Abstract
Background
In shared decision making (SDM), the patient and the physician reach decisions in partnership. We conducted a trial of SDM training for physicians who treat patients with cancer.
Methods
Physicians who treat patients with cancer were invited to participate in a cluster-randomized trial and carry out SDM together with breast or colon cancer patients who faced decisions about their treatment. Decision-related physician–patient conversations were recorded. The patients filled out questionnaires immediately after the consultations (T1) and three months later (T2). The primary endpoints were the patients’ confidence in and satisfaction with the decisions taken. The secondary endpoints were the process of decision making, anxiety, depression, quality of life, and externally assessed physician competence in SDM. The physicians in the intervention group underwent 12 hours of training in SDM, including the use of decision aids.
Results
Of the 900 physicians invited to participated in the trial, 105 answered the invitation. 86 were randomly assigned to either the intervention group or the control group (44 and 42 physicians, respectively); 33 of the 86 physicians recruited at least one patient for the trial. A total of 160 patients participated in the trial, of whom 55 were treated by physicians in the intervention group. There were no intergroup differences in the primary endpoints. Trained physicians were more competent in SDM (Cohen’s d = 0.56; p<0.05). Patients treated by trained physicians had lower anxiety and depression scores immediately after the consultation (d = -0.12 and -0.14, respectively; p<0.10), and markedly lower anxiety and depression scores three months later (d = -0.94 and -0.67, p<0.01).
Conclusion
When physicians treating cancer patients improve their competence in SDM by appropriate training, their patients may suffer less anxiety and depression. These effects merit further study.
Shared decision making has become increasingly important in oncology (1). It can be defined as a decision making process in which patient and health-care provider discuss possible treatment options and come to a joint decision (2). Studies show that decision aids support shared decision making in oncology by increasing confidence in the decision, knowledge of treatment options, and patients’ satisfaction with the decision (3– 5). Few physicians yet feel that they have received sufficient training to integrate shared decision making skills into their work (1). It is very challenging to adapt one’s conversational style to patients’ preferences regarding their involvement (Box) (6, 7).
Box. Cancer patients’ participation preferences.
Identifying a patient’s participation preferences and adapting one’s style of conversation to his or her needs is very challenging but at the same time a highly important skill for physicians: overall, whether patients prefer to take a passive, shared, or active role in decision making varies greatly between diseases, but there are also major differences between individual cancer patients. The percentage of patients who prefer to take an active role ranges from 42 to 89% in different studies (6). A study on the treatment of women with breast cancer found that at the beginning of their treatment approximately 40% of patients wished to take a passive role in decision-making, and 60% an active or shared role (7).
However, only 42% of patients felt they had a choice between various treatment options. When patients realized that they could choose, they generally wished to be actively involved in the decision making process. It was also found that patients who preferred to take a passive role were significantly more likely to suffer symptoms of depression than women who preferred to take an active role. More than half of patients (63%) felt that their decision making preferences were met by their physicians. Patients who wanted a shared role were treated according to their wishes less frequently than those who wanted an active or passive role. Patients who were treated in line with their participation preferences were more likely to report that they were satisfied with their decision than those who were less involved than they would have preferred.
In contrast to the use of decision aids, the evidence on shared decision making training programs is less clear. Two recent reviews on patient-reported outcomes (8) and health-care providers’ shared decision making strategies did not provide a uniform picture (9). Only a few studies have been able to find an effect on patient-reported outcomes or health-care providers’ shared decision making skills. Training in which decision aids for patients were also used was more effective (8). Our research group has developed two training programs in shared decision making within the context of Germany’s Federal Ministry of Health’s funding priority “The patient as partner in the medical decision-making process.” These cover depression (10) and chronic pain (11, 12). A comprehensive training manual has been published (13– 16) but does not specifically target oncology and contains no strategies for communicating risk (17, 18).
This study therefore aimed to evaluate the efficacy of a specific training program on shared decision making for physicians working in oncology. It involved patients with breast or colon cancer, as these diseases are highly prevalent (19). Our primary hypothesis was that patients who were treated by trained physicians in the intervention group would report higher confidence in and satisfaction with their decisions immediately after their consultations than those in the control group. Secondary hypotheses were that patients in the intervention group would perceive more empathy and involvement during the consultation and report less anxiety and depression and a higher quality of life. Three months after the consultations, we expected the intervention group to have more confidence in their decisions, more satisfaction with their decisions, better quality of life, and less anxiety and depression. It was also believed that the patients in the intervention group would show less regret concerning their decisions. Finally, it was expected that during consultations the observer-rated shared decision making skills of the physicians in the intervention group would be better than those of physicians in the control group.
Methods
Study design
This was a parallel-group, cluster-randomized trial. Data was gathered at two points in time: immediately following (T1) and three months after (T2) a consultation regarding a treatment decision. The study was entered in the German Clinical Trials Register (DRKS, Deutsches Register Klinischer Studien) under number DRKS00000539. The ethical approval of the Universities of Freiburg (no. 274/06) and Heidelberg (no. 377/2006) was obtained.
Participants
Initially, physicians providing inpatient and outpatient care for colon cancer and physicians working in breast cancer centers in Freiburg and Heidelberg were invited to take part in the study. Subsequently, all breast and colon cancer centers; all oncology, gynecology, and gastroenterology societies registered in Germany; and the German Association of Psycho-Social Oncology (DAPO) were contacted by telephone, mail, or e-mail. The study was also promoted at local and national conferences. The inclusion criterion for physicians was treatment of patients with breast or colon cancer during the study period. Patients could be recruited into the study if they were facing a treatment decision and if they gave their informed consent to participate. Exclusion criteria were medical contraindications for the investigated treatment decisions, secondary tumors, insufficient knowledge of German, and other medical contraindications.
Measuring tools
The patient questionnaire used at time T1 contained the following scales to document primary and secondary outcomes:
Satisfaction with Decision Scale (22)
Shared Decision Making Questionnaire (SDM-Q-9) (23)
Consultation and Relational Empathy Scale (CARE) (24)
Hospital Anxiety and Depression Scale (HADS) (25)
Cancer-specific questionnaire of the European Organization for Research and Treatment of Cancer (EORTC-QLQ-30) (26)
At time T2, the Decision Regret Scale (DRS) (27) was added to the questionnaire. Physicians documented clinical data on the progress of cancer treatment. Physicians’ shared decision making skills were assessed using the Observing Patient Involvement Scale (OPTION) (28).
Intervention
Physicians in the intervention group participated in shared decision making training consisting of 12 training units, including a unit on the use of patient decision aids. Each decision aid concerns one of four preference-sensitive decisions that were selected by experts during the study preparation (eBox). Physicians in the control group provided treatment as usual. They received neither training nor access to decision aids during the study but did have the opportunity to undergo training in shared decision making after the study had been completed.
eBox. Schedule for training in shared decision making.
-
Day 1 (8 hours)
Introductory talk: background and essentials of shared decision making
Description and explanation of shared decision making skills
Exchange of experience: observation of shared decision making skills in a demo video
Exchange of experience: exercises to develop shared decision making skills, with video feedback
Introductory talk: risk communication and decision aids
Exchange of experience: use of decision aids in role play
-
Day 2 (4 hours): Refresher
Discussion of implementation difficulties, gathering of difficult situations
Exchange of experience: exercises to develop shared decision making skills, with video feedback
Decision aids
-
Preference-sensitive decisions
Decision aids were developed in a multistage process that involved oncology physicians and cancer patients. During this process, four situations were selected that are particularly pertinent and occur frequently in the treatment of breast or colon cancer:
Neoadjuvant chemotherapy versus immediate surgery (breast cancer)
Mastectomy versus breast-conserving therapy (breast cancer)
Adjuvant chemotherapy and hormone therapy versus adjuvant hormone therapy (breast cancer)
Stage II colon cancer following R0 resection: adjuvant chemotherapy versus no adjuvant chemotherapy
-
Structure of decision aids
Decision aids were developed for use during consultation and are therefore as brief as possible. They consist mainly of graphics that explain the following content:
Treatment options: representation of the various care pathways
Advantages and disadvantages of both treatment options: table showing advantages and disadvantages of both options
10-year survival rates: diagram showing percentage survival rates for all options
Considering the arguments: table showing advantages and disadvantages of both options and space for notes and personal considerations on the various arguments
Space for notes
Study conduct
Physicians were randomized to the intervention group or control group at a ratio of 1:1 by an independent statistician, using a computer-based procedure (29). Randomization was stratified by sex, whether treatment was inpatient or outpatient, and the physician’s clinical experience. Patients were blinded to the group to which they had been randomized. Following randomization, all physicians were told which group they belonged to and how the study was to be conducted. Physicians participating in the intervention group recruited patients into the study after their training in shared decision making.
Physicians informed their patients of the study before a consultation regarding a treatment decision. Patients received written information on the study and signed a declaration of consent. Each patient’s subsequent consultation was recorded using a dictation device. Next, patients filled out the questionnaires and received the follow-up questionnaire three months later, together with a postage-paid envelope, by mail. A reminder was sent out four weeks later. No specific subsequent measures were taken to maintain patient blinding.
