration of growth factors. PRF does not require any activation prior to use and growth factors are released slowly over a sustained period of time (13). There are structural differences between the first and second generation platelet concentrates and there is no study comparing the effect of PRP and PRF on cartilage repair and regeneration.
2. Objectives
Because growth factors positively influence cartilage repair and regeneration and platelet concentrates are readily available source of growth factors, this study was undertaken with the main objective of comparing the effect of L-PRP and L-PRF as first and second generation platelet concentrates on the healing of full thickness articular cartilage defects in an animal model.
3. Materials and Methods
This experimental study was approved by the research council of the college of veterinary medicine, Islamic Azad university, Tabriz branch (research project number 2-17-5-102536 approved on 18.10.2010). All the experiments were conducted at small animal surgery division of the College of Veterinary Medicine with adherence to institutional guidelines for the care and use of laboratory animals in research based on the Guide for the care and use of laboratory animals. Eighteen skeletally mature Iranian mixed breed male dogs with the body weight of 20 - 30 kg were used in this study. The dogs were judged to be healthy based on physical examination and laboratory tests (complete blood cell count, blood biochemistry profiles and urinalysis). The stifle joint (equivalent of the human knee) of each animal was carefully examined to rule out any joint instability. Skeletal maturity was determined by radiography prior to initiation of the experiment.
A total of 72 articular cartilage defects were created on the femoral condyles of the stifle joint (4 defects per dog). In 12 randomly selected dogs, defects of the right and left hind limbs of each animal were repaired by L-PRP and L-PRF implantation, respectively. Defects in the other six dogs left without any treatment as controls.
3.1. Platelet Concentrate Preparation
L-PRP and L-PRF were prepared according to the method described by You et al. (14) and Dohan et al. (15), respectively. Briefly, 40 mL of blood was collected from the jugular vein of each animal before anesthesia. Half of the collected blood was transferred to two sterile test tubes containing sodium citrate as anticoagulant and initially centrifuged at 2400 rpm for 10 minutes to separate red blood cells from the buffy coat (BC) and acellular plasma. The acellular plasma and BC portions were collected completely and again centrifuged at 3600 rpm for 15 minutes to separate the acellular plasma from the L-PRP. Two thirds of the supernatant plasma was discarded and the rest which contained the concentrated L-PRP was activated with calcium chloride solution to form a gel prior to application. Samples of whole blood and L-PRP were taken for platelet and leukocyte counts. The other half of collected whole blood was transferred to two sterile test tubes without any anticoagulant and immediately centrifuged at 3000 rpm for 10 minutes. The resultant L-PRF clot located in the middle layer of the test tube was removed and the red blood cells at the bottom and acellular plasma at the top of the tube were discarded. The L-PRF clots were kept in sterile Petri dishes until later use during the operation.
3.2. Surgical Procedure
Food was withheld from the animals for 12 hours before the operation. Each dog was premedicated by intramuscular injection of xylazine (1 mg/kg) and atropine (0.04 mg/kg). Anesthesia was induced by intravenous injection of 2.5% solution of thiopental and maintained with halothane in oxygen following endotracheal intubation. Cefazolin (20 mg/kg) was given as preoperative antibiotic immediately following induction and lactated ringer solution (10 mL/kg/h) was infused during the operation. The animal was placed in dorsal recumbency and under aseptic conditions. The medial approach to the stifle joint with lateral patellar luxation was used to access inside the joint. The joint was fully flexed to access the weight bearing areas of the femoral condyles. Full thickness articular cartilage defects with a diameter of 6 mm and depth of 5 mm were created in the weight bearing area of each femoral condyle using a drill equipped with 6 mm drill bit. Bleeding was observed in all the defects confirming the involvement of subchondral bone and full thickness nature of the injury. The defects were thoroughly lavaged with normal saline solution. In the treatment animals, L-PRP and L-PRF were press fitted inside each designated defect to completely fill the region. Defects in the control animals left without any treatment. After completion of the procedure, the patella was reluxated to its normal position and the joint capsule, subcutaneous tissues and skin sutured routinely to close the wound. Postoperatively, penicillin (40000 IU/kg for 5 days) and ketoprofen (2.2 mg/kg for 3 days) were administered to the dogs. The animals were allowed to walk freely without any restrictions following recovery. Full weight bearing was allowed as tolerated by the dogs.