Calculation of sample size, statistical analysis
Cohen’s effect size d = 0.36 was expected (5, 30). Using a Bonferroni-corrected alpha error rate of 0.025 and a beta error rate of 0.1, assuming a coefficient of variation of 0.2, it was determined that the sample should consist of 50 physicians, who should recruit eight patients each, totaling 400 patients. In view of the clustered study design, data was analyzed using a linear mixed model. A random-intercept model was constructed with three predictors (treatment group, cancer type, interaction between treatment group and cancer type) and physician as cluster variable.
Because the sample was small, primary analysis was per-protocol. It included only physicians following the study procedure of the study group to which they had been randomized (intervention group vs. control group) and had recruited at least one patient for the outcomes to be investigated. Intention-to-treat analysis was also performed. Various strategies were used to impute missing data; all of these yielded comparable findings. Only the results of per-protocol analysis and one of the intention-to-treat analyses performed are reported here (eMethods). The significance level for the primary outcomes was set at 0.025.
eMethods. Statistical analyses and findings.
The per-protocol sample consisted of physicians in the intervention group and the control group. Those in the intervention group had undergone training. Treating physicians each recruited at least one patient into the study. A total of two per-protocol analyses was performed (eMethods: Tables 1 and 2). The first analyzed only complete data (available cases analysis). In the second, missing values for the primary outcomes at the patient level were imputed using the expectation-maximization method (imputed cases analysis).
The intention-to-treat analysis included three separate analyses which took into account all physicians in the intervention and control groups (eMethods Tables 3: and 4). The primary outcomes of missing patients, i.e. those who had not been recruited, were estimated using hierarchical (mixed) regression analyses. This involved a random-intercept model based on various physician-level predictors, in order to estimate missing patient-level data for physicians who had not recruited any patients into the study. Imputation took account of physicians’ characteristics, uncertainty caused by physician- and patient-level variation, and random variations in the size of the completed sample. Because a substantial amount of data had to be estimated, multiple imputation with 10 imputations was used (40). Analyses were performed for three scenarios, each of which was based on a different assumption:
Compliant scenario (CO): Missing values for the intervention and control groups were estimated on the basis of existing data from the groups to which the physicians had originally been allocated.
Noncompliant scenario (NC): missing values for both groups were estimated on the basis of complete data from the control group.
Most likely scenario (ML): Missing values for the control group and physicians in the intervention group who had not undergone the intervention were estimated on the basis of complete data for the control group; missing values for physicians in the intervention group who had undergone training were estimated on the basis of complete data from the intervention group.
Analysis of secondary outcomes was exploratory. Sensitivity analyses were performed using physician and patient characteristics as covariates. Statistical analysis was performed using R version 2.15.2 (31), with the lme4 package (32).
Results
Physicians
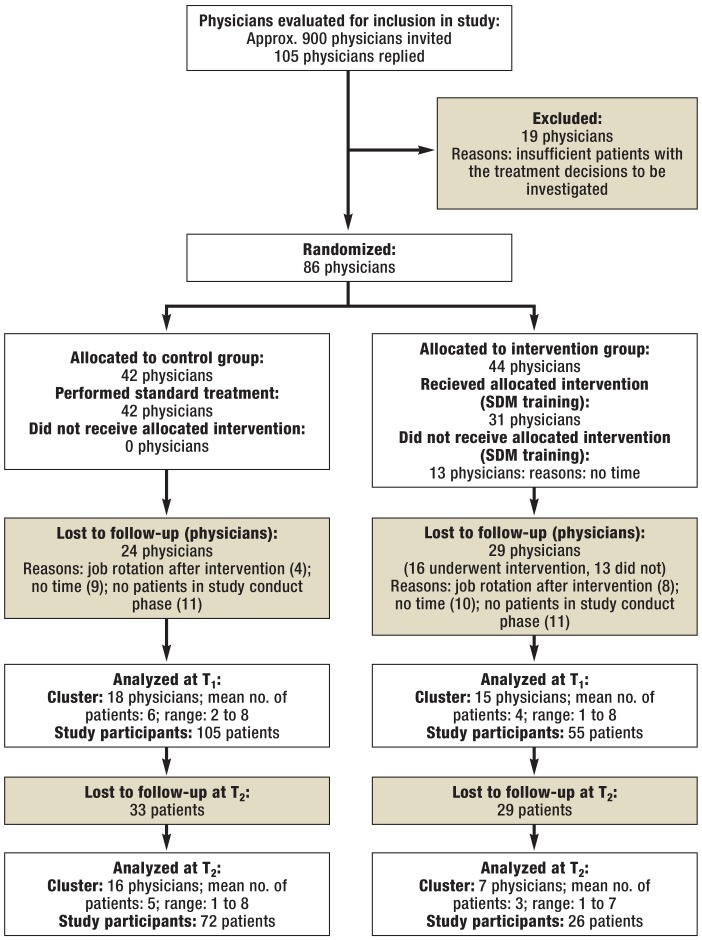
Approximately 900 physicians were invited to take part in the study between October 2008 and October 2012. Of these, 105 replied to the inquiry. A total of 86 physicians were included in the study and randomized to the intervention group (n = 44) or the control group (n= 42). After randomization, 53 physicians left the study (Figure 1).
Figure 1.
Flow diagram of patients included and excluded
SDM, shared decision making; T, time point
Of the 33 physicians who recruited patients into the study, 17 treated breast cancer patients and 16 colon cancer patients. In total, 18 physicians were male. The mean age was 36.5 years (standard deviation: 7.5). The majority had never previously undergone any psychosomatic or psycho-oncological training (eTable 1). There were no substantial differences between the physicians in the intervention group and those in the control group.
eTable 1. Sociodemographic information on physicians who recruited patients into the study.
| Control group | Intervention group | Total (n = 33) | |||||
|---|---|---|---|---|---|---|---|
| Breast cancer (n = 11) | Colon cancer (n = 7) | Total (n = 18) | Breast cancer (n = 6) | Colon cancer (n = 9) | Total (n = 15) | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Sex* | |||||||
| Male | 5 (45.5) | 4 (57.1) | 9 (50.0) | 2 (33.3) | 7 (77.8) | 9 (60.0) | 18 (54.5) |
| Female | 6 (54.5) | 3 (42.9) | 9 (50.0) | 4 (66.7) | 2 (22.2) | 6 (40.0) | 15 (45.5) |
| Age (years) | |||||||
| M (SD) | 35.7 (7.8) | 34.4 (4.5) | 35.2 (6.6) | 39.5 (9.9) | 37.0 (7.9) | 38.0 (8.5) | 36.5 (7.5) |
| Employment history* | |||||||
| 0 to 4 years | 6 (54.5) | 2 (28.6) | 8 (44.4) | 1 (16.7) | 2 (22.2) | 3 (20.0) | 11 (33.3) |
| 5 to 10 years | 1 (9.1) | 4 (57.1) | 5 (27.8) | 3 (50.0) | 3 (33.3) | 6 (40.0) | 11 (33.3) |
| >10 years | 4 (36.4) | 1 (14.3) | 5 (27.8) | 2 (33.3) | 4 (44.4) | 6 (40.0) | 11 (33.3) |
| Treatment basis* | |||||||
| Inpatient | 10 (90.9) | 7 (100.0) | 17 (94.4) | 4 (66.7) | 9 (100.0) | 13 (86.7) | 30 (90.9) |
| Outpatient | 1 (9.1) | 0 (0.0) | 1 (5.6) | 2 (33.3) | 0 (0.0) | 2 (13.3) | 3 (9.1) |
| Basic psychosomatic care | |||||||
| Yes | 2 (18.2) | 0 (0.0) | 2 (11.1) | 2 (33.3) | 4 (44.4) | 6 (40.0) | 8 (24.2) |
| No | 9 (81.8) | 7 (100.0) | 16 (88.9) | 4 (66.7) | 5 (55.6) | 9 (60.0) | 25 (75.8) |
| Continuing education in psycho-oncology | |||||||
| Yes | 2 (18.2) | 0 (0.0) | 2 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (6.1) |
| No | 9 (81.8) | 7 (100.0) | 16 (88.9) | 6 (100.0) | 9 (100.0) | 15 (100.0) | 31 (93.9) |
There was no missing data
M, mean; SD, standard deviation; n, sample size
*Variables used for stratification
Patients
Patients were recruited into the study between January 2010 and June 2012. Recruitment ended when all options for enrolling new physicians and patients had been exhausted. A total of 160 patients (nCG = 105; nIG = 55) participated, of whom 93 suffered from breast cancer and 67 from colon cancer (Table 1). Of the 160 patients, 98 (61.3%) completed the follow-up questionnaire three months after their consultation (Figure 1). Because of the difficulties in recruiting patients into the study, physicians were also asked to consider patients facing a decision other than the four treatment decisions selected in advance.
Table 1. Sociodemographic and clinical characteristics of patient sample.
| Control group | Intervention group | Total (n = 160) | |||||
|---|---|---|---|---|---|---|---|
| Breast cancer (n = 71) | Colon cancer (n = 34) | Total (n = 105) | Breast cancer (n = 22) | Colon cancer (n = 33) | Total (n = 55) | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Sex | |||||||
| Male | 0 (0.0) | 12 (42.9) | 12 (13.3) | 0 (0.0) | 12 (48.0) | 12 (27.9) | 24 (18.0) |
| Female | 62 (100.0) | 16 (57.1) | 78 (86.7) | 18 (100.0) | 13 (52.0) | 31 (72.1) | 109 (82.0) |
| Age (years) | |||||||
| M (SD) | 60.4 (13.9) | 69.5 (11.6) | 63.2 (13.8) | 55.7 (15.3) | 71.3 (10.7) | 64.8 (14.8) | 63.7 (14.1) |
| Marital status | |||||||
| Unmarried | 2 (3.9) | 3 (10.7) | 5 (6.3) | 0 (0.0) | 3 (12.0) | 3 (7.0) | 8 (6.6) |
| Married | 36 (70.6) | 15 (53.6) | 51 (64.6) | 14 (77.8) | 13 (52.0) | 27 (62.8) | 78 (63.9) |
| Divorced | 6 (11.8) | 2 (7.1) | 8 (10.1) | 2 (11.1) | 3 (12.0) | 5 (11.6) | 13 (10.7) |
| Widowed | 7 (13.7) | 8 (28.6) | 15 (19.0) | 2 (11.1) | 6 (24.0) | 8 (18.6) | 23 (18.9) |
| Schooling | |||||||
| Less than 12 years | 43 (87.8) | 25 (89.3) | 68 (88.3) | 16 (94.1) | 23 (92.0) | 32 (92.9) | 107 (89.9) |
| 12 years or more | 6 (12.2) | 3 (10.7) | 9 (11.7) | 1 (5.9) | 2 (8.0) | 3 (7.1) | 12 (10.1) |
| Cancer stage | |||||||
| I | 28 (41.8) | 2 (6.5) | 30 (30.6) | 7 (36.8) | 6 (19.4) | 13 (26.0) | 43 (29.1) |
| II | 23 (34.3) | 4 (12.9) | 27 (27.6) | 7 (36.8) | 9 (29.0) | 16 (32.0) | 43 (29.1) |
| III | 14 (20.9) | 17 (54.8) | 31 (31.6) | 3 (15.8) | 11 (35.5) | 14 (28.0) | 45 (30.4) |
| IV | 2 (3.0) | 8 (25.8) | 10 (10.2) | 2 (10.5) | 6 (16.1) | 7 (14.0) | 17 (11.5) |
Valid percentages were reported. Percentages relate to whole sample, including missing values.n , sample size; M, mean; SD, standard deviation
The per-protocol and intention-to-treat analyses did not reveal any significant differences between the intervention group and control group patients in terms of the primary outcomes (Table 2).
eMethods: Table 2. Findings of per-protocol analyses at time T2.
| Control group | InterventiDon group | Significance (p-value) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Breast cancer | Colon cancer | Total | Breast cancer | Colon cancer | Total | ||||
| M (SD); n | M (SD); n | M (SD); n | M (SD); n | M (SD); n | M (SD); n | Group | Cancer type | Interaction | |
| SwD (AC) SwD (IC) |
86.8 (13.7); 50 86.9 (13.3); 53 |
93.3 (10.5); 17 91.1 (15.3); 19 |
88.5 (13.2); 67 88.0 (13.9); 72 |
84.2 (14.6); 12 85.1 (14.4); 13 |
80.0 (15.0); 13 80.0 (15.0); 13 |
82.0 (14.7); 25 82.6 (14.6); 26 |
0.549 0.686 |
0.100*1 0.285 |
0.120 0.181 |
| DCS (AC) DCS (IC) |
82.4 (17.6); 49 81.3 (20.0); 53 |
n.d.a .n.d.a. |
82.4 (17.6); 49 81.3 (20.0); 53 |
77.5 (17.0); 12 77.9 (16.3); 13 |
20.3 (5.8); 4 20.3 (5.8); 4 |
63.2 (29.5); 16 64.3 (29.0); 17 |
0.301 0.472 |
<0.001*4 <0.001*4 |
n.c. n.c. |
| HADS-Anxiety (AC) HADS-Anxiety (IC) |
13.4 (1.6); 49 13.3 (1.6); 51 |
12.8 (1.7); 19 12.8 (1.7); 20 |
13.2 (1.6); 68 13.2 (1.6); 71 |
9.7 (4.8); 12 9.7 (4.8); 12 |
11.2 (4.4); 13 11.2 (4.4); 13 |
10.4 (4.6); 25 10.4 (4.6); 25 |
0.009*3 0.010*2 |
0.630 0.663 |
0.471 0.490 |
| HADS-Depression (AC )HADS-Depression (IC) |
7.7 (1.0); 48 7.7 (1.0); 51 |
7.5 (0.8); 19 7.5 (0.8); 20 |
7.6 (0.9); 67 7.6 (0.9); 71 |
5.3 (2.6); 12 5.3 (2.6); 12 |
7.5 (2.3); 13 7.5 (2.3); 13 |
6.4 (2.6); 25 6.4 (2.6); 25 |
<0.001*4 <0.001*4 |
0.637 0.549 |
0.005*3 0.003*3 |
| EORTC-QLQ-30 (AC) EORTC-QLQ-30 (IC) |
61.8 (22.2); 46 60.2 (23.5); 48 |
67.1 (18.3); 19 67.1 (18.3); 19 |
63.3 (21.1); 65 62.2 (22.3); 67 |
56.1 (22.1); 11 56.1 (22.1); 11 |
55.1 (25.1); 13 55.1 (25.1); 13 |
55.6 (23.3); 24 55.6 (23.3); 24 |
0.446 0.587 |
0.383 0.277 |
0.567 0.491 |
| DRS (AC) DRS (IC) |
13.4 (14.5); 48 12.8 (14.3); 52 |
11.1 (15.3); 18 11.3 (14.9); 19 |
12.8 (14.7); 66 12.4 (14.4); 71 |
17.7 (14.1); 13 17.7 (14.1); 13 |
15.0 (19.6); 13 15.0 (19.6); 13 |
16.3 (16.8); 26 16.3 (16.8); 26 |
0.388 0.311 |
0.591 0.721 |
0.961 0.868 |
*1p <0.10; *2p <0.05; *3p <0.01; *4p <0.001
M, mean; SD, standard deviation; n , sample size; AC, available cases; IC, imputed cases; n.d.a., no data available; n.c., not calculable; SwD, Satisfaction with Decision Scale; DCS, Decisional Conflict Scale (calculated as confidence in decision); HADS, Hospital Anxiety and Depression Scale; EORTC-QLQ-30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; DRS, Decision Regret Scale
At time T1, the intervention group patients overall reported less anxiety (Cohen’s d = –0.12; 95% confidence interval [CI]: –0.50 to 0.27; p<0.10) and depression (d = –0.14; 95% CI: –0.52 to 0.24; p<0.10) than those in the control group. For breast cancer patients, those in the intervention group had lower anxiety scores (d = –0.31; 95% CI: –0.85 to 0.23) and depression scores (d = –0.40; 95% CI: –0.94 to 0.4) than those in the control group, but the opposite was true for colon cancer patients (d = 0.26; 95% CI: –0.29 to 0.82 for anxiety; d = 0.24; 95% CI: –0.32 to 0.79 for depression). There were no significant differences between the two groups in terms of other secondary patient-reported outcomes (eTable 2).
eTable 2. Findings of per-protocol and intention-to-treat analyses for secondary outcomes at time t1.