3.3. Sampling Procedure and Outcome Measurement
At 4, 16 and 24 weeks following surgery, the dogs were euthanized by an overdose of thiopental sodium injection and the distal femurs were harvested for macroscopic and histological evaluations of the repair tissue. Four treatment and two control dogs were randomly assigned to each of the sampling periods, therefore the number of L-PRP and L-PRF treated and control defects were 8 at each time period. Immediately after euthanasia, digital photographs of the defect area were taken and the international cartilage repair society (ICRS) evaluation score (16) was used for macroscopic assessment of the repair tissue. Degree of defect repair, integration to border zone and macroscopic appearance evaluated in this method and the overall score ranged between 0 and 12 with 12 representing normal cartilage and 0 as severely abnormal cartilage.
Following macroscopic assessment, each femoral condyle was fixed in 10% buffered neutral formalin, decalcified and embedded in paraffin for routine histological sectioning. Sagittal sections (5 µm thick) were cut from the center of each defect and stained with hematoxylin-eosin and safraninO and examined under light microscope. Sections were blindly examined and scored according to the O’Driscoll histological grading scale (17). Nine different parameters were evaluated in this comprehensive grading method and the total score ranged between 0 and 24 with higher scores representing better histologic repair of articular cartilage.
3.4. Statistical Analysis
To compare macroscopic and microscopic scores at different time points between the groups, Kruskal-Wallis test followed by post hoc Dunn’s multiple comparison test was used. The number of platelets and leukocytes present in whole blood and L-PRP were compared using Mann-Whitney U test. P < 0.05 was considered as statistically significant. GraphPad Prism 5 software package (GraphPad Software Inc., La Jolla, CA) was used for data analysis. As stated previously, the sample size consists of 18 animals and 72 defects allocated in such a way to have 8 defects for each of the L-PRP treated, L-PRF treated and control groups at a given sampling time. The present study was a novel study, therefore sample size calculation was based on previously published literature in the field of cartilage repair conducted on large animal species and it was not possible to use specific sample size determination methods.
4. Results
4.1. Assessment of Prepared L-PRP
The mean number of counted platelets and leukocytes in whole blood were 16.5833 × 104/µL and 0.8218 × 104/µL, respectively. In L-PRP, these values were 68.425 × 104/µL and 2.7681 × 104/µL respectively indicating a 4.12 fold increase in platelets and 3.36 fold increase in leukocytes, which was statistically significant (P < 0.05).
4.2. Macroscopic Evaluations
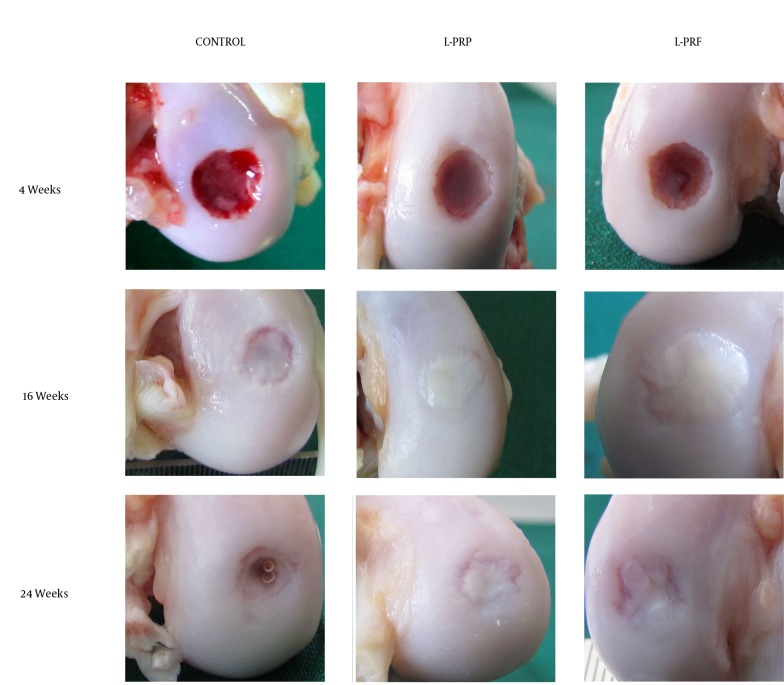
There was no significant difference in macroscopic scores at any time between L-PRP and L-PRF treated defects (Table 1). Macroscopic scores of both treatment groups were higher than untreated controls at all times. There was a significant difference (P < 0.05) between L-PRF treated and control defects at 4 weeks and between L-PRP treated and control defects at 16 weeks. No statistically significant difference was observed between treated and control groups at 24 weeks. Macroscopic appearances of the defects are shown in Figure 1.