| Control group | Intervention group | Significance | Effect size | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Breast cancer | Colon cancer | Total | Breast cancer | Colon cancer | Total | Group | Cancer type | Interaction | Group | |
| M (SD); n | M (SD); n | M (SD); n | M (SD); n | M (SD); n | M (SD); n | p | p | p | d*5 (95% CI) | |
|
SDM-Q-9*1 Per-protocol Intention-to-treat*2 |
80.3 (19.6); 48 75.8 (20.3); 141 |
72.5 (23.7); 28 69.3 (21.8); 77 |
77.4 (21.4); 76 73.5 (21.2); 219 |
72.7 (20.6); 18 73.8 (20.8); 112 |
79.4 (23.9); 24 70.9 (22.2); 87 |
76.5 (22.5); 42 72.0 (21.4); 194 |
0.378 0.759 |
0.167 0.182 |
0.138 0.497 |
–0.07 (−0.45 to 0.31) |
|
CARE* 3Per-protocol Intention-to-treat*2 |
44.8 (5.7); 51 44.3 (5.5); 142 |
42.8 (7.9); 28 43.7 (6.5); 83 |
44.1 (6.6); 79 44.1 (5.9); 222 |
41.9 (7.8); 18 43.3 (6.2); 117 |
45.7 (3.9); 24 44.6 (4.8); 86 |
44.0 (6.1); 42 43.9 (5.7); 204 |
0.253 0.545 |
0.304 0.692 |
0.079*6 0.345 |
–0.03 (−0.41 to 0.34) |
|
HADS-Anxiety*4 Per-protocol Intention-to-treat*2 |
8.9 (4.5); 48 9.2 (4.2); 143 |
6.4 (3.9); 27 6.7 (4.2); 73 |
8.0 (4.4); 75 8.4 (4.4); 218 |
6.9 (3.0); 18 7.9 (4.2);116 |
7.7 (4.1); 23 7.8 (4.1); 82 |
7.3 (3.7); 41 7.9 (4.2); 201 |
0.090*6 0.054*6 |
0.020*7 0.001*8 |
0.055*6 0.036*7 |
−0.12 (−0.50 to 0.27) |
|
HADS-Depression*4 Per-protocol Intention-to-treat*2 |
6.3 (4.2); 49 6.5 (4.3); 138 |
6.5 (3.9); 28 6.2 (4.1); 78 |
6.3 (4.1); 77 6.4 (4.3); 216 |
4.1 (2.8); 18 4.8 (4.1); 121 |
7.3 (5.0); 23 7.2 (4.4); 82 |
5.9 (4.4); 41 5.8 (4.3); 202 |
0.063*6 0.094*6 |
0.839 0.940 |
0.076*6 0.038*7 |
−0.14 (−0.52 to 0.24) |
|
EORTC-QLQ-30 Quality of Life*1 Per-protocol Intention-to-treat*2 |
59.8 (20.4); 46 58.2 (21.1); 139 |
50.9 (27.3); 28 55.2 (24.8); 75 |
56.4 (23.5); 74 56.8 (22.6); 210 |
56.0 (21.5); 18 55.5 (22.9); 118 |
41.0 (26.0); 24 44.3 (21.5); 81 |
47.7 (25.1); 42 50.7 (23.1); 204 |
0.571 0.619 |
0.128 0.733 |
0.513 0.303 |
−0.27 (−0.65 to 0.11) |
|
OPTION*1 Per-protocol Intention-to-treat*2 |
24.2 (9.9); 70 23.1 (9.6); 145 |
17.1 (5.4); 28 16.8 (8.3); 79 |
22.1 (9.4); 98 21.1 (9.7); 222 |
33.6 (11.7); 20 30.5 (11.3); 116 |
25.5 (9.9); 27 21.7 (10.2); 83 |
29.0 (11.3); 47 26.9 (11.6); 199 |
0.012*7 0.016*7 |
0.072*6 0.084*6 |
0.921 0.681 |
0.56 (0.21 to 0.91) |
Full findings reported in eMethods.
M, mean; SD, standard deviation; n, sample size; CI, confidence interval; SDM-Q-9, Shared Decision Making Questionnaire; CARE, Consultation and Relational Empathy Scale; HADS, Hospital Anxiety and Depression Scale; EORTC-QLQ-30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; OPTION, Observing Patient Involvement Scale
*1Scores range from 0 to 100. 0 is low, 100 high.
*2Intention-to-treat analysis assuming the most likely scenario: missing values for the control group were estimated on the basis of available data from the control group; missing values for physicians in the intervention group who had undergone training were estimated on the basis of available data from the intervention group; missing values for physicians in the intervention group who had not undergone training were estimated on the basis of data from the control group.
*3Scores range from 0 to 50. 0 is low, 50 high.
*4Scores range from 0 to 21. 0 is low, 21 high for anxiety/depression.
*5Cohen’s d was estimated using means and standard deviations of the intention-to-treat analysis and sample size of the per-protocol analysis.
*6 p <0.10; *7 p <0.05; *8 p <0.01
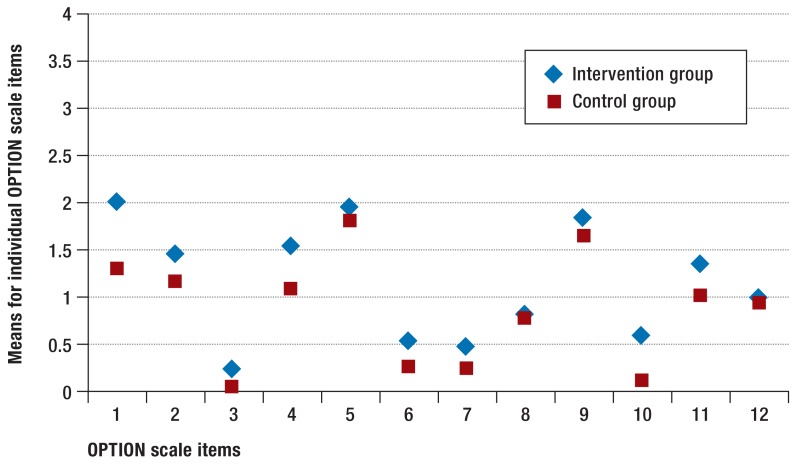
When total OPTION scores were calculated, agreement between the observers (intraclass correlation, ICC) was between 0.69 and 0.96. Physicians in the intervention group achieved higher total OPTION scores (d = 0.56; 95% CI: 0.21 to 0.91; p<0.05) (eTable 2). (Figure 2 shows the means for the 12 individual OPTION items.
Figure 2.
Means for individual items of the Observing Patient Involvement (OPTION) Scale
1: The clinician draws attention to an identified problem as one that requires a decision making process.
2: The clinician states that there is more than one way to deal with the identified problem (’equipoise’).
3: The clinician assesses patient’s preferred approach to receiving information to assist decision making (e.g. discussion in consultations, read printed material, assess graphical data, use videotapes or other media.
4: The clinician lists ’options’, which can include the choice of ’no action’.
5: The clinician explains the pros and cons of options to the patient (taking ’no action’ is an option).
6: The clinician explores the patient’s expectations (or ideas) about how the problem(s) are to be managed.
7: The clinician explores the patient’s concerns (fears) about how problem(s) are to be managed.
8: The clinician checks that the patient has understood the information.
9: The clinician offers the patient explicit opportunities to ask questions during decision making process.
10: The clinical elicits the patient’s preferred level of involvement in decision making.
11: The clinician indicates the need for a decision making (or deferring) stage.
12: The clinician indicates the need to review the decision (or deferment)
At time T2, there were no differences in terms of the primary outcomes, decision regret (DRS), or quality of life. Patients in the intervention group reported substantially less anxiety (d = –0.94; 95% CI: –1.42 to [–0.46]) and depression (d = –0.67; 95% CI: –1.14 to [–0.20]) than those in the control group (p<0.01). This effect was greater in breast cancer patients (d = –1.15; 95% CI: –1.81 to [–0.48]) than in colon cancer patients (d = –0.13; 95% CI: –0.84 to 0.58) (eTable 3). Sensitivity analyses yielded comparable results.
eTable 3. Results of per-protocol and intention-to-treat analyses for primary and secondary outcomes at time T2.
| Control group | Intervention group | Significance | Effect size | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Breast cancer | Colon cancer | Total | Breast cancer | Colon cancer | Total | Group | Cancer type | Interaction | Group | |
| M (SD); n | M (SD); n | M (SD); n | M (SD); n | M (SD); n | M (SD); n | p | p | p | d*4 (95% CI) | |
|
Confidence in decision*1 Per-protocol Intention-to-treat*2 |
82.4 (17.6); 49 84.4 (17.5); 117 |
n. d. a. 91.7 (13.5); 63 |
82.4 (17.6); 49 86.5 (16.9); 183 |
77.5 (17.0); 12 81.4 (15.5); 103 |
20.3 (5.8); 4 79.9 (20.3); 84 |
63.2 (29.5); 16 80.9 (17.7); 188 |
0.301 0.381 |
< 0.001*8 0.104 |
n. c. 0.174 |
−0.33 (−0.89 to 0.24) |
|
Satisfaction with decision*1 Per-protocol Intention-to-treat*2 |
86.8 (13.7); 50 86.3 (13.9); 115 |
93.3 (10.5); 17 92.0 (14.5); 64 |
88.5 (13.2); 67 88.2 (14.4); 184 |
84.2 (14.6); 12 81.9 (13.8); 99 |
80.0 (15.0); 13 82.0 (14.7); 84 |
82.0 (14.7); 25 81.5 (14.3); 182 |
0.549 0.155 |
0.100*5 0.043*2 |
0.120 0.211 |
–0.47 (−0.93 to 0.01) |
|
Decision regret*1 Per-protocol Intention-to-treat*2 |
13.4 (14.5); 48 13.2 (15.0); 123 |
11.1 (15.3); 18 9.6 (14.8); 60 |
12.8 (14.7); 66 11.6 (15.0); 183 |
17.7 (14.1); 13 16.3 (15.1); 104 |
15.0 (19.6); 13 10.6 (15.9); 84 |
16.3 (16.8); 26 13.6 (16.2); 186 |
0.388 0.324 |
0.591 0.287 |
0.961 0.636 |
0.13 (−0.32 to 0.58) |
|
HADS-Anxiety*3 Per-protocol Intention-to-treat*2 |
13.4 (1.6); 49 13.6 (2.4); 122 |
12.8 (1.7); 19 13.4 (2.5); 71 |
13.2 (1.6); 68 13.6 (2.5); 193 |
9.7 (4.8); 12 9.9 (4.3); 100 |
11.2 (4.4); 13 11.9 (3.3); 87 |
10.4 (4.6); 25 10.8 (4.0); 174 |
0.009*7 <0.001*8 |
0.630 0.776 |
0.471 0.135 |
−0.94 (−1.42 to −0.46) |
|
HADS-Depression*3 Per-protocol Intention-to-treat*2 |
7.7 (1.0); 48 7.6 (1.3); 113 |
7.5 (0.8); 19 7.6 (1.4); 65 |
7.6 (0.9); 67 7.6 (1.4); 177 |
5.3 (2.6); 12 5.8 (2.4); 106 |
7.5 (2.3); 13 7.4 (1.7); 83 |
6.4 (2.6); 25 6.5 (2.2); 187 |
<0.001*8 < 0.001*8 |
0.637 0.963 |
0.005*7 0.026*6 |
−0.67 (−1.14 to −0.20) |
|
EORTC-QLQ-30 Quality of Life*1 Per-protocol Intention-to-treat*2 |
61.8 (22.2); 46 85.9 (15.2); 118 |
67.1 (18.3); 19 84.9 (14.9); 63 |
63.3 (21.1); 65 85.4 (15.2); 178 |
56.1 (22.1); 11 85.1 (14.9); 96 |
55.1 (25.1); 13 83.5 (14.6); 85 |
55.6 (23.3); 24 84.6 (14.8); 179 |
0.446 0.869 |
0.383 0.677 |
0.567 0.953 |
−0.05 (−0.52 to 0.42) |
Full findings reported in eMethods
M, mean; SD, standard deviation; n, sample size; CI, confidence interval; n.d.a., no data available; n.c., not calculable; HADS, Hospital Anxiety and Depression Scale; EORTC-QLQ-30, Quality of Life Questionnaire.
*1Scores range from 0 to 100. 0 is low, 100 high.
*2Intention-to-treat analysis assuming the most likely scenario: missing values for the control group were estimated on the basis of available data from the control group; missing values for physicians in the intervention group who had undergone training were estimated on the basis of available data from the intervention group; missing values for physicians in the intervention group who had not undergone training were estimated on the basis of data from the control group.
*3Scores range from 0 to 21. 0 is low, 21 high for anxiety/depression.
*4Cohen’s d was estimated using means and standard deviations of the intention-to-treat analysis and sample size of the per-protocol analysis.
*5p <0.10;
*6 p <0.05;
*7 p <0.01; *8 p <0.001
Discussion
This study found no effect for shared decision making training on the primary outcomes, which were similar in both groups. However, training did contribute to improved observer-rated shared decision making skills in physicians and to less anxiety and depression in patients, particularly among women with breast cancer.
The small effect found may have been due to insufficient intensiveness and duration of training, as some studies suggest that there is a dose–effect relationship (33, 34). In addition, ceiling effects of the patient-reported outcomes used and pre-existing high quality of care in both groups may have played a role. The OPTION scores of the physicians in the control group, who did not undergo training, are comparable to those found in other noninterventional studies. The total OPTION scores of the physicians in the intervention group, in contrast, are lower than in comparable interventional studies (35). Examining the differences in individual OPTION items between the groups (Figure 2), both groups show similar score distribution, and overall the scores for the intervention group are slightly higher. The fears and expectations of patients in the two groups (items 6 and 7) were only slightly explored. This may indicate that physicians in the intervention group improved previously used shared decision making skills but had not made their conversational style substantially more patient-centered. The authors of earlier studies evaluating shared decision making training which also found no effect on patient-reported outcomes and a slight increase in shared decision making skills (18, 13, 34, 36) suspected that other factors such as length of consultation or a protected environment may have a greater effect than specific shared decision making skills (18, 36). In addition, the points of view of patients, physicians, and observers do not always coincide (37, 38). The choice of appropriate measuring tools to evaluate shared decision making interventions remains controversial. For example, patient-reported outcomes do not include which information was the basis for particular decisions. It is therefore impossible to rule out that patients made a particular treatment decision based on unrealistic expectations.
One of the greatest limitations of this study is its small sample size, in terms of both patients and physicians. This may have had an effect on the study’s randomization. Differences between the intervention group and the control group had to be verified using sensitivity analyses. Despite the great expense involved, only a small number of physicians and patients were ultimately recruited. The recruitment strategy had to be changed during the study, making it nationwide instead of local. It is also probable that individuals who were already positively disposed towards shared decision making and open to critical reflection on their own communication style were more likely to be recruited. The small sample size has made the statistical power of the study lower than planned.
Although we have not succeeded in finding effects for shared decision making training at patient level, training may have contributed to an objective improvement in the participating physicians’ shared decision making skills. Despite high general interest in shared decision making, there are many barriers preventing physicians from taking part in shared decision making training and evaluation studies. Training should therefore be flexibly tailored to physicians’ workplaces and working conditions. In future studies, study patient recruitment could be supported by study nurses, for example, in order to reduce costs. It may also be helpful for physicians to be recompensed for their additional expenditure.
We are currently conducting a follow-up study evaluating the efficacy of an e-learning platform and personalized coaching in shared decision making (39). Seminars and internships in shared decision making have also been integrated into a longitudinal communication curriculum in the revised iMED study program at the University Medical Center Hamburg-Eppendorf (abstract: Härter M, et al.: Das iMED-Curriculum am UKE. Klinische Untersuchungsmethoden und Kommunikationstraining [The iMED Curriculum at the University Medical Center Hamburg-Eppendorf: Clinical Research Methodology and Communication Training]. Abstracts on the 2014 DGPPN Congress in Berlin, 112).
Tablle 2. Findings of per-protocol and intention-to-treat analyses for primary outcomes “confidence in decision” and “satisfaction with decision” at time t1.
| Control group | Intervention group | Significance | Effect size | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Breast cancer | Colon cancer | Total | Breast cancer | Colon cancer | Total | Group | Cancer type | Interaction | Group | |
| M (SD); n | M (SD); n | M (SD); n | M (SD); n | M (SD); n | M (SD); n | p | p | p | d*3 (95% CI) | |
|
Confidence in decision*1 Per-protocol Intention-to-treat*2 |
83.2 (20.8); 51 82.5 (18.5); 142 |
77.2 (22.7); 28 78.0 (18.3); 79 |
81.0 (21.5); 79 81.0 (18.4); 224 |
83.7 (12.0); 18 84.2 (18.1); 103 |
78.7 (17.5); 24 78.3 (16.3); 85 |
80.9 (15.4); 42 81.5 (17.4); 190 |
0.925 0.740 |
0.204 0.123 |
0.891 0.955 |
0.03 (–0.35 to 0.40) |
|
Satisfaction with decision*1 Per-protocol Intention-to-treat*2 |
83.6 (17.4); 52 83.2 (15.3); 145 |
80.6 (20.9); 27 79.8 (16.8); 79 |
82.6 (18.6); 79 82.0 (15.7); 223 |
90.0 (11.0); 17 86.4 (13.9); 114 |
85.9 (12.6); 23 84.4 (13.4); 82 |
87.7 (12.0); 40 85.6 (14.1); 201 |
0.182 0.310 |
0.460 0.243 |
0.874 0.762 |
0.24 (–0.14 to 0.62) |
Full findings reported in eMethods.
M, mean; SD, standard deviation; n, sample size; CI, confidence interval; d , Cohen’s d
*1Scores range from 0 to 100. 0 is low, 100 high for satisfaction with decision/confidence in decision.
*2Intention-to-treat analysis assuming the most likely scenario: missing values for the control group were estimated on the basis of available data from the control group; missing values for physicians in the intervention group who had undergone training were estimated on the basis of available data from the intervention group; missing values for physicians in the intervention group who had not undergone training were estimated on the basis of data from the control group.
*3Cohen’s d was estimated on the basis of the means and standard deviations of the intention-to-treat analysis and sample size of the per-protocol analysis
Key Messages.
Shared decision making is becoming increasingly necessary in oncology. However, few physicians feel that they have received sufficient training to use shared decision making in everyday patient care.
Decision aids support shared decisions in oncology. However, to date insufficient research has been conducted into the efficacy of training measures.
Physicians’ shared decision making skills can be improved through specific training. They can also contribute to patients feeling less anxious and depressed.
There are many barriers to physicians’ participation in intensive shared decision making training and in evaluation studies.