Table 1. Macroscopic Scores of the Treatment Groups at Pre-Determined Sampling Times (Mean ± SD).
| Weeks | Control | L-PRP | L-PRF |
|---|---|---|---|
| 4 | 8.37 ± 1.18 | 9.12 ± 0.99 | 10.13 ± 0.99 |
| 16 | 8.5 ± 0.53 | 10 ± 1.19 | 9.5 ± 0.92 |
| 24 | 9 ± 1.3 | 9.25 ± 1.16 | 9.75 ± 2.05 |
Figure 1. Macroscopic Appearance of Representative Defects from Different Treatment Groups.
Note the presence of fibrous tissue in all defects at 4 weeks with a central depression, which is deeper in the control group. Later, the fibrous repair tissue was replaced by opaque white repair tissue resembling the surrounding normal cartilage, but the defect area was still distinguishable. Cystic lesion was present at the center of the repair tissue at 24 weeks in the control group.
4.3. Histological Evaluations
The results of histological evaluations indicated no significant difference in total O’Driscoll scores of L-PRP and L-PRF treated groups at any of the pre-determined sampling times (Table 2). Although significant difference (P < 0.05) in total histological score was only observed between L-PRP treated and control group at 4 and 16 weeks, but the total scores of treated groups were consistently higher than the control group at all times indicating formation of better reparative tissue in the treated groups. A significant difference (P < 0.05) between L-PRP treated and L-PRF treated groups was observed in structural integrity and freedom from degenerative changes in adjacent cartilage at 16 and 24 weeks, respectively with higher histological scores in the L-PRF group at both times. In all groups, increase in total histological scores and individual parameters was observed over time. Histologic sections of the repair tissue in different treatment groups are presented in Figures 2 and 3.
Table 2. Histological Scores of the Treatment Groups at Pre-Determined Sampling Times (Mean ± SD).
| Score Parameter | 4 Weeks Control | L-PRP | L-PRF | 16 Weeks Control | L-PRP | L-PRF | 24 Weeks Control | L-PRP | L-PRF |
|---|---|---|---|---|---|---|---|---|---|
| Cellular morphology | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.00 ± 0.00 a | 2.25 ± 1.98 a | 2.00 ± 0.00 | 3.00 ± 1.85 | 3.00 ± 1.85 |
| Matrix staining | 0.00 ± 0.00 | 0.62 ± 0.51 b | 0.25 ± 0.46 | 1.00 ± 0.00 | 1.5 ± 0.53 | 1.87 ± 0.35 a | 2.00 ± 0.00 | 2.5 ± 0.92 | 2.5 ± 0.92 |
| Surface regularity | 1.62 ± 0.91 | 2.00 ± 0.00 | 1.75 ± 0.70 | 2.5 ± 0.53 | 2.62 ± 0.51 | 2.12 ± 0.83 | 2.25 ± 0.88 | 2.25 ± 0.88 | 3.00 ± 0.00 |
| Structural integrity | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.50 ± 0.53 a,c | 0.50 ± 0.53 | 0.75 ± 0.46 | 0.75 ± 0.46 |
| Thickness of neo-formed cartilage | 0.62 ± 0.74 | 0.87 ± 0.35 | 1.12 ± 0.35 | 1.50 ± 0.53 | 1.25 ± 0.46 | 1.50 ± 0.53 | 0.25 ± 0.46 | 2.00 ± 0.00 d | 1.75 ± 0.46 d |
| Bonding to adjacent cartilage | 1.37 ± 0.91 | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 |
| Hypocellularity | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ±0 .00 | 0.00 ± 0.00 | 0.25 ± 0.46 | 1.25 ± 1.48 | 0.25 ± 0.46 | 1.50 ± 0.92 d | 1.25 ± 0.88 |
| Chondrocyte clustering | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | 1.75 ± 0.46 | 1.25 ± 0.88 | 1.75 ± 0.46 | 1.75 ± 0.46 | 1.25 ± 0.46 |
| Degenerative changes in adjacent cartilage | 2.00 ± 0.00 | 2.37 ± 0.51 | 2.50 ± 0.53 | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.50 ± 0.53 d,e |
| Total | 7.62 ± 1.99 | 9.87 ± 0.83 b | 9.62 ± 0.74 | 11.00 ± 1.06 | 13.38 ± 1.18 a | 14.75 ± 3.80 | 13.00 ± 1.30 | 17.75 ± 4.36 | 18.00 ± 3.70 |
a P < 0.05 vs. control group at 16 weeks.
b P < 0.05 vs. control group at 4 weeks.
c P < 0.05 vs. L-PRP group at 16 weeks.
d P < 0.05 vs. control group at 24 weeks.
e P < 0.05 vs. L-PRP group at 24 weeks.
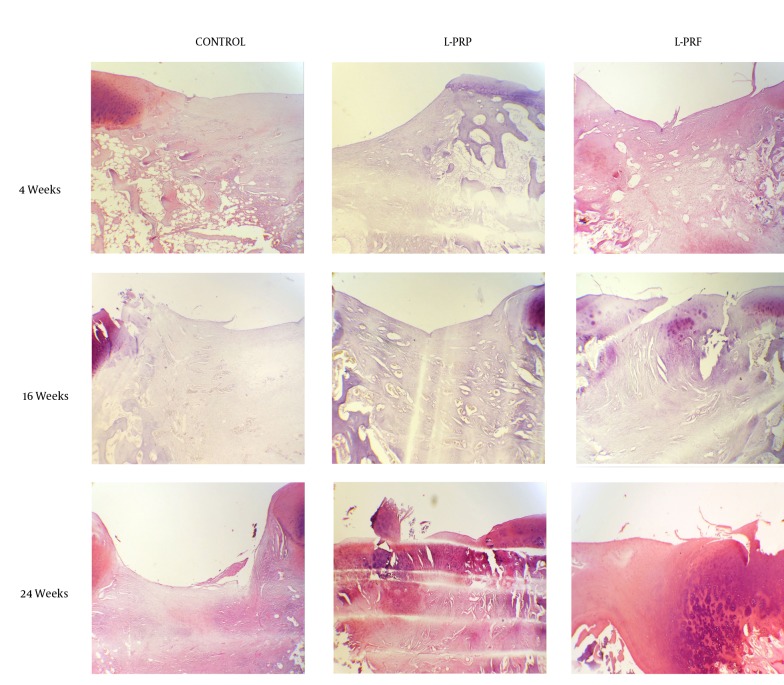
Figure 2. Safranin O Stained Histologic Sections of Representative Defects From Different Treatment Groups At the Junction of Repair Tissue and Adjacent Normal Cartilage, Original Magnification X40.
More intense staining of L-PRP and L-PRF treated groups in comparison to the control group can be seen at all sampling times.