Possible ways to promote physicians’ shared decision making skills include e-learning platforms, personalized coaching, and the integration of shared decision making into communication programs in medical schools.
eMethods: Table 1. Findings of per-protocol analyses at time T1.
| Control group | Intervention group | Significance (p-value) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Breast cancer | Colon cancer | Total | Breast cancer | Colon cancer | Total | ||||
| M (SD); n | M (SD); n | M (SD); n | M (SD); n | M (SD); n | M (SD); n | Group | Cancer type | Interaction | |
| SwD (AC) SwD (IC) |
83.6 (17.4); 52 83.7 (15.5); 71 |
80.6 (20.9); 27 79.9 (18.7); 34 |
82.6 (18.6); 79 82.5 (16.6); 105 |
90.0 (11.0); 17 88.2 (11.1); 22 |
85.9 (12.6); 23 85.0 (10.9); 33 |
87.7 (12.0); 40 86.3 (11.0); 55 |
0.182 0.330 |
0.460 0.307 |
0.874 0.858 |
| DCS (AC) DCS (IC) |
83.2 (20.8); 51 82.7 (18.2); 71 |
77.2 (22.7); 28 77.6 (20.6); 34 |
81.0 (21.5); 79 81.1 (19.1); 105 |
83.7 (12.0); 18 83.5 (11.0); 22 |
78.7 (17.5); 24 79.0 (15.2); 33 |
80.9 (15.4); 42 80.9 (13.7); 55 |
0.925 0.858 |
0.204 0.163 |
0.891 0.905 |
| SDM-Q-9 (AC) SDM-Q-9 (IC) |
80.3 (19.6); 48 79.5 (17.3); 71 |
72.5 (23.7); 28 70.2 (23.4); 34 |
77.4 (21.4); 76 76.5 (19.8); 105 |
72.7 (20.6); 18 72.3 (19.3); 22 |
79.4 (23.9); 24 77.2 (21.6); 33 |
76.5 (22.5); 42 75.3 (20.6); 55 |
0.378 0.507 |
0.167 0.079*1 |
0.138 0.102 |
| CARE (AC) CARE (IC) |
44.8 (5.7); 51 44.5 (5.1); 71 |
42.8 (7.9); 28 43.2 (7.3); 34 |
44.1 (6.6); 79 44.0 (5.9); 105 |
41.9 (7.8); 18 42.0 (7.2); 22 |
45.7 (3.9); 24 45.5 (3.4); 33 |
44.0 (6.1); 42 44.1 (5.4); 55 |
0.253 0.425 |
0.304 0.508 |
0.079*1 0.172 |
| HADS-Anxiety (AC) HADS-Anxiety (IC) |
8.9 (4.5); 48 9.0 (4.4); 52 |
6.4 (3.9); 27 6.5 (3.8); 28 |
8.0 (4.4); 75 8.1 (4.3); 80 |
6.9 (3.0); 18 6.9 (3.0); 18 |
7.7 (4.1); 23 7.7 (4.1); 23 |
7.3 (3.7); 41 7.3 (3.7); 41 |
0.090*1 0.067*1 |
0.020*2 0.015*2 |
0.055*1 0.049*2 |
| HADS-Depression (AC )HADS-Depression (IC) |
6.3 (4.2); 49 6.3 (4.1); 52 |
6.5 (3.9); 28 6.5 (3.9); 28 |
6.3 (4.1); 77 6.4 (4.0); 80 |
4.1 (2.8); 18 4.1 (2.8); 18 |
7.3 (5.0); 23 7.3 (5.0); 23 |
5.9 (4.4); 41 5.9 (4.4); 41 |
0.063*1 0.052*1 |
0.839 0.887 |
0.076*1 0.067*1 |
| EORTC-QLQ-30 (AC) EORTC-QLQ-30 (IC) |
59.8 (20.4); 46 57.4 (19.0); 71 |
50.9 (27.3); 28 54.2 (27.3); 34 |
56.4 (23.5); 74 56.4 (22.0); 105 |
56.0 (21.5); 18 57.2 (19.8); 22 |
41.0 (26.0); 24 43.1 (22.4); 33 |
47.7 (25.1); 42 48.7 (22.4); 55 |
0.571 0.872 |
0.128 0.905 |
0.513 0.278 |
| OPTION (AC) OPTION (IC) |
24.2 (9.9); 70 24.2 (9.9); 71 |
17.1 (5.4); 28 16.5 (5.5); 34 |
22.1 (9.4); 98 21.7 (9.4); 105 |
33.6 (11.7); 20 32.3 (11.9); 22 |
25.5 (9.9); 27 25.6 (9.5); 33 |
29.0 (11.3); 47 28.3 (10.9); 55 |
0.012*2 0.017*2 |
0.072*1 0.056*1 |
0.921 0.960 |
*1p <0.10; *2p <0.05
M, mean; SD, standard deviation; n, sample size; AC, available cases; IC, imputed cases; SwD, Satisfaction with Decision Scale; DCS, Decisional Conflict Scale (calculated as confidence in decision); SDM-Q-9, Shared Decision Making Questionnaire; CARE, Consultation and Relational Empathy Scale; HADS, Hospital Anxiety and Depression Scale; EORTC-QLQ-30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; OPTION, Observing Patient Involvement Scale
eMethods: Table 3. Findings of intention-to-treat analyses at time T1.
| Control group | Intervention group | Significance (p-value) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Breast cancer | Colon cancer | Total | Breast cancer | Colon cancer | Total | ||||
| M (SD); n | M (SD); n | M (SD); n | M (SD); n | M (SD); n | M (SD); n | Group | Cancer type | Interaction | |
| SwD (CO) SwD (NC) SwD (ML) |
83.9 (15.1); 147 83.9 (15.7); 146 83.2 (15.3); 145 |
80.4 (16.4); 78 79.8 (16.6); 76 79.8 (16.8); 79 |
82.6 (15.8); 226 82.4 (16.1); 221 82.0 (15.7); 223 |
89.8 (15.5); 115 84.5 (15.2); 113 86.4 (13.9); 114 |
85.4 (13.5); 82 81.5 (12.8); 76 84.4 (13.4); 82 |
87.3 (14.7); 199 83.3 (14.6); 194 85.6 (14.1); 201 |
0.097*1 0.672 0.310 |
0.324 0.246 0.243 |
0.975 0.933 0.762 |
| DCS (CO) DCS (NC) DCS (ML) |
82.6 (18.3); 144 83.2 (17.8); 142 82.5 (18.5); 142 |
77.7 (19.7); 75 77.3 (18.5); 80 78. 0 (18.3); 79 |
80.8 (18.7); 218 81.1 (18.3); 223 81.0 (18.4); 224 |
83.8 (17.7); 110 82.3 (17.4); 118 84.2 (18.1); 103 |
77.7 (16.5); 86 78.4 (16.2); 81 78.3 (16.3); 85 |
81.4 (17.5); 194 80.9 (17.1); 196 81.5 (17.4); 190 |
0.673 0.825 0.740 |
0.167 0.102 0.123 |
0.762 0.799 0.955 |
| SDM-Q-9 (CO) SDM-Q-9 NC) SDM-Q-9 (ML) |
75.6 (19.3); 143 74.6 (19.2); 142 75.8 (20.3); 141 |
68.1 (22.0); 75 69.6 (21.8); 78 69.3 (21.8); 77 |
72.8 (21.0); 210 72.9 (20.5); 216 73.5 (21.2); 219 |
72.0 (21.1); 120 76.7 (20.5); 120 73.8 (20.8); 112 |
72.0 (21.5); 80 67.6 (22.1); 83 70.9 (22.2); 87 |
72.0 (21.1); 196 72.8 (21.6); 201 72.0 (21.4); 194 |
0.762 0.747 0.759 |
0.228 0.231 0.182 |
0.340 0.956 0.497 |
| CARE (CO) CARE (NC) CARE (ML) |
44.5 (5.5); 142 44.2 (5.4); 142 44.3 (5.5); 142 |
43.1 (6.5); 77 43.4 (6.4); 85 43.7 (6.5); 83 |
44.1 (6.0); 217 44.0 (5.9); 221 44.1 (5.9); 222 |
42.6 (6.1); 112 43.9 (6.0); 119 43.3 (6.2); 117 |
45.2 (5.0); 89 45.0 (4.9); 84 44.6 (4.8); 86 |
43.8 (6.0); 197 44.2 (5.6); 203 43.9 (5.7); 204 |
0.225 0.700 0.545 |
0.367 0.468 0.692 |
0.073*1 0.323 0.345 |
| HADS-Anxiety (CO) HADS-Anxiety (NC) HADS-Anxiety (ML) |
9.2 (4.2); 139 9.2 (4.1); 147 9.2 (4.2); 143 |
6.6 (4.2); 86 6.8 (3.8); 80 6.7 (4.2); 73 |
8.2 (4.3); 222 8.4 (4.2); 223 8.4 (4.4); 218 |
7.3 (4.1); 116 8.8 (4.2); 113 7.9 (4.2); 116 |
8.2 (4.1); 81 7.1 (4.2); 80 7.8 (4.1); 82 |
7.8 (4.1); 201 8.2 (4.2); 186 7.9 (4.2); 201 |
0.009*3 0.576 0.054*1 |
0.002*3 <0.001*4 0.001*3 |
0.001*3 0.389 0.036*2 |
| HADS-Depression (CO) HADS-Depression (NC) HADS-Depression (ML) |
6.1 (4.1); 145 6.4 (4.1); 150 6.5 (4.3); 138 |
6.6 (4.0); 77 6.6 (4.1); 76 6.2 (4.1); 78 |
6.3 (4.0); 220 6.5 (4.2); 227 6.4 (4.3); 216 |
4.2 (4.0); 120 5.9 (4.0); 116 4.8 (4.1); 121 |
7.7 (4.4); 74 6.7 (4.5); 78 7.2 (4.4); 82 |
5.4 (4.5); 186 6.2 (4.2); 190 5.8 (4.3); 202 |
0.005*3 0.611 0.094*1 |
0.733 0.751 0.940 |
0.004*3 0.632 0.038*2 |
| EORTC-QLQ-30 (CO) EORTC-QLQ-30 (NC) EORTC-QLQ-30 (ML) |
56.7 (21.2); 142 57.7 (22.9); 145 58.2 (21.1); 139 |
52.8 (23.8); 78 55.2 (24.4); 82 55.2 (24.8); 75 |
55.5 (22.4); 224 56.6 (23.6); 231 56.8 (22.6); 210 |
54.3 (22.9); 110 56.6 (22.0); 112 55.5 (22.9); 118 |
41.4 (21.2); 77 51.4 (23.9); 80 44.3 (21.5); 81 |
49.4 (23.1); 192 54.7 (23.7); 197 50.7 (23.1); 204 |
0.661 0.915 0.619 |
0.679 0.998 0.733 |
0.219 0.586 0.303 |
| OPTION (CO) OPTION (NC) OPTION (ML) |
23.4 (9.8); 142 22.9 (9.8); 147 23.1 (9.6); 145 |
17.0 (8.8); 83 17.0 (9.3); 75 16.8 (8.3); 79 |
21.0 (9.8); 223 21.4 (10.2); 224 21.1 (9.7); 222 |
34.2 (10.1); 118 26.5 (10.6); 113 30.5 (11.3); 116 |
22.8 (9.4); 77 18.9 (10.9); 81 21.7 (10.2); 83 |
29.5 (11.5); 193 23.2 (11.3); 195 26.9 (11.6); 199 |
<0.001*4 0.154 0.016*2 |
0.038*2 0.100 0.084*1 |
0.184 0.882 0.681 |
*1p <0.10; *2p <0.05; *3p <0.01; *4p <0.001