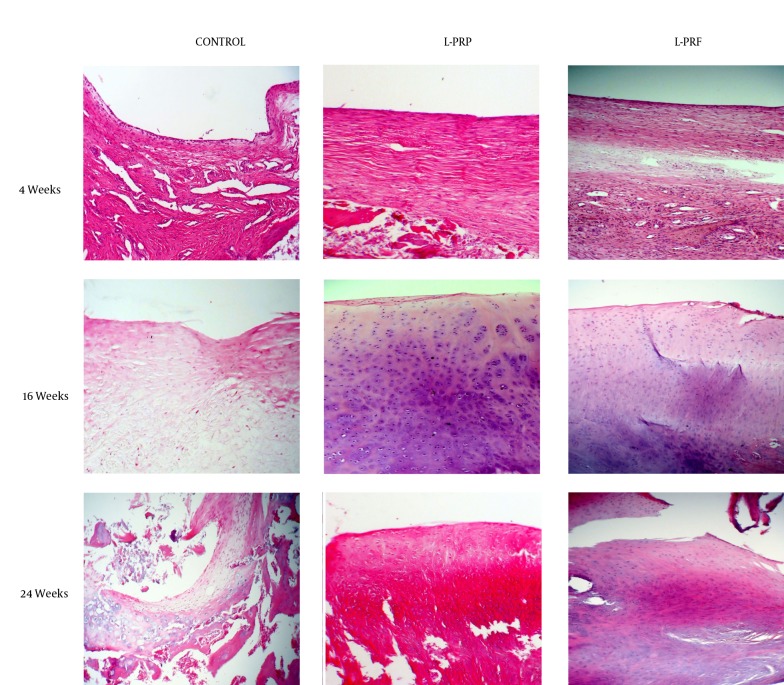
Figure 3. Hematoxylin-Eosin Stained Histologic Sections of Representative Defects From Different Treatment Groups at the Centre of Repair Tissue, Original Magnification x100.
Fibrous tissue with numerous blood vessels and fibroblasts and cystic cavities can be seen at 4 weeks in all the treatment groups. At 16 weeks, the repair tissue in L-PRP and L-PRF treated groups resembled normal articular cartilage with chondrocyte like cells and chondrocyte clustering and this pattern was also seen at 24 weeks. In the control group, the repair tissue had no similarities to normal cartilage and was obviously hypocellular at 16 weeks and at 24 weeks it contained a very large cystic cavity with tissue debris and chondrocyte like cells at the periphery of the cyst.
5. Discussion
The main rationale for the use of platelet concentrates in wound repair and articular cartilage reconstruction procedures is the presence of growth factors. Development and homeostasis of articular cartilage throughout life is regulated by growth factors (18), therefore the use of concentrated platelet products could be a promising treatment for articular cartilage regeneration. Gaissmaier et al. (19) first reported that adding human platelet supernatant to chondrocyte cultures increased cell proliferation. Subsequently, Akeda et al. (20) demonstrated that the use of PRP in culture medium increased DNA content of porcine chondrocytes and collagen and proteoglycan synthesis. Further in vitro studies also indicated that PRP increased proliferation and chondrogenic differentiation ability of bone marrow derived mesenchymal stem cells (21, 22). According to these findings, PRP could have a positive influence on cartilage repair.
The results of the present study confirmed the positive effect of PRP on cartilage repair. The repair tissue produced at the site of cartilage defects treated with L-PRP implantation was both macroscopically and histologically superior in comparison to untreated defects at all sampling times, although complete characteristics of normal hyaline articular cartilage were not achieved at the end of the 24 weeks of study period. Our results are in accordance with earlier in vivo studies indicating the positive effect of PRP on articular cartilage repair and regeneration (23-25). Similar to our study, the platelet concentrate was either implanted at the site of experimental cartilage defects or injected inside the stifle joint following surgery in these studies and the results indicated better macroscopic and histologic characteristics of repair tissue in the defects treated with PRP. Apart from the sole use of platelet concentrates for cartilage repair, combined use of PRP with other treatment methods like microfracture (16) and synthetic scaffolds (26) indicated positive influence of PRP on cartilage repair. It appears that PRP has anabolic effects on chondrocytes, synoviocytes and bone marrow derived mesenchymal stem cells resulting in increased cellular proliferation and extracellular matrix synthesis, gene expression of chondrocytes, proteoglycan and type II collagen synthesis and better cellular organization of cartilaginous tissue (10-12).
One of the limitations in PRP preparation is that the method is not standardized. Apart from the fact that PRP is classified into pure platelet rich plasma (P-PRP) and leukocyte and platelet rich plasma (L-PRP) based on the absence or presence of white blood cells (13), there are differences in the centrifugation settings and stages of PRP preparation among various studies, which could influence platelet numbers and growth factor content and ultimately the study outcome. Hence, we hypothesized that L-PRF which contains growth factors similar to PRP and could be prepared easily in a standardized manner would have a positive effect on cartilage healing through the same mechanisms described for PRP. The results of the present study confirmed this hypothesis with no significant difference between L-PRP and L-PRF treated groups, but higher macroscopic and histologic scores in L-PRF treated group at 24 weeks.
To the authors’ knowledge, this was the first study comparing the effect of these two platelet concentrates on articular cartilage healing. L-PRP was used instead of P-PRP in this study so that both platelet products would be similar regarding the presence of leukocytes in their composition. In this study, full thickness articular cartilage defects were created on the femoral condyles. This specific region was chosen because of its weight bearing role and due to the fact that most clinical lesions, both in humans and animals, are seen in this region. All the necessary mechanisms of cartilage repair, particularly cellular migration, are thought to be activated in full thickness lesions intensifying the role of growth factors in repair (25). Focal lesions were created to limit the extent of joint damage, which could negatively affect cartilage repair.
Macroscopic and histological evaluations of repair tissue are the two key parameters in the study of cartilage healing and in particular, microscopic studies are considered as the gold standard to characterize the type of repair tissue. Immunohistochemical and biomechanical studies can also be conducted to gain more information about the nature of repair tissue. In the present study, macroscopic and histological evaluations were conducted, but due to financial constraints and unavailability of equipment, characterization of collagen type through immunohistochemical methods and cartilage stiffness as a measure of its biomechanical properties were not performed. Another shortcoming of this study was that we could not increase the duration of study further i.e. up to a year to evaluate repair tissue over a longer period of time.
In conclusion, using L-PRF for the treatment of acute full thickness articular cartilage defects of the knee produced a repair tissue similar to L-PRP treated defects both macroscopically and microscopically and better than the untreated defects. These findings indicate that PRF could be used for treatment of human cartilage lesions. Further studies are required to examine the effect of this biomaterial on the healing of partial thickness and chronic articular defects and in clinical cases.
Acknowledgments
The authors would like to thank Tabriz Branch, Islamic Azad university for financial support of this research which is based on a research project contract number 2-17-5-102536.
Footnotes
Authors’ Contributions:Study concept and design: Davoud Kazemi, Acquisition of data: Davoud Kazemi and Ashraf Fakhrjou, Analysis and interpretation of data: Davoud Kazemi, Drafting of the manuscript: Davoud Kazemi, Critical revision of the manuscript for important intellectual content: Davoud Kazemi, Statistical analysis: Kazemi, Administrative, technical, and material support: Davoud Kazemi and Ashraf Fakhrjou, Study supervision: Davoud Kazemi.
Funding/Support:This study was supported by the grant number 2-17-5-102536 from Tabriz Branch, Islamic Azad University.
References
- 1.Bhosale AM, Richardson JB. Articular cartilage: structure, injuries and review of management. Br Med Bull. 2008;87:77–95. doi: 10.1093/bmb/ldn025. [DOI] [PubMed] [Google Scholar]
- 2.Shah MR, Kaplan KM, Meislin RJ, Bosco J3. Articular cartilage restoration of the knee. Bull NYU Hosp Jt Dis. 2007;65(1):51–60. [PubMed] [Google Scholar]
- 3.Memon AR, Quinlan JF. Surgical treatment of articular cartilage defects in the knee: are we winning? Adv Orthop. 2012;2012:528423. doi: 10.1155/2012/528423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706–15. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaissmaier C, Koh JL, Weise K. Growth and differentiation factors for cartilage healing and repair. Injury. 2008;39 Suppl 1:S88–96. doi: 10.1016/j.injury.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 6.Illingworth KD, Musahl V, Lorenz SG, Fu FH. Use of Fibrin Clot in the Knee. Operat Tech Orthopaed. 2010;20(2):90–7. doi: 10.1053/j.oto.2009.11.002. [DOI] [Google Scholar]