The values stated for M, SD, and n are medians of the ten imputations performed.M, mean; SD, standard deviation; n, sample size; CO, compliant scenario (missing values for the intervention and control group were estimated on the basis of existing data from the groups to which the physicians had originally been allocated); NC, noncompliant scenario (missing values for both groups were estimated on the basis of complete data from the control group); ML, most likely scenario (missing values for the control group and physicians in the intervention group who had not undergone the intervention were estimated on the basis of complete data for the control group; missing values for physicians in the intervention group who had undergone training were estimated on the basis of complete data from the intervention group); SwD, Satisfaction with Decision Scale; DCS, Decisional Conflict Scale (calculated as confidence in decision); SDM-Q-9, Shared Decision Making Questionnaire; CARE, Consultation and Relational Empathy Scale; HADS, Hospital Anxiety and Depression Scale; EORTC-QLQ-30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; OPTION, Observing Patient Involvement Scale
eMethods: Table 4. Findings of intention-to-treat analyses at time T2.
| Control group | Intervention group | Significance (p-value) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Breast cancer | Colon cancer | Total | Breast cancer | Colon cancer | Total | ||||
| M (SD); n | M (SD); n | M (SD); n | M (SD); n | M (SD); n | M (SD); n | Group | Cancer type | Interaction | |
| SwD (CO) SwD (NC) SwD (ML) |
86.2 (14.1); 117 86.1 (14.1); 124 86.3 (13.9); 115 |
90.8 (14.3); 67 90.9 (14.9); 65 92.0 (14.5); 64 |
88.1 (14.4); 183 87.7 (14.4); 190 88.2 (14.4); 184 |
79.4 (14.5); 99 85.7 (14.5); 100 81.9 (13.8); 99 |
81.1 (14.5); 80 87.3 (15.5); 76 82.0 (14.7); 84 |
80.2 (14.6); 178 86.1 (14.9); 177 81.5 (14.3); 182 |
0.042*2 0.933 0.155 |
0.128 0.061*1 0.043*2 |
0.389 0.371 0.211 |
| DCS (CO) DCS (NC) DCS (ML) |
84.3 (17.0); 112 84.1 (17.8); 112 84.4 (17.5); 117 |
92.5 (13.9); 68 91.6 (14.1); 62 91.7 (13.5); 63 |
86.6 (16.6); 173 86.4 (17.0); 175 86.5 (16.9); 181 |
79.6 (15.1); 99 84.1 (15.3); 100 81.4 (15.5); 103 |
76.7 (19.5); 78 83.8 (20.8); 88 79.9 (20.3); 84 |
78.6 (17.1); 180 83.9 (17.9); 193 80.9 (17.7); 188 |
0.147 0.933 0.381 |
0.060*1 0.126 0.104 |
0.073*1 0.136 0.174 |
| HADS-Anxiety (CO) HADS-Anxiety (NC) ADS-Anxiety (ML) |
13.4 (2.4); 118 13.4 (2.6); 123 13.6 (2.4); 122 |
12.9 (2.4); 68 13.1 (2.5); 66 13.4 (2.5); 71 |
13.2 (2.5); 184 13.5 (2.5); 189 13.6 (2.5); 193 |
8.3 (3.4); 104 12.7 (3.9); 106 9.9 (4.3); 100 |
11.0 (3.1); 79 12.6 (3.3); 85 11.9 (3.3); 87 |
9.4 (3.6); 181 12.7 (3.6); 188 10.8 (4.0); 174 |
0.001*3 0.261 <0.001*3 |
0.551 0.765 0.776 |
0.024*2 0.787 0.135 |
| HADS-Depression (CO) HADS-Depression (NC) HADS-Depression (ML) |
7.5 (1.4); 112 7.6 (1.4); 110 7.6 (1.3); 113 |
7.6 (1.4); 66 7.5 (1.4); 65 7.6 (1.4); 65 |
7.5 (1.4); 182 7.6 (1.4); 178 7.6 (1.4); 177 |
4.9 (1.9); 105 6.9 (2.2); 107 5.8 (2.4); 106 |
7.3 (1.7); 80 7.5 (1.7); 80 7.4 (1.7); 83 |
6.0 (2.2); 188 7.2 (2.0); 186 6.5 (2.2); 187 |
<0.001*3 0.079*1 <0.001*3 |
0.994 0.749 0.963 |
<0.001*3 0.273 0.026*2 |
| EORTC-QLQ-30 (CO) EORTC-QLQ-30 (NC) EORTC-QLQ-30 (ML) |
83.6 (15.4); 115 87.6 (14.7); 117 85.9 (15.2); 118 |
82.8 (14.2); 66 86.3 (14.2); 65 84.9 (14.9); 63 |
83.5 (15.2); 181 87.1 (14.8); 181 85.4 (15.2); 178 |
84.5 (14.6); 106 88.0 (14.6); 99 85.1 (14.9); 96 |
82.5 (13.9); 84 85.1 (14.9); 86 83.5 (14.6); 85 |
83.6 (13.8); 188 86.5 (14.6); 187 84.6 (14.8); 179 |
0.946 0.996 0.869 |
0.712 0.608 0.677 |
0.890 0.946 0.953 |
| DRS (CO) DRS (NC) DRS (ML) |
12.7 (15.3); 111 12.2 (15.2); 119 13.2 (15.0); 123 |
10.2 (14.8); 64 10.0 (15.6); 66 9.6 (14.8); 60 |
11.9 (15.1); 179 11.3 (15.2); 178 11.6 (15.0); 183 |
16.8 (15.0); 102 13.9 (15.7); 112 6.3 (15.1); 104 |
11.1 (16.5); 78 11.6 (16.0); 79 10.6 (15.9); 84 |
14.4 (16.0); 179 12.3 (16.1); 185 13.6 (16.2); 186 |
0.110 0.346 0.324 |
0.414 0.530 0.287 |
0.475 0.679 0.636 |
*1p <0.10; *2p <0.05; *3p <0.01
The values stated for M, SD, and n are medians of the ten imputations performed. M, mean; SD, standard deviation; n, sample size; CO, compliant scenario (missing values for the intervention and control group were estimated on the basis of existing data from the groups to which the physicians had originally been allocated); NC, noncompliant scenario (missing values for both groups were estimated on the basis of complete data from the control group); ML: Most likely scenario (missing values for the control group and physicians in the intervention group who had not undergone the intervention were estimated on the basis of complete data for the control group; missing values for physicians in the intervention group who had undergone training were estimated on the basis of complete data from the intervention group); SwD, Satisfaction with Decision Scale; DCS, Decisional Conflict Scale (calculated as confidence in decision); HADS, Hospital Anxiety and Depression Scale; EORTC-QLQ-30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; DRS, Decision Regret Scale
Acknowledgments
Translated from the original German by Caroline Shimakawa-Devitt, M.A.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Politi MC, Studts JL, Hayslip JW. Shared decision making in oncology practice: what do oncologists need to know? Oncologist. 2012;17 doi: 10.1634/theoncologist.2011-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60:301–312. doi: 10.1016/j.pec.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;10 doi: 10.1002/14651858.CD001431.pub3. CD001431. [DOI] [PubMed] [Google Scholar]
- 4.Waljee JF, Rogers MAM, Alderman AK. Decision aids and breast cancer: do they influence choice for surgery and knowledge of treatment options? J Clin Oncol. 2007;25:1067–1073. doi: 10.1200/JCO.2006.08.5472. [DOI] [PubMed] [Google Scholar]
- 5.Whelan T, Levine M, Willan A, et al. Effect of a decision aid knowledge and treatment decision making for breast cancer surgery. A randomized trial. JAMA. 2004;292:435–441. doi: 10.1001/jama.292.4.435. [DOI] [PubMed] [Google Scholar]
- 6.Vogel BA, Helmes AW, Bengel J. Arzt-Patient-Kommunikation in der Tumorbehandlung: Erwartungen und Erfahrungen aus Patientensicht. Z Med Psychol. 2006;15:149–161. [Google Scholar]
- 7.Vogel BA, Helmes AW, Hasenburg A. Concordance between patients’ desired and actual decision making roles in breast cancer care. Psychooncology. 2008;17:182–189. doi: 10.1002/pon.1215. [DOI] [PubMed] [Google Scholar]
- 8.Légaré F, Turcotte S, Stacey D, Ratté S, Kryworuchko J, Graham ID. Patients’ perceptions of sharing in decisions. A systematic review of interventions to enhance shared decision making in routine clinical practice. Patient. 2012;5 doi: 10.2165/11592180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Légaré F, Ratté S, Stacey D, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2010 12. 2010;9 doi: 10.1002/14651858.CD006732.pub2. CD006732. [DOI] [PubMed] [Google Scholar]