- 7.Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1(3-4):165–74. doi: 10.1007/s12178-008-9032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrams GD, Frank RM, Fortier LA, Cole BJ. Platelet-rich plasma for articular cartilage repair. Sports Med Arthrosc. 2013;21(4):213–9. doi: 10.1097/JSA.0b013e3182999740. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad Z, Howard D, Brooks RA, Wardale J, Henson FM, Getgood A, et al. The role of platelet rich plasma in musculoskeletal science. JRSM Short Rep. 2012;3(6):40. doi: 10.1258/shorts.2011.011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortier LA, Hackett CH, Cole BJ. The Effects of Platelet-Rich Plasma on Cartilage: Basic Science and Clinical Application. Operative Techniques in Sports Medicine. 2011;19(3):154–9. doi: 10.1053/j.otsm.2011.03.004. [DOI] [Google Scholar]
- 11.Smyth NA, Murawski CD, Fortier LA, Cole BJ, Kennedy JG. Platelet-rich plasma in the pathologic processes of cartilage: review of basic science evidence. Arthroscopy. 2013;29(8):1399–409. doi: 10.1016/j.arthro.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Yuan M, Meng HY, Wang AY, Guo QY, Wang Y, et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthritis Cartilage. 2013;21(11):1627–37. doi: 10.1016/j.joca.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158–67. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 14.You TM, Choi BH, Li J, Jung JH, Lee HJ, Lee SH, et al. The effect of platelet-rich plasma on bone healing around implants placed in bone defects treated with Bio-Oss: a pilot study in the dog tibia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(4):e8–12. doi: 10.1016/j.tripleo.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 15.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Milano G, Sanna Passino E, Deriu L, Careddu G, Manunta L, Manunta A, et al. The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects: an experimental study in a sheep model. Osteoarthritis Cartilage. 2010;18(7):971–80. doi: 10.1016/j.joca.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Moojen DJ, Saris DB, Auw Yang KG, Dhert WJ, Verbout AJ. The correlation and reproducibility of histological scoring systems in cartilage repair. Tissue Eng. 2002;8(4):627–34. doi: 10.1089/107632702760240544. [DOI] [PubMed] [Google Scholar]
- 18.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97(1):33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 19.Gaissmaier C, Fritz J, Krackhardt T, Flesch I, Aicher WK, Ashammakhi N. Effect of human platelet supernatant on proliferation and matrix synthesis of human articular chondrocytes in monolayer and three-dimensional alginate cultures. Biomaterials. 2005;26(14):1953–60. doi: 10.1016/j.biomaterials.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 20.Akeda K, An HS, Okuma M, Attawia M, Miyamoto K, Thonar EJ, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14(12):1272–80. doi: 10.1016/j.joca.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Mishra A, Tummala P, King A, Lee B, Kraus M, Tse V, et al. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods. 2009;15(3):431–5. doi: 10.1089/ten.tec.2008.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaky SH, Ottonello A, Strada P, Cancedda R, Mastrogiacomo M. Platelet lysate favours in vitro expansion of human bone marrow stromal cells for bone and cartilage engineering. J Tissue Eng Regen Med. 2008;2(8):472–81. doi: 10.1002/term.119. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Alvarez F, Castiella T, Guallar E, Grasa JM, Gomez-Barrena E, Lacleriga A. Influence of platelet time activation on articular cartilage growth in the rabbit knee: preliminary study. Knee. 2008;15(4):314–7. doi: 10.1016/j.knee.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 24.García-Álvarez F, Castiella T, Val S, Gómez-Arrue J, Grasa JM, Viloria A, et al. Influence of concentrated platelets on the reconstruction of cartilage defects in the lamb knee joint. Revista Española de Cirugía Ortopédica y Traumatología (English Edition). 2010;54(6):378–82. doi: 10.1016/s1988-8856(10)70265-0. [DOI] [Google Scholar]
- 25.Serra CI, Soler C, Carrillo JM, Sopena JJ, Redondo JI, Cugat R. Effect of autologous platelet-rich plasma on the repair of full-thickness articular defects in rabbits. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1730–6. doi: 10.1007/s00167-012-2141-0. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Feng Y, Zhang CQ, Chen SB, Cheng XG. The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int Orthop. 2010;34(4):589–97. doi: 10.1007/s00264-009-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]