- 10.Loh A, Simon D, Wills CE, Kriston L, Niebling W, Härter M. The effects of a shared decision-making intervention in primary care of depression: a cluster-randomized controlled trial. Patient Educ Couns. 2007;67:324–332. doi: 10.1016/j.pec.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Bieber C, Müller KG, Blumenstiel K, et al. Long-term effects of a shared decision-making intervention on physician-patient interaction and outcome in fibromyalgia. A qualitative and quantitative 1 year follow-up of a randomized controlled trial. Patient Educ Couns. 2006;63:357–366. doi: 10.1016/j.pec.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Bieber C, Müller KG, Blumenstiel K, et al. A shared decision-making communication training program for physicians treating fibromyalgia patients: effects of a randomized controlled trial. J Psychosom Res. 2008;64:13–20. doi: 10.1016/j.jpsychores.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Bieber C, Loh A, Ringel N, Eich W, Härter M. Manual zur Partizipativen Entscheidungsfindung (Shared Decision-making) Heidelberg: Selbstverlag; 2007. Patientenbeteiligung bei medizinischen Entscheidungen. [Google Scholar]
- 14.Loh A, Simon D, Rockenbauch K, Härter M. Partizipative Entscheidungsfindung - Stellenwert und Verbreitung in der medizinischen Ausbildung. Z Med Psychol. 2006;15 8:7–92. [Google Scholar]
- 15.Hölzel L, Vollmer M, Kriston L, Siegel A, Härter M. [Patient participation in medical decision making within an integrated health care system in Germany: results of a controlled cohort study] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55:1524–1533. doi: 10.1007/s00103-012-1567-3. [DOI] [PubMed] [Google Scholar]
- 16.Bieber C, Nicolai J, Hartmann M, et al. Training physicians in shared decision-making-who can be reached and what is achieved? Patient Educ Couns. 2009;77:48–54. doi: 10.1016/j.pec.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Gaissmaier W, Gigerenzer G. Statistical illiteracy undermines informed shared decision making. Ger J Evid Qual Heal Care. 2008;102:411–413. doi: 10.1016/j.zefq.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Elwyn G, Edwards A, Hood K, et al. Achieving involvement: process outcomes from a cluster randomized trial of shared decision making skill development and use of risk communication aids in general practice. Fam Pract. 2004;21:337–346. doi: 10.1093/fampra/cmh401. [DOI] [PubMed] [Google Scholar]
- 19.Baker SK, Mayer DK, Esposito N. The contralateral prophylactic mastectomy decision-making process. Plast Surg Nurs. 2013;33:11–21. doi: 10.1097/PSN.0b013e3182842424. [DOI] [PubMed] [Google Scholar]
- 20.Connor AM. Decisional Conflict Scale 1993 (updated 2005) https://decisionaid.ohri.ca/eval_dcs.html. (last accessed on 10 July 2015) [Google Scholar]
- 21.Buchholz A, Hölzel L, Kriston L, Simon D, Härter M. Die Decisional Conflict Scale in deutscher Sprache (DCS-D) - Dimensionale Struktur in einer Stichprobe von Hausarztpatienten. Klin Diagnostik und Eval. 2011;4:15–30. [Google Scholar]
- 22.Holmes-Rovner M, Kroll J, Schmitt N, et al. Patient satisfaction with health care decisions: the satisfaction with decision scale. Med Decis Mak. 1996;16:58–64. doi: 10.1177/0272989X9601600114. [DOI] [PubMed] [Google Scholar]
- 23.Kriston L, Scholl I, Hölzel L, Simon D, Loh A, Härter M. The 9-item Shared Decision Making Questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample. Patient Educ Couns. 2010;80:94–99. doi: 10.1016/j.pec.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 24.Neumann M, Wirtz M, Bollschweiler E, Warm M, Wolf J, Pfaff H. Psychometrische Evaluation der deutschen Version des Messinstruments “Consultation and Relational Empathy” (CARE) am Beispiel von Krebspatienten. Psychother Psychosom Med Psychol. 2008;58:5–15. doi: 10.1055/s-2007-970791. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann-Lingen C, Buss U, Snaith RP. Hospital Anxiety and Depression Scale - Deutsche Version. Bern PN>Verlag Hans Huber: 2005. HADS-D. [Google Scholar]
- 26.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 27.Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Mak. 2003;23:281–292. doi: 10.1177/0272989X03256005. [DOI] [PubMed] [Google Scholar]
- 28.Elwyn G, Hutchings H, Edwards A, et al. The OPTION scale: measuring the extent that clinicians involve patients in decision-making tasks. Health Expect. 2005;8:34–42. doi: 10.1111/j.1369-7625.2004.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman D, Bland J. Treatment allocation by minimisation. BMJ. 2005;330 doi: 10.1136/bmj.330.7495.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayes RJ, Bennett S. Simple sample size calculation for cluster randomized trials. Int J Epidemiol. 1999;28:319–326. doi: 10.1093/ije/28.2.319. [DOI] [PubMed] [Google Scholar]
- 31.R Core Team. www.r-project.org. Vienna, Austria: R Foundation for Statistical Computing; 2012. A language and environment for statistical computing. (last accessed on 12 July 2015) [Google Scholar]
- 32.Bates DM. Luxemburg, Berlin: Springer; 2010. lme4: Mixed-effects modeling with rle. [Google Scholar]
- 33.Bernhard J, Butow P, Aldridge J, Juraskova I, Ribi K, Brown R. Communication about standard treatment options and clinical trials: can we teach doctors new skills to improve patient outcomes? Psychooncology. 2012;21:1265–1274. doi: 10.1002/pon.2044. [DOI] [PubMed] [Google Scholar]
- 34.Butow P, Brown R, Aldridge J, et al. Can consultation skills training change doctors’ behaviour to increase involvement of patients in making decisions about standard treatment and clinical trials: a random-ized controlled trial. Health Expect. 2014 doi: 10.1111/hex.12229. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Couët N, Desroches S, Robitaille H, et al. Assessments of the extent to which health-care providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect Mar. 2013 doi: 10.1111/hex.12054. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards A, Elwyn G, Hood K, et al. Patient-based outcome results from a cluster randomized controlled trial of shared decision making skill development and use of risk communication aids in general practice. Fam Pract. 2004;21:345–352. doi: 10.1093/fampra/cmh402. [DOI] [PubMed] [Google Scholar]
- 37.Scholl I, Kriston L, Dirmaier J, Härter M. Comparing the nine-item shared decision-making questionnaire to the OPTION Scale—an attempt to establish convergent validity. Health Expect. 2015;18:137–150. doi: 10.1111/hex.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasper J, Heesen C, Köpke S, Fulcher G, Geiger F. Patients and observers perceptions of involvement differ. Validation study on inter-relating measures for shared decision making. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bieber C, Bergelt C, Nicolai J, Nikendei C, Eich W, Härter M. Randomisiert-kontrollierte Studie (RCT) zu innovativen Disseminationsstrate_gien einer Shared Decision Making (SDM)-Kurzintervention für Onkologen: Web-basiertes SDM-Online-Training und individualisiertes, kontextbezogenes SDM-Einzel-Training. Abstractband zur 13. PSO-Jahrestagung in Heidelberg. 2014;18 [Google Scholar]
- 40.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]